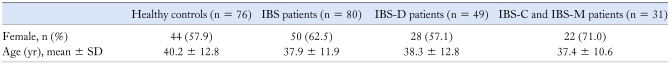Abstract
Introduction
Cholecystokinin (CCK) belongs to a group of endogenous molecules known as brain-gut neuropeptides and functions as a neuropeptide as well as a gut hormone. It remains unclear whether genetic variation of the CCK receptor plays a role in irritable bowel syndrome (IBS). The aim of this study was to determine and compare the allele and genotype frequencies of the CCK1 receptor polymorphisms between healthy controls and patients with IBS.
Methods
Genotyping of 80 patients with IBS (who met the Rome III criteria) and 76 healthy controls was performed. We performed PCR amplification for the CCK1 receptor intron 1 779 T > C and Exon 1 G > A. We confirmed polymorphisms by direct sequencing method.
Results
There was a significantly different trend for genotypic distributions of the CCK1 receptor polymorphism between patients with IBS and healthy controls (p for trend = 0.048). The CCK1 receptor intron 1 779 T >C polymorphic type was more common in patients with 'IBS-constipation predominant (IBS-C) and IBS-mixed (IBS-M) forms' (19/31, 61.3%) than healthy controls 32/76, 42.1% adjusted odd ratio 2.43, 95% Confidence interval 1.01-5.86). The genotypic distributions of the CCK1 receptor exon 1 polymorphism were not significantly different between the two groups (p for trend = 0.223).
Conclusions
CCK1 receptor polymorphisms were associated with IBS. In particular, the CCK1 receptor intron 1 779 T > C polymorphic type was associated with 'IBS-C and IBS-M'. Further studies are needed in larger number of patients with an even distribution of IBS subtypes.
Keywords: Irritable bowel syndromes, Receptor, Cholecystokinin, Polymorphism, Genetic
Introduction
Irritable bowel syndrome (IBS) is characterized by the presence of abdominal discomfort or pain associated with disturbed defecation. IBS is important because of its high prevalence, substantial morbidity, and enormous cost. In clinical practice of gastroenterology, more than one third of the patients have functional gastrointestinal disorders, and IBS is the most common diagnosis.1 However, the etiology and physiology of IBS are not fully understood. A number of different mechanisms have been implicated in the pathogenesis of IBS including abnormal motility, visceral hypersensitivity, low-grade inflammation, and stress.2 No single pathophysiological mechanism explains the clinical manifestation of IBS entirely. Current evidence suggests that an altered brain-gut axis is the key mechanism associated with disordered motility, visceral hypersensitivity, and autonomic dysfunction. Regulation of these connections occurs via numerous neurotransmitters such as cholecystokinin (CCK), vasoactive intestinal peptide (VIP), substance P, serotonin, and corticotrophin releasing hormone (CRH).3 Cholecystokinin (CCK) is a neuropeptide released by endocrine I cells within the duodenal and jejunal mucosa in response to a variety of nutrients, notably the digestion products of fat, and its secretion is associated with contraction of the gallbladder, pancreas enzyme secretion, and inhibition of gastric emptying.4 Two receptor subtypes of CCK have been identified, CCK1 and CCK2, which were initially classified pharmacologically on the basis of their affinity for the endogenous peptides CCK and gastrin and several selective non-peptide antagonists.5 Both CCK1 and CCK2 belong to a single family of G-coupled receptors (GPCRs): the CCK1 is predominantly located in the gastrointestinal (GI) track (previously called CCKA, where A = alimentary), whereas the CCK2 receptor is distributed widely throughout the central nervous system (CNS) (previously called CCKB, where B = brain).6 Blocking of the CCK1 receptors in the GI tract has been proposed as a novel approach to stimulate gut motility and to change colonic transit time in patients with constipation-predominant functional bowel disorders.7 CCK1 receptor polymorphism in IBS patients with constipation has been associated with slower gastric emptying.7 However, it remains unclear whether genetic variation of the CCK receptor plays a role in the pathogenesis of IBS. Thus, the aim of this study was to determine and compare the allele and genotype frequencies for the CCK1 receptor polymorphism between healthy controls and patients with IBS.
Materials and Methods
1. Subjects
This was a case-control study. The subjects in this study were recruited between March 2007 and February 2008. Eighty outpatients with IBS, who were referred to a tertiary hospital and 76 healthy controls, who visited the health promotion center for a routine checkup were enrolled. The subjects with IBS were diagnosed according to the Rome III criteria.8 Healthy controls had no prior history of disease or symptoms related to the gastrointestinal system in the study. IBS subjects were excluded if they had an unstable medical disorder, another gastrointestinal disorder, a major psychiatric disorder, or a history of substance abuse within the previous two years.
This study was approved by the institutional review board of the Chonnam National University Hospital, Gwangju, Korea. All subjects were provided with written information on the project, and informed consent was obtained as well.
2. Genotyping
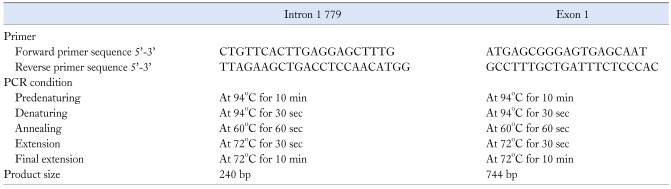
Venous blood drawn from a forearm vein was stored at -70℃. DNA was extracted from whole blood by the alkaline lysis method using the QIAamp DNA Midi Kit (Qiagen Inc., Valencia, CA, USA). We examined the DNA for the CCK1 receptor polymorphisms in intron 1 and exon 1. We performed PCR amplification using GeneAmp 2700 for the CCK1 receptor intron 1 779 T > C and Exon 1 G > A. The forward primers and reverse primers used for the CCK1 receptor intron 1 779 T > C were 5'-CTG TTC ACT TGA GGA GCT TTG-3' and 5'-TTA GAA ACT GAC CTC CAA CAT GG-3'. The forward primers and reverse primers used for Exon 1 G > A were 5'-ATG AGC CGG GAG TGA GCA AT-3' and 5'-GCC TTT GCT GAT TTC TCC CAC-3' (GeneBank accession number #U23427). Amplification of intron 1 779 T > C and Exon 1 G > A was performed in a 25 reaction volume containing 100 ng of genomic DNA, Tris-HCl (pH 9.0), (NH4)2SO4, 20 mM MgCl2, PCR enhancers in reaction buffer 2.5 µL, DW 16.5 µL, and Exprime Taq polymerase (Genet BIO Teageon, Korea) 0.5 µL. DNA was predenatured at 94℃ for 10 min, and subjected to 35 cycles of 30 sec of denaturation at 94℃, 1 min of annealing at 60℃, 30 sec of extension at 72℃, and 10 min of final extension at 72℃ (Table 1). The amplification products were resolved by electrophoresis on 1.8% SeaKem LE agarose (Cambrex Bio Science Rockland, Inc., Rockland, ME, USA) next to a DNA molecular weight standard marker 100 bp ladder (TAKARA BIO Inc., Otsu, Shiga, Japan) and visualized with ethidium bromide staining. We confirmed polymorphisms by direct sequencing with ABI version 3.1 Sequence Analysis software.
Table 1.
Nucleotide Sequences for Assay Primer and PCR Condition for Polymorphisms in the CCK1 Receptor Polymorphisms
3. Statistical methods
Statistical analysis was performed using the SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). The statistical significance of the differences between the subjects with IBS and healthy controls was estimated by logistic regression analysis. Adjusted odds ratios (aORs) were calculated with a logistic regression model that were controlled by gender and age with 95% confidence intervals (CI). A p < 0.05 was considered as the level of significance. Mann-Whitney U test were used to determine whether there were difference in the demographic data of the IBS patients and healthy controls. Subjects with wild type genotypes (intron 779 TT, exon 1 GG) were considered to be at baseline risk. The expected frequency of healthy control's genotypes was assessed by Hardy-Weinberg equilibrium test.9 Linkage disequilibrium was calculated using the SNP Analyzer program (Istech Ltd., Goyang-si, Korea).
Results
1. Clinical characteristics of the study population
In the IBS patients, the median age was 35 years (range 22-67 years) and female patients comprised 62.5%. In healthy controls, the median age was 38 years (range 22-69 years) and female subjects comprised 57.9%. There were no statistical difference between the IBS patients and healthy controls in the age or sex (p > 0.05) (Table 2). Forty-nine of the IBS subjects had a diarrhea-predominant bowel pattern (IBS-D), 15 had a constipation-predominant bowel pattern (IBS-C), and 16 had a mixed pattern (IBS-M).
Table 2.
Clinical Characteristics of the IBS Patients and Healthy Controls
IBS, irritable bowel syndrome; IBS-D, IBS with diarrhea-predominant pattern; IBS-C, IBS with constipation-predominant bowel pattern; IBS-M, IBS with mixed pattern.
2. CCK1 receptor intron 1 779 T > C and exon 1 G > A polymorphism in the IBS patients and in healthy controls
The distribution of the CCK1 receptor intron 779 and exon 1 polymorphisms in healthy controls was in Hardy-Weinberg equilibrium (Intron 779; p = 0.505, exon 1; p = 0.496).
1) CCK1 receptor intron 1 779 T > C polymorphism in the IBS patients and in healthy controls
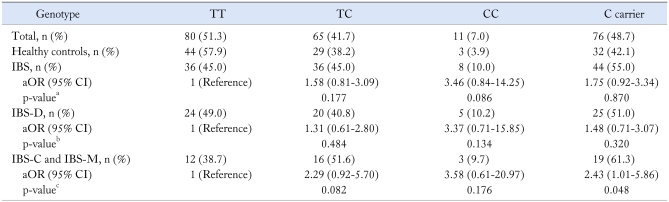
Table 3 shows the genotype distributions and allele frequencies of the CCK1 receptor intron 1 779 T > C polymorphism in the IBS patients and healthy controls. There was a significantly different trend for genotypic distributions of CCK1 receptor intron 1 779 T > C polymorphism between the IBS patients and healthy controls (p for trend = 0.048). However, no significant difference was observed in C carrier between the two groups (p = 0.870). The TC genotype was 45% (36/80) in IBS patients and 38.2% (29/76) in controls (p = 0.177, aOR 1.58, 95% CI 0.81-3.09). The CC genotype was 10.0% (8/80) in IBS patients and 3.9% (3/76) in healthy control (p = 0.086, aOR 3.46, 95% CI 0.84-14.25).
Table 3.
Genotype Frequency for CCK1 Receptor Intron 1 779 T > C in the IBS Patients and in Healthy Controls
ap for trend = 0.048, bp for trend = 0.151, cp for trend = 0.045.
CCK, cholecystokinin; IBS, irritable bowel syndrome; IBS, irritable bowel syndrome; IBS-D, IBS with diarrhea-predominant pattern; IBS-C, IBS with constipation-predominant bowel pattern; IBS-M, IBS with mixed pattern; OR, odds ratio; CI, confidence interval.
OR and 95% CI were estimated using multiple logistic regression and adjusted for sex and age.
The CCK1 receptor intron 1 779 T > C polymorphic type (TC genotype and CC genotype) was more common in patients with 'Non IBS-D (IBS-C and IBS-M)' (19/31, 61.3%) than it was in healthy controls (32/76, 42.1%, aOR 2.43 95% CI 1.01-5.86) (Table 3).
2) CCK1 receptor exon 1 G > A polymorphism in the IBS patients and in healthy controls
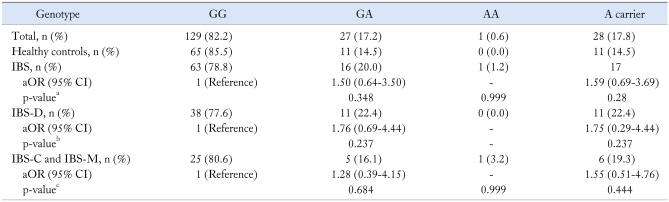
The genotypic distributions (p for trend = 0.223) and A carrier frequencies (p = 0.28) of the CCK1 receptor exon 1 polymorphism were not significantly different between the two groups (Table 4).
Table 4.
Genotype Frequency for CCK1 Receptor Exon 1 G > A in the IBS Patients and in Healthy Controls
ap for trend = 0.223, bp for trend = 0.237, cp for trend = 0.269.
CCK, cholecystokinin; IBS, irritable bowel syndrome; IBS-D, IBS with diarrhea-predominant pattern; IBS-C, IBS with constipation-predominant bowel pattern; IBS-M, IBS with mixed pattern; OR, odds ratio; CI, confidence interval.
OR and 95% CI were estimated using multiple logistic regression and adjusted for sex and age.
Discussion
CCK belongs to the group of endogenous molecules known as 'brain-gut neuropeptides' and, due to its anatomical location, it functions as a neuropeptide as well as a gut hormone. CCK is also found in the nerves of the circular muscle layer of the colon, to some extent in the ileum, and it is abundant in the celiac plexus and vagus nerves.10 Due to these multiple anatomical locations it is not surprising that CCK has been implicated in different physiological functions: gallbladder contraction and pancreatic enzyme secretion, as well as motor and sensory functions at various levels of the gastrointestinal tract such as gastric emptying and colonic motility.11 The role of CCK was first reported by Harvey et al. who have observed CCK to cause an abnormal increase in colonic motility in patients with IBS.12 Further studies have shown that the release of CCK, induced by a fatty meal, acts as a mediator of the gastrocolonic response.13 CCK infusion appears to unmask intestinal dysmotility in patients with IBS.14 Intraduodenal lipids increased visceral sensitivity in both IBS-D and IBS-C patients suggesting a role for CCK at the neuronal level. In patients with functional abdominal pain syndromes, infusion of CCK8 correlate with significantly higher pain scores compared to control subjects.15 Therefore, the promising potential use of CCK1 antagonists for the treatment of a variety of functional gastrointestinal disorders including IBS will be expected.
Recent reports in human subjects have shown an association between CCK1 receptor gene polymorphism and schizophrenia,16 alcohol dependence,17 panic disorder,18 gallstone disease,19 and postprandial dyspepsia.20 However, there have been no reports on the association between the CCK1 receptor polymorphism and IBS. The results of present study suggest that the CCK1 receptor intron 1 779 T > C polymorphism may be functionally important, since the genotypic distribution of the CCK1 receptor intron 1 779 T > C polymorphism in IBS patients had a significantly different trend compared to that in healthy controls (p for trend = 0.048). Moreover, the findings of this study showed that the CCK1 receptor intron 1 779 T > C polymorphic type (TC genotype and CC genotype) was associated with an increased odds ratio for 'non-IBS-D (IBS-C and IBS-M)' (aOR 2.43, 95% CI 1.01-5.86). However, we did not find any association between the CCK1 receptor intron 1 779 T > C polymorphic type and patients with IBS-D, implying that the genetic variation was only associated with IBS patients of 'non IBS-D' type from our study. In a study of Caucasian patients, the CCK1 receptor intron 1 779 T > C polymorphism in patients with IBS-C, was associated with slower gastric emptying. This suggested that, compared to the 779 T variant, the 779 C substitution resulted in an increased response to endogenous CCK (like CCK agonist), which was followed by delaying gastric emptying.7 From the viewpoint of sensory functions at various levels of the gastrointestinal tract, our results are consistent with the original hypothesis that suggested that the CCK1 receptor intron 1 779 T > C polymorphic type might have an increased response to endogenous CCK and be associated with visceral hypersensitivity. However, from the viewpoint of colon motility, present study showed an opposite trend to our expectation that the CCK1 779 C substitution would be related with accelerating colon transit time, like CCK agonist. Therefore, characterization of the CCK1 receptor polymorphism and its clinical significance require further study.
The limitations of the present study include the following. First, this study was limited by the small sample size and uneven distribution of IBS subtypes. Second, present study did not show clinical significance for the CCK1 receptor polymorphism in regard to visceral hypersensitivity and colon motility. Further studies are needed with larger patient samples with an even distribution of IBS subtypes. However, our findings suggest that the CCK1 intron 1 779 T > C polymorphism might have a significant association with IBS.
The presence of CCK1 receptor polymorphisms may indeed provide new opportunities for predicting how patients will respond to a particular treatment according to their genetic profile, and therefore may have an impact on current management strategies. Therefore, pharmacogenomic studies on CCK1 receptor polymorphisms and the CCK antagonist might be of significant interest and potential clinical relevance.
In summary, we have shown that CCK1 receptor polymorphisms are associated with IBS. In particular, the CCK1 receptor intron 1 779 T > C polymorphic type was associated with non-IBS-D. However, further studies are needed in larger patient samples with an even distribution of IBS subtypes. In addition, further studies for the characterization of CCK1 receptor polymorphisms and understanding of its clinical significance by associations with clinical outcomes are also needed.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Feldman M, Friedman LS, Brandt LJ. Irritable blowel syndrome. In: Nicholas J, editor. Sleisenger & Fordtran's gastrointestinal and liver disease. 8th ed. Volume 2. Philadelphia: Elsevier; 2006. pp. 2633–2702. [Google Scholar]
- 2.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 3.Hotoleanu C, Popp R, Trifa AP, Nedelcu L, Dumitrascu DL. Genetic determination of irritable bowel syndrome. World J Gastroenterol. 2008;14:6636–6640. doi: 10.3748/wjg.14.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990;99:409–415. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- 5.Noble F, Wank SA, Crawley JN, et al. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- 6.Peter SA, D'Amato M, Beglinger C. CCK1 antagonists: are they ready for clinical use? Dig Dis. 2006;24:70–82. doi: 10.1159/000090310. [DOI] [PubMed] [Google Scholar]
- 7.Cremonini F, Camilleri M, McKinzie S, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am J Gastroenterol. 2005;100:652–663. doi: 10.1111/j.1572-0241.2005.41081.x. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Lin DY, Zeng D, Millikan R. Maximum likelihood estimation of haplotype effects and haplotype-environment interactions in association studies. Genet Epidemiol. 2005;29:299–312. doi: 10.1002/gepi.20098. [DOI] [PubMed] [Google Scholar]
- 10.Liddle RA. Cholecystokinin. In: Walsh JH, Dockray GJ, editors. Gut Peptides. Biochemistry and physiology. New York: Raven; 1994. p. 175. [Google Scholar]
- 11.Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 12.Harvey RF, Read AE. Effect of cholecystokinin on colonic motility and symptoms in patients with the irritable-bowel syndrome. Lancet. 1973;1:1–3. doi: 10.1016/s0140-6736(73)91219-1. [DOI] [PubMed] [Google Scholar]
- 13.Renny A, Snape WJ, Jr, Sun EA, London R, Cohen S. Role of cholecystokinin in the gastrocolonic response to a fat meal. Gastroenterology. 1983;85:17–21. [PubMed] [Google Scholar]
- 14.Kellow JE, Phillips SF, Miller LJ, Zinsmeister AR. Dysmotility of the small intestine in irritable bowel syndrome. Gut. 1988;29:1236–1243. doi: 10.1136/gut.29.9.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts-Thomson IC, Fettman MJ, Jonsson JR, Frewin DB. Responses to cholecystokinin octapeptide in patients with functional abdominal pain syndromes. J Gastroenterol Hepatol. 1992;7:293–297. doi: 10.1111/j.1440-1746.1992.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 16.Minato T, Tochigi M, Kato N, Sasaki T. Association study between the cholecystokinin A receptor gene and schizophrenia in the Japanese population. Psychiatr Genet. 2007;17:117–119. doi: 10.1097/YPG.0b013e328011c02e. [DOI] [PubMed] [Google Scholar]
- 17.Miyasaka K, Yoshida Y, Matsushita S, et al. Association of cholecystokinin-A receptor gene polymorphism with alcohol dependence in a Japanese population. Alcohol Alcohol. 2004;39:25–28. doi: 10.1093/alcalc/agh002. [DOI] [PubMed] [Google Scholar]
- 18.Miyasaka K, Yoshida Y, Matsushita S, et al. Association of cholecystokinin-A receptor gene polymorphisms and panic disorder in Japanese. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:78–80. doi: 10.1002/ajmg.b.20160. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava A, Pandey SN, Dixit M, Choudhuri G, Mittal B. Cholecystokinin receptor A gene polymorphism in gallstone disease and gallbladder cancer. J Gastroenterol Hepatol. 2008;23:970–975. doi: 10.1111/j.1440-1746.2007.05170.x. [DOI] [PubMed] [Google Scholar]
- 20.Tahara T, Arisawa T, Shibata T, et al. 779 TC of CCK-1 intron 1 is associated with postprandial syndrome (PDS) in Japanese male subjects. Hepatogastroenterology. 2009;56:1245–1248. [PubMed] [Google Scholar]