Abstract
The GTPase dynamin is essential for clathrin-mediated endocytosis (CME), but its exact function and mechanism of action has been controversial. Here we review findings that have led to current models for dynamin function, either as a mechanochemical enzyme driving membrane fission or as a regulatory GTPase monitoring rate limiting steps in CME. However, these models are not mutually exclusive and subsequent studies have provided evidence for both dynamin functions. Here we present recent evidence derived from divergent in vivo and in vitro approaches that dynamin plays a dual role in CME, functioning at early stages as a fidelity monitor to regulate clathrin-coated pit maturation and at later stages to directly catalyze membrane fission and clathrin-coated vesicle formation.
Keywords: clathrin-mediated endocytosis, dynamin, fission, vesicle formation
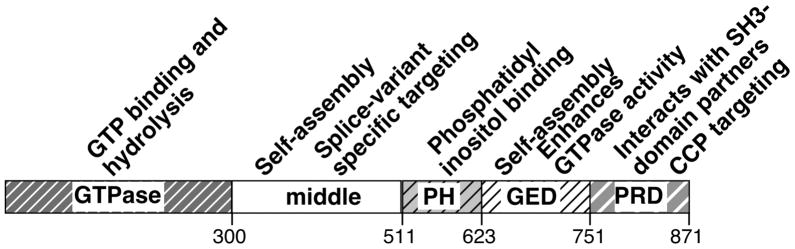
The GTPase dynamin controls several distinct endocytic pathways, with clathrin-mediated endocytosis (CME) being the best studied. These dynamin-mediated endocytic pathways function critically in many aspects of cell and organismal physiology, including nutrient uptake, immune presentation, regulation of signal transduction pathways, synaptic vesicle recycling, and regulation of surface transporters to control serum homeostasis [1]. Dynamin is a 96kD, multi-domain GTPase (Fig 1) that is composed of an N-terminal catalytic GTPase domain, a middle domain involved in assembly [2] and intracellular targeting [3], a pleckstrin-homology (PH) domain that binds to PI4,5P2 and other phosphatidylinositol lipids [4], a GTPase effector domain (GED) required for self-assembly and assembly-stimulated GTPase activity [5] and a C-terminal proline, arginine-rich domain (PRD) that binds to several SH3 domain containing protein partners [6]. Dynamin is an atypical GTPase that binds GTP with low affinity and hydrolyzes GTP at a robust basal rate of ~1 min−1[7]. Dynamin’s GTPase activity can be stimulated >100-fold upon self-assembly into helical arrays on lipid templates [8]. Despite dynamin’s clear importance in CME and extensive research over the past 15 years, a clear consensus regarding its exact role and mechanism of action has yet to emerge.
Figure 1. Domain structure and function of dynamin.
Dynamin is a multidomain GTPase, containing an N-terminal GTPase domain, a middle domain, a pleckstrin homology (PH) domain, a GTPase effector domain (GED) and a C-terminal proline-arginine rich domain (PRD), whose functions are indicated. Dynamin exists in solution as a tetramer.
Dynamin was first identified as a MT-based motor protein
‘Dynamin’ (derived from ‘dynamic’) was named to reflect its molecular motor-like properties [9]. Indeed, it was first isolated as a microtubule (MT)-binding protein that was released in the presence of ATP and exhibited MT-dependent ATPase activity. Dynamin bundled MTs and upon addition of ATP caused them to slide past each other [9], although this latter activity required the presence of a side fraction later suspected to harbor nucleoside diphosphokinase activity, as well as other motor contaminants [10]. Two surprises followed this initial study. Cloning and sequencing of dynamin revealed it to be a member of the GTPase superfamily, and not an ATP-dependent motor protein as originally thought [11]. Subsequently, dynamin was found to be 70% identical to shibire [12,13], a Drosophila gene product linked to synaptic vesicle recycling and endocytosis. As MTs are not present in the synapse, a requirement for a MT-based motor in synaptic vesicle endocytosis was unexpected.
To determine whether mammalian dynamin was indeed required for endocytosis and whether its function was related to potential MT motor activities, we cloned human dynamin and generated and characterized the effects of overexpression of the now widely used dyn(K44A) mutant [14]. We chose this mutation because Lys44 is present in the active site P-loop conserved in both GTPases and ATP-dependent motor proteins: K->A mutations decrease nucleotide binding and generate dominant-negative mutants of both classes of proteins. In the case of motor proteins, like kinesin, the protein is locked in the ‘rigor’ state and binds tightly to MTs when expressed in vivo [15]. Our analyses of stable cell lines expressing dyn(K44A) under inducible tetracycline-expression system revealed that dynamin was selectively required for CME and not for general fluid phase uptake [16]. Immuno-electron microscopy revealed that endogenous dynamin was localized to clathrin coated pits on the cell surface. Importantly, we found no effect on MT structure and no association of dyn(K44A) with MTs in these cells, suggesting that this in vitro interaction may not be relevant in vivo.
Two models for dynamin function in endocytosis
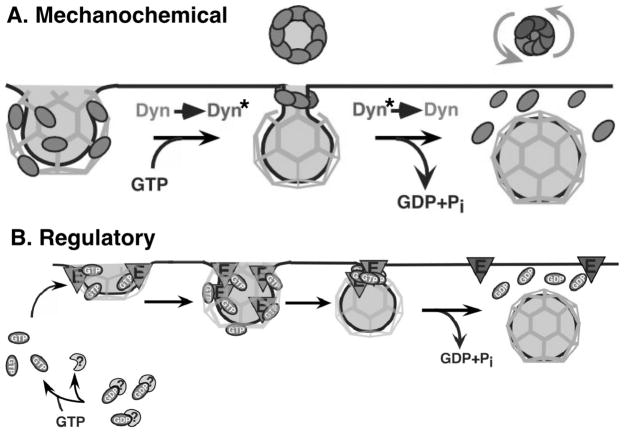
Having firmly established dynamin’s role in CME, we and others have since endeavored to understand its mechanism of action. The discovery that purified recombinant dynamin self-assembles into rings and helical stacks of rings [17] and the localization of dynamin to collar-like structures that accumulate on membranes of permeabilized synaptosomes treated with the nonhydrolyzable GTP analogue, GTPγS [18] led us to propose a model (Figure 2A) in which dynamin, triggered by GTP binding, assembles at the necks of invaginated coated pits causing constriction. We proposed that subsequent assembly-stimulated GTP hydrolysis causes a concerted conformational change to generate force required for membrane fission [17,19]. This model was consistent with previous findings that nonhydrolyzable GTP analogues supported the formation of constricted coated pits in perforated cells, and that GTP hydrolysis was required for vesicle formation [20].
Figure 2. Two models for dynamin function in clathrin mediated endocytosis.
(A) Model 1: Dynamin functions as a mechanochemical GTPase. GTP binding triggers dynamin assembly into collars and coordinated, assembly-stimulated GTP hydrolysis triggers a concerted conformation change to mediate fission. (B) Model 2: Dynamin functions as a regulatory GTPase. GTP-bound dynamin controls effectors that mediate vesicle formation. Self-assembly activates an internal GAP domain (GED) that negatively regulates dynamin function and terminates dynamin-effector interactions.
In a series of studies to test this hypothesis and further explore the mechanism of dynamin function, we showed that dynamin self-assembly in solution indeed stimulates its GTPase activity, that self-assembly is regulated by GTP binding and hydrolysis, and that guanine nucleotides regulate the distribution of dynamin on clathrin coated pits [21–23]. Through limited proteolysis, we identified a distal domain of dynamin, termed the GTPase effector domain (GED) that was required for self-assembly and assembly-stimulated GTPase activity [24] and showed that isolated recombinant GED could stimulate dynamin’s GTPase activity [5]. Using this E. coli-expressed GED as a facile platform for mutagenesis, we were able to identify two GED mutants K694A and R725A that selectively impaired self-assembly and hence dynamin’s assembly-stimulated GTPase activity without affecting GTP binding or dynamin’s basal rate of GTP hydrolysis [5]. The assembly-dependent activation of GED provided a mechanism by which self-assembly could be coupled to GTP hydrolysis to generate the mechanochemical force we had hypothesized was required for membrane fission. However, we were once again surprised to find that overexpression of these GED mutants (DynK694A and DynR725A) stimulated rather than inhibited CME [5,25]. These findings strongly suggested that dynamin, like most other GTPase superfamily members, was negatively regulated by self-assembly and GED-accelerated GTP hydrolysis. Based on these unexpected findings we proposed a second model in which dynamin functions as a regulatory GTPase (Figure 2B).
As originally formulated, these two models were seemingly incompatible and consequently there has been much debate regarding their respective merits [26–30]. However, these models are not mutually exclusive. Indeed, considerable evidence has now accumulated to support both aspects of dynamin function.
Experimental evidence supporting dynamin’s role in membrane fission
In support of a mechanochemical function in fission, the Hinshaw and DeCamilli labs showed that dynamin binds to liposomes, causes tubulation and undergoes force-generating conformational changes upon GTP binding and hydrolysis [31,32]. The nature of the conformational change observed, however, was dependent on the assay conditions. Hence, when bound either to phosphatidylserine liposomes [31] or to liposomes composed of brain polar lipids supplemented with PI4,5P2, assembled dynamin underwent a constriction that apparently resulted in vesiculation of the dynamin-coated tubules as detected by changes in light scattering and electron microscopy [31,33]. However, later studies showed that the vesiculation observed after EM analysis may have been an artifact due to constraining the tubules on an adherent surface [34,35], and that the changes in light scattering may have reflected GTP-dependent dissociation of dynamin from the membrane tubules (Ramachandran and Schmid, 2008). A distinct global conformation change was observed when dynamin was assembled onto more rigid, PI4,5P2-containing lipid nanotubules. In this case, GTP hydrolysis caused an increase in helical pitch suggested to cause vesicle release [8]. Finally, a third mechanochemical activity of dynamin was observed in which GTP hydrolysis caused assembled dynamin molecules to undergo a rotation around coated membrane tubules. This twisting action was proposed to drive fission of the underlying membrane [35], but only if external tension was applied to hold the membrane tubule taut. Despite their differences, a clear consensus emerged from these studies that dynamin is a mechanochemical enzyme, but that this mechanochemical activity on its own was insufficient to drive membrane fission.
Each of these nucleotide-driven conformational changes were observed upon addition of GTP after preassembling long dynamin spirals on lipid templates. Using fluorescence-based assays to study the dynamic interaction of dynamin with liposomes, we found that GTP triggered the rapid disassembly of preassembled dynamin (Ramachandran and Schmid, 2008). Under more physiological conditions, in the constant presence of GTP, dynamin undergoes continuous cycles of assembly, GTP hydrolysis and disassembly. To examine dynamin’s potential role in membrane fission, we developed a versatile new assay using supported lipid bilayers with excess membrane reservoir on 5 μm silica beads, or SUPER templates. These templates enable us to both visualize dynamin’s effects on the adsorbed lipid bilayer and to quantitatively measure membrane fission by the release of fluorescently-labeled lipids into the supernatant of a low speed spin. We found that when dynamin was added to SUPER templates in the constant presence of GTP–mirroring physiological conditions–it catalyzed multiple rounds of fission and the release of small vesicles (70–90 nm in diameter) into the supernatant. By contrast, if we incubated dynamin with SUPER templates in the absence of nucleotides, dynamin generated long membrane tubules that remained attached to the beads. Addition of GTP led to the rapid dissociation of dynamin and retraction of the tubules without fission, unless they were tethered and hence ruptured upon retraction [36]. Dynamin could be visualized during fission events and it was seen to form short collar-like structures at the necks of budding vesicles prior to fission. Complementary electrophysiological studies using membrane tubules drawn from a lipid reservoir and theoretical analyses confirmed that preassembled dynamin was unable to mediate fission and that dynamin-catalyzed membrane fission occurred stochastically during cycles of squeezing and relaxation of short dynamin collars (Bashkirov et al., 2008).
Together these studies establish a direct role for dynamin in catalyzing membrane fission. It is likely that other factors, such as coat proteins and potentially curvature generating and/or stabilizing BAR-domain containing accessory factors function together with dynamin to regulate membrane fission and to enhance its efficiency.
Experimental Evidence Supporting a Role for Dynamin in Regulating CME
CME requires dynamin’s GTPase activity and its ability to undergo GTP-dependent conformational changes [37–39]. It has been argued that these requirements support a mechanochemical role for dynamin in CME [39]; however, all GTPases, including signaling GTPases such as ras and Gα require these properties for correct cellular function. What distinguishes signaling GTPases from mechanochemical enzymes is that their activities are negatively regulated by accelerated GTP hydrolysis. Thus, the strongest evidence for a regulatory role for dynamin in endocytosis derives from genetic studies in Drosophila. In a screen for suppressors of the shits2 allele, Ramaswami and colleagues isolated 4 suppressor of shibire (Sushi) mutants that mapped to the shibire allele [40]. The original shits2 mutation corresponds to G146S in the conserved switch 2 region of the GTPase domain that, for other GTPases, is involved in nucleotide-dependent interactions with effectors and/or GAPs. Strikingly, two second site Sushi mutations that fully rescue dynamin function in vivo, mapped to dynamin’s GTPase effector domain, GED [41]. Biochemical analysis of purified dynamin bearing the single ts2 GTPase domain mutant revealed a temperature-sensitive defect in GTP binding that correlated with the temperature-sensitive loss of function in vivo [41]. As expected, neither of the second site Sushi mutations in GED (i.e. G146S/A738T or G146S/T749I) corrected the GTP binding defect, instead these mutations impaired dynamin’s basal and assembly-stimulated GTPase activities without altering dynamin’s ability to self-assemble. The finding that reduced GTPase activity restores function of a GTP-binding impaired mutant protein suggests, as for other regulatory GTPases, that accelerated GTP hydrolysis, which is dependent on GED function, negatively regulates dynamin function in vivo.
Dynamin’s function in regulating early stages of endocytosis has also been directly detected using quantitative live cell total internal reflection fluorescence microscopy (TIR-FM) to monitor CCP maturation and CCV formation. Using new particle tracking and gap-closing algorithms [42] we were able to establish an unbiased and complete inventory of CCP trajectories visible by TIR-FM [43]. Statistical analyses of the life-time distributions identified three dynamically distinct CCP subpopulations: two short-lived subpopulations corresponding to aborted intermediates, and one longer-lived productive subpopulation. The proportion of productive CCPs increased upon overexpression of transferrin receptor, a model cargo molecule, at the expense of abortive species and in a manner dependent on AP2 adaptor protein concentration. Partial knock-down of dynamin-2 prolonged the lifetime of productive CCPs, consistent with previous findings showing that dynamin controlled rate-limiting steps in CME [25]. Interestingly, dynamin knock-down also prolonged the life time of abortive CCPs and reconstitution with wt and mutant dynamin molecules increased or decreased the turnover of abortive CCPs in a manner dependent on basal GTP binding and hydrolysis properties. From these data we have inferred the existence of an endocytic ‘restriction-’ or ‘check-point’ that monitors and gates CCP maturation and governs the rate of CME [43]. In the course of CCV formation, dynamin interacts with numerous SH3 domain-containing proteins, whose other domains interact with coat proteins (e.g. amphiphysin, SNX9), cargo molecules (e.g. Grb2, SNX9) and/or sense and generate membrane curvature (e.g. amphiphysin, endophilin, SNX9, syndapin). Thus, we propose that these binding partners function as sensors that respond to upstream molecular events and determine progression beyond the dynamin-governed restriction point.
A dual role for dynamin in endocytic clathrin-coated vesicle formation
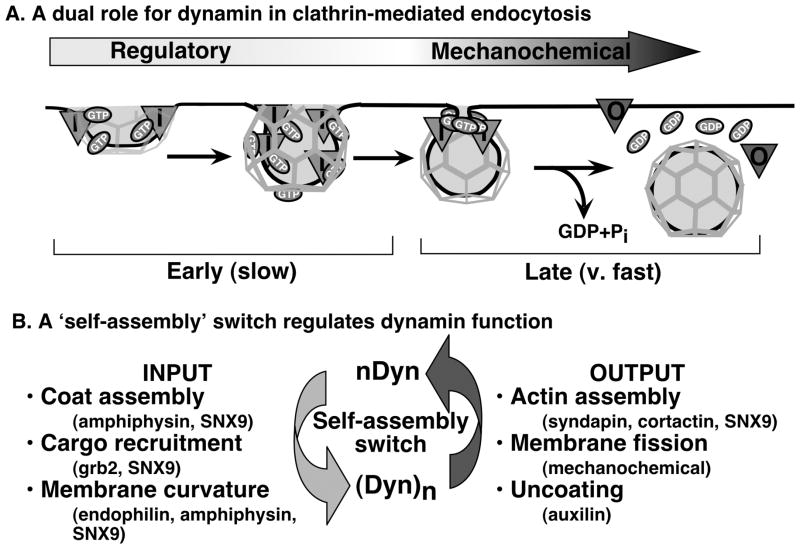
To reconcile these collective findings we propose a model in which dynamin plays a dual role in CME (Figure 3). We suggest that early, rate-limiting steps of endocytosis are controlled by unassembled dynamin, which is targeted to coated pits and functions either as a timer, a fidelity monitor and/or to ensure vectoriality over the processes of coat assembly, cargo capture and membrane curvature formation. This early function of unassembled dynamin depends on its basal rate of GTP hydrolysis and is negatively regulated by GED. At late stages of CCV formation, dynamin self-assembles into a short, transient collar around the neck of deeply invaginated, fully mature coated pits and catalyzes membrane fission. Implicit in this model is that the ‘switch’ of dynamin from one functional state to another corresponds not to GDP dissociation, GTP binding and hydrolysis, as for classical GTPases, but to an acute transition from the unassembled to the assembled state. This assembly switch should be tightly regulated and further work is necessary to identify factors, which presumably include dynamin’s SH3 domain-containing partners, that impinge on this critical transition.
Figure 3. Dynamin plays a dual role in clathrin mediated function.
(A) We propose a new reconciliatory model for dynamin function and suggest that dynamin plays a dual role in CME with sequentially function. Dynamin functions in early, rate-limiting stages of clathrin coated pit (CCP) maturation as a regulatory GTPase/fidelity monitor that receives input from SH3 domain-containing partners that monitor coat assembly, cargo concentration and curvature generation. Subsequently, dynamin functions as a self-limited, assembly-stimulated GTPase collar that catalyzes membrane fission. (B) We propose that self-assembly – the ‘switch’ between dynamin functional states – is regulated by SH3 domain-containing dynamin partners that sense critical events in CCV maturation. Through other partners, dynamin might also trigger late events in CME such as actin polymerization or uncoating.
Acknowledgments
Funding
Work in the Schmid laboratory was supported by NIH grants GM42455, GM73165 and MH61345. M. Mettlen and R. Ramachandran were supported by fellowships from the American Heart Association, T. Pucadyil was supported by a fellowship from the Leukemia and Lymphoma Society. This is TSRI manuscript number
Abbreviations
- CME
clathrin-mediated endocytosis
- CCP
clathrin-coated pit
- CCV
clathrin-coated vesicle
- TIR-FM
total internal reflection fluorescence microscopy
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran R, Surka M, Chappie JS, Fowler DM, Foss TR, Song BD, Schmid SL. The dynamin middle domain is critical for tetramerization and higher-order self-assembly. EMBO J. 2007;26:559–566. doi: 10.1038/sj.emboj.7601491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu YW, Surka MC, Schroeter T, Lukiyanchuk V, Schmid SL. Isoform and splice-variant specific functions of dynamin-2 revealed by analysis of conditional knock-out cells. Mol Biol Cell. 2008;19:5347–5359. doi: 10.1091/mbc.E08-08-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarar D, Surka MC, Leonard MC, Schmid SL. Snx9 activities are regulated by multiple phosphoinositides through both px and bar domains. Traffic. 2008;9:133–146. doi: 10.1111/j.1600-0854.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 5.Sever S, Muhlberg AB, Schmid SL. Impairment of dynamin’s GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–486. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]
- 6.Schmid SL, McNiven MA, De Camilli P. Dynamin and its partners: A progress report. Curr Opin Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- 7.Song BD, Schmid SL. A molecular motor or a regulator? Dynamin’s in a class of its own. Biochemistry. 2003;42:1369–1376. doi: 10.1021/bi027062h. [DOI] [PubMed] [Google Scholar]
- 8.Stowell MHB, Marks B, Wigge P, McMahon HT. Nucleotide-dependent conformational changes in dynamin: Evidence for a mechanochemical molecular spring. Nat Cell Biol. 1999;1:27–32. doi: 10.1038/8997. [DOI] [PubMed] [Google Scholar]
- 9.Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 10.Maeda K, Nakata T, Noda Y, Sato-Yoshitake R, Hirokawa N. Interaction of dynamin with microtubules: Its structure and GTPase activity investigated by using highly purified dynamin. Mol Biol Cell. 1992;3:1181–1194. doi: 10.1091/mbc.3.10.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obar RA, Collins CA, Hammarback JA, Shpetner HS, Vallee RB. Molecular cloning of the microtubule associated mechanochemical enzyme dynamin reveals homology with a new family of gtp binding proteins. Nature. 1990;347:256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- 12.Chen MS, Ober RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 13.van der Bliek AM, Meyerowitz EM. Dynamin like protein encoded by the drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 14.van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meluh PB, Rose MD. Kar3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990;60:1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- 16.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinshaw JE, Schmid SL. Dynamin self assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 18.Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTPγs in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 19.Warnock DE, Schmid SL. Dynamin GTPase, a force generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- 20.Carter LL, Redelmeier TE, Woollenweber LA, Schmid SL. Multiple gtp-binding proteins participate in clathrin-coated vesicle-mediate endocytosis. J Cell Biol. 1993;120:37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warnock DE, Baba T, Schmid SL. Ubiquitously expressed dynamin-II has a higher intrinsic GTPase activity and a greater propensity for self-assembly than neuronal dynamin-I. Mol Biol Cell. 1997;8:2553–2562. doi: 10.1091/mbc.8.12.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warnock DE, Hinshaw JE, Schmid SL. Dynamin self assembly stimulates its GTPase activity. J Biol Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- 23.Warnock DE, Terlecky LJ, Schmid SL. Dynamin GTPase is stimulated by crosslinking through the C terminal proline rich domain. EMBO J. 1995;14:1322–1328. doi: 10.1002/j.1460-2075.1995.tb07118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhlberg AB, Warnock DE, Schmid SL. Domain structure and intramolecular regulation of dynamin GTPase. EMBO J. 1997;16:6676–6683. doi: 10.1093/emboj/16.22.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sever S, Damke H, Schmid SL. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J Cell Biol. 2000;150:1137–1148. doi: 10.1083/jcb.150.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Cerione RA. Endocytosis: Is dynamin a ‘blue collar’ or ‘white collar’ worker? Curr Biol. 1999;9:R511–514. doi: 10.1016/s0960-9822(99)80323-6. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhausen T. Boa constrictor or rattlesnake? Nature. 1999;398:470–471. doi: 10.1038/18989. [DOI] [PubMed] [Google Scholar]
- 28.van der Bliek AM. Is dynamin a regular motor or a master regulator? Trends Cell Biol. 1999;9:253–254. doi: 10.1016/s0962-8924(99)01591-3. [DOI] [PubMed] [Google Scholar]
- 29.McNiven MA, Cao H, Pitts KR, Yoon Y. The dynamin family of mechanoenzymes: Pinching in new places. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 30.Sever S, Damke H, Schmid SL. Garrotes, springs, ratchets and whips: Putting dynamin models to the test. Traffic. 2000;1:385–392. doi: 10.1034/j.1600-0854.2000.010503.x. [DOI] [PubMed] [Google Scholar]
- 31.Sweitzer S, Hinshaw J. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 32.Takei K, Mundigl O, Daniell L, De CP. The synaptic vesicle cycle: A single vesicle budding step involving clathrin and dynamin. J Cell Biology. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin- mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 34.Danino D, Moon KH, Hinshaw JE. Rapid constriction of lipid bilayers by the mechanochemical enzyme dynamin. J Struct Biol. 2004;147:259–267. doi: 10.1016/j.jsb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Roux A, Uyhazi K, Frost A, De Camilli P. Gtp-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 36.Pucadyil TJ, Schmid SL. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–1275. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damke H, Binns DD, Ueda H, Schmid SL, Baba T. Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell. 2001;12:2578–2589. doi: 10.1091/mbc.12.9.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marks B, Stowell MHB, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–235. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 40.Ramaswami M, Rao S, van der Bliek A, Kelly RB, Krishnan KS. Genetic studies on dynamin function in Drosophila. J Neurogenetics. 1993;9:73–87. doi: 10.3109/01677069309083451. [DOI] [PubMed] [Google Scholar]
- 41.Narayanan R, Leonard M, Song BD, Schmid SL, Ramaswami M. An internal GAP domain negatively regulates presynaptic dynamin in vivo: A two-step model of dynamin function. J Cell Biol. 2005;169:117–126. doi: 10.1083/jcb.200502042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaqaman K, Loerke D, Mettlen M, Kuwata H, Grinstein S, Schmid SL, Danuser G. Robust single-particle tracking in live-cell time-lapse sequences. Nature methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, Schmid SL. Cargo and dynamin regulate clathrin coated pit maturation. PLoS Biology. 2009 doi: 10.1371/journal.pbio.1000057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]