Abstract
We have described a line of VH “knockin” mice termed HKIR in which the transgenic Igh locus partially encodes “dual reactive” anti-chromatin and anti-arsonate (Ars) BCRs. HKIR B cells termed canonical, expressing a particular Vκ light chain, evade central tolerance by down regulating BCR levels. Canonical HKIR B cells can be recruited into the primary GC and AFC compartments via Ars immunization. However, their participation in the GC response rapidly wanes and they do not efficiently contribute to the memory compartment, indicating they are regulated by a GC tolerance checkpoint. We analyzed the influence of the Sle1 genetic interval, shown to break tolerance of chromatin reactive B cells, on the behavior of HKIR B cells during the anti-Ars response. Canonical B cells from congenic HKIR.Sle1 mice gave rise to elevated short and long-lived AFC responses, and the attenuated GC and memory responses characteristic of these B cells were relieved in adoptive, wild type recipients. HKIR GC B cells containing Sle1 expressed increased levels of Bcl-2 and c-FLIP and decreased levels of Fas RNA as compared to HKIR controls, suggesting direct alteration of the regulation of the GC response by Sle1. High titers of canonical and anti-dsDNA antibodies spontaneously developed in many aged HKIR.Sle1 mice. Together, these data indicate that Sle1 perturbs the action of peripheral tolerance checkpoints operative on antinuclear antigen B cells in both the AFC and GC pathways in a cell autonomous fashion.
Keywords: Rodent, B cells, autoimmunity, Systemic Lupus Erythematosus, antibody forming cell response, germinal center response
INTRODUCTION
During their development, B cells expressing B cell antigen receptors (BCRs) reactive to auto antigens may be deleted in the bone marrow by apoptosis (1, 2) undergo receptor editing (3, 4) or become anergic (5-7) processes that play key roles in tolerance. However, not all autoreactive B cells are eliminated by these central tolerance mechanisms, as the mature peripheral B cell pool contains multi-reactive B cells, that are cross-reactive with auto antigens (8, 9). B cells with BCRs that are “dual reactive” for both auto and foreign antigens could be recruited into a foreign-antigen driven immune response. In addition, the variable (V) regions of immunoglobulin (Ig) genes undergo somatic hypermutation in germinal centers (GCs) during T-dependent (TD) immune responses, resulting in de novo generation of autoreactive B cells (10, 11). Therefore, it has been postulated that tolerance mechanisms must operate during primary peripheral B cell development and B cell immune responses (6, 12, 13). Studies of auto reactive BCR transgenic mouse models (14-18) and in humans (19-21) have supported the existence of such peripheral tolerance mechanisms, but these are as yet not well defined.
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by the production of anti-nuclear antibodies (ANAs) affecting multiple organs. The NZB/NZW-derived NZM2410 mouse strain develops a disease that resembles human SLE. Genetic linkage studies on this strain have indicated that lupus pathogenesis is a multi-step and multi-factorial process. Three major genomic intervals for autoimmune susceptibility (Sle1, Sle2 and Sle3/Sle5) were identified in the NZM2410 strain (22). C57BL/6 (B6) mice congenic for each of these loci exhibit different component phenotypes (23-25). For instance, B6.Sle1 mice spontaneously develop high titers of ANAs but these can mediate high penetrance of severe glomerulonephritis only in combination with other susceptibility loci (Sle2, Sle3/Sle5, Yaa or lpr) (26). Congenic recombination of the Sle1 locus has resulted in three sub-loci named Sle1a, Sle1b and Sle1c (27). The presence of each of these sub loci alone in B6 mice results in only partial autoimmune phenotypes, with the Sle1b sub region appearing to be primarily responsible for loss of B cell tolerance to nuclear autoantigens (28).
We have utilized an immunoglobulin (Ig) variable heavy chain (VH) knock-in line termed HKIR (29, 30) that generates DNA and chromatin-reactive B cells to study peripheral B cell tolerance checkpoints. The HKIR VH transgene, in combination with a single endogenous κ light (L) chain gene, encodes BCRs with specificity for both the hapten arsonate (Ars) and nuclear autoAgs. We term these dual-reactive B cells “canonical”. Whereas ANA B cells in other BCR transgenic models such as 3H9 (anti-chromatin) and 2-12H (anti-Smith/ssDNA) undergo receptor editing or anergy (3, 31) HKIR B cells escape these fates by down regulating their BCRs, resulting in reduced avidity for nuclear autoantigens (29, 30). These B cells develop to mature follicular phenotype (13, 29, 30) and stably reside in the follicles of peripheral lymphoid organs. Therefore, the HKIR model allows us to study the role and mechanisms of peripheral tolerance checkpoints in regulation of ANA B cell activity.
Due to their dual reactivity, canonical HKIR B cells can be recruited into the GC and AFC responses via immunization with Ars-conjugated to foreign Ag. However, canonical HKIR B cells participate in the early but not the late GC response and do not efficiently seed the memory B cell compartment, suggesting that these cells are regulated by GC/memory tolerance checkpoints (12, 13). To investigate the factors operative in these checkpoints, we previously evaluated the influence of intrinsic deficiencies of the inhibitory Fc receptor FcγRIIB, and the Fas death receptor on canonical HKIR B cell participation in the GC/memory B cell pathway (13, 32). The FcγRIIB deficiency increased the participation of canonical HKIR B cells in the primary AFC response, but neither deficiency augmented the participation of these B cells in the late GC or memory responses.
We also previously showed that in B6 mice congenic for the Sle1 genomic interval, GC B cells fail to up regulate the expression of FcγRIIB, as takes place in non autoimmune-prone strains of mice (33). In subsequent studies, we demonstrated that this failed up regulation mapped to a small sub interval of the Sle1 locus containing the NZW allele of the FcγRIIB gene (34). B6 mice congenic for an Sle1 sub interval including this NZW FcγRIIB allele and much of the Sle1a sub interval, but lacking any contribution from the Sle1b sub interval, displayed enhanced primary AFC responses, thus phenocopying B6.FcγRIIB and HKIR.FcγRIIB deficient B cells in this regard (13). Given these results, we wished to determine if the presence of the NZW FcγRIIB allele in canonical HKIR B cells increased their participation in the late GC and memory B cell responses. However, given our past data showing that a complete FcγRIIB deficiency did not result in such rescue (13), combined with previous results showing that the Sle1b subinterval appears primarily responsible for loss of tolerance to nuclear auto antigens (28), we elected to first evaluate the influence of the entire Sle1 genomic interval on participation of canonical HKIR B cells in the GC and memory B cell responses.
As such, we generated HKIR.Sle1 mice on a B6 background and analyzed the primary development and Ars-KLH driven immune response of their B cells. No major influence of Sle1 on the primary development of these B cells was detected. However, when transferred into syngenic normal mice, Sle1-bearing canonical HKIR B cells gave rise to enhanced primary anti-Ars AFC responses. More interestingly, the attenuated late anti-Ars GC and memory responses characteristic of HKIR B cells were reversed when these B cells contained Sle1. In addition, we observed high serum titers of spontaneously produced anti-dsDNA and canonical antibodies in aged HKIR mice bearing Sle1. Taken together, our results indicate that the presence of the Sle1 interval perturbs both AFC and GC/memory tolerance checkpoints normally operative on ANA B cells, and that these alterations function in a cell autonomous fashion. These data are the first to show that a lupus susceptibility locus can alter GC/memory tolerance checkpoints in this manner.
Materials and Methods
Mice
C57BL/6 (B6) and C57BL/6.SJL-Ptprca Pepcb/BoyJ (B6.CD45.1) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and then bred in house. The immunoglobulin (Ig) variable heavy chain (VH) knock-in mouse lines HKI65 and HKIR were described previously (29, 30). B6.Sle1 mice were also described previously (35). All mice were maintained in a pathogen-free barrier facility, given only autoclaved food and water and were 7-9 weeks old when used in all experiments except the aging studies. These studies have been reviewed and approved by an appropriate institutional review committee.
Adoptive transfer and immunizations
Two adoptive transfer protocols were used. In one protocol, B6.CD45.1 or B6 recipient mice were immunized i.p. with 100 μg of Ars-KLH (in alum) one week prior to transfer (i.v., in PBS) of splenic B cells from transgenic donor mice. Chimeric mice were then injected i.p. with 50 μg of Ars-KLH in PBS immediately after cell transfer. In another protocol, recipient mice were immunized with 100 μg of Ars-KLH in alum i.p. 12-24 hrs after transfer of splenic B cells from transgenic mice. For secondary immune responses, mice were boosted 6-8 weeks after cell transfer and primary immunization following the second protocol. In all experiments, donor B cells were MACS purified using either the E4 mAb, specific for canonical BCRs, or an anti-B220 mAb.
Antibodies and other reagents
Antibodies and other reagents used for flow cytometry and immunohistology included: FITC-GL7; PE, FITC and PE-Texas red-anti-B220 (RA3-6B2); PE-anti-IgD (11-26); streptavidin-CyChrome; FITC-anti-CD21/35 (7G6); PE-anti-CD23 (B3B4); PE-anti-TCR beta; PE-anti-CD69 (H1.2F3); PE-anti-CD80 (16-10A1); FITC-anti-CD86 (GL-1, BD Pharmingen, San Diego, CA); FITC-MOMA-1 (Serotec, Raleigh, NC); biotin-anti-IgD (11-26, Southern Biotechnology Associates, Birmingham, AL); PE and biotin-anti-mouse CD45.2 (104, eBioscience, San Diego, CA ); streptavidin (SA)-PE; SA-alexafluor 633 (Molecular probes, Eugene, OR); FITC-PNA; FITC-donkey anti-mouse IgM (Jackson Immunoresearch Laboratories, West Grove, PA) and a biotinylated form of the anti-idiotypic mAb E4 (prepared in-house).
Immunohistology
Spleen cryostat sections (5-6 microns) were prepared as described (36). Immunohistology was performed using the Abs listed above and the stained sections were analyzed using a fluorescence microscopy (Leica microsystems) and images were captured as described (37).
Flow cytometry
Three and four color flow cytometric analysis was done on cell suspensions prepared from spleens of naïve and immunized mice stained with multiple combinations of the Abs listed above. Biotinylated Abs were detected with streptavidin-CyChrome. Stained cells were analyzed using a Coulter Epics XL/MCL analyzer. Data were analyzed using the FlowJo software (Treestar, San Carlos, CA).
ELISpot assays for primary and secondary AFCs
Splenocyte suspensions from chimeric mice were plated at 1 × 106 cells/well and diluted serially (1:2) in multiscreen 96-well filtration plates (Millipore, Bedford, MA) coated with goat anti-mouse IgM (μ-specific) or goat anti-mouse IgG (γ-specific, CALTAG laboratories, Burlingame, CA) for 6 hr at 37°C. E4+ canonical IgM and IgG Abs produced by AFCs were detected using biotinylated anti-clonotypic mAb E4 (prepared in-house) and streptavidin (SA)-alkaline phosphatase (Vector Laboratories, Burlingame, CA). Plates were developed using the Vector Blue Alkaline-phosphatase Substrate kit III (Vector laboratories). ELISpots were counted using a computerized imaging video system (Cellular Technology, Cleveland, OH).
ELISA
Anti-Ars, anti-DNA, and clonotype-specific (E4) serum Abs were measured in sera from naïve and immunized mice by solid-phase ELISA on 96-well plates (Immulon-4; Thermo Electron) as previously described (10).
GC B cell sorting, DNA extraction and somatic mutation analysis
B220+, E4+, PNA+ GC B cells were purified on day five of the donor B cell response using a MoFlo fluorescent activated high-speed sorter (DakoCytomation, Glostrup, Denmark). Genomic DNA was prepared from these cells using the DNeasy tissue kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. PCR amplification of VH Tgs, PCR product purification, cloning, sequencing and mutation analyses were done as described (13).
GC B cell sorting, RNA extraction and real time RT-PCR
B220+, E4+, PNA+ GC B cells were purified on day five of the donor B cell response using a MoFlo FACS as described above. RNA from LPS-stimulated E4+ canonical B cells were used as control. RNA purification, reverse transcription of RNA, real time RT-PCR and generating raw RQ (relative quantification) values for gene expression were performed as described (13).
RESULTS
Primary development of HKIR B cells is not altered by the Sle1 locus
We generated HKI65.Sle1 and HKIR.Sle1 mice by crossing B6.Sle1 congenic mice with the HKI65 and HKIR lines (backcrossed to the B6 background for at least 20 generations), respectively. The HKI65 Igh knockin locus is identical to the HKIR Igh knockin locus with the exception of a single amino acid codon difference at position 55 in the CDR2 region of the VH gene. In the HKIR locus this codon specifies arginine (R) and in the HKI65 locus it encodes asparagine (N). The position 55R substantially increases the reactivity of canonical antibodies to chromatin and dsDNA, making canonical HKIR B cells far more autoreactive than their HKI65 counterparts (38). However, both types of canonical antibodies have similar affinity for Ars, and both can be specifically detected using an anti-idiotypic antibody termed E4 (29, 30, 38).
We first evaluated whether bone marrow (BM) development of B cells from both HKI65 (left panels, Figure 1A) and HKIR (right panels, Figure 1A) mice was altered in the presence of Sle1. We found no major differences in the percentage of B220low IgMneg pre-pro B, B220int IgMhi immature and B220hi IgMlow recirculating mature B cells in HKI65.Sle1 and HKIR.Sle1 mice as compared to HKI65 and HKIR controls.
Figure 1. Primary development of HKIR and HKI65 B cells in the presence or absence of Sle1.
Panel A) Flow cytometric analysis was performed on bone marrow cells obtained from HKI65.Sle1 and HKIR.Sle1, and HKI65 and HKIR controls after staining with B220 and anti-IgM. The percentage of B220low IgMneg pre-pro B, B220Int IgMhi immature and B220hi IgMlow mature B cells are shown in three separate gates. (B) The percentage of canonical IgMlow E4+ cells in the spleens of mice of the indicated genotypes as determined by flow cytometry is shown in rectangular gates. (C) Results of flow cytometric analysis of total (B220+) and canonical (E4+) B cells for surface IgM and IgD (upper and middle rows) and CD21/35 and CD23 levels (lower row). The gates encompass FO B cells (left) and transitional and MZ B cells (right). (D) Spleen sections obtained from naïve mice of the indicated genotypes were stained with MOMA-1 (green), anti-TCR-beta (red) and E4 (blue) and images captured by fluorescence microscopy. Original magnification of images was 200X. (E) Levels of activation markers CD69, CD80 and CD86 were examined by flow cytometry on B220+ E4+ B cells from naïve HKIR (blue line) and HKIR.Sle1 (red line) mice (upper row). All data are representative of those obtained from three independent experiments.
Both HKI65 and HKIR BCRs promote efficient development of canonical B cell precursors to mature FO but not to marginal zone (MZ) B cells (13). As shown in Table I and Figure 1B, the total number of B220+ cells, and the total number and frequency of canonical E4+ cells in HKI65.Sle1 and HKIR.Sle1 were comparable to HKI65 and HKIR mice. In addition, nearly all E4+ cells (99%) from mice of all four genotypes were IgMlowIgD+ (Figure 1C, second row) and CD21lowCD23+ (Figure 1C, third row) FO B cells. The profile of bulk B220+ IgMhighIgDlow cells containing transitional (T) and MZ B cells and IgMlowIgDhigh FO B cells in HKI65.Sle1 and HKIR.Sle1 mice were similar to HKI65 and HKIR controls, respectively (Figure 1C, first row). The lower percentage of IgMhighIgDlow T plus MZ, and slightly higher percentage of IgMlow IgDhigh FO B cells observed in HKIR and HKIR.Sle1 mice resulted from the more pronounced reduction of the MZ B population in HKIR mice, as described previously (13). We also observed substantially lower levels of surface BCR (both IgM and IgD) on canonical HKIR and HKIR.Sle1 E4+ B cells as compared to their HKI65 counterparts (Figure 1C, second row), indicating that the adaptive central tolerance pathway that results in reduced avidity of canonical HKIR B cells for autoantigen was not influenced by Sle1.
Table I.
Absolute number of B220+ and E4+, and frequency of E4+ cells in the presence or absence of Sle1
| Total number of B cells (x106)b |
Total number of E4+ cells (x106)c |
Frequency of E4+ cells (%)d |
|
|---|---|---|---|
| knock-in mice a | |||
| HKI65 | 15.0 ± 3.3 | 0.7 ± 0.2 | 4.9 ± 0.2 |
| HKI65.Sle1 | 16.5 ± 5.5 | 0.8 ± 0.3 | 4.9 ± 0.1 |
| HKIR | 16.7 ± 4.7 | 0.8 ± 0.2 | 5.1 ± 0.1 |
| HKIR.Sle1 | 15.6 ± 4.3 | 0.9 ± 0.3 | 5.9 ± 0.4 |
HKI65+/− and HKIR+/− knock-in mice bearing or not bearing Sle1
Total number of B (B220+) cells on lymphocyte gate from three to four mice of each genotype analyzed
Total number of E4+ cells out of total lymphocytes
The frequency of E4+ cells out of total lymphocytes
We next performed immunohistological analysis of spleen sections obtained from naive mice by staining with E4 (blue), anti-TCR-β (red) and MOMA-1 (green) (Figure 1D). MOMA-1 stains for metallophillic cells located at the border of follicles and the MZ. E4+ cells were confined mostly to B cell areas in the white pulp in HKI65.Sle1 (upper right) and HKIR.Sle1 (lower right) mice with few E4+ cells in the T cell and MZ areas, similar to that observed in HKI65 (upper left) and HKIR (lower left) controls.
We also examined the surface levels of the activation/co-stimulatory markers CD69, CD80 and CD86 to determine whether HKIR B cells were pre-activated in the presence of Sle1. The levels of these markers on ex vivo HKIR.Sle1 naïve E4+ cells (Figure 1E, red lines, upper row) were comparable to HKIR controls (Figure 1E, blue lines, upper row).
Enhanced primary (short-lived) AFC responses of canonical HKIR cells in the presence of Sle1
Due to the dual-reactive (Ars and ANA) nature of canonical HKIR cells, they can be recruited into the GC and AFC responses with Ars-conjugated foreign Ag. By performing adoptive transfer experiments we previously showed that during the ensuing anti-Ars AFC response, there was no significant difference between canonical HKI65 and HKIR B cell differentiation into IgM and IgG-producing primary AFCs (12, 13). There are several reasons why we do not examine the GC and AFC responses in the primary mice. First, the precursor frequency of canonical clonotype B cells in HKI65 and HKIR primary mice is very high (approximately a million E4+, Ars+ B cells in the spleen). Apparently as a consequence, even high doses of Ars antigen do not elicit normal levels of AFCs or GCs and these mice fail to develop memory. In our transfer experiments, we inject 2×106 total purified B cells containing at the most 1×105 E4+ donor B cells. Second, adoptive transfer allows us to study B cell intrinsic effects of Sle1, e.g. by transferring B cells expressing Sle1 into wild type recipients. Third, using allotype markers we can distinguish responding donor cells from recipient cells, and this allows the recipient cells to be used as “internal controls” (e.g. for the magnitude of the overall GC response).
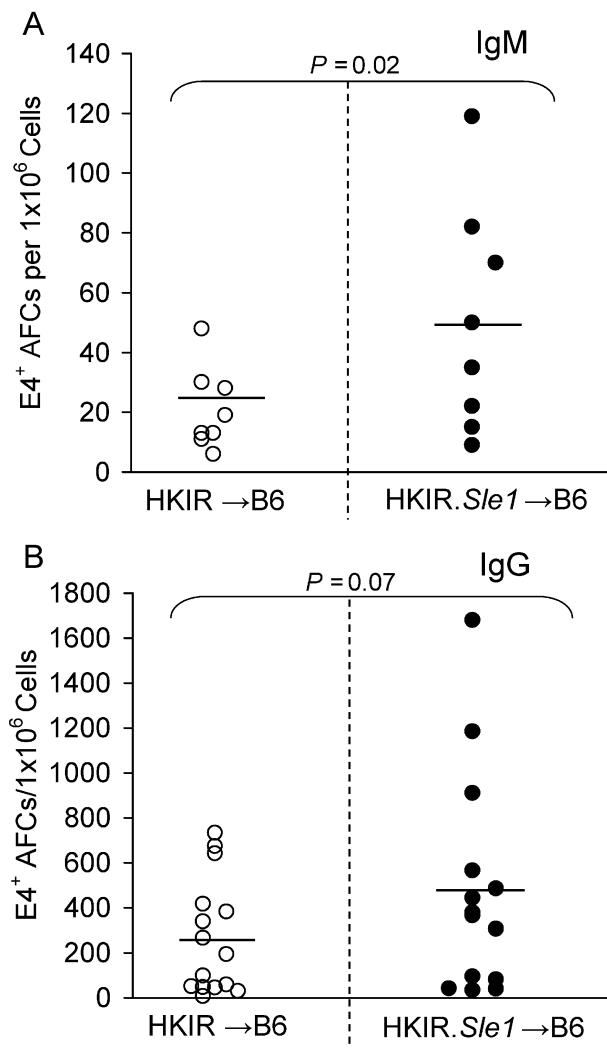
Two ×106 B cells (containing approximately 105 E4+ cells) from HKIR.Sle1 and HKIR control mice were adoptively transferred to syngeneic B6 recipients. B6 mice lack the VH gene necessary to encode canonical, E4+ antibodies (39). We then immunized the resulting chimeric mice with Ars-KLH 12-24 hours later and donor cell derived E4+ AFCs were quantified by ELISpot assay on day 6 post-immunization. Numbers of IgM producing E4+ AFCs in mice receiving HKIR.Sle1 B cells were significantly higher as compared to HKIR controls (Figure 2A). In addition, IgG producing E4+ AFCs (Figure 2B) in mice receiving HKIR.Sle1 B cells were almost two-fold more frequent on average and approximately 30% of these mice had significantly higher numbers of E4+ AFCs compared to controls. As discussed in more detail below, this variability might be explained by the incomplete penetrance of the Sle1 locus on the AFC phenotype in B cells derived from young mice.
Figure 2. Primary IgM and IgG AFC responses of HKIR canonical B cells in the presence or absence of Sle1.
HKIR and HKIR.Sle1 canonical B cells were transferred to B6 recipients that were subsequently immunized with Ars-KLH (see Materials and Methods). The number of splenic E4+ IgM (A, upper panel) and IgG (B, lower panel) secreting AFCs were measured by ELISpot assay 6 days after immunization. Each circle represents the number of E4+ AFCs per 1×106 splenocytes obtained from an individual chimeric mouse. Open and closed circles represent data from HKIR and HKIR.Sle1 chimeric mice, respectively. Horizontal bars represent the average number of E4+ AFCs. Statistical analysis was performed by Student’s t-test. The data for IgG producing AFCs were obtained from three independent experiments and the data for IgM producing AFCs were obtained from two independent experiments.
Reduced participation of HKIR E4+ autoreactive B cells in the foreign-Ag driven GC and memory responses
We previously have shown reduced participation of HKIR E4+ as compared to HKI65 E4+ B cells in the anti-Ars GC response (13), in which we adoptively transferred splenocytes into syngeneic recipients that were pre-immunized one week prior to cell transfer. In these previous studies, the presence of canonical E4+ B cells in GCs was detected using the anti-clonotypic mAb E4 alone. However, BCR levels on primary canonical HKIR B cells are low, and GC B cells further reduce BCR levels (40-42). Therefore, in order to improve detection of canonical HKIR B cells in GCs and to study the B cell autonomous effect of Sle1 in subsequent experiments, we MACS-purified canonical splenic B cells (B220+, E4+, CD45.2+) that were then transferred into B6.CD45.1 congenic recipients. This allowed the use of both anti-clonotypic (E4) and anti-CD45.2 Abs to distinguish canonical donor from host B cells. The B6.CD45.1 recipients were immunized with Ars-KLH one week prior to transfer of E4+ B cells. This protocol results in a highly synchronized immune response from the input donor B cells and, thus, greatly facilitates analysis of the GC response of canonical E4+ B cells in the chimeric mice.
Consistent with our previously published data where we transferred splenocytes (12, 13), we found similar results with transferring purified E4+ B cells. Figure 3A illustrates histological data showing that in HKIR→CD45.1 chimeras, CD45.2+ (red) canonical HKIR B cells clearly enter splenic GCs (stained with GL7, green) as evidenced by the presence of CD45.2+ cells within GL7+ B cell foci (yellow overlap staining, Figure 3A). Therefore, the reduced participation of canonical HKIR B cells in GC and memory responses cannot be attributed to these cells being excluded from GCs, which might be the case for anergic B cells. However, the expansion of these B cells in GCs appeared limited. We observed mostly small CD45.2+ GCs with a reduced number of CD45.2+ cells in HKIR→B6.CD45.1 GCs compared to HKI65→B6.CD45.1 controls.
Figure 3. Reduced anti-Ars GC and memory responses of HKIR B cells.
Panel (A): Spleen sections, obtained from Ars-KLH pre immunized B6.CD45.1 five days after transfer of HKI65 or HKIR CD45.2+ B cells (see Materials and Methods), were stained with anti-CD45.2 (red) and the GC marker GL7 (green). Two representative GCs from each type of mouse are shown. (B) The number of CD45.2+ B cells per GC were counted in eight randomly chosen small and medium sized CD45.2+ GCs per mouse. Each circle indicates the number of CD45.2+ cells counted from a particular GC in HKI65 →CD45.1 (blue) and HKIR→CD45.1 (red) chimeric mice. (C) Donor cell derived E4+ IgG secreting secondary AFCs were measured by ELISpot assay on day four post secondary immunization. Chimeric mice were created by transfer of HKIR or HKI65 splenic B cells followed by Ars-KLH immunization. Mice were rested for 8 weeks prior to boosting. Each circle represents the number of E4+ AFCs per 106 splenocytes obtained from an individual chimeric mouse. Blue and red circles represent the data from mice receiving HKI65 and HKIR donor cells, respectively. (D) Anti-Ars (left two panels) and E4 (right two panels) Ab serum titers were measured by ELISA on day four post secondary immunization. The serum samples assayed in (D) were obtained from the same mice described in (C). Horizontal bars represent averages of the data set in section B-D. Statistical analysis was performed by Student’s t-test.
As such, we next performed semi-quantitative analysis of the numbers of CD45.2+ B cells in splenic GCs in HKIR→B6.CD45.1 and HKI65→B6.CD45.1 mice five days after cell transfer. GC sizes were determined by counting the number of PNA+ cell diameters at 100× magnification in the largest GC dimension. GCs were categorized into three groups: small (10-25 cell diameters), medium (26-39 diameters) and large (40 or more diameters). Very few large GCs were observed in either type of chimera. The frequency of CD45.2+ GCs in HKIR →B6.CD45.1 chimeras was similar to that observed in HKI65 →B6.CD45.1 mice (data not shown) but the number of CD45.2+ cells per GC differed. CD45.2+ cells in randomly chosen small and medium GCs were counted. The number of CD45.2+ cells per HKIR→B6.CD45.1 CD45.2+ GC was significantly reduced by an average of nearly two-fold (Figure 3B) as compared to that found in HKI65 →B6.CD45.1 CD45.2+ GCs.
To study the differences in anti-Ars memory responses of HKIR versus HKI65 canonical B cells, chimeric mice were rested for two months post transfer of 2 ×106 B cells (containing approximately 105 E4+ cells) from HKIR or HKI65 mice and immunization with Ars-KLH. IgG producing E4+ AFCs were quantified by ELISpot assay on day four after secondary immunization with Ars-KLH in saline. The anamnestic response of HKIR E4+ cells (red) was three to four-fold lower compared to HKI65 controls (blue, Figure 3C). In agreement with these AFC data, anti-Ars and E4+ total serum Ig titers in HKIR→B6 mice (red) were substantially reduced as compared to HKI65→B6 controls (blue, Fig. 3D). Altogether, our previously published data in which we transferred splenocytes (13) are consistent with the current data generated via adoptive transfer of B cells. This allows us to study the B cell-specific effect of Sle1 on the anti-Ars AFC and GC responses by transferring HKIR B cells expressing Sle1.
The GC response of canonical HKIR B cells is augmented in the presence of Sle1
To study the influence of Sle1 on the action of the GC tolerance checkpoint indicated by the data above, 2×105 purified canonical HKIR or HKIR.Sle1 B cells (B220+, E4+ and CD45.2+) were transferred to B6.CD45.1 recipients that had been immunized with Ars-KLH one week earlier. Flow cytometry analysis of splenocytes obtained on day five of the donor B cell response revealed a significant increase in the percentage of donor-derived CD45.2+ PNA+ GC B cells in HKIR.Sle1→B6.CD45.1 mice compared to HKIR→B6.CD45.1 controls (Figure 4A and B). These data are consistent with immunohistology results (Figure 4C) illustrating that more CD45.2+ cells (red and yellow overlap staining) per GC were observed in HKIR.Sle1→B6.CD45.1 mice (right two panels) as compared to HKIR→B6.CD45.1 controls (left two panels).
Figure 4. Sle1 alters anti-Ars primary GC and Ab responses of canonical HKIR cells.
Panel (A): Flow cytometry analysis was performed on splenocytes obtained from Ars-KLH pre immunized HKIR→B6.CD45.1 (upper panel) and HKIR.Sle1 →B6.CD45.1 (lower panel) mice on day 5 after cell transfer. The percentage (with standard error) of B220hi CD45.2+ PNA+ GC B cells is shown in rectangular gates. (B) The percentage of CD45.2+ PNA+ donor-derived GC B cells from HKIR → B6.CD45.1 (blue circle) and HKIR.Sle1 → B6.CD45.1 (red circle) mice described in (A). Each circle indicates data from one mouse. (C) Spleen sections, obtained on day 5 after cell transfer from HKIR→ B6.CD45.1 (left two panels) and HKIR.Sle1→ B6.CD45.1 (right two panels) mice generated as described in (A) were stained with anti-CD45.2 mAb (red) and the GC marker GL7 (green). Two representative images from each type of chimeric mouse are shown. (D) The number of donor-derived CD45.2+, E4+ cells counted in 60-90 representative GCs from 3-4 HKIR→ B6.CD45.1 (blue) and HKIR.Sle1→B6.CD45.1 (red) mice, generated as described in (A) on day 5 after cell transfer in shown. (E) The total number of CD45.2+, E4+ GCs counted and shown in (D) was categorized into small (gray), medium (brown) and large (white) as described (36, 37). The percentage of different sizes of GCs is illustrated. (F) B6 and B6.Sle1 mice were immunized with sheep red blood cells (SRBCs) and spleen sections obtained on day 9 of the response from these mice were stained with the GC marker PNA. The number of small, medium and large GCs per 100X microscope field was counted from randomly chosen areas on spleens from B6 (blue) and B6.Sle1 (red) mice. Horizontal bars indicate the average value for each data set. GCs in two randomly chosen fields were counted in each type of mouse. Anti-Ars (G) and E4 (H) serum Ig titers were measured by ELISA in sera collected on day 5 after cell transfer from the chimeric mice described in panels A-E. These data were obtained from multiple mice of each genotype.
Semi-quantitative analysis of CD45.2+ cells in histologically defined GCs, conducted as described above, corroborated these findings. The number of CD45.2+ cells per HKIR.Sle1→B6.CD45.1 GC was significantly higher compared to that observed in HKIR→B6.CD45.1 GCs (Figure. 4D). In addition, while we found few large CD45.2+ GCs in HKIR→B6.CD45.1 mice, 10% of CD45.2+ GCs in HKIR.Sle1→B6.CD45.1 were large (white bar, Figure 4E) indicating enhanced expansion of canonical HKIR GC B cells bearing the Sle1 locus. We did not, however, observe that HKIR B cells formed or participated in extrafollicular GC-like reactions, as previously observed in MRL/lpr mice (43).
To determine if the difference in the GC response between canonical HKIR and HKIR.Sle1 cells might result from a generalized defect in all B cells expressing Sle1, GC responses induced by the TD Ag sheep red blood cells (SRBC) were evaluated in B6 (blue) and B6.Sle1 (red) mice (Figure 4F). As described above, splenic GCs in these mice defined histologically were counted and sorted into small, medium and large categories. This analysis revealed that the number of small, medium and large GCs in SRBC immunized B6 and B6.Sle1 mice did not significantly differ. Consistent with these data the average number of secondary NP-specific IgG1 AFCs was also similar in both B6 and B6.Sle1 mice described below (Fig. 7E) in which mice were immunized with another TD-Ag (4-hydroxy-3-nitrophenyl) acetyl (NP)-chicken gamma globulin (CGG).
Figure 7. Anti-Ars primary IgG AFC and GC responses of canonical B cells in B6.Sle1+/− recipients.
Canonical B cells from HKI65 and HKIR mice expressing or not-expressing Sle1 were transferred to B6.Sle1+/− recipients that had been pre-immunized one week prior to transfer (see Materials and Methods). (A) Secondary splenic E4+ IgG AFCs were measured by ELISpot assay four days after boosting with Ars-KLH, in HKIR→B6.Sle1+/− (blue circles) and HKIR.Sle1→B6.Sle1+/− (red circles) mice. (B) Anti-Ars and (C) E4 serum Ig titers were measured in the indicated types of chimeric mice four days after boosting. The mice were generated as described in the legend to Figure 7A. Five days after cell transfer, in experiment 1, the number of splenic E4+ IgG secreting AFCs (D) and the percentage of total E4+ PNA+ cells in GCs (E) were measured in HKI65 →B6.Sle1+/− (blue), HKI65.Sle1 → B6.Sle1+/− (green), HKIR → B6.Sle1+/− (red) and HKIR.Sle1 → B6.Sle1+/− (black) mice by ELISpot and flow cytometry assays, respectively. Five days after cell transfer, in experiment 2, the percentage of total E4+ PNA+ cells in GCs (F) and the number of splenic E4+ IgG secreting AFCs (G) were measured in HKIR →B6.Sle1+/− (blue) and HKIR.Sle1 → B6.Sle1+/− (red) mice by flow cytometry and ELISpot assays, respectively. Each circle represents the number of E4+ IgG AFCs per 1× 106 splenocytes (A, D and G) or the percentage of total E4+ PNA+ cells in GCs (E and F) obtained from an individual chimeric mouse. Horizontal bars represent the averages of each data set. Statistical analysis was performed by Student’s t-test. These data were obtained from five to six mice of each genotype.
Finally, to test whether the augmented anti-Ars AFC and GC responses of canonical HKIR B cells containing Sle1 could lead to increased titers of serum Abs derived from these cells, we measured the levels of anti-Ars (Figure 4G) and E4+ Abs in the sera of the chimeric mice described in Figure 4 A-E and found that 30% of HKIR.Sle1→B6.CD45.1 mice had significantly higher anti-Ars and E4+ titers than those of HKIR→B6.CD45.1 mice, again consistent with an incomplete penetrance of the effect of the Sle1 locus on this response.
Augmented secondary (long-lived) AFC response of HKIR E4+ cells in the presence of Sle1
Some AFCs generated during TD immune responses migrate to and reside in the BM where they are relatively long-lived. Therefore, we next examined whether the Sle1 locus led to an increased number of long lived BM E4+ AFCs derived from canonical HKIR B cells. For this purpose, 2×106 B cells (containing approximately105 E4+ cells) from HKIR.Sle1 or HKIR control mice were transferred to B6 recipients that were immunized 12 hours later with Ars-KLH in alum. Long-lived E4+ AFCs were quantified in the BM by ELISpot assay on day 30 post-immunization. Numbers of both IgM (Figure 5A) and IgG (Figure 5B) producing E4+ BM AFCs in mice receiving HKIR.Sle1 B cells were found to be significantly higher as compared to HKIR controls.
Figure 5. Elevated bone marrow long-lived IgM and IgG AFC responses from canonical HKIR cells bearing Sle1.
MACS-purified HKIR and HKIR.Sle1 total (2× 106) B cells were transferred into B6 mice, the mice were immunized with Ars-KLH and the number of E4+ IgM (A, top) and E4+ IgG (B, bottom) AFCs were measured by ELISpot 30 days post-transfer/immunization. Each circle represents data from an individual chimeric mouse. Horizontal bars represent the average number of E4+ AFCs in each data set. All statistical analyses were performed by Student’s t-test.
The influence of Sle1 in post-GC memory pathways
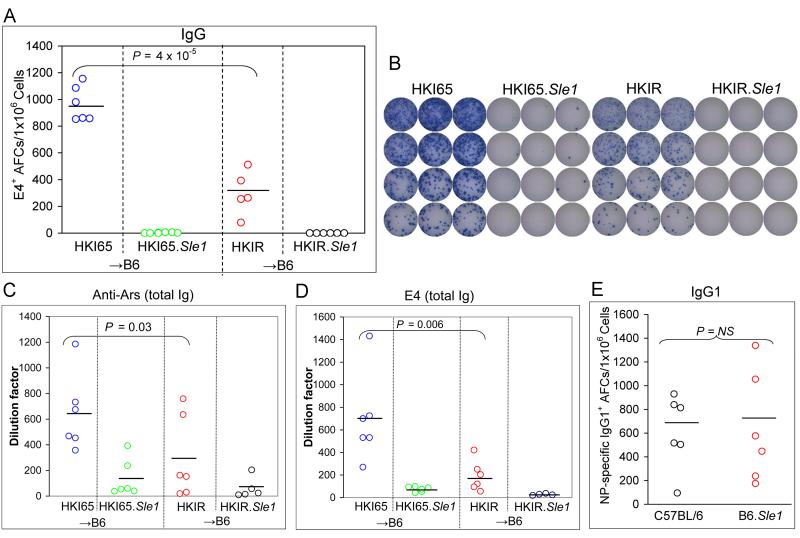
To evaluate whether Sle1 alters post-GC memory pathways taken by ANA B cells, we transferred purified B cells (2 ×106) from HKI65.Sle1 and HKIR.Sle1 mice, and HKI65 and HKIR controls into B6 mice that were immunized with Ars-KLH 12 hours later. Chimeric mice were rested for two months and memory responses were evaluated by quantifying secondary E4+ IgG splenic AFCs in these mice by ELISpot assay four days after boosting with Ars-KLH in saline. In accordance with our previous data (13) and the data shown in Figure 3C, we found a significant reduction in the number of E4+ secondary IgG AFCs (Figure 6A and B) in HKIR→B6 mice (red circles) compared to HKI65→B6 control (blue circles). Surprisingly, we found a complete absence of E4+ secondary IgG AFCs in both HKI65.Sle1→B6 (green circles) and HKIR.Sle1→B6 (black circles) mice (Figure 6A and B).
Figure 6. Influence of Sle1 on memory responses from canonical B cells.
Panel A: Secondary splenic E4+ AFCs were measured by ELISpot assay four days after Ars-KLH boosting, in HKI65.Sle1→B6 (green circles), HKIR.Sle1→B6 (black circles), HKI65 →B6 (blue circles) and HKIR →B6 (red circles) mice. The mice were generated by transfer of 2×106 donor B cells, immunization with Ars-KLH 12 hours later, followed by a six to eight week rest period. (B) Image of representative ELISpot plates used to generate the data illustrated in (A). (C) Anti-Ars and (D) E4 serum Ig titers in the chimeric mice described in (A) were measured by ELISA on day four post secondary immunization. (E) NP-specific IgG1-producing AFCs were measured by ELISpot assay after primary immunization (100 μg NP-CGG in alum) and boosting (50 μg NP-CGG/PBS) of B6 (black circles) and B6.Sle1 (red circles) mice. Horizontal bars represent the averages of each data set.
To determine if this was due to a defect in IgH class switching leading to the accumulation of secondary AFCs producing IgM, we quantified IgM-producing E4+ AFCs and obtained analogous results (data not shown). We also measured serum Ab titers by ELISA and found low to undetectable levels of anti-Ars (Figure 6C) and E4 (Figure 6D) Abs in secondary sera from both HKI65.Sle1→B6 (green circles) and HKIR.Sle1→B6 (black circles) mice. Finally, to investigate a potential pleiotropic defect in the generation of secondary AFCs due to Sle1, we immunized B6 and B6.Sle1 mice with the TD-Ag (4-hydroxy-3-nitrophenyl) acetyl (NP)-chicken gamma globulin (CGG, in alum). Mice were rested for two months before boosting with NP-CGG in PBS. We examined the secondary NP-specific IgG1 AFC response by ELISpot assay four days after boosting. Figure 6E shows that the average number of secondary NP-specific IgG1 AFCs was similar in both B6 (black circles) and B6.Sle1 (red circles) mice, indicating that there was no global influence of the Sle1 locus on the secondary AFC response.
The Sle1 interval is a 37 cM long genomic segment of NZW origin (22, 35) that encodes at least 100 transcripts. Therefore, we next considered whether the complete absence of E4+ secondary AFCs in HKI65.Sle1→B6 and HKIR.Sle1→B6 mice might result from rejection of memory B cells bearing Sle1 by the B6 recipient mice. To test this possibility, we transferred B cells from HKIR.Sle1 mice into B6 and B6.Sle1 mice and quantified E4+ secondary AFC responses as described above. While we found a complete lack of such responses in HKIR.Sle1→B6 mice, secondary E4+ IgG AFCs in HKIR.Sle1→B6.Sle1 mice were readily observed (data not shown) indicating that HKIR.Sle1 B cells are rejected or suppressed by the B6 hosts due to allotype differences in an antigen(s) encoded in the Sle1 locus.
As such, we next transferred B cells from HKIR and HKIR.Sle1 mice into B6 × B6.Sle1 F1 (termed B6.Sle1+/−) mice. Previous studies have indicated that the influence of the Sle1 locus on T and accessory cell function is recessive (44, 45). We evaluated memory responses in these chimeric mice by quantifying numbers of secondary splenic AFCs as described above and found that the number of secondary E4+ IgG AFCs on average was two-fold higher in HKIR.Sle1→B6.Sle1+/− mice as compared to HKIR→ B6.Sle1+/− controls (Figure 7A). While the p value between these two groups did not reach statistical significance, 30% of B6.Sle1+/− mice receiving HKIR.Sle1 B cells had a 5-6 fold larger number of E4+ IgG AFCs compared to HKIR→ B6.Sle1+/− controls. These data are consistent with the primary AFC data shown in Figure 2 indicating that the influence of the Sle1 locus on the AFC response of HKIR B cells is incompletely penetrant. In addition, anti-Ars (Figure 7B) and E4+ (Figure 7C) total serum Ig titers in HKIR.Sle1→B6.Sle1+/− mice (red) were also significantly higher compared to HKIR→B6.Sle1+/− controls.
Finally, we considered the possibility that allogeneic effects that resulted in rejection or suppression of HKIR.Sle1 memory B cells in B6 hosts might be responsible for the enhanced primary AFC and GC responses of HKIR.Sle1 B cells in HKIR.Sle1→B6 chimeric mice (Figures 2, 4 and 5). However, chimeric mice created by transfer of HKIR.Sle1 B cells into B6.Sle1+/− hosts also gave rise to enhanced primary AFC (Figure 7D) and GC (Figure 7E) responses. In contrast, the same responses of HKI65 B cells in B6.Sle1+/− hosts were not enhanced by the presence of Sle1 in these donor B cells. To confirm these data, in another set of experiments we transferred HKIR and HKIR.Sle1 B cells into B6.Sle1+/− hosts and performed similar analysis, and obtained analogous results for GC responses (Fig. 7F). Interestingly, we observed significantly increased IgG-producing E4+ AFCs in B6.Sle1+/− mice receiving HKIR.Sle1 B cells compared to HKIR controls (Fig. 7G). In total, these results suggest that alloantigenic differences between HKIR.Sle1 B cells and B6 hosts do not overtly influence the outcome of the primary immune response produced by these B cells.
Sle1 alters expression of genes regulating GC B cell survival but not differentiation
In normal mice, the transcription factors Blimp-1 and Xbp-1 are upregulated in B cells committed to the AFC pathway and drive development to secretory phenotype. Conversely, most GC B cells express very low levels of these factors but express high levels of the transcription factor Bcl-6, which appears to suppress the expression of Blimp-1 and Xbp-1 (46-49). Elevated levels of Blimp-1/Xbp-1 expression in GC B cells resulting from the influence of the Sle1 locus could potentially promote the development of these cells to AFCs. To test this idea, 2×106 B cells from HKIR or HKIR.Sle1 mice were transferred into B6 mice that had been immunized with Ars-KLH one week earlier. Five days post transfer, E4+ GC B cells (B220hi E4+ PNA+) were purified by FACS from both types of chimeric mice and RNA was extracted and used to perform qPCR for levels of Blimp-1, Xbp-1 and Bcl-6 transcripts. Bcl-6 RNA was up regulated to a similar extent and the levels of Blimp-1 and Xbp-1 RNA were comparably low in both types of GC B cells (Figure 8A). These data indicate that the enhanced AFC response of canonical HKIR.Sle1 B cells was not due to increased expression of Blimp-1/Xbp-1 by these cells during the GC response.
Figure 8. Survival and differentiation gene RNA levels in canonical HKIR GC B cells.
RNA was extracted from pooled samples of FACS-purified B220highE4+PNA+ GC B cells, obtained on day 5 post-transfer of 2×106 B cells of the indicated genotypes into Ars-KLH preimmunized B6 mice. RNA extracted from in vitro LPS-activated (72 hr) MACS-purified E4+ cells were used as a control. (A) Raw RQ values of Blimp-1 (left), Bcl-6 (middle) and Xbp-1 (right) RNA levels in GC B cells and LPS-activated HKIR canonical B cells were obtained via Q-PCR. (B) Raw RQ values of Bcl-2 (left), Bcl-xL (second left), c-FLIP (second right) and Fas (right) RNA levels in GC B cells. Amplifications were done in triplicate. These data are representative of those obtained from three to four mice in two independent experiments.
Next, to examine whether the augmented anti-Ars GC response of HKIR B cells expressing Sle1 resulted from alteration of expression of genes that regulate GC B cell survival, we performed qPCR for Bcl-2, Bcl-xL, c-FLIP and Fas transcripts in GC B cells in the presence or absence of Sle1 using the same RNA samples used to generate the data shown in Figure 8A. Interestingly, we found Bcl-2 and c-FLIP transcript levels were upregulated four to five-fold in HKIR E4+ GC B cells containing the Sle1 interval compared to follicular or HKIR GC B cells without Sle1 (Figure 8B). Conversely, we observed decreased levels of Fas transcripts in HKIR.Sle1 GC B cells compared to controls (Figure 8B). We performed similar experiments using RNA samples obtained from HKIR→ B6.Sle1+/− and HKIR.Sle1 → B6.Sle1+/− GC B cells which revealed analogous results (data not shown).
Increased frequency of somatic mutations in the V regions of HKIR.Sle1 canonical GC B cells
Next we determined whether the augmented anti-Ars GC response of canonical B cells correlated with the increased frequency of somatic mutations in the variable regions of transgenes in mice receiving HKIR.Sle1 B cells compared to HKIR controls. B220+E4+PNA+ GC B cells were purified by FACS sorter on day five of donor B cell responses in pre-immunized B6.Sle1+/− recipients. Variable regions of heavy chain knock-in transgenes were amplified, cloned and sequenced as previously described (13). As shown in Table II, somatic hypermutation frequency in Ig heavy chain transgene in GC B cells obtained from HKIR.Sle1 →B6.Sle1+/− mice (0.5%) was equal if not higher than that observed in HKIR →B6.Sle1+/− control GCs (0.3%).
Table II.
Somatic hypermutation frequency in variable region of Ig heavy chain knock-in transgenes in E4+ GC B cells from chimeric mice
| Total Mutationsa | Total Sequenceb | Frequency (%)c | |
|---|---|---|---|
| HKIR→ B6.Sle1+/− | 33 | 9540 | 0.34 |
| HKIR.Sle1→B6.Sle1+/− | 50 | 9540 | 0.52 |
Variable regions of heavy chain knock-in transgenes were amplified from sorted B220+E4+PNA+ GC B cells in Ars-KLH immunized C57BL/6 (B6).Sle1+/− recipients after adoptively transferring HKIR and HKIR.Sle1 B cells.
Total number of somatic mutations that was observed in total number of sequences analyzed
Total nucleotide number in sequences that were amplified, sequenced and analyzed from 9-11 plasmid clones, pooled samples of five mice of each genotype
Mutation frequency of Ig heavy chain knock-in gene in B220+E4+PNA+ GC B cells
High titers of spontaneously produced canonical and anti-dsDNA antibodies in the presence of Sle1
To test whether data obtained through the adoptive transfer and immunization protocols described were reflective of events that might take place in HKIR mice that were not overtly immunized, we measured anti-dsDNA, E4 and anti-Ars serum Ab titers by ELISA (10) in naïve HKIR mice at 3 and 6 months of age and found low to undetectable levels of these Abs (black circles, Figure 9). In contrast, we observed increased titers of anti-dsDNA, E4 and anti-Ars Abs in most 3 and 6 month old HKIR.Sle1 mice, consistent with the data obtained from chimeric mice and indicating that the operation of a peripheral B cell tolerance checkpoint(s) is impaired in unmanipulated HKIR mice in the presence of Sle1.
Figure 9. Elevated clonotype-specific and anti-dsDNA serum antibody titers in the presence of Sle1.
Sera were collected from three (left panels) and six (right panels) month old naïve HKIR (black) and HKIR.Sle1 (red) mice. Anti-dsDNA (A) and E4 (B) specific total Ig titers were measured by ELISA. Each circle represents the dilution factor generated from a single mouse and horizontal bars indicate averages.
Discussion
Extensive previous studies by Wakeland and colleagues have shown that of the three major NZW derived loci that contribute to lupus-like disease in mice with a C57BL/6 (B6) background: Sle1, 2 and 3; only Sle1 appears to cause major intrinsic changes in B cell tolerance and immunoregulatory pathways, particularly to chromatin-based autoantigens (23, 28, 50). This insight led these investigators to propose an epistatic model for the development of autoimmune disease in this system in which the first step is mediated by genes in the Sle1 interval, resulting in a loss of B cell tolerance. This is followed by secondary events mediated by genes in the Sle2 and Sle3 regions, resulting in loss of T cell tolerance, epitope spreading, and the development of autoantigen-driven production of pathological IgG antibodies (51-53). Nonetheless, further genetic dissection of the Sle1 locus revealed three sub regions termed Sle1a, Sle1b and Sle1c that produce different component autoimmune phenotypes (27). Subsequently it has been shown that the B cell intrinsic effects conferred by the Sle1 interval mainly map to the Sle1b sub region (28, 54), while Sle1a and Sle1c regions were reported to be largely influencing CD4 T cell tolerance and regulatory T cell numbers and activity (45, 55). As such, in this study we chose to perform adoptive transfers of chromatin-Ars “dual-reactive”, Sle1-bearing HKIR B cells into B6 hosts. There are caveats associated with the use of the pre-immunization/adoptive transfer protocol that need to be taken into account when interpreting the significance of the data to a more general understanding of the role of the GC tolerance checkpoint in the regulation of the development of autoimmunity. These include the pre-existing T cell help and GC responses generated by recipient cells and the high precursor frequency of donor cells specific for one antigen. Nonetheless, we have previously shown using this protocol that canonical HKI65 B cells produce Ars-driven early primary responses that are qualitatively indistinguishable from those produced by canonical B cell clonotypes in normal (i.e. A/J) mice (12). The distinct advantage of using this protocol over and above allowing the participation of ANA B cells in immune responses to be directly evaluated via techniques such as flow cytometry and histology is that we can study how various genes and genetic loci influence the action of B cell tolerance pathways in a B cell autonomous manner. In the case of the Sle1 locus this is particularly important, as sub regions of this locus have been shown to influence the behavior of both T cells and myeloid cells (45, 55).
Our studies were also motivated by our previous findings that a deficiency in FcγRIIB, whose gene maps to a location between the Sle1a and Sle1b sub regions, generically perturbed the activity of both strongly and weakly autoreactive B cells in the AFC, but not the GC pathway (13). Moreover, analysis of a newly developed congenic line of mice containing the Sle1-derived FcγRIIB gene, but lacking the other major sub regions of the Sle1 locus, demonstrated that B cell tolerance was maintained in these mice, but short lived AFC responses to foreign antigens were amplified, particularly for IgG production (34). In total, these data suggested that other genes in the Sle1 interval must be responsible for loss of B cell tolerance to chromatin-based autoantigens. Indeed, we have shown that genes of the SLAM/CD2 family present in this interval result in perturbed deletion, receptor editing and anergy induction in the soluble-hen egg-lysozyme (HEL)/anti-HEL Ig transgenic model of B cell tolerance (28). In particular, these phenotypes correlated with expression of a particular allele of a SLAM/CD2 gene termed Ly108.1, which appears to attenuate BCR signaling.
Since canonical HKIR B cells evade central and early peripheral tolerance pathways via adaptive down regulation of BCR levels, the HKIR model afforded us the potential to study the effects of the Sle1 interval on tolerance pathways operative during antigen-driven immune responses and beyond. This potential was fulfilled, as analysis of the primary development of canonical and other B cells in HKIR.Sle1 mice showed no major differences in BCR down regulation and progression to mature, follicular phenotype and locale, strongly suggesting that genes in the Sle1 interval do not perturb the primary adaptive tolerance pathway taken by these B cells. Moreover, these results demonstrate that the previously described alterations in T cell development and activity conferred by the Sle1 interval (45, 54-56) do not indirectly influence the primary development and tolerance of HKIR B cells.
In contrast, analysis of the Ars-driven immune response in chimeric mice created by injection of HKIR.Sle1 B cells into B6 hosts revealed significant perturbation of the activity of these B cells in both the AFC and GC pathways. Importantly, this did not appear to result from a generic alteration of the GC response, as the presence of the Sle1 interval did not change the number and size of GCs in SRBC immunized B6.Sle1 mice as compared to B6 or the participation of HKI65 B cells in the GC response. Anti-NP secondary responses in B6.Sle1 mice were also similar to B6 controls, suggesting that the GC response induced by this antigen was not quantitatively altered by Sle1.
Given our previous results, the most likely candidate gene that results in the amplified AFC response characteristic of HKIR.Sle1 B cells is the NZW FcγRIIB allele. We and others have shown that this allele fails to be up regulated on GC B cells (33, 57, 58). While we have not yet detected any impact of this failed up regulation on the quantity or quality of the GC response, lower levels of expression of this Fc receptor on B cells in the AFC pathway would be expected to perturb the immune-complex mediated feedback that controls the number and activity of B cells in this pathway (59). Indeed, our previous studies of B6 congenic mice bearing the NZW FcγRIIB allele strongly support this possibility (34). Interestingly, however, only a subset of HKIR.Sle1→B6 chimeric mice displayed increased primary and anamnestic AFC responses, indicating that the penetrance of the NZW FcγRIIB allele on AFC feedback regulation is incomplete and is influenced by stochastic, perhaps environmental factors. This is in contrast to our previous findings on analogous chimeric mice generated using FcγRIIB deficient HKIR B cells (13), suggesting that the effects of the NZB FcγRIIB allele do not completely phenocopy an FcγRIIB deficiency, perhaps because this allele is indeed expressed, albeit at a lower level than in wild type mice.
In contrast, the elevated GC and memory responses produced by HKIR.Sle1 as compared to HKIR B cells are likely not due to the NZW FcγRIIB allele alone, as we previously showed that a complete FcγRIIB deficiency in HKIR B cells did not influence the substantially reduced participation of these auto reactive B cells in the GC and anamnestic responses (13). Given that the SLAM/CD2 Ly108.1 allele has been shown to attenuate BCR signaling (28), it is possible that reduced signaling through the BCR complex in HKIR.Sle1 GC B cells spares them from the action of an autoantigen-driven GC tolerance checkpoint that normally mediates their deletion. Lack of a direct influence of SLAM alleles on the magnitude of the primary AFC response might be explained by the fact that the BCR is down regulated on GC B cells, creating a situation where levels of BCR signaling become limiting and placing particular importance on the efficient functioning of signaling components that act downstream of the BCR. Testing the above ideas will require the development of new lines of HKIR congenic mice containing various subintervals of the Sle1 locus including Sle1b and the region between Sle1a and Sle1b containing the NZW FcγRIIB allele.
These are the first data demonstrating that a lupus susceptibility locus can alter GC tolerance checkpoints in a B cell autonomous fashion. While the mechanism of perturbation of the GC tolerance checkpoint by Sle1 remains to be clarified, our data suggest that this is taking place via alteration of GC B cell survival. We found that the levels of expression of Blimp-1, Xbp-1 and Bcl-6, genes involved the regulation of B cell differentiation, in GC B cells were not altered by Sle1. However, expression of Bcl-2 and c-FLIP RNA was increased and Fas RNA expression was decreased in GC B cells in the presence of Sle1. While the Bcl-2 and c-FLIP genes are located on the same chromosome (chromosome 1) as Sle1, they are 20 and 40 cM upstream of the Sle1 locus, respectively. Thus, Bcl-2, c-FLIP and Fas are likely to act downstream of genes in the Sle1 interval.
Our studies also revealed an unanticipated caveat to the use of congenic mice in adoptive transfer studies for the analysis of the genes that contribute to the development of autoimmunity. While HKIR.Sle1→B6 chimeras gave rise to primary immune responses that were equivalent or elevated as compared to HKIR→B6 mice, the former produced weak to undetectable anamnestic responses. However, when analogous chimeras were generated using Sle1 homozygous or heterozygous recipients, the anamnestic responses produced by HKIR.Sle1 B cells were at least equivalent, and often more robust than those developed by HKIR B cells. These results strongly suggest that alloantigens encoded in the Sle1 interval activate recipient T cells, resulting in the eventual killing or suppression of donor B cells by these primed T cells. Clearly, such allogeneic effects could either enhance, or inhibit the action of various autoimmunity-influencing gene products in other contexts, confounding interpretation of the role of such gene products in the development of autoimmunity. However, such allogeneic effects appeared to have little or no influence on the primary immune responses of HKI65.Sle1 and HKIR.Sle1 B cells in B6 hosts (Figure 7).
Finally, our analyses showed that HKIR.Sle1 congenic mice spontaneously develop significant titers of anti-DNA and canonical serum antibodies by three months of age, and serum levels of such antibodies are further increased at six months of age. This suggests that even in the absence of overt immunization, defects in peripheral tolerance checkpoints due to the presence of Sle1 allow activation and eventual anti-DNA autoantibody production by HKIR B cells. Given the results we obtained from Ars-immunized HKIR.Sle1 B cell chimeric mice, it is tempting to speculate that the production of serum autoantibodies in HKIR.Sle1 mice is driven, at least initially, by environmental antigens cross reactive with HKIR BCRs. This perspective leads to a new kinetic model for how genetic susceptibility loci like Sle1 may sometimes operate to promote the development of autoimmunity. In this model, the influence of these loci would be most prominent not before, but after an autoreactive B cell was recruited into an immune response.
ACKNOWLEDGMENTS
We thank Scot Fenn and Tahsin Khan for technical support and all members of the Manser and Rahman laboratories for their indirect contributions to this work.
Non standard abbreviations
- GC
germinal center
- AFC
antibody forming cell
- IC
immune complex
- FO
follicular
- MZ
marginal zone
- MOMA-1
metallophillic macrophage-1
- BCR
B cell antigen receptor
- Ars
p-azophenylarsonate
- KLH
Keyhole limpet hemocyanin
- PNA
peanut lectin (agglutinin)
- RQ
relative quantification
- Blimp-1
B lymphocyte-induced maturation protein-1
- qPCR
quantitative polymerase chain reaction
- TD
thymus dependent
- ANA
anti-nuclear autoantibody
Footnotes
These studies were supported by grants from the NIH to T.M. (AI038965) and Z.S.M.R. (AR055701) and to Z.S.M.R. from the Arthritis National Research Foundation.
References
- 1.Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, Weigert M. The site and stage of anti-DNA B-cell deletion. Nature. 1995;373:252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 2.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 3.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nossal GJ. A lifetime’s flirtation with repertoire purging. Res. Immunol. 1992;143:268–272. doi: 10.1016/s0923-2494(92)80117-4. [DOI] [PubMed] [Google Scholar]
- 6.Klinman NR. The “clonal selection hypothesis” and current concepts of B cell tolerance. Immunity. 1996;5:189–195. doi: 10.1016/s1074-7613(00)80314-3. [DOI] [PubMed] [Google Scholar]
- 7.Goodnow CC, Cyster JG, Hartley SB, Bell SE, Cooke MP, Healy JI, Akkaraju S, Rathmell JC, Pogue SL, Shokat KP. Self-tolerance checkpoints in B lymphocyte development. Adv. Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 8.Limpanasithikul W, Ray S, Diamond B. Cross-reactive antibodies have both protective and pathogenic potential. J. Immunol. 1995;155:967–973. [PubMed] [Google Scholar]
- 9.Rousseau PG, Mallett CP, Smith-Gill SJ. A substantial proportion of the adult BALB/c available B cell repertoire consists of multireactive B cells. Mol. Immunol. 1989;26:993–1006. doi: 10.1016/0161-5890(89)90118-1. [DOI] [PubMed] [Google Scholar]
- 10.Casson LP, Manser T. Random mutagenesis of two complementarity determining region amino acids yields an unexpectedly high frequency of antibodies with increased affinity for both cognate antigen and autoantigen. J. Exp. Med. 1995;182:743–750. doi: 10.1084/jem.182.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond B, Katz JB, Paul E, Aranow C, Lustgarten D, Scharff MD. The role of somatic mutation in the pathogenic anti-DNA response. Annu. Rev. Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- 12.Alabyev B, Rahman ZS, Manser T. Quantitatively reduced participation of anti-nuclear antigen B cells that down-regulate B cell receptor during primary development in the germinal center/memory B cell response to foreign antigen. J. Immunol. 2007;178:5623–5634. doi: 10.4049/jimmunol.178.9.5623. [DOI] [PubMed] [Google Scholar]
- 13.Rahman ZS, Alabyev B, Manser T. FcgammaRIIB regulates autoreactive primary antibody-forming cell, but not germinal center B cell, activity. J. Immunol. 2007;178:897–907. doi: 10.4049/jimmunol.178.2.897. [DOI] [PubMed] [Google Scholar]
- 14.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J. Exp. Med. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santulli-Marotto S, Retter MW, Gee R, Mamula MJ, Clarke SH. Autoreactive B cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 1998;8:209–219. doi: 10.1016/s1074-7613(00)80473-2. [DOI] [PubMed] [Google Scholar]
- 16.Paul E, Lutz J, Erikson J, Carroll MC. Germinal center checkpoints in B cell tolerance in 3H9 transgenic mice. Int. Immunol. 2004;16:377–384. doi: 10.1093/intimm/dxh035. [DOI] [PubMed] [Google Scholar]
- 17.William J, Euler C, Primarolo N, Shlomchik MJ. B cell tolerance checkpoints that restrict pathways of antigen-driven differentiation. J. Immunol. 2006;176:2142–2151. doi: 10.4049/jimmunol.176.4.2142. [DOI] [PubMed] [Google Scholar]
- 18.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat. Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 19.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J. Exp. Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J. Clin. Invest. 1998;101:1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis: Sle2 on murine chromosome 4 leads to B cell hyperactivity. J. Immunol. 1997;159:454–465. [PubMed] [Google Scholar]
- 25.Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of Sle pathogenesis: Sle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. J. Immunol. 1999;162:6492–6502. [PubMed] [Google Scholar]
- 26.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 29.Heltemes-Harris L, Liu X, Manser T. Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype. J. Immunol. 2004;172:823–833. doi: 10.4049/jimmunol.172.2.823. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Manser T. Antinuclear antigen B cells that down-regulate surface B cell receptor during development to mature, follicular phenotype do not display features of anergy in vitro. J. Immunol. 2005;174:4505–4515. doi: 10.4049/jimmunol.174.8.4505. [DOI] [PubMed] [Google Scholar]
- 31.Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J. Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Alabyev B, Vuyyuru R, Manser T. Influence of Fas on the regulation of the response of an anti-nuclear antigen B cell clonotype to foreign antigen. Int. Immunol. 2008;20:1279–1287. doi: 10.1093/intimm/dxn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman ZSM, Manser T. Failed up-regulation of the inhibitory IgG Fc receptor FcγRIIB on germinal center B cells in autoimmune-prone mice is not associated with deletion polymorphisms in the promoter region of the FcγRIIB gene. J. Immunol. 2005;175:1440–1449. doi: 10.4049/jimmunol.175.3.1440. [DOI] [PubMed] [Google Scholar]
- 34.Rahman ZS, Niu H, Perry D, Wakeland E, Manser T, Morel L. Expression of the autoimmune Fcgr2b NZW allele fails to be upregulated in germinal center B cells and is associated with increased IgG production. Genes Immun. 2007;8:604–612. doi: 10.1038/sj.gene.6364423. [DOI] [PubMed] [Google Scholar]
- 35.Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J. Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- 36.Vora KA, Tumas-Brundage KM, Lentz VM, Cranston A, Fishel R, Manser T. Severe attenuation of the B cell immune response in Msh2-deficient mice. J. Exp. Med. 1999;189:471–482. doi: 10.1084/jem.189.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman ZSM, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J. Exp. Med. 2003;198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Notidis E, Heltemes L, Manser T. Dominant, hierarchical induction of peripheral tolerance during foreign antigen-driven B cell development. Immunity. 2002;17:317–327. doi: 10.1016/s1074-7613(02)00392-8. [DOI] [PubMed] [Google Scholar]
- 39.Siekevitz M, Gefter ML, Brodeur P, Riblet R, Marshak-Rothstein A. The genetic basis of antibody production: the dominant anti-arsonate idiotype response of the strain A mouse. Eur. J. Immunol. 1982;12:1023–1032. doi: 10.1002/eji.1830121208. [DOI] [PubMed] [Google Scholar]
- 40.MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 41.Rahman ZSM, Manser T. B cells expressing Bcl-2 and a signaling-impaired BAFF-specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J. Immunol. 2004;173:6179–6188. doi: 10.4049/jimmunol.173.10.6179. [DOI] [PubMed] [Google Scholar]
- 42.Camacho SA, Kosco-Vilbois MH, Berek C. The dynamic structure of the germinal center. Immunol. Today. 1998;19:511–514. doi: 10.1016/s0167-5699(98)01327-9. [DOI] [PubMed] [Google Scholar]
- 43.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 44.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J. Immunol. 2002;169:2694–2700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 45.Cuda CM, Wan S, Sobel ES, Croker BP, Morel L. Murine lupus susceptibility locus Sle1a controls regulatory T cell number and function through multiple mechanisms. J. Immunol. 2007;179:7439–7447. doi: 10.4049/jimmunol.179.11.7439. [DOI] [PubMed] [Google Scholar]
- 46.Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- 47.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J. Immunol. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 48.Chang CC, Ye BH, Chaganti RS, Dalla-Favera R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6947–6952. doi: 10.1073/pnas.93.14.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 50.Sobel ES, Mohan C, Morel L, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J. Immunol. 1999;162:2415–2421. [PubMed] [Google Scholar]
- 51.Morel L, Tian XH, Croker BP, Wakeland EK. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 1999;11:131–139. doi: 10.1016/s1074-7613(00)80088-6. [DOI] [PubMed] [Google Scholar]
- 52.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 53.Wakeland EK, Wandstrat AE, Liu K, Morel L. Genetic dissection of systemic lupus erythematosus. Curr. Opin. Immunol. 1999;11:701–707. doi: 10.1016/s0952-7915(99)00039-4. [DOI] [PubMed] [Google Scholar]
- 54.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Cuda C, Morel L. Genetic determination of T cell help in loss of tolerance to nuclear antigens. J. Immunol. 2005;174:7692–7702. doi: 10.4049/jimmunol.174.12.7692. [DOI] [PubMed] [Google Scholar]
- 56.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J. Immunol. 2002;169:2694–2700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Y, Hirose S, Sanokawa-Akakura R, Abe M, Mi X, Li N, Miura Y, Shirai J, Zhang D, Hamano Y, Shirai T. Genetically determined aberrant down-regulation of FcgammaRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int. Immunol. 1999;11:1685–1691. doi: 10.1093/intimm/11.10.1685. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Y, Hirose S, Abe M, Sanokawa-Akakura R, Ohtsuji M, Mi X, Li N, Xiu Y, Zhang D, Shirai J, Hamano Y, Fujii H, Shirai T. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics. 2000;51:429–435. doi: 10.1007/s002510050641. [DOI] [PubMed] [Google Scholar]
- 59.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]