Abstract
Initiation of an adaptive cellular immune response depends on intimate interactions with antigen-presenting cells and naive T lymphocytes. We have previously reported that activation of naive Mycobacterium tuberculosis-specific CD4+ T cells depends on dendritic cell (DC) transport of live bacteria from the lungs to the mediastinal lymph node (MDLN). Since the migratory paths of DCs are largely governed by the chemokine receptor CCR7, which is expressed on DCs upon maturation by proinflammatory stimuli, we examined the quantitative contribution of CCR7-dependent DC migration in the context of tuberculosis, and found that early trafficking of DCs from the lungs to the MDLN depended on CCR7-mediated signaling, but alternative mechanism(s) are employed later in infection. Impaired migration of DCs in CCR7−/− mice resulted in delayed dissemination of bacteria to MDLN and spleen, and in delayed kinetics of activation of adoptively-transferred Ag85B-specific CD4+ T cells. Furthermore, in contrast to control mice, we found that naive Ag85B-specific CD4+ T cells are activated to proliferate in the lungs of CCR7−/− mice and, when infected with higher doses of bacteria, resistance to M. tuberculosis infection in CCR7−/− mice is compromised compared to wild type mice.
Keywords: dendritic cells, bacterial, chemokines, cell activation, cell trafficking
Introduction
The protective response to M. tuberculosis relies on cell mediated immunity. Humans and experimental animals infected with M. tuberculosis exhibit robust Ag-specific CD4+ Th1 and CD8+ T lymphocyte responses to M. tuberculosis Ags (1–6), implying that the requisite steps for priming and differentiation of T lymphocytes are functional in the setting of tuberculosis.
One characteristic of the adaptive immune response to tuberculosis is the long interval required for its development compared with the response to immunization or to other infections. Using live smallpox and yellow fever vaccines as models of acute viral infection in humans Miller et al (7) have recently shown that antiviral T cell responses occur rapidly, peaking around 2 weeks after immunization. In contrast, development of adaptive immunity to tuberculosis in humans, assayed by tuberculin skin test reactivity, requires up to 5–6 weeks after infection (8). In mouse models of infection, we and others have shown that the earliest M. tuberculosis-specific CD4+ T cell response develops in the lung-draining mediastinal lymph nodes (MDLN) between 10–12 days after aerosol infection and only after dissemination of live M. tuberculosis to the MDLN has taken place (2, 9–11). These studies imply that initiation of an adaptive immune response to M. tuberculosis depends on transport of live bacteria from the lungs to the mediastinal lymph node. In an effort to elucidate the mechanisms of dissemination of the bacteria, we have recently reported evidence that dendritic cells (DCs) transport M. tuberculosis from the lungs to the MDLN (12).
Migration of antigen presenting cells (APCs) and T cells, which converge in the T cell zones of secondary lymphoid organs, is largely governed by the chemokine receptor CCR7 (13), therefore, CCR7 has been suggested to play an important role in the initiation of adaptive immune responses. CCR7 is expressed on B cells, naive T cells and on mature DCs (14–16) and its two chemokine ligands, CCL19 and CCL21, are highly expressed by stromal cells in the T cell-rich lymph node areas (17, 18).
The requirement for CCR7 expression in mounting an immune response against distinct pathogens and antigens varies (Reviewed in (19)). For example, although CCR7−/− mice develop normal antibody responses upon infection with vesicular stomatitis virus (VSV), CCR7 is crucial for virus neutralizing B cell responses after immunization with limiting amounts of antigen (20). CCR7−/− mice also develop protective immune responses to lymphocytic choriomeningitis virus (LCMV) (21) and are relatively resistant to primary and secondary infection with Listeria monocytogenes (22). Regarding infection with M. tuberculosis, it was recently reported that mice lacking CCR7 are capable of controlling pulmonary tuberculosis and that unique characteristics of lymphoid-organ functionality are induced in the lungs of these mice during chronic pulmonary tuberculosis (23). The expression of the homeostatic chemokine CXCL13 within these structures and the presence of follicular DCs (FDCs) and HEVs suggested that M. tuberculosis induces lymphoid neo-organogenesis in the lungs of CCR7−/− mice (23). While that study established that CCR7-deficient mice can generate an immune response to M. tuberculosis, it did not determine the mechanisms underlying the initiation of the immune response. Since the adaptive immune response to M. tuberculosis in immunocompetent mice is initiated in the mediastinal lymph node and not in the lungs (9, 10) and since CCR7 is believed to coordinate migration of T cells and dendritic cells during cellular immune responses (24), we investigated the mechanisms underlying initiation of an antigen-specific CD4+ T cell response in CCR7−/− mice infected with M. tuberculosis.
Material and Methods
Mice
P25TCR-Tg mice, whose CD4+ T cells express a transgenic T-cell antigen receptor that recognizes peptide 25 (aa 240–254) of M. tuberculosis Antigen 85B bound to I-Ab were on a C57BL/6 background, as previously described (25) and bred in the New York University School of Medicine animal facilities. C57BL/6 CCR7+/+ mice were purchased from The Jackson Laboratory or were bred and housed in the New York University School of Medicine (New York, NY) animal facilities. C57BL/6 CCR7−/− mice were obtained from A. Erlebacher, NYU School of Medicine. All mice were specific pathogen-free, and were used for experiments at 8–12 weeks of age. All animal experiments were done in accordance with procedures approved by the New York University School of Medicine Institutional Animal Care and Use Committee.
Antibodies
All Abs were purchased from BD Pharmingen unless otherwise stated. Anti-CD11c PerCP (H3L) was a custom conjugate from BD Pharmingen, and other Ab conjugates used were anti-CD11b APC and anti-CD4 PerCp. Purified anti-KN7 was provided by Dr. Kiyoshi Takatsu (University of Tokyo) and was labeled with Alexa Fluor 647 by using a mAb conjugation kit from Molecular Probes.
P25TCR-Tg CD4+ T cell isolation and labeling
P25TCR-Tg CD4+ T cells were isolated as described (9). Briefly, P25TCR-Tg mice were killed according to approved laboratory animal procedures. Lymph nodes and spleen were aseptically removed. CD4+ T cells were magnetically isolated using a CD4+ T Cell Isolation kit and an AutoMACS (Miltenyi Biotech). CD4+ T cell purity was routinely >90% as assessed by flow cytometry. For in vivo proliferation assays, CD4+ T cells were labeled with CFSE.
Adoptive transfer and infection
Mice routinely received 2.5–3 × 106 CFSE-labeled CD4+ P25TCR-Tg T cells by tail vein injection in 100 µl of sterile PBS (9). After 24 h, mice were infected with M.tuberculosis H37Rv by the aerosol route using an Inhalation Exposure Unit (Glas-Col), as previously described (12). To determine the infection dose, 4–5 mice were killed 1 day after infection and lungs were harvested and homogenized in PBS/0.5% Tween-80 and plated on 7H11 agar plates.
Tissue processing and flow cytometry
At designated time points, 4 infected mice in each group were killed, and tissues were used to prepare single-cell suspensions, as previously described (12). 5 × 106 cells were stained with anti-CD4 and anti-KN7 or with anti-CD11b and anti-CD11c antibody mixtures at a density of 1.5 × 107 cells/ml in FACS buffer (PBS, 1% FCS, 0.1% sodium azide, and 1 mM EDTA) and incubated at 4°C for 20–30 min. Cells were washed and fixed in 1% PFA overnight at 4°C. Data were acquired on a FACSCalibur. The number of cells of a specific phenotype was determined by taking the percentage of that cell type determined by flow cytometry multiplied by the number of total cells. The percentage of proliferating cells was defined as the percentage of all P25TCR-Tg CD4+ T cells that had undergone at least one cycle of replication.
Determination of bacterial load
At every time point, each tissue from each mouse was assessed for bacterial load as previously described (9).
Statistical analysis
Comparison of the number of P25TCR-Tg T cells and the number of dendritic cells in lung and MDLN and the number of bacteria in lungs, MDLN and spleen of CCR7+/+ and CCR7−/− mice was performed by unpaired Students’ t test, using Prism 4 for Macintosh (version 4.0a) from GraphPad Software. Survival results are expressed as Kaplan-Meier curves, and the values were determined using log-rank test. P values of <0.05 were considered significant.
Results
Impaired migration of dendritic cells from the lungs to the MDLN in CCR7−/− mice
To determine the role of CCR7-dependent cell migration in the context of tuberculosis, we first compared the trafficking of dendritic cells to the lungs and MDLN of CCR7+/+ and CCR7−/− mice following aerosol infection with a low dose (~100 bacteria) of M. tuberculosis.
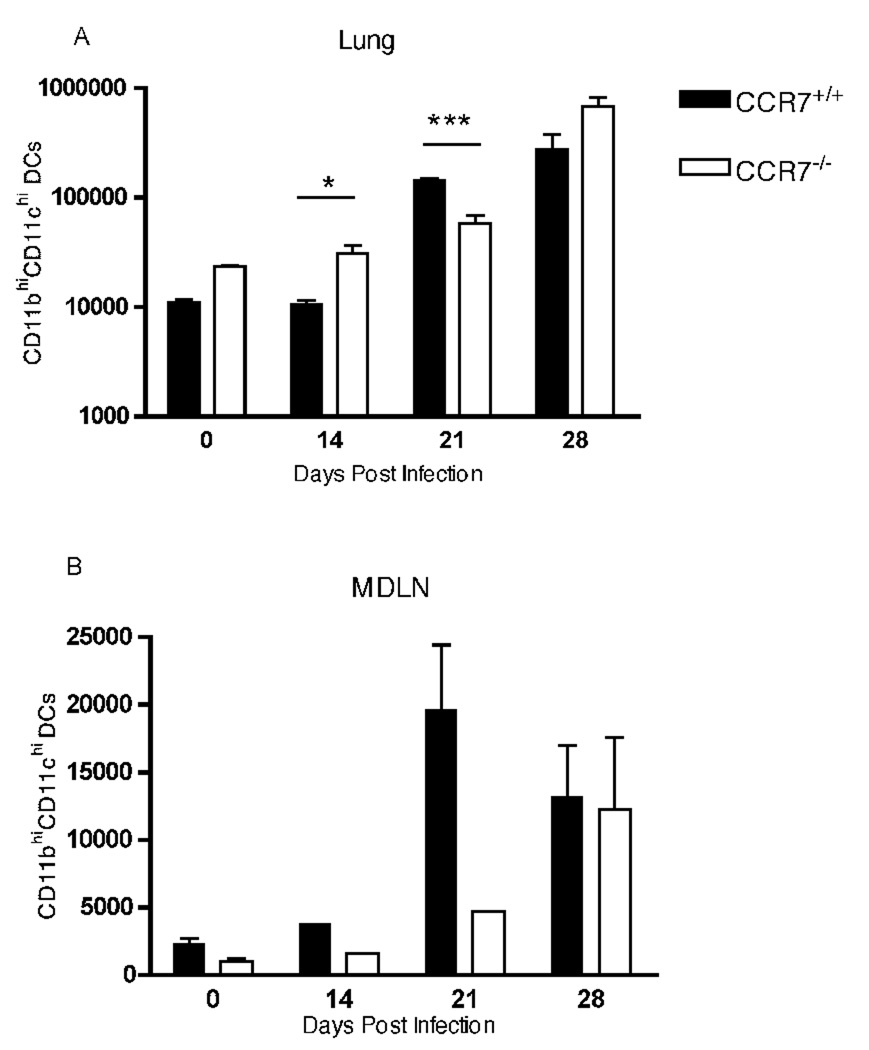
As we and others (26, 27) have previously reported, CD11chighCD11bhigh conventional dendritic cells (cDC) were recruited to the lungs after infection with M. tuberculosis (Fig. 1A). We found that there were more cDC in the lungs, and fewer cDC in the MDLN, of CCR7−/− compared with CCR7+/+ mice; this pattern persisted in the lungs, with the exception of day 21 post infection. Consistent with a recent study that demonstrated a role for CCR7 in the migration of latex bead-labeled lung DCs to the local draining lymph nodes in plt mice (28), we found that trafficking of dendritic cells from the lungs to MDLN was impaired in CCR7−/− mice (Fig. 1B). On day 14 post infection the number of CD11b+CD11c+ cells in the MDLN of CCR7−/− mice was ~ 2.5-fold lower than the number of CD11b+CD11c+ cells in the MDLN of wild type mice: 1525 cDC in CCR7−/− MDLN vs 3744 cDC in CCR7+/+ MDLN (mean cell numbers in a pool of 4 mice per group). Likewise, 21 days after infection CCR7−/− mice had 4-fold fewer DCs in the MDLN than did CCR7+/+ controls: 4639 cDC in CCR7−/− MDLN (pool of 4 mice) vs 19612 ± 9542 cDC in CCR7+/+ MDLN (mean ± SD of four mice per group). However, by day 28 post infection, the number of CD11b+CD11c+ cells in MDLN of CCR7−/− mice reached the same number as in infected CCR7+/+ mice, indicating that one or more CCR7 independent mechanisms exist for recruitment of DCs to the mediastinal lymph node, but that the alternative mechanism(s) are only employed during a later phase of infection.
FIGURE 1.
Impaired trafficking of dendritic cells from lungs to MDLN in CCR7−/− mice. CD11b+CD11c+ dendritic cells were quantitated in lungs and mediastinal lymph nodes of CCR7+/+ and CCR7−/− mice at days 14, 21 and 28 after aerosol infection with a low dose of M. tuberculosis. Unpaired Student’s t test was performed, * P = 0.035, ***P = 0.0005. In an additional independent experiment, we found similar results, and we found that the number of lung and MDLN DCs did not differ between CCR7−/− and CCR7+/+ at day 35 post infection.
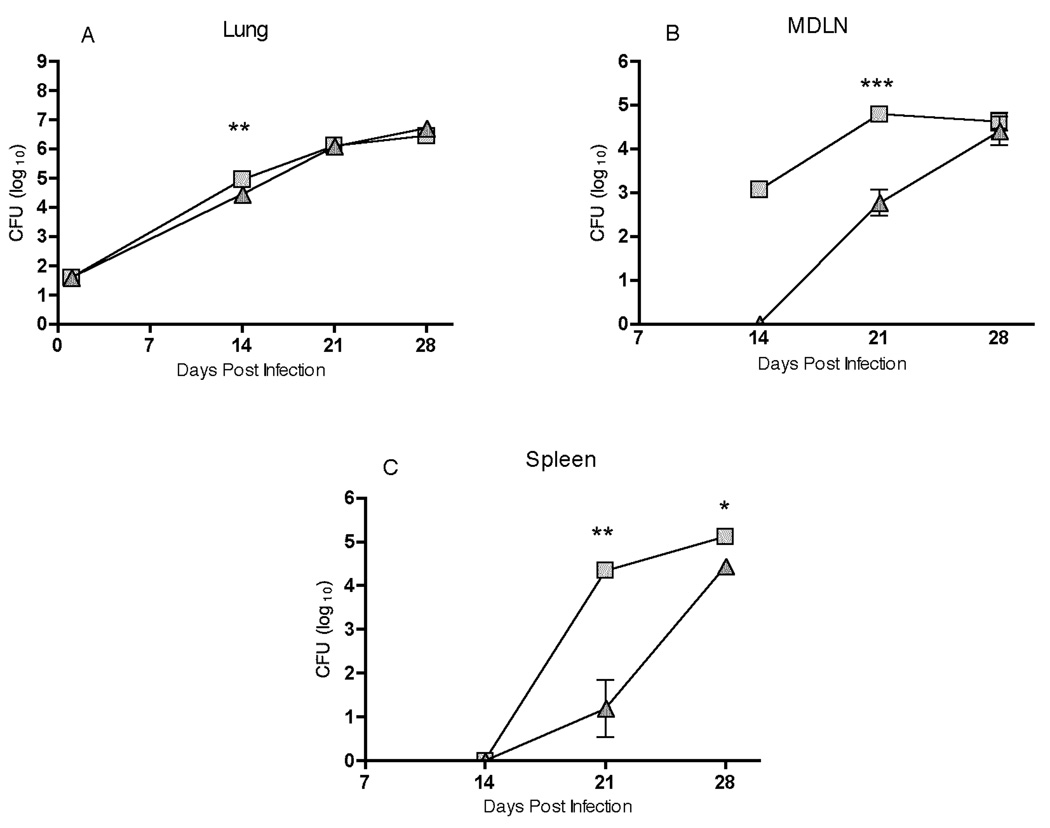
Impaired migration of DCs results in delayed dissemination of M. tuberculosis to MDLN and spleen in CCR7−/− mice
We have previously reported that DCs transport live M. tuberculosis from the lungs to the mediastinal lymph node, and that transport of the bacteria during the first 14 days of infection was defective in plt mice, which lack the CCR7 ligands CCL19 and two of the three CCL21 isoforms (12). To extend these observations, we assessed trafficking of M. tuberculosis from the lungs to the MDLN and spleen of CCR7+/+ and CCR7−/− mice after aerosol infection (Fig. 2). We observed the expected increase in bacterial numbers in the lungs of CCR7+/+ mice for the first 21 days post-infection, followed by a plateau between day 21 and day 28; the same pattern of bacterial growth was observed in lungs of CCR7−/− mice (Fig. 2A). In contrast, we observed that trafficking of M. tuberculosis to the mediastinal lymph node and the spleen was delayed in CCR7−/− compared to CCR7+/+ mice (Fig. 2B and 2C). While ~103 bacteria were detected by day 14 in the MDLN of CCR7+/+ mice, there were fewer than 10 (the lower limit of detection) bacteria in the MDLN of CCR7−/− mice at that time point. Bacteria were detectable in the MDLN of CCR7−/− mice by day 21, but there were approximately 100-fold fewer bacteria than in the MDLN of CCR7+/+ mice on day 21. Despite the initial delay observed in bacterial dissemination in CCR7−/− mice, by day 28 of infection the bacterial loads in the MDLN of both groups were indistinguishable (Fig. 2B). Delayed spread of M. tuberculosis from the lungs to the MDLN in CCR7−/− mice was also accompanied by delayed dissemination to the spleen. On day 28 of infection, the bacterial load in spleen of CCR7−/− mice was still significantly lower than in spleen of CCR7+/+ mice (Fig. 2C). These results support our previous observation that early dissemination of M. tuberculosis from the lungs to the mediastinal lymph node is due to transport by migrating DCs. They also indicate that dissemination of M. tuberculosis to the spleen does not occur until after dissemination to the mediastinal lymph node.
FIGURE 2.
Trafficking of M. tuberculosis from the lungs to the MDLN and spleen is delayed in CCR7−/− mice compared with controls. CCR7+/+ and CCR7−/− mice were sacrificed at the indicated time points after aerosol infection with a low dose of M. tuberculosis and colony-forming units were determined in homogenates of lungs, mediastinal lymph node, and spleen. Data are represented as mean ± SD of four mice per group and per time point. * P< 0.05, ** P<0.005, *** P<0.001 by unpaired Student’s t test.
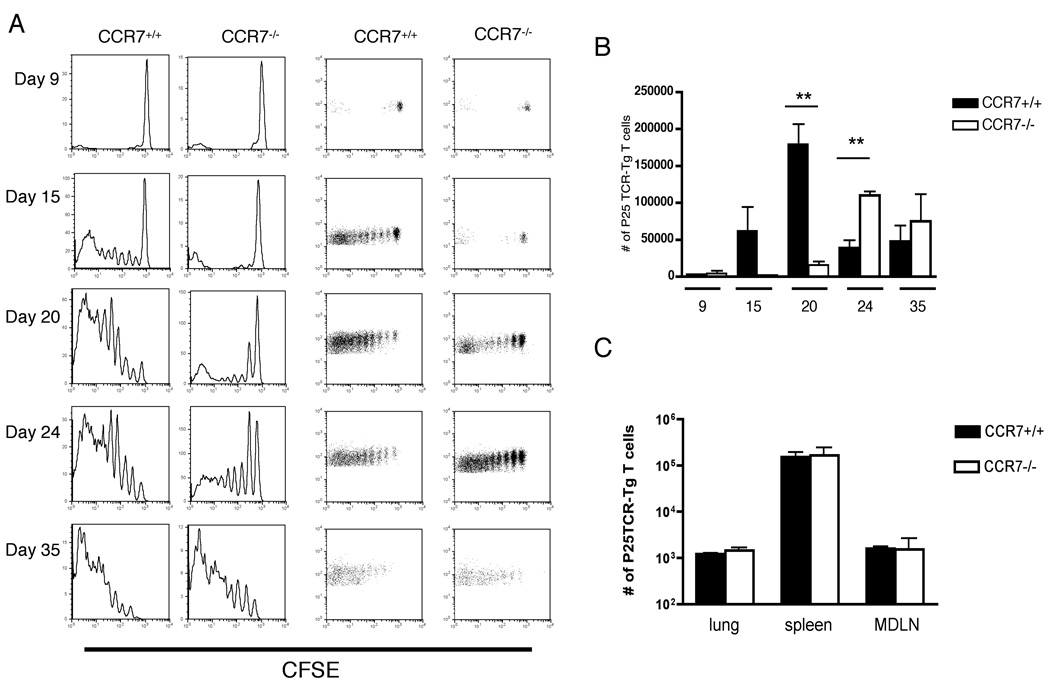
Activation of M. tuberculosis Ag85B–specific CD4+ T cells is delayed in MDLN of CCR7−/− compared to wild type mice
We have previously reported that initiation of the adaptive immune response to M. tuberculosis antigen 85B requires transport of live bacteria from the lungs to the local draining lymph node (9). Since we found that trafficking of M. tuberculosis from the lung to MDLN is delayed in CCR7−/− compared to CCR7+/+ mice, we characterized the kinetics of activation of M. tuberculosis Ag85B-specific CD4+ T cells during M. tuberculosis infection. We adoptively transferred CFSE-labeled P25TCR-Tg CD4+ T cells into CCR7+/+ and CCR7−/− mice, then infected them with a low dose of M. tuberculosis (~100 cfu/mouse). When examined 9 days after infection, fewer than 15% of the P25TCR-Tg CD4+ T cells in the MDLN of CCR7+/+ and CCR7−/− mice were CFSEdim. By day 15, proliferation of Ag85B-specific CD4+ T cells was detectable in the mediastinal lymph node of CCR7+/+ mice; more than 70% of the P25TCR-Tg CD4+ population had undergone up to 5 cycles of replication. In contrast, in the MDLN of CCR7−/− mice, fewer than 40% of the P25TCR-Tg CD4+ T population exhibited dilution of CFSE, and none had divided beyond one to two cycles of proliferation. The majority of the P25TCR-Tg CD4+ T cells in the MDLN in CCR7−/− mice remained CFSEhigh on day 20-post infection, while by day 35 of infection more than 90% of the P25TCR-Tg CD4+ T cells had proliferated in mediastinal lymph node of both wild type and CCR7−/− mice (Fig. 3A). This delay in activation was also reflected in the number of P25TCR-Tg CD4+ T cells in the mediastinal lymph node: the number of P25TCR-Tg CD4+ T cells reached a peak in the lymph nodes of CCR7+/+ mice on day 20, while the peak in the lymph nodes of CCR7−/− mice occurred on day 24 (Fig. 3B), followed by a decrease of the P25TCR-Tg CD4+ T cell numbers by day 35 in both groups of mice.
FIGURE 3.
Proliferation of M. tuberculosis Ag85B–specific CD4+ T cells is delayed in MLN of CCR7−/− compared to wild type mice. (A) CFSE dilution profile of cells in the mediastinal lymph node of CCR7+/+ and CCR7−/− mice over the course of infection with M. tuberculosis. Plots are representative of four mice per group at each time point. In uninfected CCR7−/− (but not CCR7+/+) mice, a small fraction of adoptively-transferred P25TCR-Tg CD4+ T cells exhibited fluoresence half as intense as that of undivided cells; the cause of this is unknown. (B) Total number of P25TCR-Tg CD4+ T cells was calculated within the CD4+ population in mediastinal lymph node of CCR7+/+ and CCR7−/− mice. Data are represented as mean ± SD of four mice per group and per time point. ** P < 0.005 by unpaired Student’s t test. (C) 2×106 CFSE-labeled P25TCR-Tg CD4+ T cells were transferred either into naive CCR7+/+ or CCR7−/− recipients; 4 hours later, mice were euthanized and CFSE+ P25TCR-Tg CD4+ T cells were isolated from different organs (mean ± SD; n = 4 mice per group).
To assess the possibility that the differences in the kinetics of antigen-specific CD4+ T cell proliferation that we observed could be due to differences in trafficking of (CCR7+/+) P25TCR-Tg CD4+ T cells in CCR7+/+ and CCR7−/− recipients, we transferred 2×106 naive P25TCR-Tg CD4+ T cells into uninfected CCR7+/+ and CCR7−/− mice and 4 hours later determined the frequency of donor T cells in different organs. As shown in Figure 3C, P25TCR-Tg CD4+ T cells populate the lungs, spleen, and mediastinal lymph node at similar frequencies in both groups of mice. Similar numbers of donor cells were also recovered from lungs, spleen, and mediastinal lymph node of CCR7+/+ or CCR7−/− mice by 24 hours after P25TCR-Tg CD4+ T cell transfer (not shown).
These results indicate that the delayed activation of P25TCR-Tg CD4+ T cells in CCR7−/− mice is not due to deficient homing of donor T cells to the MDLN, but rather due to defective antigen presentation secondary to delayed trafficking of DCs and delivery of M. tuberculosis from the lungs to the mediastinal lymph node.
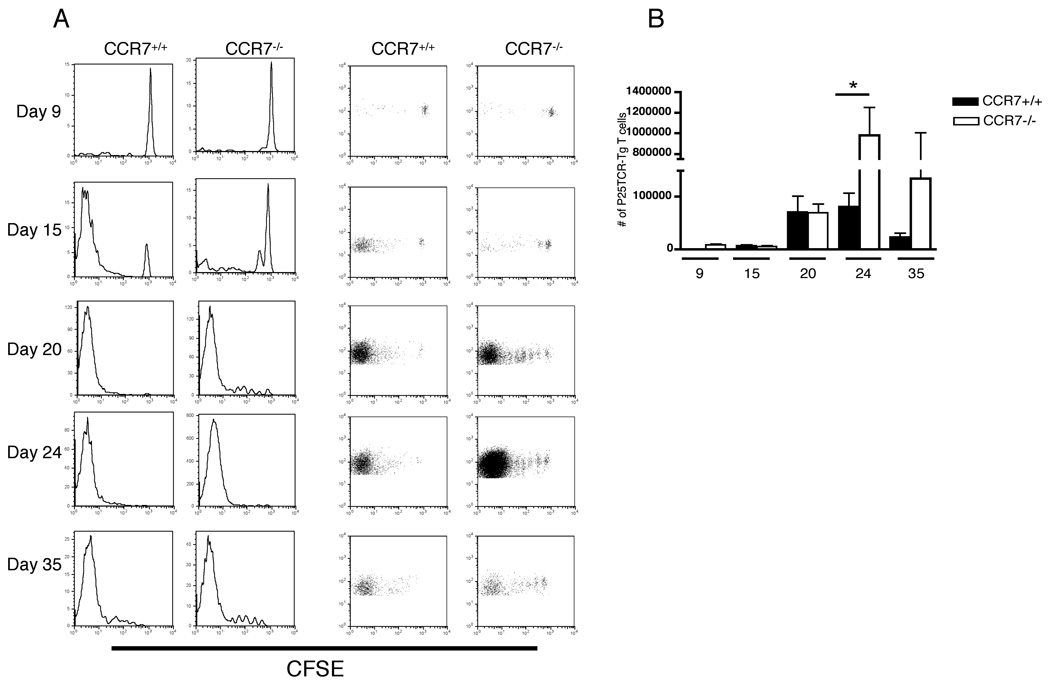
Ectopic proliferation of adoptively-transferred M. tuberculosis Ag85B-specific CD4+ T cells in lungs of CCR7−/− mice
Since we observed defective trafficking of DCs from the lungs to the mediastinal lymph node in CCR7−/− mice after M. tuberculosis infection, we considered the possibility that mature DCs retained in the lungs might be capable of activating Ag85B-specific CD4+ T cells in the lungs of CCR7−/− mice. Consistent with our previous observations (9) there was no detectable proliferation of Ag85B-specific CD4+ T cells in lungs of infected wild type mice (Fig. 4). By day 9 of infection, few P25TCR-Tg CD4+ cells were present the lungs of either CCR7+/+ or CCR7−/− mice (Fig. 4A). On days 15 and 20 of infection, when between 70 and 90% of P25TCR-Tg CD4+ cells had proliferated in mediastinal lymph nodes of wild type mice, over 90% of the P25TCR-Tg CD4+ T cells in the lungs of wild type mice were CFSElow, with no P25TCR-Tg CD4+ cells of CFSE intermediate intensity. The absence of cells with intermediate levels of CFSE indicates that the P25TCR-Tg CD4+ CFSElow population found in lungs of wild type mice had migrated there after proliferating in the MDLN. In contrast, in CCR7−/− mice, we found that some of the Ag85B-specific P25TCR-Tg CD4+ proliferated in the lungs, in addition to their proliferation in the mediastinal lymph node. By day 20 of infection, more than 90% of the P25TCR-Tg CD4+ in lungs of CCR7−/− mice had undergone 1–5 rounds of cell division, and this included subpopulations with incremental dilution of CFSE, indicating that they were proliferating in the lungs (Fig. 4A). P25TCR-Tg CD4+ T cells with intermediate levels of CFSE were also detected in lungs of CCR7−/− mice on day 24 and 35 of infection (Fig. 4A). Ectopic proliferation of the adoptively-transferred P25TCR-Tg CD4+ T cells in the lungs contributed to a markedly higher accumulation of these cells in the lungs of CCR7−/− mice: by day 24 post infection, the number of P25TCR-Tg CD4+ T cells was 12-fold higher in the lungs of CCR7−/− mice than in the lungs of wild-type mice (Fig. 4B). This excess is likely to be due to local proliferation in the lungs, since the number of P25TCR-Tg cells in the mediastinal lymph nodes of CCR7−/− mice did not exceed that in lymph nodes of CCR7+/+ mice (Fig. 3B).
FIGURE 4.
M. tuberculosis Ag85B–specific CD4+ T cells proliferate in lungs of CCR7−/− mice. (A) CFSE dilution profile of P25TCR-Tg CD4+ T cells in the lungs of CCR7+/+ and CCR7−/− mice over the course of infection with M. tuberculosis. Plots are representative of four mice per group at each time point. (B) Total number of P25TCR-Tg CD4+ T cells was calculated within the CD4+ population in lung of CCR7+/+ and CCR7−/− mice. Data are represented as mean ± SD of four mice per group and per time point. * P = 0.016 by unpaired Student’s t test.
Survival of M. tuberculosis infected CCR7−/−mice
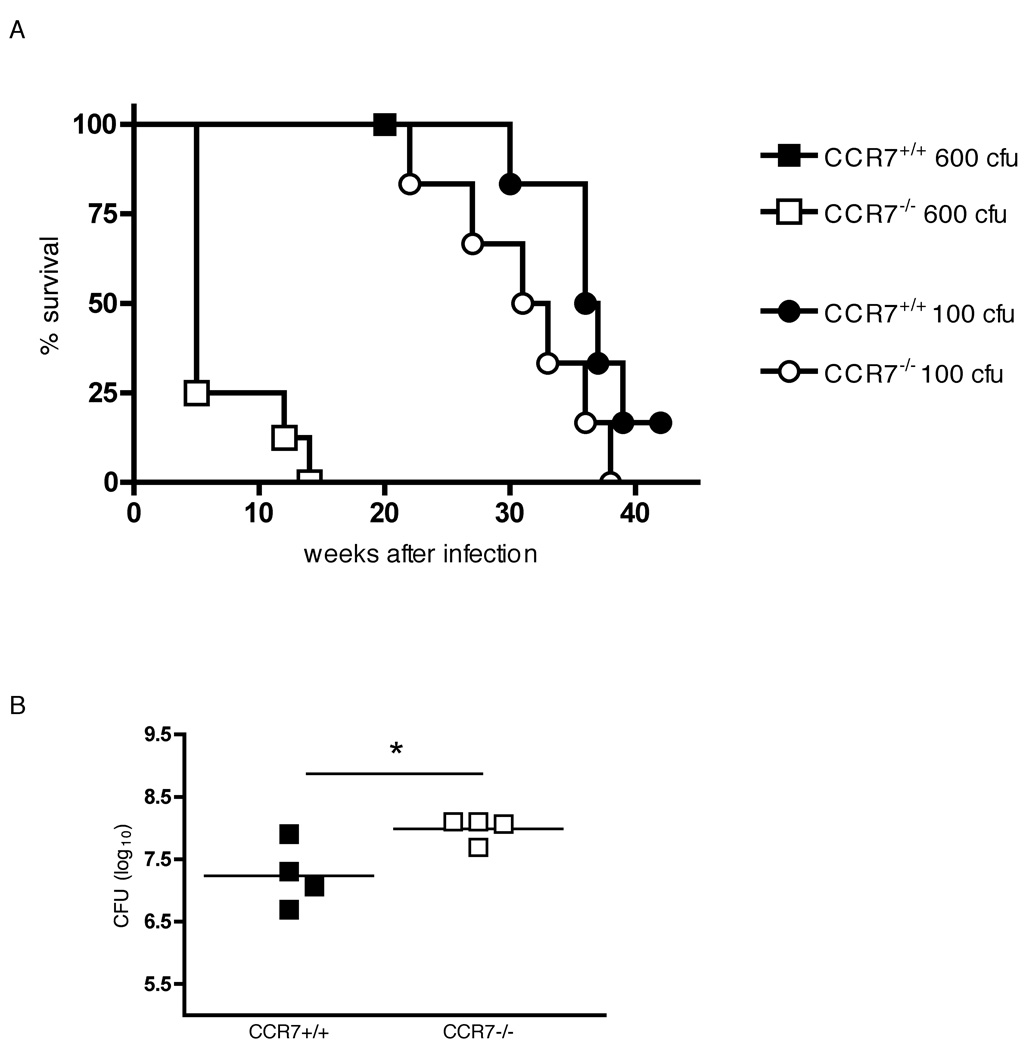
Finally, to determine the impact of CCR7 deficiency in the outcome of tuberculosis we infected CCR7+/+ and CCR7−/− mice with a low dose (~100 cfu) or a high dose (~600 cfu) of M. tuberculosis and monitored survival of the mice. After week 24 of infection CCR7−/− mice infected with a low inoculum succumbed gradually to infection and were found to be somewhat more susceptible than CCR7+/+ mice, however, by week 40 of infection the survival curve of CCR7−/− and wild type mice receiving a 100 cfu was not significantly different (Fig. 5A). Nevertheless, while CCR7+/+ mice were resistant to a higher infection dose (~600 cfu) and none had died by week 20 post-infection (when they were killed), all CCR7−/− mice succumbed to infection between weeks 5 and 14 (Fig. 5 A). 28 days after a high dose infection (~600 cfu), the bacterial burden was significantly higher in CCR7−/− lungs than in CCR7+/+ lungs (Fig. 5B), consistent with the higher susceptibility to a higher inoculum infection shown by the CCR7-deficient mice. Therefore, the resistance to M. tuberculosis infection in CCR7−/− mice is compromised compared to wild type mice, and the difference in susceptibility is more apparent with a higher inoculum of bacteria.
FIGURE 5.
Survival of M. tuberculosis-infected CCR7−/− mice. (A) CCR7+/+ and CCR7−/− mice were aerosol infected with a low dose (~100 cfu) or a high dose (~600 cfu) of M. tuberculosis and survival was monitored. n = 6–8 mice per group. (B) CCR7+/+ and CCR7−/− mice were sacrificed 28 days after aerosol infection with a high dose of M. tuberculosis (~600cfu) and colony-forming units were determined in lung homogenates. * P = 0.033 by unpaired Student’s t test.
Discussion
In the studies reported here, we examined the quantitative contribution of CCR7-dependent DC migration in the context of tuberculosis. We found that trafficking of DCs from the lungs to the mediastinal lymph node was significantly delayed in CCR7−/− mice compared with controls after aerosol infection with M. tuberculosis, a finding that is in accord with a prominent role for CCR7-dependent DC trafficking in initiation of adaptive immune responses. We also found that trafficking of live M. tuberculosis from the lungs to the MDLN was delayed in CCR7-deficient mice compared with controls, in agreement with our previously-published evidence that DCs transport M. tuberculosis from the lungs to the MDLN for initiation of adaptive immune responses (9). Moreover, we found that dissemination of M. tuberculosis from the lungs to the spleen was also delayed in CCR7-deficient mice compared with controls, and that dissemination to the spleen was preceded by dissemination to the MDLN. This suggests that dissemination of M. tuberculosis from the lungs to the spleen does not occur through direct entry of the bacteria into the bloodstream in the lungs, but depends on one or more DC-dependent processes. We also found that, while trafficking of DCs and of M. tuberculosis from the lungs to the mediastinal lymph node clearly depended on CCR7-mediated signaling during the first 3 weeks of infection, at a later time point (28 days), there were equivalent numbers of both DCs and of M. tuberculosis in the mediastinal lymph node of CCR7-deficient and control mice, which provides clear evidence for one or more CCR7-independent mechanisms of DC and bacterial trafficking during infection with M. tuberculosis. In addition, we found that M. tuberculosis antigen 85B-specific naive CD4+ T cells underwent initial activation and proliferation in the lungs of CCR7-deficient, but not CCR7-sufficient mice, indicating that the organized cellular aggregates resembling secondary lymphoid organs previously reported in the lungs of M. tuberculosis infected CCR7−/− mice may be potential sites for activation of naive CD4+ T cells (23). CCR7−/− mice on a BALB/c background have been reported to be capable of controlling low-dose (100–200 cfu) infection with M. tuberculosis for up to 120 days (23). In the present report, we monitored survival beyond 200 days and found that CCR7−/− mice infected with a low dose (~100 cfu) of M. tuberculosis succumbed earlier than CCR7+/+ mice, although the difference was not statistically significant. However, CCR7-deficient mice infected with a higher inoculum (~600 cfu) of M. tuberculosis succumbed significantly earlier than CCR7-sufficient control mice, and this susceptibility to a high dose infection was associated with poorer control of M. tuberculosis in CCR7−/− mice (Figure 5). These results indicate that, while alternative mechanisms of DC trafficking and naive CD4+ T cell activation exist in the context of tuberculosis, optimal protective immunity depends on CCR7-dependent cell trafficking.
Certain of our findings in mice infected with M. tuberculosis differ from those in other experimental systems in which CCR7−/− DC failed to migrate to lymph nodes. For example, CCR7−/− DC fail to leave dermal tissue and migrate to draining lymph nodes in response to in vivo mobilization stimuli such as contact sensitization by fluorescein isothiocyanate (FITC) skin painting (24) and DC differentiated from the bone marrow of CCR7−/− mice do not migrate to the draining lymph nodes following subcutaneous injection or intra-tracheal instillation (14, 29, 30). However, these were short term studies, which corresponds to the defective CCR7−/− DC trafficking from lung to the MDLN that we observe at the earliest time points after M. tuberculosis infection (Figure 1A). At later time points, CCR7-independent mechanisms clearly account for the DC recruitment to MDLN in CCR7−/− mice during M. tuberculosis infection. Recent findings provide evidence of a role for CCL5 and CCR5 in inducing DC maturation and allowing subsequent CCR7 dependent homing of lung DCs to lymph nodes during viral infection (31), suggesting that migration of lung DCs might be controlled by a multi-step chemokine-driven mechanism. However, the relevance of this mechanism in tuberculosis is questionable. While it has been suggested that CCR5 may play a role in the migration of DCs to and from the MDLN after the acute phase of M. tuberculosis infection (32), the results of that same study provide evidence that CCR5 is not required for the initiation of the Th1 immune response to M. tuberculosis (32). At least one other mechanism that contributes to DC trafficking from the lungs to the MDLN has been described (28). Jakubzick et al have shown that migration of DC carrying fluorescent particles from the lungs to the MDLN was substantially but not fully impaired in CCR8 KO mice, revealing that both CCR7 and CCR8 may mediate lung DC migration (28). We cannot exclude the possibility that a subset of DCs in the MDLN is acquired by mechanisms other than migration from the lungs. Nakano and colleagues found that after viral infection or immunization, inflammatory monocytes were recruited into lymph nodes directly from the blood to become inflammatory DCs that potently stimulated TH1 responses (33). While blood-derived inflammatory DCs could contribute to DC accumulation in MDLN of CCR7−/− mice infected with M. tuberculosis, recruitment from the blood would not account for the concurrent appearance of bacteria in the MDLN, since the lungs are the predominant site of bacterial replication (they contain 100-fold more bacteria than the MDLN (12)), and since few, if any, bacteria are detected in the blood.
Our finding that CCR7−/− mice can generate antigen-specific immune responses to M. tuberculosis and survive low-dose infection was not expected, given the central role in adaptive immune responses that has been assigned to CCR7 (19). However, in certain other contexts, the requirement for CCR7 in protective immune responses has not been absolute. In keeping with our results, it has been shown that CCR7−/− mice generate a delayed but complete cytotoxic T lymphocyte response to infection with lypmhocytic choriomeningitis virus (LCMV) (21), indicating that CCR7-dependent cell migration and structural differentiation of the lymphoid T cell zone is not essential for the generation and maintenance of antiviral CTL responses. Moreover, CCR7−/− mice are relatively resistant to primary and secondary infection with Listeria monocytogenes (22); in this model the priming of naive but not memory MHC class Ia-restricted CD8+ cells requires CCR7, whereas naive MHC class Ib-restricted CD8+ T cells or MHC class II-restricted CD4 T cells were found to be less dependent on the presence of this chemokine receptor, indicating that different T cell subtypes and maturation stages have discrete requirements for CCR7 (22). A differential requirement of CCR7-mediated active Ag transport for CD4+ and CD8+ T cell expansion has also been described during influenza infection: CCR7-mediated migration of dendritic cells was more crucial for CD8+ T cell than CD4+ T cell responses (34). While no influenza-specific CD8+ T cell response could be detected in CCR7-deficient mice, a small influenza-specific CD4+ T cell population was detectable in the MDLN, suggesting the existence of a CCR7-independent mechanism for Ag transport to the MDLN that accounts for the expansion of influenza specific CD4+ T cells.
An additional finding from our present studies was the ectopic proliferation of Ag85B-specific CD4+ T cells observed in lungs of CCR7−/− mice following infection with M. tuberculosis (Figure 4). The defective DC trafficking from the lung to the MDLN observed in CCR7−/− mice (Figure 1B) led us to speculate that DC retained in the lungs of CCR7−/− mice might be capable of activating Ag85B-specific CD4+ T cells in that tissue. Although it is known that the activation of naive T cells during localized infection takes place in the local draining lymph nodes, the development of neolymphoid structures in the lungs of M. tuberculosis-infected CCR7−/− mice have been described (23). Our finding that the lungs of CCR7−/− mice can serve as alternative priming sites for T cells agrees with previous reports demonstrating that CCR7−/− mice spontaneously develop organized tertiary lymphoid structures at mucosal sites such as the lung, stomach and colon (35, 36). Kocks et al have shown that CCR7−/− mice develop highly organized bronchus-associated lymphoid tissue (BALT) independently of the presence of inflammatory stimuli such as pathogen-associated molecular patterns (36). While our studies indicate that adoptive-transferred Ag85B-specific CD4+ T cells can be activated to proliferate in the lungs of M. tuberculosis-infected CCR7−/− mice, they do not reveal the precise location in the lungs (ie, BALT, or the neolymphoid structures resembling granulomas). Ectopic proliferation of adoptively-transferred P25TCR-Tg CD4+ T cells in the lungs contributed at least in part to a 12-fold larger number of these cells in the lungs of CCR7−/− compared with CCR7+/+ mice; it is very likely that proliferation in the lungs contributed to this excess, since no differences were observed in the peak number of P25TCR-Tg CD4+ T cells in the mediastinal lymph nodes of CCR7−/− vs CCR7+/+ mice. However, there is no apparent relationship between the ectopic proliferation of Ag85B-specific CD4+ T cells observed in lungs of CCR7−/− mice and their ability to control M. tuberculosis replication. 28 days after infection the bacterial burden is similar in the lungs of CCR7−/− and CCR7+/+ mice infected with low doses of M. tuberculosis (Figure 2A); moreover, when infected with a higher dose of M. tuberculosis, CCR7−/− mice have higher lung bacterial burdens than CCR7+/+ mice (Figure 5B) and succumb to infection (Figure 5A), despite ectopic proliferation of Ag85B-specific CD4+ T cells in the lungs. In addition to our finding that M. tuberculosis antigen-specific CD4+ T cells undergo proliferation in the lungs, at least one alternative mechanism may also have contributed to the excessive accumulation of these cells in the lungs. Mori et al. have shown that in plt mice, which lack 2 of the 3 ligands for CCR7, have defects in the migration of naive T cells and mature DC into the T cell zone of lymphoid organs, the responses to contact sensitization and subcutaneous immunization are delayed, but ultimately enhanced compared to those seen in wild type mice. These findings were interpreted as evidence that the activation of T cells within the thymus dependent areas of secondary lymphoid organs is required for the contraction phase of an immune response (37). Moreover, augmented T cell-mediated immune responses to contact antigens exceeding those seen in wild type mice, have been shown to develop in CCR7−/− mice; this was attributed to defective trafficking of regulatory T cells to the local draining lymph nodes (38) (Reviewed in (39)). Whether defective regulatory T cell trafficking contributes to the markedly higher expansion of Ag85B-specific CD4+ T cells in the lungs of M. tuberculosis-infected mice will require additional study. The findings reported here provide evidence for roles of CCR7-dependent and CCR7-independent cell trafficking in the immune response to M. tuberculosis in mice. It will be of considerable interest to identify the CCR7-independent mechanisms that contribute to initiation of adaptive immune responses to M. tuberculosis, and to determine the extent to which they contribute to the variable outcomes of tuberculosis in humans.
Acknowledgments
We thank Dr. Adrian Erlebacher and Mary Collins for generously providing the CCR7−/− mice. We also thank Tawania Fergus for excellent technical assistance.
This work was supported by National Institutes of Health Grant R01 AI051242.
Abbreviations used in this paper
- MDLN
mediastinal lymph node
- DCs
dendritic cells
- cDCs
conventional dendritic cells
- Tg
transgenic
References
- 1.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winslow GM, Roberts AD, Blackman MA, Woodland DL. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J Immunol. 2003;170:2046–2052. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- 4.Woodworth JS, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev Immunol. 2006;26:317–352. doi: 10.1615/critrevimmunol.v26.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, Walker AT, Friedland GH. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 7.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Poulsen A. Some clinical features of tuberculosis. 1. Incubation period. Acta Tuberc Scand. 1950;24:311–346. [PubMed] [Google Scholar]
- 9.Wolf AJ, Desvignes L, Linas B, Banaie N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, Martino CA, Roberts AD, Cooper AM, Winslow GM, Woodland DL. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Sanchez N, Riol-Blanco L, Rodriguez-Fernandez JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol. 2006;176:5153–5159. doi: 10.4049/jimmunol.176.9.5153. [DOI] [PubMed] [Google Scholar]
- 14.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 17.Gunn MD. Chemokine mediated control of dendritic cell migration and function. Semin Immunol. 2003;15:271–276. doi: 10.1016/j.smim.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 19.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 20.Scandella E, Fink K, Junt T, Senn BM, Lattmann E, Forster R, Hengartner H, Ludewig B. Dendritic cell-independent B cell activation during acute virus infection: a role for early CCR7-driven B-T helper cell collaboration. J Immunol. 2007;178:1468–1476. doi: 10.4049/jimmunol.178.3.1468. [DOI] [PubMed] [Google Scholar]
- 21.Junt T, Scandella E, Forster R, Krebs P, Krautwald S, Lipp M, Hengartner H, Ludewig B. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J Immunol. 2004;173:6684–6693. doi: 10.4049/jimmunol.173.11.6684. [DOI] [PubMed] [Google Scholar]
- 22.Kursar M, Hopken UE, Koch M, Kohler A, Lipp M, Kaufmann SH, Mittrucker HW. Differential requirements for the chemokine receptor CCR7 in T cell activation during Listeria monocytogenes infection. J Exp Med. 2005;201:1447–1457. doi: 10.1084/jem.20041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis. 2007;195:46–54. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- 24.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 25.Tamura T, Ariga H, Kinashi T, Uehara S, Kikuchi T, Nakada M, Tokunaga T, Xu W, Kariyone A, Saito T, Kitamura T, Maxwell G, Takaki S, Takatsu K. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int Immunol. 2004;16:1691–1699. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- 26.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott HM, Flynn JL. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun. 2002;70:5946–5954. doi: 10.1128/IAI.70.11.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 29.Hintzen G, Ohl L, del Rio ML, Rodriguez-Barbosa JI, Pabst O, Kocks JR, Krege J, Hardtke S, Forster R. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177:7346–7354. doi: 10.4049/jimmunol.177.10.7346. [DOI] [PubMed] [Google Scholar]
- 30.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 32.Algood HM, Flynn JL. CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J Immunol. 2004;173:3287–3296. doi: 10.4049/jimmunol.173.5.3287. [DOI] [PubMed] [Google Scholar]
- 33.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nature immunology. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heer AK, Harris NL, Kopf M, Marsland BJ. CD4+ and CD8+ T cells exhibit differential requirements for CCR7-mediated antigen transport during influenza infection. J Immunol. 2008;181:6984–6994. doi: 10.4049/jimmunol.181.10.6984. [DOI] [PubMed] [Google Scholar]
- 35.Hopken UE, Wengner AM, Loddenkemper C, Stein H, Heimesaat MM, Rehm A, Lipp M. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 2007;109:886–895. doi: 10.1182/blood-2006-03-013532. [DOI] [PubMed] [Google Scholar]
- 36.Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J Exp Med. 2001;193:207–218. doi: 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pabst O, Bernhardt G, Forster R. The impact of cell-bound antigen transport on mucosal tolerance induction. Journal of leukocyte biology. 2007;82:795–800. doi: 10.1189/jlb.0307144. [DOI] [PubMed] [Google Scholar]