Abstract
This topically limited review explores the relationship between the immune system and insulin-like growth factors (IGF-I and IGF-II) and the proteins through which they act, including IGF-I receptor (IGF-IR) and the IGF-I binding proteins. The IGF/IGF-IR pathway plays important and diverse roles in tissue development and function. It regulates cell cycle progression, apoptosis, and the translation of proteins. Many of the consequences ascribed to IGF-IR activation result from its association with several accessory proteins that are either identical or closely related to those involved in insulin receptor signaling. Relatively recent awareness that IGF-I and IGF-IR regulate immune function has cast this pathway in an unexpected light; it may represent an important switch governing the quality and amplitude of immune responses. IGF-I/IGF-IR signaling may also participate in the pathogenesis of autoimmune diseases, although its relationship with these processes seems complex and relatively unexplored. On the one hand, IGF-I seems to protect experimental animals from developing insulin-deficient diabetes mellitus. In contrast, activating antibodies directed at IGF-IR have been detected in patients with Graves' disease, where the receptor is overexpressed by multiple cell types. The frequency of IGF-IR+ B and T cells is substantially increased in patients with that disease. Potential involvement of IGF-I and IGF-IR in the pathogenesis of autoimmune diseases suggests that this pathway might constitute an attractive therapeutic target. IGF-IR has been targeted in efforts directed toward drug development for cancer, employing both small-molecule and monoclonal antibody approaches. These have been generally well-tolerated. Recognizing the broader role of IGF-IR in regulating both normal and pathological immune responses may offer important opportunities for therapeutic intervention in several allied diseases that have proven particularly difficult to treat.
I. Introduction
Insulin-like growth factors (IGF-I1 and IGF-II), their binding proteins (IGFBPs), and the receptors mediating their signaling (types I and II IGF-IR), play critical roles in normal development, growth, metabolism, and homeostasis (Adams et al., 2000; De Meyts and Whittaker, 2002). The IGF-I pathway exerts such diverse influence on mammalian biology that the scope of its function is only now beginning to be understood. It has been insinuated in fundamental processes such as determining life span and coping with oxidative stress in rodents (Holzenberger et al., 2003). IGF-IR bears both structural and functional resemblance to other closely related tyrosine kinase receptors, such as InR in Drosophila melanogaster (Kennington et al., 2006) and DAF-2 in Caenorhabditis elegans (Kenyon et al., 1993; Dorman et al., 1995; Kennington et al., 2007). It begins functioning during fetal development and retains its importance throughout life, although the consequences of its normal or abnormal activation change with aging. IGF-IR and its related proteins have been implicated in many diseases, including growth abnormalities, metabolic disorders, and several forms of cancer (Baserga et al., 2003; Kant et al., 2007; Frasca et al., 2008). Thus, this pathway continues to attract interest as a potentially useful target for therapeutic design (Clemmons, 2007).
Detection of IGF-I and IGF-IR mRNAs and the proteins they encode in peripheral blood mononuclear cells suggests that this pathway might serve some regulatory function in the “professional” immune system. Moreover, IGF-I production, action, and intracellular signaling can be influenced by multiple cytokines and the pathways they use. IGF-IR expression on the surface of T lymphocytes can be down-regulated after cell activation (Schillaci et al., 1998). IGF-I enhances diverse aspects of bone marrow function, including lymphocyte maturation (Clark et al., 1993), granulopoiesis (Merchav et al., 1988), and erythropoiesis (Kurtz et al., 1982). Growth hormone (GH), which drives much of the IGF-I generation occurring in liver, promotes hematopoietic growth (Murphy et al., 1992a,b,c). Its effects are substantial in that they can attenuate the myelosuppressive effects of powerful chemotherapeutic agents such as azidothymidine (Murphy et al., 1992a,b,c). Administration of GH and IGF-I or driving the production of IGF-I and IGF-II using transgenic approaches in animals promotes both B and T cell development. Thus, there is reason to explore the potential for this endocrine pathway as a regulator of immunity. Moreover, targeting IGF-I and IGF-IR signaling as a strategy for altering the natural course of chronic inflammation may become an attractive means of managing autoimmune disease.
This review attempts to describe recent findings implying that the IGF-I/IGF-IR pathway plays diverse roles in regulating immune function. These new insights become particularly important in the context of therapy discovery. A number of biological agents, both small molecules and monoclonal antibodies, are entering the late stages of development. They have been examined as potential treatment for neoplastic diseases (Baserga et al., 2003; Clemmons, 2007). The widening scope of activities recently ascribed to IGF-I should provoke a search for broader applications for agents that can disrupt IGF-IR signaling through a variety of mechanisms. If IGF-I/IGF-IR regulates immune function, autoimmune diseases might represent unanticipated indications for drugs targeting this pathway.
II. Structure and Biology of Insulin-Like Growth Factor-I, Insulin-Like Growth Factor Receptor, and Insulin-Like Growth Factor-I Binding Proteins
A. Insulin-Like Growth Factor-I
IGF-I represents one of several structurally related polypeptides that also include IGF-II, insulin, and relaxin (Bryant-Greenwood and Schwabe, 1994). It comprises 70 amino acids organized into A and B chains connected by disulfide bonds. (Fig. 1) The amino acid sequence of human IGF-I was first reported by Rinderknecht and Humbel (1978). IGF-I possesses a connecting or C-peptide region of 12 amino acids. This region has been shown to determine the high-affinity binding of IGF-I to the type I IGF-IR (Bayne et al., 1989). An eight-amino acid D-region peptide forms an extension of the carboxyl terminus (Brissenden et al., 1984).
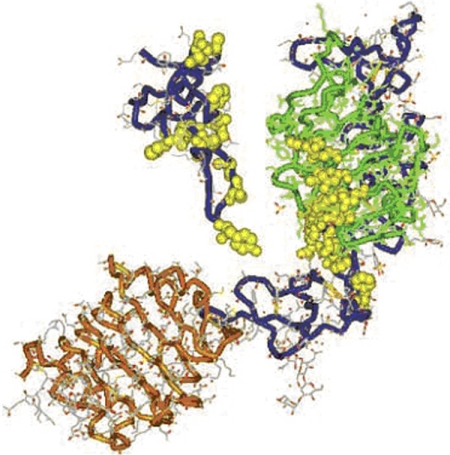
Fig. 1.
Structures of the IGF-I receptor and its ligand. Three-dimensional structure of large domain 1 (L1)–Cys-rich (CR)-L2 domain of IGF-IR determined by X-ray crystallography. An extended bilobed structure (40 × 48 × 105 Å) comprises the two globular L-domains with a new type of right-handed β-helix fold that flanks the CR domain. They seem to be part of the leucine-rich-repeat superfamily. Although L1 (residues 1–15; green) contacts the CR domain (blue) along its length, there is minimal contact with L2 (residues 300–460; orange). Flexibility between CR domain and L2 could affect ligand binding. A −30-Å diameter cavity represents a potential binding pocket. The amino acids that have been determined by alanine-scanning mutagenesis to be important for ligand binding are shown in yellow as van der Waals spheres. Three-dimensional IGF-I structure is based on the X-ray coordinates from Brzozowski et al. (2002). The backbone is shown in blue. [Reproduced from De Meyts P and Whittaker J (2002) Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov 1:769–783. Copyright © 2002 Nature Publishing Group. Used with permission.]
IGF-I and IGF-II circulate in the plasma as complexes formed with IGFBPs that apparently serve several biological functions. The vast majority of IGF-I (99%) is bound to IGFBP3 or IGFBP5 and is coupled with a glycoprotein called the acid labile subunit (Baxter, 1993; Twigg and Baxter, 1998). However, the full repertoire of biological implications ascribed to “free” versus bound IGF-I has yet to be determined.
Two distinct tissue sources of IGF-I production separate its functions. First, the liver generates IGF-I, which acts as an extension of the GH axis by virtue of tonic pituitary stimulation of hepatic synthesis (Maiter et al., 1988). In fact, GH, in concert with nutritional factors, represents the major determinant of circulating IGF-I levels (Oster et al., 1995). In this regard, IGF-I functions in a manner characteristic of other endocrine pathways. Second, IGF-I is also produced locally by many peripheral cell types under basal conditions and in response to inflammatory cues. In this case, IGF-I acts on peripheral tissues as an autocrine or paracrine factor resembling cytokines and other growth factors.
Whatever the source of IGF-I, responses to it are frequently mediated through the moderately high-affinity association it displays for IGF-IR (LeRoith et al., 1995; Adams et al., 2000). In other situations, members of the IGFBP family bind IGF-I, in some cases at higher affinities than those occurring with IGF-IR. IGF-I/IGFBP complex formation can produce signal initiation. Alternatively, formation of these complexes can limit the occupation by IGF-I of IGF-IR and impose limits on cellular responses dependent on IGF-IR activation. Thus, IGFBPs, IGF-I, and IGF-IR form an important pathway that can exert substantial self-regulation and can form endocrine, paracrine, and autocrine loops through which these molecules exert their biological impact. A number of factors influence the turnover of IGFs. Among these are the relative levels of both proteases and protease inhibitors found in the microenvironment of target cells. Proteases directed at IGF-I and specific IGFBPs can promote the degradation and clearance of the growth factor (Roth et al., 1984; Bhaumick and Bala, 1987; Misbin and Almira, 1989; Cwyfan Hughes et al., 1992; Myers et al., 1993; Timmins et al., 1996; Skjaerbaek et al., 1998). Some of these proteases are regulated by IGF-I itself (Myers et al., 1993). Modulation of these enzymes must then be considered potentially important determinants of the biological activity of IGF-I. The IGF-I structural variant, IGF-I Des 1–3, can also be generated as a consequence of proteolytic digestion (Maake et al., 1997). This analog represents an IGF-IR-specific activator that lacks the N-terminal three amino acids (Bagley et al., 1989; Ross et al., 1989; Yamamoto and Murphy, 1995; Jansson et al., 1997). It exhibits high affinity for the type I receptor but does not bind or activate the IGFBPs. Moreover, it is more active than IGF-I in terms of its receptor-dependent signaling (Jansson et al., 1997). The proteolytic fragmentation of IGF-I into Des 1–3 seems to be regulated in part by the serine protease inhibitor Spi 2.1, which is down-regulated in GH-deficient rodents (Maake et al., 1997). This finding suggests a potential mechanism for regulating the availability of IGF-I in GH deficiency.
B. Insulin-Like Growth Factor-I Receptors
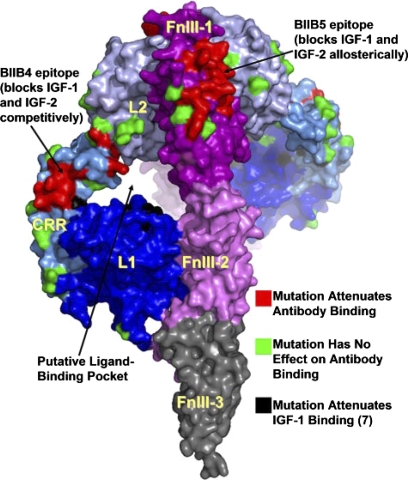
Type I IGF-IR consists of 1368 amino acids (Fig. 2) and belongs to a family of relatively large transmembrane tyrosine kinase receptors. These include the insulin receptor (IR) and a third, orphan member, namely IR-related receptor, the endogenous ligand for which has yet to be identified (LeRoith et al., 1995). These proteins share considerable structural similarities (Lawrence et al., 2007). The extracellular domain of IGF-IR, which is the site of constitutive receptor dimerization, can be subdivided into six protein domains. These include two L domains (L1 and L2) located in the N terminus, a cysteine-rich domain, and three fibronectin domains, termed FnIII. The second of the fibronectin domains contains a cleavage site between residues 708 and 710. Cleavage at this site results in the formation of two polypeptides, IGF-IRα and IGF-IRβ, that are linked by disulfide bonds. The residues determining IGF-I and IGF-II binding apparently reside in the L1 and second FnIII domains (Whittaker et al., 2001; Sørensen et al., 2004). IGF-IR was established as a protein distinct from IR some 35 years ago (Megyesi et al., 1975). Its critical importance to normal development and physiology is underscored by the neonatal lethality resulting from a complete absence of IGF-IR (Liu et al., 1993). On the other hand, after the conclusion of linear growth in mammals, its functions seem less critical. At that stage, IGF-IR serves other important functions, such as secondarily regulating carbohydrate metabolism and perhaps influencing immune function. Among the potential ligands, it binds IGF-I with the highest affinity but also displays appreciable avidity for IGF-II and insulin. The affinities for these latter two ligands are 1 and 2 orders of magnitude lower, respectively, than the affinity for IGF-I. Type II IGF-IR, which has been shown to be identical to the cation-independent mannose 6 phosphate receptor (Kornfeld, 1992; Hassan, 2003), binds IGF-II with the greatest avidity but can also bind IGF-I. Unlike the type I receptor, type II IGF-IR fails to bind insulin. Its signaling potential is considered relatively minor. Rather, it may function to promote ligand clearance. It represents a single membrane-spanning domain-containing glycoprotein (Ghosh et al., 2003). The extracellular domain comprises 15 cysteine-rich repeats, whereas the carboxyl terminus is quite short. Two distinct binding sites accommodate IGF-II and mannose-6-phosphate (Braulke, 1999). Type I and II receptors may mediate Erk 1/2 phosphorylation provoked by IGF-I and IGF-II, respectively (El-Shewy et al., 2007). By knocking down type I IGF-IR, the activation of Erk p42/44 elicited by IGF-I is substantially abrogated, whereas that of IGF-II persists. In contrast, interfering with the type II receptor had little effect on IGF-I signaling to Erk, whereas the activities of IGF-II on the activation of this kinase were reduced. Thus, type I and II receptors might function independently with regard to Erk activation, and IGF-II might exert at least some of its actions through the type II receptor (El-Shewy et al., 2007). The signaling initiated through IGF-IR begins with a conformational change provoked by receptor ligation and involves a number of well used pathways in tissues in which IGF-I exerts its actions. In solution, type I IGF-IR can bind three molecules of IGF-I (Whitten et al., 2009). Moreover, binding of the ligand to this receptor results in relatively little structural movement and may be limited to local rotation of protein domains.
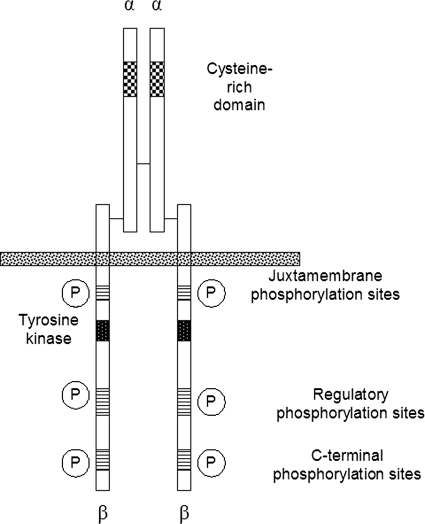
Fig. 2.
Schematic of the IGF-IR dimer demonstrating the distribution of domains across α and β chains and the location of α-β disulfides and α-α dimer disulfide bonds. [Adapted from Clemmons DR (2001) IGF-I receptor-mediated signal transduction, in Targets for Growth Hormone and IGF-I Action (Bouillon R ed), pp 17–28, Bioscientifica Ltd., Bristol, UK. Copyright © 2001 Bioscientifica Ltd. Used with permission.]
Cell-surface IGF-IR levels are regulated by the relative expression of its gene (LeRoith et al., 1995; Werner et al., 1995). A number of factors seem to determine expression, depending on the cell-type (Du et al., 1999, 2001; Maile and Clemmons, 2003). In turn, those levels of receptor expression govern key cellular processes such as apoptosis. For instance, in vascular smooth-muscle cells, oxidative stress diminishes receptor levels through a mechanism involving enhanced association of p53 with the IGF-IR gene promoter (Kavurma et al., 2007). IGF-IR signals to multiple antiapoptotic pathways, and its overexpression generally enhances cell survival. Moreover, IGF-IR seems necessary for malignant cell transformation in some models of carcinogenesis, such as the Ewing's family of tumors (Toretsky et al., 1997). In prostate cancer cells, IGF-IR activation leads to the initiation of downstream mTOR signaling regulating the expression of survivin (Vaira et al., 2007), a member of the inhibitor of apoptosis gene family and an important regulator of cell proliferation and viability (Ambrosini et al., 1997). By introducing the dominant-negative mutant 486/STOP IGF-IR into M12 prostate cancer cells expressing high levels of wild-type IGF-IR, Wu et al. (2003) enhanced apoptosis through actions mediated by p38 mitogen activated protein kinase (MAPK).
Recent studies reveal that the levels of cell-surface IGF-IR are also governed by regulatory events occurring at the surface of the plasma membrane. As with multiple other tyrosine kinase receptors, ligand-induced endocytosis serves an important function in signaling through the recruitment of several proteins, including the adaptor protein 2 (AP-2) complex, dynamin, endophilin, and clathrin (Mellman, 1996; Schmid et al., 1998). The requisite recognition determinates for complex assembly have been localized. They are contained in the EH domain of the N terminus of epidermal growth factor receptor (EGFR) pathway substrate, Eps15, a 100-amino acid signature that is repeated three times (Salcini et al., 1997). EPS15 has been linked to EGFR endocytosis (Benmerah et al., 1998). A family of four EH domain-containing proteins has been identified, termed EHD1-EHD4 (Mintz et al., 1999). These EHD domains are located in the C termini. Rotem-Yehudar et al. (2001) have implicated EHD1 in the endocytosis of IGF-IR, in association with SNAP29. In their study, the authors demonstrate that a complex containing clathrin, α-adaptin of AP-2, small nuclear RNA-activating protein, and EHD1 colocalize to the endocytic vesicles. Over-expression of EHD1 retards the phosphorylation of mitogen-activated protein kinase and Akt and dampens IGF-I-provoked signaling substantially in transfected Chinese hamster ovary cells (Rotem-Yehudar et al., 2001).
The immediate consequence of IGF-IR activation involves tyrosine autophosphorylation at several residues resulting from intrinsic tyrosine kinase activity in the β subunit (Kato et al., 1993). Phosphorylation of tyrosine residues 1131, 1135, and 1136 plays important roles in the canonical signaling attributed to the receptor (Grønborg et al., 1993; Kato et al., 1994). These protein modifications in turn create binding sites for multiple docking proteins (Craparo et al., 1995; Dey et al., 1996). Among these are the insulin receptor substrates (IRS)-1, -2, -3, and -4 and the Src homology and collagen domain protein p66 Shc (Fig. 3). IRS-1 contains 21 tyrosine residues and serves a prominent role by interacting with several Src homology-2 (SH-2)-containing proteins involved in downstream signaling. The phosphorylation of IRS-1 leads to phosphoinositol kinase 3/AKT activation and the adaptor protein Grb-2, which contains both SH-2 and SH-3 domains. Phosphoinositol kinase 3/AKT can associate with IRS-1, and the Grb-2 can bind the guanine nucleotide-releasing protein son-of-sevenless, which in turn participates in Ras activation (Egan et al., 1993), leading to phosphorylation of the serine/threonine kinase, Raf-1, and various components of the mitogen-activated protein (MAP) kinase pathway (Kecha et al., 2000). In addition, several phosphotyrosine phosphatases have been implicated in regulating IGF-I signaling. For instance, SH-2-containing phosphotyrosine phosphatase-2 (SHP-2) governs the duration of IGF-IR phosphorylation in smooth muscle cells (Maile and Clemmons, 2002a,b,c).
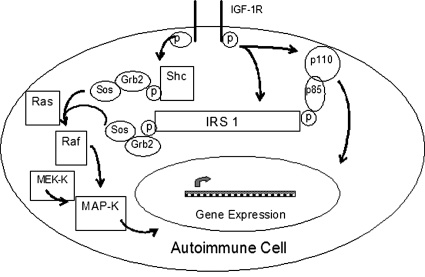
Fig. 3.
Major components of IGF-R-linked signaling pathways. IRS-1 represents a central docking protein involved in the activation of MAP kinase and PI3 kinase pathways. Like IRS, Shc can be phosphorylated directly as a consequence of the receptor kinase. GF-R, growth factor receptor; MEK-K, MEK kinase; p, phosphorylation; Sos, Son of Sevenless. [Reprinted from Clemmons DR (2001) IGF-1 receptor-mediated signal transduction, in Targets for Growth Hormone and IGF-1 Action (Bouillon R ed), pp 17–28, Bioscientifica Ltd., Bristol, UK. Copyright © 2001 Bioscientifica Ltd. Used with permission.]
Multiple factors exert powerful regulatory influences on IGF-I-mediated signaling, insights that remain incompletely explored (Nagaoka et al., 1990; Lecka-Czernik et al., 2007; Martin and Baxter, 2007; O'Connor et al., 2008). Notable among them are abundant components of the extracellular matrix. When fibroblasts are cultured on fibronectin-coated culture surfaces, the abundance of IRS-1 increases (Lebrun et al., 2000), whereas a substratum rich in vitronectin facilitates its phosphorylation through interactions with focal adhesion kinase pp125 (Lebrun et al., 1998). Apparently, IGF-IR can associate with multiple integrins, and these interactions are cell-specific. The activation of α3β1 by IGF-I in breast cancer cells can be up-regulated by plating them on a substratum enriched with thrombospondin (Chandrasekaran et al., 1999). The integrin receptor αVβ3 regulates IGF-IR phosphorylation by influencing the rate of SHP-2 recruitment to the receptor complex (Maile and Clemmons, 2002a,b,c). A dynamic interplay exists between IGF-IR and the transmembrane proteins SHPS-1, a docking protein, and the αVβ3 integrin (Clemmons and Maile, 2005). In lens epithelium, the receptor coprecipitates with α6 (Walker et al., 2002), whereas in human chondrocytes, it associates with α1β1 and α5β1 (Shakibaei et al., 1999). It seems that αVβ3 must be ligated to allow IGF-IR to fully influence vascular smooth muscle cell proliferation and migration. Blocking αVβ3 with the monoclonal antibody LM609 attenuates IGF-I-dependent cell migration. A critical component to this signaling concerns the recruitment of SHP-2. SHP-2 is subsequently transferred to SHPS-1. This reaction requires that the latter become tyrosine-phosphorylated through an IGF-IR-dependent event (Pollak et al., 2004). Blocking the interaction between ligands and αVβ3 enhances SHP-2 binding to IGF-IR, causing dephosphorylation of the receptor's tyrosine residues and dampening the signaling mediated through MAP kinase and phosphatidylinositol 3 kinase pathways (Maile and Clemmons, 2002a,b,c). The phosphorylation of tyrosines 785 and 773 on β3 seems critical to IGF-I-dependent MAP kinase signaling and cell proliferation (Ling et al., 2003). Conversely, IGF-I activity enhances the avidity with which αVβ3 binds ligands without altering maximum receptor binding capacity (Jones et al., 1996).
Another regulatory phosphatase, protein tyrosine phosphatase 1B, can also reduce the levels of IGF-IR phosphorylation (Buckley et al., 2002). Unlike the closely related EGFR and platelet-derived growth factor receptor, IR and IGF-IR fail to bind SH-2-domain-containing proteins but instead drive the phosphorylation of IRS and Shc proteins (White, 1997). Moreover, a number of potentially seminal findings have suggested that a cooperative relationship between IGF-IR and EGFR in signaling patterns exists and may prove to be cell type-specific (Roudabush et al., 2000). These receptors, separately and in aggregate, may form “clearing houses” for converging signals derived from a wide array of cross-talking pathways, including those involved in the actions of multiple hormones, cytokines, growth factors, agents of cell stress, and oxidative events (Rosen and Greenberg, 1996; Rosette and Karin, 1996; Moro et al., 1998; Carpenter, 1999; Hackel et al., 1999; Luttrell et al., 1999). In particular, IGF-I can promote Erk phosphorylation through the intermediate activation of Shc. This series of events might require IGF-IR dependent EGFR trans-activation (Roudabush et al., 2000). It is noteworthy that El-Shewy et al. (2004) have recently proposed a model in which the generation of EGFR ligands provoked by IGF-IR activation results in the trans-activation signaling of EGFR. This, in turn, leads to cell type-specific downstream signaling events. By transfecting an expression plasmid encoding heparin binding-epidermal growth factor-like growth factor/influenza virus hemagglutinin/Myc into human embryonic kidney 293 cells, they demonstrated that IGF-I elicited the rapid proteolysis of the fusion protein. Furthermore, IGF-I, EGF, and heparin binding-EGF-like growth factor could all enhance Tyr-1068 phosphorylation of endogenous EGFR and mimic EGF in driving EGFR internalization from the cell surface (El-Shewy et al., 2004). The paracrine nature of this relationship was established by demonstrating that IGF-IR+ cells could respond to stimulation as did IGF-IR− cells in coculture. Responses of these receptor-null cells were abolished by inhibiting matrix metalloproteinases and EGFR activation.
IGF-IR may also serve as a substrate for γ-secretase. McElroy et al. (2007) have demonstrated that a 52-kDa C-terminal fragment of IGF-IR is generated both constitutively and at an increased level after treatment of mouse embryo fibroblasts with phorbol 12-myristate 13-acetate. Generation of the fragment, an appropriate substrate for γ-secretase, is presumed to be proceeded by the shedding of IGF-IR from the membrane surface. This would be mediated by one or more metalloproteinases, such as those belonging to the disintegrin and metalloproteinase domain-containing protein (ADAMs) family. The authors tested whether the putative C-terminal fragment of IGF-IR was indeed a γ-secretase substrate by treating cells with compound E, a specific inhibitor of the enzyme. The compound enhanced accumulation of the 52-kDa protein but limited production of the expected 50-kDa intracellular domain fragment that should result from γ-secretase cleavage activity (McElroy et al., 2007). In aggregate, based on these studies, IGF-IR signaling activity and abundance at the cell- surface seems to be regulated through a number of mechanisms involving interactions with a diverse array of molecules. These would include proteases involved in protein cleavage and recruitment of docking proteins.
C. Insulin-Like Growth Factor-I Binding Proteins
In addition to the relative concentrations of IGFs and the cell-surface density of IGF-IR, the abundance and profile of IGFBPs serve as important determinants of signaling by influencing IGF availability for binding to the receptor. The IGFBP family comprises six proteins exhibiting relatively high affinity for IGFs. In fact, their affinities for IGF-I generally exceed that of IGF-IR. They are synthesized by many tissues and cell types, and their relative levels are under hormonal control. For instance, sex steroids regulate IGFBP synthesis in breast, granulosa cells, and cultured osteoblasts (Mondschein et al., 1990). In addition to their relative levels determining the biological impact of IGF-I, IGFBPs can undergo post-translational processing, such as phosphorylation, glycosylation, and ubiquitination. Each of these protein modifications can profoundly alter IGFBP binding activity. Besides their functions as IGF-I carrier proteins, IGFPBs exert actions on target cells, either as ligated or unligated molecules (Hwa et al., 1999). Notable among them is IGFBP3, which has been implicated in the pathogenesis of several forms of cancer, including that of the prostate (Pollak et al., 2004). It is by virtue of the wide array of proteases associated with prostate cancer that IGFBP3 is degraded, freeing IGF-I in the process. Specific proteases have been identified for each IGFBP. Among these are cathepsin, various matrix metalloproteinases, stromelysins, and kallikreins (Blat et al., 1994; Mañes et al., 1997, 1999).
IGFBPs generally serve to modulate growth factor activity, in large part by sequestering IGF-I and therefore determining the fraction that is available to act on target cells (Rosenfeld et al., 2000). Most of these circulate as approximately 150-kDa complexes containing an acid-labile subunit, IGFBP, and IGF-I. After the dissociation of this three-component aggregate, IGF-I/IGFBP comes out of circulation and crosses the endothelium to associate with target cell surface IGF-IR. With regard to its abundance and contribution to IGF-I carrying capacity, IGFBP3 is the most important (Firth and Baxter, 2002). IGFBPs can also enhance IGF-I activity, perhaps by facilitating its delivery to target cells (Wetterau et al., 1999). As a complex, IGF-I survival in the circulation is prolonged, its interactions with IGF-IR are modulated, and the IGFBPs might help target specific cells for IGF-I action. Moreover, IGFBPs help create concentration gradients for IGF-I and therefore determine the factor's impact on microenvironments surrounding target cells.
While a number of the biological effects ascribed to IGFBPs are dependent upon their association with IGF-I or IGF-II (Jones and Clemmons, 1995; Rajaram et al., 1997), increasing interest has driven further investigation into those actions that are IGF-I-independent. In addition to their roles as chaperones for IGF-I, at least some of the IGFBPs seem to act in the absence of bound IGF-I (i.e., in an unligated state). They bind multiple extracellular matrix components (Firth and Baxter, 2002). For instance, IGFBP1 contains an Arg-Gly-Asp (RGD) integrin recognition site situated in the carboxyl terminus (Drop et al., 1992) through which it can bind to the fibronectin receptor α5β1 (Jones et al., 1993). IGFBP2 possesses a similar motif. In addition, a basic heparin-binding sequence in the thyroglobulin-like domains resembles those found in IGFBP-3, IGFBP-5, and IGFBP-6. IGFBP-3 interacts with the glycosaminoglycan moiety of proteoglycans (Baxter and Firth, 1995; Fowlkes and Serra, 1996). Similar domains on other IGFBPs allow analogous interactions on cell surfaces and within the extracellular matrix (Parker et al., 1996, 1998), although little insight currently exists concerning their biological function. One potential consequence of this basic heparin-binding domain concerns modulating the degradation of another related protein, IGFBP-4, the effects of which seem to be opposed by IGF-I (Verschure et al., 1996). It is possible that the basic region of IGFBPs inhibits protease activity (Fowlkes et al., 1997).
IGFBP3-provoked signaling seems to use retinoic acid receptor X, a nuclear transcription factor, to which it binds with relatively high affinity (Lee and Cohen, 2002). This binding protein complexes with RXR-α within the cell nucleus (Liu et al., 2000). Moreover, IGFBP3-induced apoptosis is abolished in cells in which RXR-α is knocked out. Thus, the aggregate of RXR and IGFBP3 proteins conveys functional importance. In addition, specific membrane binding of IGFBP3 has been demonstrated in chick embryonic cells (Delbé et al., 1991), Hs578T (Oh et al., 1993) and MCF-7 breast cancer cells (Ricort et al., 2002). The type V TGF-β receptor can bind IGFBP3 (Leal et al., 1999). A putative 420-kDa membrane receptor for IGFBP5 that exhibits serine kinase activity has been demonstrated on osteoblasts (Andress, 1995; Berfield et al., 2000). IGFBP5 can also associate with TS-1, which in turn attenuates the integrin associated protein-TS-1 complex and therefore diminishes IGF-IR phosphorylation and activity (Moralez et al., 2005). IGFBP3 directly induces phosphotyrosine phosphatase activity in MCF-7 cells (Ricort and Binoux, 2002). Thus, it is possible that this protein exerts its modulating influence on IGF-I signaling by dampening tyrosine phosphorylation (Ricort and Binoux, 2001).
III. Emerging Insights into Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-I Receptor: Roles in Immune Integration
Although endocrine function is intimately intertwined with growth and development, the potential relationship between immune function and growth factors such as IGF-I has remained poorly characterized until relatively recently. With the growing realization that diverse regulatory pathways often converge, a number of studies have demonstrated the importance of GH, IGF-I, and IGF-IR to many aspects of immune function. In addition, immune reactions and the inflammation with which they are often linked have been shown to affect normal growth and patterns of tissue remodeling occurring in wound repair. Although these interactions could have been predicted from the well known deleterious effects of chronic inflammatory disease on child growth and development, we now have gained critical insights into their mechanistic basis. Linking these biological functions is the complex interplay between cytokines and growth factors, including IGF-I. This topic has been reviewed recently (O'Connor et al., 2008). In brief, pro-inflammatory cytokines seem to dampen several components of the IGF-I pathway. Many of the cytokines share common signaling components, such as the Erk 1/2 MAP kinase. Thus, molecular events initiating signaling down a common pathway can modify the availability and activities of shared postreceptor docking proteins and thereby influence the magnitude and quality of cellular responses emanating from receptor/ligand interactions. As an example, levels of IGF-I trend downward as a consequence of aging and chronic disease (Moldawer and Copeland, 1997; Grounds, 2002). Thus, if all other factors remain constant, the influence exerted by the endogenous IGF-I/IGF-IR pathway on immune function and inflammation mediated through the signaling pathways shared with IGF-IR might diminish as an individual ages or becomes chronically ill. Conversely, children diagnosed with one of several diseases associated with chronic illness exhibit alterations in growth and development attributable to GH/IGF-I dysfunction.
A. Hematopoiesis
Soon et al. (1999) reported findings from studies examining how IGF-IR and IL-4R signaling interact in 32D myeloid precursor cells. These IL-3-dependent cells express IR but lack both IRS-1 and IRS-2. IL-4 fails to elicit any response in 32D cells. However, when either IRS-1 or IRS-2 is coexpressed with IL-4R, the cells respond to IL-4 robustly (Wang et al., 1993). When IGF-IR is overexpressed in 32D cells, the activated receptor initiates DNA synthesis. It is noteworthy that IGF-IR transfectants were also responsive to IL-4 in the absence of either IRS-1 or IRS-2 (Soon et al., 1999). The effects of IGF-I and IL-4 seemed synergistic and were unrelated to changes in PI3 kinase activity, but the Src-homology-collagen/Grb2/MAPK pathway played a critical role (Soon et al., 1999). Moreover, c-myc gene up-regulation and enhanced Erk2 and STAT6 activities were positively correlated with the effect of IL-4 on cell proliferation in these IGF-IR transfectants. hIGF-I promotes hematopoietic growth in vivo in mice (Tsarfaty et al., 1994). In mouse syngeneic bone marrow transplant models, GH and IGF-I administration enhances reconstitution of the immune system (Murphy et al., 1992a,b,c; de Mello-Coelho et al., 1997). Recombinant GH also enhances hematopoietic reconstitution after syngeneic bone marrow transplantation in mice (Tian et al., 1998). Using three different but extensively characterized mouse models of allogeneic bone marrow transplantation, Alpdogan et al. (2003) demonstrated that CD4−CD8−CD3−Thy−1.2+ lymphoid and myeloid reconstitution after marrow transplantation was enhanced by IGF-I. In that study, thymic precursor cell populations were expanded by IGF-I administration (Alpdogan et al., 2003). The CD25+CD44+CD4−CD8−CD3−Thy−1.2+ and CD25+CD44−CD4−CD8−CD3−Thy−1.2+ subsets were increased. On the other hand, overall thymic cellularity was unaffected. IGF-I increased the frequency of splenic myeloid cells and pro-B, pre-B, and mature B cells in allogeneic bone marrow transplant recipients. Donor-derived peripheral splenic CD3+T cells became more abundant and exhibited enhanced proliferative responses to mitogens. Moreover, the effects of IL-7 on B cells were substantially promoted by IGF-I but not those actions exerted on T cells. The study also examined the impact of IGF-I on the development of graft-versus-host reactions in both MHC matched and mismatched hosts and found no effects on either morbidity or mortality among the animals (Alpdogan et al., 2003).
B. Thymus Development and Function
Regulation of thymic development and physiologic function involves numerous intersecting molecular signals. Although it was thought to function only in childhood, much evidence now supports the concept that thymic activity persists well into adulthood (Poulin et al., 1999). Moreover, certain pathological states seem to promote increased activity within the thymus (Choyke et al., 1987; McCune et al., 1998). Among the supporting factors, IGF-I and GH target thymic epithelial cells, where they synergistically promote the action of anti-CD3 in stimulating proliferation (Savino et al., 2002). Kooijman et al. (1995a,b,c) reported that human thymocytes display 257 ± 28 IGF-I binding sites/cell with a Kd of 0.12 ± 0.01 nM. Precursor thymocytes represent pluripotential CD3−CD4−CD8− cells that differentiate into mature CD3+CD4+CD8− or CD3+CD4−CD8+ phenotypes. Mature thymocytes express IGF-IR. Immature cells (CD4−CD8−) display 3- to 4-fold higher receptor levels than do immature CD3−/lowCD4+CD8+ cells and mature CD4+CD8− or CD4−CD8+ cells. IGF-I directly stimulates DNA synthesis in human thymocytes (Kooijman et al., 1995a,b,c). Besides thymocytes, GH and IGF-I have been shown to regulate nonlymphoid components of the thymus. Thymic epithelial cells from human and murine sources express IGF-IR mRNA, as do established cell lines (de Mello-Coelho et al., 1997). In culture, IGF-I induces the synthesis of the zinc-binding nanopeptide serum thymic factor (i.e., thymulin) in human thymic epithelial cells (Timsit et al., 1992; de Mello-Coelho et al., 1997). The authors of these studies found that the plasma levels of thymulin were increased significantly in 21 patients with acromegaly compared with 30 control subjects (Timsit et al., 1992). The effects of IGF-I on the thymic cell population are time-dependent and seem specific. Moreover, the cross-talk between the thymic epithelium and lymphoid cells can be modulated by GH through IGF-I (de Mello-Coelho et al., 1997). IGF-I and IGF-IR mRNA can be detected in murine and human thymic epithelial cells (de Mello Coelho et al., 2002). GH enhances IGF-I synthesis in murine and human thymic epithelial cells. Moreover, treatment of these cells with IGF-I results in increased CD4−CD8−CD90+ (Thy-1+) T cell adhesion, suggesting the possibility that the growth factor might indirectly influence intrathymic T cell differentiation and migration (de Mello Coelho et al., 2002). Atrophy of the thymus is a recognized consequence of substantial insulin deficits in diabetes mellitus. The thymic atrophy found in animal models of insulin-deficient diabetes can be reversed with exogenous IGF-I at doses insufficient for restoring glycemic control (Binz et al., 1990). That result suggests the possibility that IGF-I, and perhaps insulin, might exert actions on the thymus that are independent of their impact on blood glucose levels. Both IGF-I and IGF-II can increase the number of CD4+CD8+ immature T cells in rat thymus and spleen (Hinton et al., 1998). They also enhance repopulation of atrophic thymus after cyclosporine treatment (Beschorner et al., 1991). Although IGF-I failed to prevent glucocorticoid-induced thymocyte apoptosis in rats, it reduced the death rate among lymphocytes in the spleen and protected modestly splenic B and T cell loss (Hinton et al., 1998). It also enhanced the recovery of CD4+CD8+ immature intrathymic T cells and depressed the abundance of CD8+ cells (Hinton et al., 1998). When administered to 9-month-old male mice, both splenic and thymic weights increased, and these tissues contained substantially increased numbers of CD4+ T cells (Clark et al., 1993). In addition, splenic B cells were also more numerous in these animals. GH helps reverse age-related decline of thymopoiesis in animal models (Clark et al., 1993; Kelley et al., 1996; Clark, 1997). In adult patients infected with HIV-1, GH increased thymic mass and the abundance of circulating CD4+ T cells (Napolitano et al., 2002). In a considerably more comprehensive prospective clinical trial, these same investigators re-examined GH effects in adults infected with HIV-1 (Napolitano et al., 2008). They concluded that the thymic output was increased because the frequency of circulating naive and total T cells was elevated, as was T cell receptor rearrangement excision circles (Napolitano et al., 2008). Those findings imply that GH might reverse thymic involution in immunodeficient human subjects and therefore its administration might constitute therapy. Although the administration of hGH and rIGF-I in patients infected with HIV failed to influence significantly the abundance of CD4+ T cells, alter the profile of RA and RO CD4+ subsets, or affect several other immunologic endpoints, the authors of a pilot study concluded that these agents could modestly improve HIV-specific immune function (Nguyen et al., 1998). These observations could carry broader implications concerning a strategy for preserving immune function in the context of various disease processes such as retroviral illness and the decline resulting from normal aging.
C. Immunocoordination
Besides their impact on discrete components of the immune system, IGF-I and IGF-II seem to modify several aspects of inflammation, at least in part by influencing the actions of cytokines and other small molecule mediators. These same mediators can in turn alter the abundance of IGF-I and modulate its actions on target tissues. Thus various components of the inflammatory machinery and the IGF-I pathway share a complex relationship that manifests in several ways. As an example, IGF-I can increase survival in rats treated with d-galactosamine and LPS, a strategy used to induce experimental acute hepatic failure (Hijikawa et al., 2008). When administered before the d-galactosamine and LPS, IGF-I prevented the biochemical stigmata of liver failure, such as elevations in bilirubin and transaminases. Upon histologic examination, the growth factor seemed to decrease hepatic apoptosis and neutrophil infiltration. This effect seems to result from IGF-I blocking the elevation of IL-1β, TNF-α, and neutrophil chemoattractant 1 associated with d-galactosamine and LPS administration. IGF-I additionally reduces the production of nitrous oxide by inhibiting the inductive effects of LPS and d-galactosamine on nitric-oxide synthase mRNA and protein levels in the liver. These effects seem to be independent of nuclear factor-κB (Hijikawa et al., 2008). When TNF-α signaling in skeletal muscle is activated through the c-Jun N-terminal kinase, the signal activity provoked by IGF-I can be attenuated through changes induced in the conformation of IRS-1 and its phosphorylation (Grounds et al., 2008). In children with extensive thermal burns, the administration of IGF-I in combination with IGFBP3 as a continuous infusion at a rate of 1 to 4 mg/kg/day, reduced serum levels of IL-1β, TNF-α, C-reactive protein (CRP), α1-acid glycoprotein, and complement C-3 (Jeschke et al., 2000). In contrast, the serum levels of retinol-binding proteins, prealbumin, and transferrin were increased by the infusion. The inflammatory status of patients with peripheral arterial disease affects serum levels of IGF-I and IGFBP-3 (Brevetti et al., 2008). In patients manifesting either ulcerative colitis or Crohn's disease, circulating levels of IGF-I are inversely correlated with erythrocyte sedimentation rate and C-reactive protein (Street et al., 2003). On the other hand, IGFBP-2 levels are positively correlated with erythrocyte sedimentation rate and IL-1β levels. In black male smokers, IGF-I levels are inversely correlated with those of C-reactive protein (Colangelo et al., 2009).
IV. Impact of Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-I Receptor on Immune Cell Lineages
A. T Lymphocytes
T cells display many different surface growth factor receptors, including IGF-IR (Schillaci et al., 1998). IGF-I, IGF-II, and insulin bind to the surface of T and B cells. High-affinity binding sites for insulin (Kd = 2 × 10−10 M) were found by Helderman and Strom (1978) on activated B and T cells, and IR was proposed by the authors to represent a marker of lymphocyte activation. Lee et al. (1986) were among the first to demonstrate IGF-IR expression by some, but not all, T cell lines derived from patients with lymphoid malignancies. High-affinity, saturable binding of 125I-IGF-I was subsequently demonstrated on both activated and resting human T cells with a Kd of 1.2 × 10−10 M (Tapson et al., 1988). The number of binding sites increases from approximately 45 high-affinity sites per resting cell to 330 sites per activated cell. Moreover, those studies demonstrated that IGF-I can induce T-cell proliferation and chemotaxis (Tapson et al., 1988). The binding capacity for IGF-I is greater on rat CD4+ compared with CD8+ T cells. Moreover, binding sites on CD8+ T cells seem to exhibit a relatively lower affinity for the ligand than those on their CD4+ counterparts (Xu et al., 1995). IGF-IR levels displayed by rat lymphocytes increase after activation with concanavalin A in both CD4+ and CD8+ T cell subsets.
The consequences of IGF-IR expression to lymphocyte function have been examined in several different studies employing a wide array of experimental models and technical approaches. Yang et al. (2002) studied human cord blood lymphocytes and the impact of knocking down IGF-IR expression with antisense oligonucleotides on cell function. They found that receptor abundance was increased by cell activation with either phytohemagglutinin or pokeweed mitogen and that reducing IGF-IR levels resulted in depressed cell proliferation and reduced IgM production and cytokine expression (Yang et al., 2002). Their studies apparently were conducted in culture medium supplemented with 10% fetal calf serum but otherwise in the absence of IGF-I. T-cell generation in neonates focuses on maintaining a preset optimal clonal size and is not necessarily geared toward attaining maximal repertoire diversity (Schönland et al., 2003). Thus, naive T cells remain capable of cellular replication, even late in life. A host of factors allowing expansion supports attainment of the preset post-thymic T cell clonal size. Among them, IL-7 plays an important role, especially during neonatal life, but may also exert an influence during adulthood (Schönland et al., 2003). Whether IGF-I and its signaling pathways also play some role in determining how T-cell compartments are filled throughout life remains an open question. However, given the importance of IL-7 in that process and how IGF-I potentiates the actions of IL-7 in pro-B cell expansion (Gibson et al., 1993), a similar influence on T cells seems likely.
Both IGF-I and IGF-II play important roles in the development and function of T cells. IGF-I can activate T cell Akt and thereby enhance lymphocyte survival (Walsh et al., 2002). However, both IGF-I and insulin can also suppress immune responses. In C57BL/6 mouse splenocytes, IGF-I profoundly blocked IL-2-dependent cell growth (Hunt and Eardley, 1986). Although not as potent, insulin had similar effects. These were evident in both T and B cell-enriched splenocyte preparations treated with IL-2 and were also observed in unfractionated splenocytes treated with Concanavalin A and LPS. The EL4 thymoma cell line exhibited growth retardation in response to IGF-I similar to that observed in primary splenocytes, where the growth factor was 100 to 1000 times more potent than insulin. Proliferation of the mouse T cell line CTLL2 cells is dependent on IL-2 but is inhibited by IGF-I. These cells were less sensitive to the inhibitory actions of IGF-I than were splenocytes. With regard to responses in vitro, in a plaque-forming cell assay, IGF-I and insulin suppressed antibody production (Hunt and Eardley, 1986). The suppressive effects of IGF-I on IL-2-dependent cell proliferation could not be overcome with increasing concentrations of the latter agent. The suppressive effects were time-dependent and evolved over many hours of incubation. Thus, IGF-I and insulin, although generally enhancing lymphocyte proliferation (Heulin et al., 1982; Schimpff et al., 1983; Walsh et al., 2002), can also block IL-2-dependent lymphocyte growth and function.
Thymic IGF-I, IGF-II, and IGF-IR mRNAs are expressed as early as fetal day 14 in mice (Kecha et al., 2000). The IGF-II transcript declined in the postnatal period, but a weak reverse transcription-polymerase chain reaction signal remained detectable at week 7 (Kecha et al., 2000). In that report, culture-based studies of fetal thymic organ suggested that interruption of the IGF-I and IGF-II pathways results in potentially important and divergent effects on the differentiation of double-negative CD4−CD8− cells compared with CD4+CD8+ cells and after their progression to mature CD4+CD8− and CD4−CD8+ phenotypes (Kecha et al., 2000). When administered to animals, IGF-I expands the T-cell population (Clark et al., 1993). IGF-II also enhances T-cell development, as was strongly suggested in studies using transgenic mice overexpressing that protein where human IGF-II production was under the control of the H2Kb gene promoter (Kooijman et al., 1995a,b,c). Transgenic thymus in 1-week-old mice from the two different lines contained 36 and 68% more thymocytes than did control animals (Kooijman et al., 1995a,b,c). At 4 weeks of age, IGF-II expression resulted in enhanced thymic cellularity. This increase could be accounted for by the emergence of more numerous early CD4−CD8− and CD4−CD8dim cells, intermediate CD4+CD8+ cells, and mature thymocytes with the CD3+CD4+CD8− or CD3++CD4−CD8+ phenotypes. As the mice continued to age, differences between thymocyte populations in transgenic and control animals disappeared.
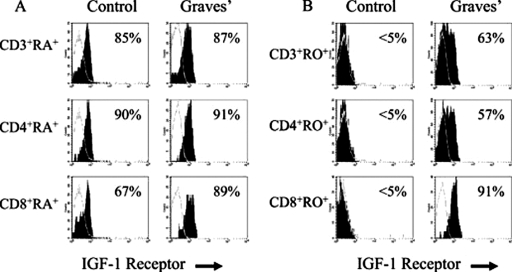
Stage of maturation determines the level of IGF-IR displayed by developing T cells, as assessed by recognition with the specific monoclonal antibody αIR3. Receptor density was quantified on human peripheral T cells (Kooijman et al., 1995a,b,c). Eighty-seven percent of CD4+CD45RA+ cells and 66% of CD8+CD45RA+ cells stained for the receptor (Kooijman et al., 1995a,b,c). In contrast, 37 and 38% of the CD4+CD45RO+ and CD8+CD45RO+ memory T cells, respectively, exhibited the IGF-IR+ phenotype (Kooijman et al., 1995a,b,c). T-cell expression of IGF-IR undergoes down-regulation as differentiation proceeds. Thus double-negative CD4−CD8− cells exhibit 3- to 4-fold higher levels of surface receptor display than do either double-positive or single-positive cells. IGF-IR levels were markedly lower in activated T cells, both in vitro and in vivo. CD4+CD45RO+ cells activated by phytohemagglutinin or after exposure to recall antigens in vitro display considerably lower levels of IGF-IR than do inactive lymphocytes (Kooijman et al., 1995a,b,c; Schillaci et al., 1998; Segretin et al., 2003). This reduction may be transient and associated with depressed steady-state IGF-IR mRNA levels (Segretin et al., 2003). A similar finding could be demonstrated in Jurkat cells, and addition of IGF-I to the culture medium enhanced this effect (Schillaci et al., 1998). These findings contrast with those reported by Tapson et al. (1988) and may reflect differences in experimental conditions or how cell maturation studies were performed. In addition, monocyte-depleted peripheral human T cells activated with immobilized anti-CD3 were found to display up-regulated IGF-IR, IGF-IIR, and IR (Johnson et al., 1992). Those cells proliferated in response to both IGF-I and IGF-II, effects that could be blocked with the IGF-IR-blocking antibody αIR3. IGF-IR expression can also be up-regulated in T cells through CD28 receptor cross-linking or by activating the CD80/CD86 pathway (Walsh and O'Connor, 2000). Moreover, blocking IGF-IR on TCR- and CD28-engaged T cells decreases lymphocyte survival in the presence of IL-2 (Walsh and O'Connor, 2000). CD28 activation enhances IGF-IR display on Jurkat cells and treatment with IGF-I conveys resistance to Fas-mediated apoptosis (Walsh and O'Connor, 2000). IGF-I enhances the maturation of T cells collected from cord blood (Tu et al., 2000) and blocks spontaneous apoptosis and the programmed cell death induced by phytohemagglutinin. It down-regulates interferon γ R2 chain display on the surface of human T cells (Bernabei et al., 2003), resulting in desensitization of these cells to interferon γ-dependent STAT-1 signaling. It also activates Akt (aka PKB) and c-Jun N-terminal kinases, resulting in resistance to Fas-mediated apoptosis (Walsh et al., 2002). On the other hand, IGF-I elicits the production of IL-10 in human T cells through a modest increase in IL-10 mRNA (Kooijman and Coppens, 2004). In cultured 32D hematopoietic cells overexpressing IGF-IR, treatment with IGF-I and IL-4 enhanced DNA synthesis in the absence of IRS expression (Soon et al., 1999). The SH-2/Grb2/MAPK pathway seems crucial to the mitogenic effects of both agents.
Activation of the signaling pathways downstream from IGF-IR and IR increases lymphocyte metabolism. The overall capacity for increased energy turnover accompanying T-cell activation is regulated by insulin (Frauwirth and Thompson, 2004). Two signals provoke cell activation: primary signals are directed through the TCR/CD3 complex, and secondary signals are provided by activated costimulatory receptors such as CD28 (Parry et al., 1997; Frauwirth et al., 2002). Enhanced glucose uptake is mediated through the transcriptional up-regulation of the Glut1 transporter accompanied by the translocation of Glut4 transporter to the cell surface (Barthel et al., 1999; Wang et al., 1999). Moreover there is an overall shift to aerobic glycolysis (Buttgereit et al., 2000). Oxygen demand after activation occurs rapidly but is overshadowed by a shift to glycolysis. A major consequence of the metabolic profile associated with cell activation is accelerated lactate generation, similar to that observed in tumor cells. The signaling downstream from both IR and IGF-IR is mediated through Akt/PKB (Barthel et al., 1999). CD28 activation, like that of IR and IGF-IR, culminates in the recruitment of Akt to the cell surface by phosphatidyl inositol triphosphate, where it becomes phosphorylated through the actions of PI3 kinase-responsive kinase PDK-1.
Aging rodents exhibit diminished responsiveness to pathogens. This shift is associated with reduced cellularity and frank involution of the thymus (Hadden et al., 1992; Miller, 1996; Montecino-Rodriguez and Dorshkind, 1997; Tian et al., 1998). A potential strategy for reversing these senile changes in thymic vitality involves administration of either GH or IGF-I. These agents have been examined for their potential to expand T-cell populations in animals. Eighteen-month-old mice were administered IGF-I, bone marrow cells from younger mice, or a combination of the two (Montecino-Rodriguez et al., 1998). Cellularity of the thymus in animals receiving both was enhanced considerably more than in those receiving only one of these treatments. This report contained studies conducted in vitro, demonstrating that IGF-I might potentiate thymic colonization by T cells derived from the bone marrow (Montecino-Rodriguez et al., 1998). Thus, alteration of the hematopoietic as well as endocrine defects associated with normal aging might reverse thymic involution.
DW/J dwarf mice produce less prolactin and GH than is found in control mice (Duquesnoy and Pedersen, 1981). They also manifest abnormalities in T-cell development (Fabris et al., 1971), including deficits in the abundance of CD4+CD8+ thymic cells (Murphy et al., 1992a,b,c). Treatment of these animals with GH could restore the deficient T-cell progenitor pool within the thymus, enlarge this gland, and enhance peripheral T-cell function. These authors explored the impact of prolactin treatment in the same DW/J mice and reported that its effects were markedly distinct from those of GH (Murphy et al., 1993). Prolactin decreased further thymic cellularity but enhanced both the frequency and activity of peripheral T lymphocytes in heterozygous and dwarf mice. In contrast, GH administration had little influence on antigen-specific responses (Murphy et al., 1993). GH did enhance human T-cell engraftment in SCID mice (Murphy et al., 1992a,b,c). This was related to increased resting and anti-CD3-activated T cell adhesion to intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and fibronectin (Taub et al., 1994). Moreover, analysis of the mechanisms involved revealed that β1 integrin mediates binding to vascular cell adhesion molecule-1 and fibronectin, whereas β2 is necessary for adhesion to intercellular adhesion molecule-1. Furthermore, the impact of GH on increasing human T cell engraftment could be blocked following preincubation with anti-β1 and anti-β2 antibodies (Taub et al., 1994). The hormone could also up-regulate the random migration of resting and activated human T cells, but the effects were substantially less pronounced than those elicited by regulated upon activation, normal T-cell expressed (RANTES), even when the concentration of RANTES was 100-fold lower.
Like many other regulatory molecules, states of resistance to the actions of IGF-I have been described, usually in the context of growth abnormalities (Jain et al., 1998). In a series of studies, Geffner et al. (1993, 1995) established a number of T and B cell lines derived from adult Efe Pygmies or from Lese farmers who are their neighbors in Zaire. The basis for the growth abnormalities found in Efe pigmies concerns a substantially diminished compliment of IGF-IR molecules on the cell-surface and an absence of receptor autophosphorylation in response to physiological concentrations of IGF-I (Hattori et al., 1996). The stature attained by these Lese farmers is intermediate between those of urban black Africans and Pygmies. Interbreeding between these populations is known to occur. The investigators performed clonal proliferation assays in which T cells from the two populations and a third control cohort from North America were incubated with GH, IGF-I, insulin, or appropriate controls and cultures were enumerated after 4 to 7 days. T cells from the Efe Pygmies were found to be completely resistant to the actions of IGF-I. Lese farmer-derived clones exhibited intermediate responses. On the other hand, responses to insulin were indistinguishable among the three T cell donor sources, even when the concentrations used were extremely high. The absence of attenuation in the clones from Pygmies was surprising to the authors because they had demonstrated that responses to high concentrations of insulin are usually mediated through the promiscuous activation of IGF-IR (Geffner et al., 1987, 1992). However, in the case of the Pygmy-derived clones, IGF-IR apparently was uninvolved in the insulin response (Geffner et al., 1993). Similar studies conducted in B cells from these same Efe Pygmy donors revealed similar results: IGF-I failed to elicit responses, even at extremely high concentrations (Cortez et al., 1996), despite the comparable responses in cells from all three sources to phorbol 12-myristate 13-acetate.
B. B Lymphocytes
B cells play diverse roles in immune function by virtue of their further differentiation into immunoglobulin-secreting plasma cells, generation of cytokines, and their importance in antigen presentation. A critical component of B-cell development concerns how committed precursor cells from the hematopoietic lineage undergo immunoglobulin heavy chain gene rearrangement (Kincade et al., 1989). This leads to the expression of light chains and ultimately to the release of mature B cells from the bone marrow. A number of exogenous factors emanating from marrow stromal cells provide molecular support for early B-cell proliferation. Specifically, B-lineage progenitor cells in which Ig gene rearrangement has already been initiated, but before any detectable Ig light or heavy chains are produced, can undergo expansion in the presence of IL-7 when cocultured with stromal cells. Among these stromal factors, IGF-I drives B-cell differentiation (Landreth et al., 1992). In addition, IGF-I enhances IL-7-dependent B-cell proliferation in concert with c-kit ligand (Landreth et al., 1992) and potentiates IL-7 promotion of pro-B-cell expansion (Gibson et al., 1993). Cell lines derived from 14-day-old mouse fetal liver cells proliferate when cocultured with stromal cells cloned from S10 in the presence of IL-7. The proliferation of these lines could be enhanced with the addition of either c-kit ligand or IGF-I (Gibson et al., 1993). Moreover, the effects of c-kit ligand and IGF-I were additive, reflecting the distinct signaling pathways each used to activate these cells. Signaling components lying downstream from IGF-IR may condition B-cell function and responses to additional factors. IRS-1 overexpression in B lymphocytes derived from transgenic mice alters the density-dependent production of IgE and IgG1 in vitro and enhances IgE responses in those animals (Kelly-Welch et al., 2004). In contrast, IL-4-mediated proliferation and its impact on apoptosis were unaffected in mice harboring the IGF-IR transgene. When administered in vivo, IGF-I enhances the population of intrasplenic B cells through increased proliferation of mature cells (Clark et al., 1993; Jardieu et al., 1994). In bone marrow, the number of B cells increased after IGF-I administration in normal adult mice and those receiving lethal irradiation followed by reconstitution with syngeneic bone marrow (Jardieu et al., 1994). Studies conducted in vitro disclose that the differentiation of human CD34+ bone marrow cells was substantially impeded by down-regulating the IGF-I pathway of MS-5 cells in coculture (Taguchi et al., 2006). These studies implicate IGFBP-6 as a necessary component of IGF-I-dependent B cell differentiation, whereas IGFBP-3 acted as an inhibitor of that process.
Plasma cells also respond to IGF-I. DNA synthesis increases substantially in four myeloma cell lines treated with IGF-I in vitro in the absence of IL-6 (Jelinek et al., 1997). In addition, both IGF-I and IGF-II enhance the proliferative action of IL-6 in three of these cell lines. The effects were absent in normal B cells, suggesting that the actions of IGF-I on B cells may diverge in malignant cells. Both IGF-IR and IR are expressed at higher levels by myeloma cell lines than those found in B lymphoblastoid lines (Freund et al., 1994). Plasma cell metabolism may also be targeted by IGF-I. In the RPMI 8226 line, cells express high-affinity binding sites for both insulin and IGF-I (Freund et al., 1993). IGF-I increases IGF-IR phosphorylation and PI3 kinase activation, enhances DNA synthesis, and up-regulates lactate production in these cells. In the IL-6Rα-expressing human myeloma cell line NOP2, IL-6 induces phosphorylation of IGF-IR, an action that cannot be blocked with Janus kinase 2 inhibitors but is duplicated in IL-6Rα-transfected U266 cells (Abroun et al., 2004). Moreover, IL-6Rα colocalizes with IGF-IR in lipid rafts. The authors of the study also reported that IL-6 leads to the activation of STAT3, Erk1/2, and Akt/PKB, which they suggest is a consequence of IGF-IR phosphorylation. These findings identify a potential molecular basis for Janus kinase 2-independent IL-6 signaling mediated through IGF-IR. Moreover, they define a mechanism for cross-talk between two receptors that had been previously shown to independently support myeloma cells and their biosynthetic activities.
IGF-I can also influence antibody expression and class switching by plasma cells. For example, administration of IGF-I to mice results in elevated levels of antibodies (Robbins et al., 1994). Peripheral human B cells selectively bind IGF-I (Stuart et al., 1991). The binding is saturable and can be displaced with unlabeled IGF-I and by high concentrations of insulin. IGF-I binds to a 130-kDa protein in B cells. Human lymphoblasts synthesize IGFBP2 and IGFBP4 (Neely et al., 1991). Baudler et al. (2005) reconstituted Rag2-deficient C57BL/6 mice with fetal liver cells from IGF-IR(−/−) mice. T-cell-independent humoral responses to the type 2 antigen 4-hydroxy-3-nitrophenyl acetyl Ficoll were substantially diminished, whereas those against the T-cell-dependent antigen 4-hydroxy-3-nitrophenyl acetyl-chicken globulin were unaltered. B-cell development remained normal in IGF-IR-deficient chimeras, as did T-cell differentiation. In these animals, IGF-I enhanced immunoglobulin production, an effect that proved independent of B-cell proliferation (Baudler et al., 2005). Kimata and Yoshida (1994a,b) examined human B cells from individuals undergoing tonsillectomies for chronic tonsillitis. In addition, they analyzed several Epstein-Barr virus-transformed lymphoblastoid cell lines, including GM-1056, CESS, GM-1500, GM-3332, SKW, and CBL, and found that both GH and IGF-I enhanced Ig production in all of these cell types. It is noteworthy that the actions of GH were not dependent on an intermediate production of IGF-I (Kimata and Yoshida, 1994a,b). These same authors reported that GH and IGF-I induce IgG4 and IgE production in tonsillar B cells from control donors depleted of sIgE+, treatments that left IgGM, IgG1, IgG2, IgG3, and IgA production unaffected (Kimata and Fujimoto, 1994). Unlike IGF-I, IGF-II and insulin failed to affect IgG4 or IgE synthesis. In these studies, too, the actions of GH seem to be unrelated to IGF-I production, because neutralizing antibodies directed against the growth factor failed to attenuate the impact of GH. It would seem, therefore, that IGF-I and GH may selectively induce IgG4 and IgE production through a class-switching mechanism (Kimata and Fujimoto, 1994). It is possible that GH signals through pathways that are independent of those involving the production of IGF-I. However, the absence of attenuation in studies in which anti-IGF-I antibodies are used to block GH actions does not entirely rule out a role for IGF-I. Interferons α and β could block the induction by IL-4 and IL-13 of IgG4 and IgE but not that of GH or IGF-I. Moreover, anti-CD40 antibodies failed to block the induction of Ig by either GH or IGF-I (Kimata and Fujimoto, 1994). Thus, it would seem that IGF-I and GH induce Ig class-switching in a manner that does not involve the IL-4 or IL-13 signaling pathways.
C. Monocyte-Macrophage Lineage
Human macrophages and granulocytes display IGF-IR. A number of IGF-I binding sites (1390 ± 467) were detected possessing a Kd of 2.3 ± 0.9 nM (Kooijman et al., 2002). IGF-I attenuated spontaneous apoptosis in these cells. Activated macrophages also express IGF-IR, whereas binding sites for 125I-labeled IGF-I were absent on cells before activation (Rom and Pääkkö, 1991). This binding was quenched with excess unlabeled growth factor or with anti-IGF-IR monoclonal antibodies, attesting to its specificity. Alveolar macrophages, obtained from patients with idiopathic pulmonary fibrosis, produce a polypeptide growth factor that promotes fibroblast proliferation (Bitterman et al., 1983). This factor was subsequently identified as IGF-I (Rom et al., 1988). In that study, the 26-kDa macrophage-produced polypeptide was neutralized with an anti-IGF-I monoclonal antibody, could displace 125I-IGF-I from IGF-IR on the surface of human lung fibroblasts, and provoked the phosphorylation of a tyrosine residue on an artificial IGF-IR substrate. In contrast, circulating monocytes fail to generate IGF-I, but tissue-infiltrating cells can be provoked to produce the growth factor by a number of agents (Rom et al., 1988; Kirstein et al., 1992). These act on macrophages through multiple signaling pathways. For instance, prostaglandin E2 induces IGF-I expression through the generation of cAMP and the activation of protein kinase A (Fournier et al., 1995). Colony-stimulating factors also induce in macrophages the synthesis of IGF-I at a pretranslational level (Arkins et al., 1993), as does TNF-α (Fournier et al., 1995).
It seems that in idiopathic pulmonary fibrosis, a disease associated with increased fibroblast proliferation, IGF-I levels correlate well with disease severity. The production of IGF-I by macrophages in this disease may determine, at least in part, disease severity (Uh et al., 1998). This is controlled by several factors, including hyaluronan, acting through the surface receptor, CD44 (Noble et al., 1993). Induction of IGF-I by glycosaminoglycan was found to be mediated through an intermediate up-regulation of TNF-α, an action that was enhanced by IL-1β. On the other hand, interferon γ attenuates IGF-I synthesis in rat macrophages by lowering levels of its steady-state mRNA. These actions are mediated at the level of IGF-I gene transcription rather than as a result of alterations in mRNA stability (Arkins et al., 1995a,b,c). Activation of the human macrophage-like cell line U937 with either the Ca2+ ionophore calcimycin (A23187) or phorbol acetate, results in the up-regulation of IGF-I gene transcription, as assessed by nuclear run-off assays (Nagaoka et al., 1990). These actions require ongoing intermediate protein synthesis. On the other hand, steady-state levels of cytoplasmic IGF-I mRNA declined with macrophage activation, and this effect could be blocked with an inhibitor of protein kinase C. The release of IGF-I protein from activated cells is rapid and resistant to protein synthesis inhibition, suggesting that the released protein comes from a substantial preformed pool of molecules in macrophages (Nagaoka et al., 1990). IL-4 and IL-13 induce IGF-I production in mouse macrophages through the enhancement of gene transcription (Wynes and Riches, 2003). Moreover, interferon γ could block the actions of these Th2 cytokines. STAT6 mediated the actions of IL-4 and IL-13, whereas STAT1 was necessary for the attenuating effects exerted by interferon γ (Wynes and Riches, 2003). In a growth factor withdrawal model of lung fibrosis, IL-4-induced macrophage-derived IGF-I protects CCL39 myofibroblasts from apoptosis, an action attenuated with IGF-I-specific neutralizing antibodies (Wynes et al., 2004). Macrophages and monocytes express receptors for advanced glycosylation end-products. When these receptors become activated, IGF-I mRNA is up-regulated in monocytes and IGF-I protein is released (Kirstein et al., 1992). Levels of IGF-I mRNA and IGFBP-4 increased in a time-dependent manner in cultured bone marrow-derived murine macrophages subjected to macrophage colony stimulating factor-1. These experimental conditions promote cell proliferation and differentiation (Long et al., 1998). Those studies suggested that IGFBP-4 might exert a modulating effect on IGF-I-dependent macrophage differentiation and proliferation.
D. Neutrophils and Other Granulocytes
Other members of the immune system also seem to share important relationships with IGF-I and its signaling pathway. Notable among these are neutrophils, which serve as important effector cells in innate immunity. They function as an early defense against microorganisms. Neutrophils undergo spontaneous apoptosis, a process that seems to be slowed by IGF-I (Kooijman et al., 2002). In a later study, these same investigators demonstrated that IGF-I can block Fas-mediated apoptosis (Himpe et al., 2008). The pro-survival effects of the growth factor are mediated through the phosphatidylinositol-3 kinase pathway. Moreover, the presence of cytokines failed to alter the antiapoptotic actions of IGF-I, suggesting that they may play a dominant role, even within the context of active inflammation. In contrast, IGF-I seems to attenuate the impact of stress on mouse gastric mucosal injury by inhibiting neutrophil activation (Zhao et al., 2009). IGF-I mRNA is developmentally regulated in mononuclear phagocytic cells (Arkins et al., 1993, 1995a,b,c).
V. Implications of Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-I Receptor in Immune Modulation
What does the realization that a growth factor pathway can influence immune function teach us about integrative human biology? Why should molecules playing dominant roles in the regulation of metabolism, growth, and development also influence the function of the immune system? What advantage to the organism does such integration provide with respect to the overall energetic economy, survival, and host defense? Is nature “double-dipping” and using a well traveled set of signaling pathways for multiple unrelated purposes? Or does the logic emerging from a better understanding of evolution help us to identify the pressures that are driving such an overlap?
Great wisdom may be found in this sharing of common pathways. First, growth factors have now been implicated in normal and pathological wound healing and in tissue remodeling under a variety of clinical circumstances. The IR and its downstream signaling targets, including Akt, are now thought to regulate the metabolic activity of T cells (Frauwirth and Thompson, 2004). Insulin itself has been shown to exhibit a set of chemoattractant properties (Berman and Center, 1987). Phytohemagglutinin-activated human T cells are 100-fold more responsive to porcine insulin than are resting lymphocytes. Moreover, both CD4+ and CD8+ cells behave identically when treated with insulin (Berman and Center, 1987). PI3 kinase mediates many of the actions of T-cell costimulatory molecules including CD28. Activation of CD28, with TCR, can coordinately up-regulate glucose availability and metabolism and can therefore accommodate the increased energetic needs associated with cell activation. A specific role for the IGF-I pathway in influencing metabolic activity in lymphocytes has yet to be explored, but its similarities to that of insulin suggest that both may modulate energy turnover in immune responses.
Taken in aggregate, these studies tell us that substantial cross-talk occurs between a complex array of pathways that have proven integral to energy turnover, growth, and development. These pathways, however, seem to determine, at least in part, the amplitude and quality of immune responses. A thread common to these biological functions relates to the high energetic costs of each. It seems logical and even likely, therefore, that a selection advantage underlies the coordination between these aspects of survival. However, because these components are overlapping, interrupting any one of them might have untoward consequences and thus might limit therapeutic strategies targeting these pathways. Therein lies the particular attraction associated with the therapeutic targeting of receptors such as IGF-IR. These lie upstream from the intermediate signaling kinases that participate in multiple signaling pathways.
VI. Insulin-Like Growth Factor-I, Insulin-Like Growth Factor-I Receptor, and Autoimmunity
The constellation of autoimmune diseases continues to represent a particularly vexing group of maladies that are tied together by common etiologic features with uncertain identities. They are thought to emerge from both genetic and environmental factors. Genetic predisposition to autoimmunity was demonstrated nearly 40 years ago by Vladutiu and Rose (1971). There is reason to suspect that shared susceptibility genes participate in many, because they cluster in families. Moreover, patients often manifest multiple diseases. Most but not all exhibit strong female gender predilection (Fairweather et al., 2008). Moreover, the general features of autoimmunity occurring in men and women differ. At the center of these diseases are many of the same pathological features associated with other chronic processes in which inflammation gives way to tissue remodeling. The findings to date concerning the putative role of IGF-I/IGF-IR in regulating immune function have begun to suggest its potential involvement in autoimmunity. Specifically, IGF-I influences the physiological behavior of lymphocytes and other professional immune cells through its activation of IGF-IR. Responses to growth factors and the display of their receptors at relatively high levels could underlie the participation of these cells in chronic inflammatory disease. T cell activation seems to be coupled to increased IGF-I responses (Berman and Center, 1987). Thus, a potential involvement of the IGF-I pathway might help explain several aspects of human autoimmunity. These lines of evidence will be reviewed.
A. Systemic Inflammation
Several potential connections have been made recently between the loss of tolerance to self-antigens and the actions of IGF-I. Of particular interest is the potential for IGF-I to influence the functions and regulation of inflammatory effector cells, particularly professional phagocytes. For instance, cathelicidin hCAP-18/LL37, an antimicrobial peptide involved in innate immunity, can be detected in alveolar macrophages, neutrophils, and macrophages after infection with Mycobacterium tuberculosis (Drop et al., 1992). It is constitutively expressed or can be induced in these cells through toll-like receptors 9, 4, and 2 (Rivas-Santiago et al., 2008). LL-37 complexes with DNA and RNA and, in doing so, elicits immune responses to microbial nucleic acids. Cathelicidin hCAP-18/LL37 is also expressed by A549 epithelial cells and, in this context, has been implicated in the pathogenesis of psoriasis (Lande et al., 2007) through a mechanism involving plasmacytoid dendritic cells becoming activated to host DNA. Human keratinocytes express LL-37 as well as human β-defensin 3, neutrophil gelatinase-associated lipocalin, and secretory leukocyte protease inhibitor in response to IGF-I and TGF-α (Sørensen et al., 2003). Is it thus possible that the up-regulation of LL-37 by IGF-I and the pro-inflammatory cytokine, TGF2α, elicits autoimmune responses through inappropriate reactivity to host DNA or RNA? Could these insights provide important clues regarding IGF-I activation of innate immunity during tissue injury? LL-37 might also possess inhibitory activity against proteases, potentially resulting in a breakdown of immune tolerance.
B. Graves' Disease
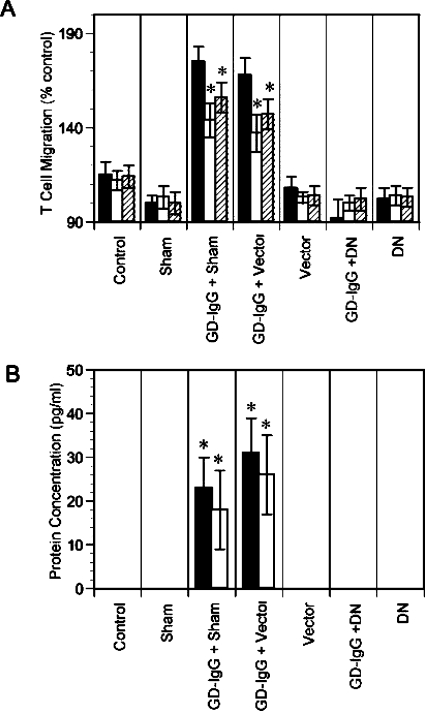
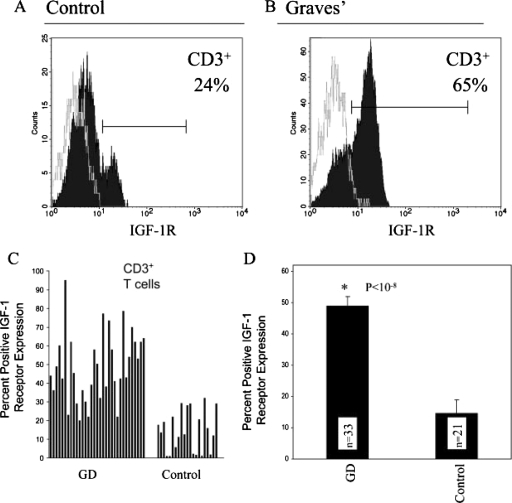
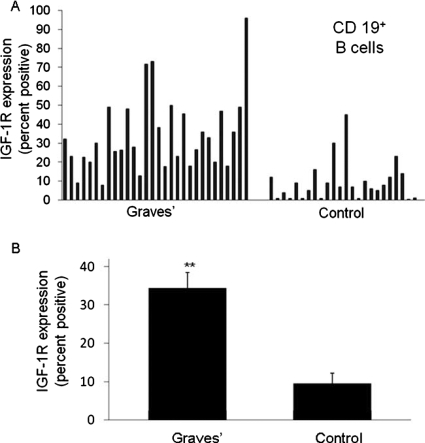
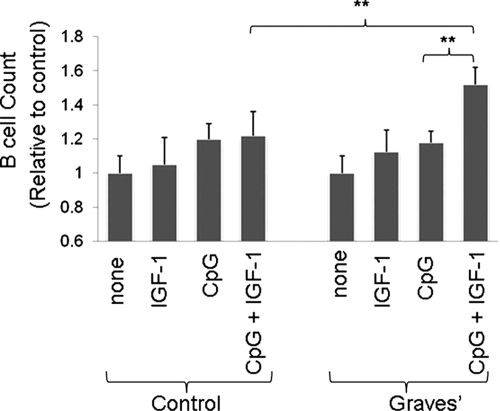
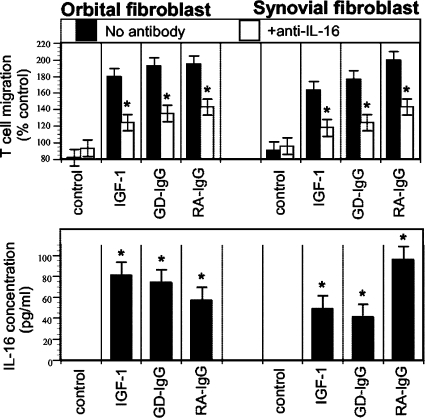
Graves' disease has recently been examined as potentially involving the IGF-I/IGF-IR pathway. It represents an autoimmune syndrome with multiple components, the most frequent of which involves thyroid gland overactivity and enlargement (Davies, 1996). In addition, the tissues surrounding the eye, called the orbit, become activated in a substantial fraction of patients with Graves' disease in a process known as thyroid-associated ophthalmopathy (TAO). The activation of orbital fibroblasts seems to be at the center of TAO pathogenesis. These cells have a phenotype distinct from fibroblasts inhabiting other connective tissues (Smith et al., 1995, 2002).
Graves' disease is a prototypic example of antibody-driven autoimmunity. Central to promoting the hyperthyroid state and thyroid enlargement in this disease are activating antibodies directed against the thyroid-stimulating hormone receptor (thyrotropin receptor or TSHR) (Zakarija et al., 1988). These antibodies, termed thyroid-stimulating immunoglobulins, override the trophic control of thyroid function normally imposed by the hypothalamic/anterior pituitary axis through its elaboration by thyrotrophs of TSH. TSH ordinarily acts as the dominant regulator of the biosynthetic activities of thyroid epithelial cells. This axis is regulated by a sensitive feedback loop that responds to the circulating levels of the thyroid hormones thyroxine and triiodothyronine. Adequate levels of these hormones turn off the production and release of TSH in states of thyroid hormone sufficiency. TSHR represents a seven membrane-spanning G protein-coupled receptor (Parmentier et al., 1989). Its expression was originally thought to be limited to the thyroid epithelium. More recently, it has been found to be displayed on fat cells in several connective tissue depots, and unexpectedly by CD11b− bone marrow cells in mice (Bell et al., 2000; Wang and Klein, 2001; Klein, 2003). Of potential importance to the pathogenesis of TAO is the demonstration of TSHR mRNA in orbital tissues (Feliciello et al., 1993) and by orbital fibroblasts (Heufelder et al., 1993).
An intriguing relationship between thyroid function and IGF-I was first recognized more than 20 years ago, when the rat clonal thyroid epithelial cell line FRTL-5 was found to exhibit far greater responses to TSH in the presence of either insulin or IGF-I in the culture medium (Tramontano et al., 1986, 1987, 1988a,b). Ingbar et al. found that both IGF-I and TSH could enhance FRTL-5 cell proliferation and DNA synthesis in a concentration-dependent manner and that the actions of these agents were synergistic (Tramontano et al., 1986). In contrast, IGF-I does not influence TSH-dependent cAMP generation in these FRTL-5 cells (Tramontano et al., 1986, 1987, 1988a,b). When IGF-I is replaced by insulin, equivalent effects are seen. Both insulin and IGF-I down-regulate major histocompatibility complex class I gene expression (Giuliani et al., 2006). These growth factors depress the activity of the intact PD1 gene promoter but up-regulate those of mutant promoter constructs lacking the tissue-specific region but retaining a gene enhancer sequence. Moreover, IGF-I and insulin facilitate the interactions between promoter regions with transcription factors, including activator protein-1 and nuclear factor-κB (Giuliani et al., 2006). Clément et al. (2001) demonstrated that the conditional overexpression of both IGF-I and IGF-IR in the thyroids of double-transgenic mice increases thyroid gland weight and follicular lumen volumes in vivo. A number of reports have suggested that substantial overlap exists between the signaling elicited by TSH and IGF-I in thyroid epithelium. Specifically, several signaling events downstream from TSHR are mediated through the PI3 kinase pathway and involve activation of the Akt/FRAP/mTOR/p70s6k pathway (Cass and Meinkoth, 1998; Park et al., 2000a,b, 2005). This pathway mediates a number of the growth-promoting actions of IGF-I in many responsive cell types. In addition, it regulates the translation of proteins encoded for by preformed mRNAs containing an oligopyrimidine tract at the transcriptional start site (Jefferies et al., 1997).
It has been proposed that IGF-IR and TSHR might participate together in the pathogenesis of Graves' disease through their physical and functional interactions. Activation of each receptor results in the utilization of common downstream signaling pathways. Specifically, TSHR and IGF-IR seem to act in concert to regulate discrete metabolic activities within the thyroid, including cellular proliferation and apoptosis. Recently, a report suggested that TSHR and IGF-IR colocalize in orbital fibroblasts and in human thyroid epithelial cells in culture (Tsui et al., 2008). Moreover, these investigators found that both IGF-IRβ and TSHR could be pulled down by antibodies specific for either protein. It is noteworthy that that report also suggested that interrupting IGF-IR signaling by incubating cells with a blocking monoclonal antibody directed against IGF-IRα (1H7) could attenuate the activation of Erk provoked by TSH (Tsui et al., 2008). Those findings imply a functional complex comprising TSHR and IGF-IR and that IGF-IR transactivation could mediate at least some aspects of TSHR signaling. Whether this relationship between the two receptors in any way relates to the pathogenesis of Graves' disease remains uncertain.
Activation of the orbital connective tissue in TAO is characterized by often intense inflammatory responses involving T-, B-, and mast-cell infiltration of orbital tissues and their dramatic remodeling (Prabhakar et al., 2003). Although the antibodies generated against the TSHR help explain the aberrant growth of thyroid tissue and its overproduction of thyroid hormone, any role for that receptor and the activating antibodies generated against it in TAO remains incompletely understood. Reconciling the tissue reactivity in the orbit with the thyroid pathology on the basis of a one-antigen/one antibody paradigm has been difficult to accomplish. Other antigens besides TSHR have been examined for their potential involvement in TAO. Among them, the IGF-I pathway was first implicated in studies examining whether IgG from patients with Graves' disease (GD-IgG) could displace 125I-IGF-I from the surface of human fibroblasts (Weightman et al., 1993). Those studies demonstrated a dose-dependent competition with radiolabeled IGF-I from fibroblast surfaces. Although this study was incomplete in that it neglected to explore the identity of the binding site(s), it broke new ground in implicating the IGF-I pathway in Graves' disease. The study also fell short of characterizing whether GD-IgG could either block occupancy or activate the receptor. The basis for loss of peripheral immune tolerance to IGF-IR underlying the generation of the IGF-IR-activating antibodies in Graves' disease remains unknown.
More recently, a study has demonstrated increased density of IGF-IR on the surface of orbital fibroblasts from patients with Graves' disease (Pritchard et al., 2003). In addition, the frequency of fibroblasts expressing the receptor protein is also elevated above that found in control cultures derived from healthy tissue. The levels of IGF-IR are approximately 3- to 4-fold higher than those found in control fibroblasts. Moreover, GD-IgGs could activate fibroblasts from patients with Graves' disease from several connective tissue depots (Pritchard et al., 2003). These include orbital fat, neck skin, abdominal skin, and pretibium. When treated with GD-IgG or with IGF-I, these fibroblasts expressed two powerful T-cell chemoattractants, namely IL-16 and RANTES (Pritchard et al., 2002). IL-16 specifically targets CD4+-bearing T cells and therefore serves as a chemoattractant for only one subset of lymphocytes (Klimiuk et al., 1999). It is expressed by both CD4+ and CD8+ lymphocytes, epithelial cells, fibroblasts, and mast cells (Center et al., 1997, Sciaky et al., 2000). The mechanisms involved in the synthesis of IL-16 and regulation of its expression vary among these cell types. In fibroblasts, relatively high levels of untranslated IL-16 mRNA can be detected under basal culture conditions. Cell activation with IGF-I leads to the caspase-3-dependent production of IL-16 protein (Zhang et al., 1998). RANTES, a C-C chemokine, is also expressed by several cell types and exerts its biological actions through multiple G protein-coupled receptors, including CCR4 and CCR5 (Schall et al., 1990). RANTES targets several subpopulations of lymphocytes. Both IL-16 and RANTES have been implicated previously in other autoimmune diseases (Klimiuk et al., 1999; Simchen et al., 2000) and elevated levels of both have been detected in the general circulation of patients with chronic inflammatory disease (Blaschke et al., 2001; Christodoulakos et al., 2007).
The induction of IL-16 by IGF-I in fibroblasts from patients with Graves' disease involves the activation of the Akt/mTOR/FRAP/p70s6k pathway and can be attenuated with rapamycin, a macrolide exhibiting both antifungal and immunosuppressive activities (Pritchard et al., 2002). Rapamycin specifically targets mTOR, one of the terminal kinases coupling mitogenic stimulation to the serine/threonine phosphorylation of the eukaryotic initiation factor (eIF)-4E-binding protein, PHAS-I (Pritchard et al., 2002). Physiological concentrations of glucocorticoids also block the induction by IGF-I of IL-16 and RANTES (Pritchard et al., 2002). In contrast, the induction of RANTES seems unaffected by rapamycin and, unlike that of IL-16, involves IGF-I actions mediated at the pretranslational level (Pritchard et al., 2002). The mechanism for IGF-I and GD-IgG induction of these chemoattractants remains incompletely described. The antibody(s) was found to bind to IGF-IR (Pritchard et al., 2003). An IGF-IR blocking monoclonal antibody, 1H7, completely abrogates the signaling and induction of IL-16 and RANTES-dependent T-cell migration activity generated by fibroblasts treated with either IGF-I or GD-IgG (Fig. 4) (Pritchard et al., 2003). Moreover, transfection of TAO orbital fibroblasts with a mutant dominant-negative IGF-IR, designated 486 STOP (Reiss et al., 2001), could also block IL-16 and RANTES synthesis resulting from the actions of IGF-I and GD-IgG (Fig. 5). Subsequently, GD-IgG and IGF-I were found to also induce the synthesis of hyaluronan in orbital fibroblasts from donors with TAO (Smith and Hoa, 2004). It is noteworthy that control orbital fibroblasts from persons without autoimmune diseases failed to respond to either IGF-I or GD-IgG (Pritchard et al., 2002, 2003; Smith and Hoa, 2004). This same pattern of IL-16 and RANTES induction by IGF-I was subsequently described in cultured primary human thyroid epithelial cells (Gianoukakis et al., 2006). Unlike control fibroblasts, thyrocytes from donors without Graves' disease or other autoimmune thyroid processes also responded to IGF-I and GD-IgG. What has emerged from these studies conducted in cultured fibroblasts and thyrocytes is the concept that IGF-IR might play a critical role in activating T-cell trafficking signals in infiltrated tissues.
Fig. 4.
The effects of IL-1β, IGF-I, and GD-IgG, without or with anti-IGF-IR antibody 1H7, on T-cell chemotactic activity (A) and IL-16 (■) and the RANTES (□) protein expression (B) in fibroblasts from donors with GD. Cultures were treated with IL-1β (10 ng/ml), IGF-I (10 nM), and GD IgG (100 ng/ml), without or with antibody 1H7 (5 μg/ml) for 24 h, then the media were subjected to T-cell migration assays or specific enzyme-linked immunosorbent assays. Samples used for chemotaxis analysis were then treated with no Ab (■) or anti-IL-16 (clone 14.1, 5 μg/ml; □) or anti-RANTES (5 μg/ml; ▨) neutralizing antibodies, as indicated. Migratory data are expressed as a percentage compared with unstimulated (random) migration, which is designated 100%. *, statistically different migration in the presence of neutralizing antibodies (A) or protein production (B) at the 5% confidence level. [Reprinted from Pritchard J, Han R, Horst N, Cruikshank WW, and Smith TJ (2003) Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol 170:6348–6354. Copyright © 2003 The American Association of Immunologists, Inc. Used with permission.]
Fig. 5.
Expression of a DN mutant IGF-IR in GD fibroblasts can block the effects of GD-IgG on T-cell chemoattractant activity (A) and IL-16 (■) and RANTES (□) protein expression (B). Confluent cultures of fibroblasts from a patient with GD were transiently transfected with a plasmid containing the dominant-negative mutant IGF-IR designated 486/STOP or with empty vector (as control). Cultures were then treated with GD-IgG (100 ng/ml) or nothing (control) for 24 h. Media were collected and analyzed for T-cell migratory activity without (■) or with either anti-IL-16 (□) or anti-RANTES (▨) neutralizing antibodies (5 μg/ml) or for IL-16 and RANTES protein expression. The migratory data are expressed as a percentage compared with unstimulated (random) migration, which is designated 100%. *, statistically different migration in the presence of neutralizing antibodies (A) or protein production (B) at the 5% confidence level. [Reprinted from Pritchard J, Han R, Horst N, Cruikshank WW, and Smith TJ (2003) Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol 170:6348–6354. Copyright © 2003 The American Association of Immunologists, Inc. Used with permission.]
Finding more numerous IGF-IR+ fibroblasts with increased receptor density raised questions concerning whether other cell types might also overexpress the protein. Moreover, this abnormal pattern of expression could help explain loss of peripheral immune tolerance to IGF-IR. Examination of circulating T cells from patients with Graves' disease has disclosed an over-abundance of IGF-IR+ lymphocytes (Fig. 6) (Douglas et al., 2007). The frequency of receptor-harboring T cells was found to be increased from controls, in which 15 ± 3% of CD3+ T cells expressed the receptor compared with 48 ± 5% (n = 33, p ≤ 10−8 versus control) among those from patients. This divergence does not vary with the stage of the disease, treatment, or duration of illness. The phenotypic skew toward IGF-IR+ T cells segregates unequally among several lymphocyte subsets. It is noteworthy that CD4+CD45RO+IGF-IR+ and CD8+CD45RO+IGF-IR+ memory T cell populations are extraordinarily rare in healthy persons without the disease (<5%) (Fig. 7). However, they become predominant among memory T cells in donors with Graves' disease, whether or not the patient manifests clinically important TAO (Douglas et al., 2007). In some patients, more than 95% of CD8+CD45RO+ T cells display IGF-IR. Unlike TAO-derived orbital fibroblasts, in which cell-surface IGF-IR densities are 3- to 4-fold higher than those on control cells, receptor levels on T cells from patients with Graves' disease are similar to those found on control lymphocytes (Douglas et al., 2007). As with fibroblasts, T cells bearing the IGF-IR+ phenotype exhibit preferential resistance to apoptosis and a growth advantage in vitro. Orbital T cells from those patients manifesting severe TAO also exhibit the skewed phenotype. Studies examining the phenotype of peripheral and orbital B cells from these patients suggest that they too are skewed toward the IGF-IR+ phenotype. Of the B cells from donors with Graves' disease (n = 30), 34 ± 4% (mean ± S.E.) display IGF-IR, whereas the receptor was detected on 9 ± 3% of B cells from 24 control donors (Fig. 8) (Douglas et al., 2008). Analogous to T cells, the phenotype of B cells with the IGF-IR+ skew also seems durable in patients observed over many months. Moreover, it seems to remain, even in those whose diagnosis was made years before their participation in the study. IGF-I treatment promotes B cell survival in vitro and seems to act synergistically with CpG to enhance total IgG production (Fig. 9). EBV-transformed IGF-IR+ B cells consistently produced anti-TSHR antibodies, suggesting that the skew toward receptor expression might underlie autoantibody production. Future examination of other phenotypic attributes displayed by IGF-IR+ B cell subsets might prove enlightening. These include determining the impact of IGF-IR display and activation on antigen presentation, cytokine production, and T-cell-dependent B-cell activation.
Fig. 6.
Increased fraction of peripheral blood T cells from patients with GD display IGF-IR compared with those from control donors. Peripheral blood mononuclear cells were stained with anti-CD3 and IGF-IR antibodies and subjected to multiparameter flow cytometry. A and B, the open histograms represent staining with isotype control antibodies. Data are derived from single, representative samples from each source. C, fraction of IGF-IR+ CD3+ T cells from individual patients with GD and control donors. D, analysis of IGF-IR display in T cells from the aggregate of multiple patients with GD and control donors; 48 ± 4% GD T cells (n = 33) display IGF-IR compared with 15 ± 3% control T cells (n = 21; p < 10−8). Data are expressed as mean ± S.E. [Reprinted from Douglas RS, Gianoukakis AG, Kamat S, and Smith TJ (2007) Aberrant expression of the IGF-1 receptor by T cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. J Immunol 178:3281–3287. Copyright © 2007 The American Association of Immunologists, Inc. Used with permission.]
Fig. 7.
Disproportionate IGF-IR+CD45RO+ memory T cells from patients with GD. The fraction of CD3+, CD4+, and CD8+ T lymphocytes expressing IGF-IR was determined using multiparameter flow cytometry by gating on populations of CD3+, CD4+, or CD8+, CD45RA+, or CD45RO+ T cells and is represented as a histogram (filled) compared with isotype controls (open). A, naive CD45RA+ lymphocytes from a patient with GD and a control donor demonstrate a similar, frequent display of IGF-IR. B, the fraction of memory CD45RO+ lymphocytes expressing IGF-IR is dramatically greater in lymphocytes from a patient with GD compared with control. GD CD8+CD45RO+ T lymphocytes uniformly express IGF-IR, compared with infrequent control CD8+CD45RO+ cells. T-cell expression of IGF-IR was representative of our aggregate observations. [Reprinted from Douglas RS, Gianoukakis AG, Kamat S, and Smith TJ (2007) Aberrant expression of the IGF-1 receptor by T cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. J Immunol 178:3281–3287. Copyright © 2007 The American Association of Immunologists, Inc. Used with permission.]
Fig. 8.
A disproportionate fraction of peripheral blood B cells from 30 patients with GD express IGF-IR compared with that found in 24 control donors. A, individual data sets demonstrating fractional IGF-IR+ B cells. B, analysis of IGF-IR display in B cells as an aggregate of multiple patients with GD versus control donors [34 ± 4% IGF-IR+ B cells (mean ± S.E., n = 30) versus 9 ± 3% IGF-IR+ control B cells (n = 24)]. Data are expressed as means ± S.E. (**, p < 10−6). [Reprinted from Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, and Smith TJ (2008) B cells from patients with Graves' disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol 181:5768–5774. Copyright © 2008 The American Association of Immunologists, Inc. Used with permission.]
Fig. 9.
IGF-I potentiates B-cell expansion when added together with a concentration of CpG, yielding a submaximal response. Number of B cells was assessed after 5 days in culture with CpG (2 μg/ml) and IGF-I (10 nM) as single agents or in combination. Data were derived from five independent experiments (mean ± S.E.; **, p < 0.02). [Reprinted from Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, and Smith TJ (2008) B cells from patients with Graves' disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol 181:5768–5774. Copyright © 2008 The American Association of Immunologists, Inc. Used with permission.]
Both genetic and acquired factors are known to contribute to the pathogenesis of Graves' disease (Brix et al., 1998). Much investigation has focused on the identification of a gene locus or multiple loci that could help explain susceptibility to the disease. A number of candidates have been found (Ban and Tomer, 2005). Nevertheless, no single gene seems likely to explain the pattern of inheritance of Graves' disease in large cohorts of patients and their families. Indeed, substantial evidence supports the importance of acquired factors in disease development layered on to as yet unidentified genetic determinants (Prummel et al., 2004). These conclusions rest on data from population-based twin studies, especially those using the Danish Twin cohort. Examination of monozygotic twins discordant for Graves' disease suggests that 70% of the cause of the disorder comes from environmental aspects of disease acquisition (Brix et al., 1998). Douglas et al. (2009) found that the majority of clinically healthy, unaffected monozygotic twins in discordant pairs failed to exhibit an IGF-IR+ skewed population of circulating T and B cells compared with their twin with Graves' disease. The study replicated observations made earlier by the same authors in a separate North American cohort of patients with sporadic Graves' disease (Douglas et al., 2007, 2008). It is noteworthy that this recent study demonstrates that the more frequent display of IGF-IR on T and B cells, associated with Graves' disease, seems to derive from acquired rather than genetic factors. Thus, for the first time in the context of Graves' disease, a discrete cellular attribute might be ascribed to acquired factors. Whether the skew toward IGF-IR+ phenotype in B and T cells might be shared with other chronic autoimmune diseases or is specific to Graves' disease remains to be determined.
These findings identify IGF-IR as a potentially attractive target for interrupting early disease-related processes. That strategy would have the goal of preventing local trafficking of lymphocyte subsets responsible for driving tissue reactivity and remodeling. Such an approach could be based on the use of antibodies that block receptor binding or small molecules that interfere specifically with IGF-IR phosphorylation or its interaction with relevant docking proteins. Whatever treatment approach proves most rewarding, IGF-IR has emerged as a potentially important therapeutic target for attenuating connective tissue activation in Graves' disease. This is especially true of those disease manifestations that remain ineffectively treated, such as TAO. Early experiences with the anti-IGF-IR blocking antibodies currently being examined for treating various forms of cancer suggest that many of these might prove well tolerated. The absence of complete and robust preclinical models of Graves' disease (Baker et al., 2005) in which to test the efficacy of this approach imposes a considerable obstacle to efficiently identifying new therapies.
C. Other Autoimmune Diseases
A number of autoimmune diseases have been superficially examined for their potential association with abnormalities in the IGF-I/IGF-IR pathway. A substantial link between this pathway and the pathogenesis of these diseases has yet to be established. However, a limited body of information thus far generated suggests that IGF-I, its receptors, and its binding proteins might ultimately prove relevant to the mechanistic underpinnings of several allied diseases. The insights recently generated concerning the involvement of IGF-IR in Graves' disease would seem to make similar efforts in these other autoimmune processes potentially rewarding.
1. Diabetes Mellitus.
Like the other common human autoimmune diseases, type 1 DM represents a convergence of multiple genetic and environmental factors (Eisenbarth et al., 1994). Its pathogenesis is linked to innate immunity and inflammatory dysfunction of β islet cells of the pancreas (Eizirik et al., 2009). At least 10 genetic loci have been associated with the disease, including cytotoxic T lymphocyte-associated protein 4, human leukocyte antigen class II genes, IL-2 receptor α, protein tyrosine phosphatase nonreceptor 22, and interferon-induced helicase C domain 1. Although most of these have been extensively examined, additional candidates continue to be identified (Todd et al., 2007). A number of polymorphisms of the IGF-I gene have been identified, and the implications of these genetic variations to the development of diabetes are currently being explored (Vella et al., 2008).
A number of aspects of both normal and pathological endocrine pancreatic function have been examined with regard to the insulin and IGF-I signaling pathways as regulators of immune function and dysfunction. Xuan et al. (2002) reported that tissue-specific, conditional mutagenesis of IGF-IR, resulting in its silence, failed to alter β cell mass. On the other hand, ablation of IGF-IR signaling resulted in an age-dependent decline in glucose and arginine-dependent insulin release, underscoring the importance of this receptor in normal pancreatic function. Therefore, carbohydrate intolerance might result from altered function of the IGF-I pathway. In the Goto-Kakizaki rat model of type II DM, defective IGF-II and IGF-IR expression in the embryonic pancreas results in diminished β-cell mass and precedes hyperglycemia (Calderari et al., 2007). Insulin itself exhibits substantial chemotactic activity to T cells (Berman and Center, 1987). In phytohemagglutinin-activated T cells, the chemotactic response to insulin was enhanced compared with that exhibited by resting lymphocytes. Moreover, CD4+ and CD8+ cells responded identically. The kinetics suggested that high-affinity IR was mediating this response (Berman and Center, 1987). T cells express and display IR after activation and treatment with insulin enhances intermediary metabolism (Krug et al., 1972; Helderman and Strom, 1977), progression through the cell cycle (Snow et al., 1980; Helderman, 1981; Kumagai et al., 1981), and an up-regulated effector function (Brown et al., 1983). Thus, it is entirely possible that elevated serum insulin levels, resulting from states of insulin resistance such as those found in type II DM, might result in a substantial and direct impact on T cells. This is true of lymphocytes in the circulation as well as those infiltrating the pancreas.
IGF-I and IGF-II play integral roles in the development and function of β-islet cells, which produce both proteins and respond to them through IGF-IR (Van Schravendijk et al., 1987; Zhang et al., 1997). IGF-I promotes islet growth, an action mediated by IRS-1 (Bonner-Weir and Smith, 1994). Conditional knockout of IGF-IR in β cells results in elevated blood insulin levels and glucose intolerance but fails to influence β-cell development or mass (Kulkarni et al., 2002). In contrast, global interruption of IGF-IR, such as that found in Igf1r(−/−) mice, results in reduced β-cell mass (Withers et al., 1999). IGF-I can protect against diabetes in a NOD mouse model, where autoreactive T cells were adoptively transferred and donors treated with either saline or the growth factor (Bergerot et al., 1995). IGF-I reduced the incidence of diabetes, abrogated insulitis, and increased the abundance of intact islets. Transgenic mice, the β-islet cells of which overexpress IGF-I, exhibit enhanced recovery from cytotoxicity after treatments with streptozotocin (Fig. 10) (George et al., 2002). The Fas and β2-microglobulin hyperexpression and lymphocytic infiltration observed in interferon β single transgenic animals was abrogated in double-transgenic animals harboring β cells overexpressing both IGF-I and interferon β (Casellas et al., 2006). Moreover, the susceptibility of the single transgenic animals to streptozotocin-induced diabetes was markedly reduced. Administration of IGF-I, either as a free molecule or complexed with IGFBP3, reduced the severity and delayed the onset of diabetes in NOD mice (Chen et al., 2004). This protective effect is mediated by increased CCL4 production and dampened CCL3 expression. Within the β cell, IGF-I activates Akt signaling, resulting in enhanced proliferation and resistance to apoptosis. Human β cells transfected with the IGF-I gene also exhibit resistance to apoptosis induced by IL-1β (Giannoukakis et al., 2000).
Fig. 10.
Analysis using immunohistochemistry of insulin expression by islets from N4 CD-1 mice. a–f, pancreata from 2-month-old, untreated nontransgenic (Con) (a) and transgenic (Tg) (d) mice, nontranstgenic (b) and transgenic (e) mice treated with streptozotocin (STZ) for 2 weeks, whereas other nontransgenic (c) and transgenic (f) mice were treated for 4 months. Magnification, 400×. g–j, analysis of 6-month-old, untreated nontransgenic (g) and transgenic (h) mice and nontransgenic (i) and transgenic (j) mice treated for 4 months with STZ. Magnification, 40×. [Reproduced from George M, Ayuso E, Casellas A, Costa C, Devedjian JC, and Bosch F (2002) β Cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest 109:1153–1163. Copyright © 2002 American Society for Clinical Investigation. Used with permission.]
Because of its antiapoptotic effects on β islet cells, and its promotion of insulin secretion (Kido et al., 2002; Xuan et al., 2002), IGF-I has been proposed as a potential therapy for DM, especially when associated with insulin resistance (Murphy, 2006). Its administration has been shown to result in enhanced insulin sensitivity and improved glycemic control in both type I and II DM (Clemmons et al., 2000, 2005). Pennisi et al. (2006), using MKR mice in which defective IGF-IR dimerizes with endogenous IGF-IR and IR (Le Roith et al., 2002), have reported that administration of IGF-I results in reduced glucose levels. This effect can be attributed to reduced endogenous glucose production rather than enhanced whole-body insulin sensitivity. The findings of this and similar studies have reinvigorated consideration of IGF-I and its analogs in the therapy of DM, a strategy largely abandoned because of the potential side effects associated with its administration.
2. Crohn's Disease.
Inflammatory diseases affecting the gut continue to plague society. The etiologies of the two principal forms, ulcerative colitis and Crohn's disease, remain enigmatic, as does the pathogenic relationship between the two. Both are believed to represent an overdetermined immune reactivity to commensal bacteria in persons with a susceptibility to chronic inflammatory diseases (Xavier and Podolsky, 2007). The exact nature of susceptibility to either process remains uncertain but many inciting factors seem to be shared by the two diseases (Cho, 2008). Ulcerative colitis and Crohn's disease present with distinctly different patterns of tissue involvement, affect different segments of the gut, and seem to be based on divergent genetic abnormalities. They share genetic variations in the IL-23 receptor, STAT3, NKX2–3, and IL-12B genes (Fisher et al., 2008; Franke et al., 2008). The immunological underpinnings of Crohn's disease can be divided into those genetic factors contributing to innate immunity and those involved in adaptive responses. With regard to the former, gene polymorphisms of NOD2, ATG16L1, and IRGM appear to contribute to disease pathogenesis, as these may play important roles in the processing of bacterial-derived factors. Participation of the GH/IGF-I pathway in the pathogenesis of either Crohn's disease or ulcerative colitis has yet to be firmly established, but important alterations in it have been well documented in patients with active inflammatory bowel disease. GH can dampen disease activity and enhance healing in patients with Crohn's disease (Slonim et al., 2000). The GH receptor has been detected in the gut of the rat (Lobie et al., 1990), and thus it may exert direct effects on intestinal inflammation. Han et al. (2005) reported that GH reduces colitis activity in an experimental model using C3H/HeJBir IL-10(−/−) mice, animals exhibiting a propensity for relatively severe forms of large bowel inflammation. GH inhibits apoptosis and enhances the proliferation of crypt epithelial cells and increases mononuclear cell death in the lamina propria. The mechanism underlying the effects of GH on disease activity in this experimental model may involve an increased association between gp130 and SHP-2 and down-regulation of constitutively active STAT3 (Han et al., 2005). The investigators involved in this study also reported, using the same animal model, that blocking the actions of TNF-α with a neutralizing antibody could up-regulate hepatic IGF-I production and GH receptor expression and enhance GH-dependent STAT5 activation (DiFedele et al., 2005). In contrast, TNF-α reduced the abundance of GH receptors and attenuated STAT5 phosphorylation in cultured rat hepatocytes (DiFedele et al., 2005). Thus, it would seem that a complex interplay between pro-inflammatory cytokines and the GH/IGF-I pathway might condition the inflammatory environment of the gut. Moreover, these patterns of molecular cross-talk seem to regulate gut responses to mediators of chronic diseases in these experimental preclinical models. What these findings in animals and cultured cells suggest about human disease remains to be firmly established.
In a study examining 13 patients with Crohn's disease and 7 subjects with ulcerative colitis, IGF-I mRNA was found to be elevated in involved ileum and colon segments from patients with Crohn's disease (Pucilowska et al., 2000). However, the same does not seem to be true for involved colon tissue derived from patients with ulcerative colitis. IGF-I and procollagen αI mRNAs exhibited overlapping distribution in fibrotic submucosa and muscularis propria in Crohn's disease. Moreover, the numbers of IGF-I-expressing mesenchymal cells types was increased in involved regions of bowel in patients with Crohn's disease (Pucilowska et al., 2000). In another study, Zimmermann et al. (2001) reported that IGF-I and IGFBP5 mRNA levels were increased in areas of tissue affected by disease that had been resected from patients with Crohn's disease compared with normal-appearing bowel.
Using immunohistochemistry, El Yafi et al. (2005) detected transmural expression of IGF-IR in inflammatory cells and in smooth muscle cells identified in specimens from patients with ulcerative colitis, diverticulitis, and Crohn's disease (Fig. 11). Infiltrating IGF-IR+ mononuclear cells are far more numerous in the mucosa and submucosa in Crohn's disease. In contrast, the increased frequency of these cells seems to be confined to the mucosa in ulcerative colitis (Fig. 12). In addition, fibroblasts, adipocytes, and hypertrophic nervous plexi displaying IGF-IR seemed peculiar to Crohn's disease (El Yafi et al., 2005). These findings suggest that IGF-IR might play disease-specific roles in the pattern of tissue reactivity and remodeling in inflammatory bowel diseases.
Fig. 11.
Evidence of increased abundance of IGF-IR+ cells in Crohn's disease: a, inflammatory cells; b, fibroblastoid cell; c, adipocytes; d, hypertrophied nerve plexus. Note the relative frequencies of IGF-IR+ cells in the lamina propria in uninvolved (e) and disease-involved (f) areas. [Reproduced from El Yafi F, Winkler R, Delvenne P, Boussif N, Belaiche J, and Louis E (2005) Altered expression of type I insulin-like growth factor receptor in Crohn's disease. Clin Exp Immunol 139:526–533. Copyright © 2005 British Society for Immunology. Used with permission.]
Fig. 12.
Immunohistochemical analysis of human bowel. a, increased number of IGF-IR positive inflammatory cells (in brown) in the mucosa and the submucosal in an involved area of Crohn's disease. b, increased number of IGF-IR positive inflammatory cells limited to the mucosa in an involved area of ulcerative colitis. c, no overexpression of IGF-IR in diverticulitis. d, immunohistochemistry with an anti-active caspase 3 antibody: positive inflammatory cells (in red) undergoing apoptosis in an uninvolved area of Crohn's disease. [Reproduced from El Yafi F, Winkler R, Delvenne P, Boussif N, Belaiche J, and Louis E (2005) Altered expression of type I insulin-like growth factor receptor in Crohn's disease. Clin Exp Immunol 139:526–533. Copyright © 2005 British Society for Immunology. Used with permission.].
Children with Crohn's disease often exhibit depressed systemic IGF-I levels. The factors underlying this finding remain uncertain. Altered nutrition, such as reduced caloric intake, is frequently encountered in patients with active Crohn's disease and could account for the low IGF-I levels (Kirschner and Sutton, 1986). Twenty-nine children with active disease were examined before and after treatment with either systemic steroids or an elemental diet (Thomas et al., 1993). Median serum IGF-I levels, as determined by radioimmunoassay, were lower among this cohort with disease than those found in matched healthy control subjects and lower than those in children with growth-stunting from other causes. Insulin and IGFBP1 levels were unaffected. IGF-I levels increased after 4 weeks of either dietary intervention or steroid therapy. In another study, children and adolescents with Crohn's disease were assigned to either of two treatment groups (Beattie et al., 1998). The first cohort of 14 subjects received enteral nutrition, whereas the second group of nine underwent surgical intestinal resection (Beattie et al., 1998). In those patients treated with diet modification, CRP fell from pretreatment levels. Median circulating total IGF-I levels, as determined by a formic acid-acetone extraction and radioimmunoassay method, increased rapidly within 2 weeks of treatment initiation, as did serum IGFBP3 levels. Similar trends were observed in the subjects undergoing surgical resections. Serum IGF-I levels increased, whereas CRP concentrations trended downward, but these changes failed to reach statistical significance after 6 months after surgery (Beattie et al., 1998). Studies in adults have demonstrated many of these same effects of bowel inflammation on the IGF-I pathway. In a series of 22 consecutive patients with inflammatory bowel disease, 10 were diagnosed with Crohn's disease, whereas 12 had ulcerative colitis (Katsanos et al., 2001). Serum IGF-I levels were similarly depressed in patients with either disease compared with control subjects. IGFBP3 levels were also lower, whereas the mean serum IL-6 concentration was elevated. In 13 patients with refractory Crohn's disease, total IGF-I and IGFBP3 levels were depressed by 36 and 27%, respectively, before therapy (Eivindson et al., 2007). Both serum markers normalized during treatment with infliximab. In contrast, free IGF-I levels, determined using ultrafiltration, were reduced by 47% pretreatment but failed to normalize. IGFBP2 levels were elevated at baseline by 2.3-fold over controls and fell with therapy. Other markers, such as CRP and serum albumin, also normalized as a consequence of therapy (Eivindson et al., 2007).
Whether the IGF-I/IGF-IR pathway and GH play primary roles in the pathogenesis of inflammatory bowel disease remains uncertain. Although several lines of investigation have identified specific abnormalities in these pathways, especially in Crohn's disease, alterations in many other laboratory-based parameters have also been demonstrated. Some of these are most likely a secondary consequence of the often-severe malnutrition resulting from bowel disfunction. Distinguishing these nonspecific effects from those metabolic and immunologic derangements underlying disease pathogenesis will require further investigation.
3. Rheumatoid Arthritis and Allied Connective Tissue Diseases.
IGF-I plays an important role in the regulation of articular connective tissues under normal physiological conditions as well as those associated with disease (Verschure et al., 1996). A hallmark of rheumatoid arthritis (RA), the progressive formation and growth of abnormal tissues, such as panus, suggests that growth factor production and/or activity might play some role in the disease process. Indeed, IGF-I can be detected in synovial fluid from patients with active RA but at levels that do not differ from those found in healthy donors (Matsumoto et al., 1996). Using in situ hybridization, Keyszer et al. (1995) detected transcripts encoding IGF-I and IGF-II in synovial tissues, both from patients with RA and from those with osteoarthritis. The greatest signal intensity was localized to the synovial lining and subsynovial layer. Inflammatory mononuclear infiltrates rarely stained with probes for either growth factor (Keyszer et al., 1995). IGF-I present in synovial fluid may regulate the synthesis of proteoglycans in chondrocytes (Schalkwijk et al., 1989). Neidel et al. (1997) reported elevated levels of serum and synovial fluid IGFBP-2 and IGFBP-3 in patients with RA compared with those found in patients with osteoarthritis. They also found equivalent levels of IGF-I and IGF-II in synovial fluid from control subjects and patients with RA. These stimulated proteoglycan synthesis in cartilage in vitro, activity that could be partially blocked with anti-IGF-I neutralizing monoclonal antibodies (Neidel et al., 1997). Fernihough et al. (1996), on the other hand, reported elevated IGF-I and IGFBP3 levels both in patients with RA and in those with osteoarthritis. Moreover, they found that CRP levels in patients with RA correlated with those of IGF-I, IGF-II, and IGFBP-3 in the synovial fluid. Matsumoto et al. (1996) reported that IGF-I levels as well as those of IGFBP-1, IGFBP-2, IGFBP-3, and IGFBP-4 were elevated in synovial fluid in patients with RA. This same group of investigators reported that serum IGF-I levels were lower in RA, whereas serum IGFBP-3 levels exceeded those found in control subjects (Matsumoto and Tsurumoto, 2002). Pritchard et al. (2004) found that either IGF-I or IgGs collected from patients with active RA could activate disease-derived synovial fibroblasts to produce IL-16 and RANTES. Both cytokines exhibited biological activity promoting chemotaxis in CD4+ T cells in vitro. In contrast, fibroblasts from patients with osteoarthritis, serving as controls, failed to respond to either the disease-derived IgGs or to IGF-I. It is noteworthy that the effects in RA fibroblasts were mediated through IGF-IR and could also be elicited by GD-IgG from patients with Graves' disease (Fig. 13). Moreover, IgGs from these patients with RA could reciprocally induce IL-16 and RANTES in fibroblasts from donors with Graves' disease (Pritchard et al., 2004). These findings imply that a common disease mechanism might underlie T-cell trafficking in multiple autoimmune disorders, such as Graves' disease and RA. Moreover, the incidence of these diseases is known to cluster in certain families and to cosegregate with greater frequency in susceptible persons. Their findings imply disease specificity in that donors with osteoarthritis, who manifest a chronic inflammatory disease albeit of a nonautoimmune nature, fail to respond to either IGF-I or disease-derived IgG. It is thus possible that the breakdown of peripheral tolerance to IGF-IR results from its overexpression in populations at risk for autoimmune disease.
Fig. 13.
IL-16-dependent chemoattraction and cytokine expression in GD orbital fibroblasts and RA synovial fibroblasts are induced by both GD-IgG and RA-IgG. Cultures were treated with nothing (control), IGF-I (10 nM), GD-IgG (100 ng/ml), or RA-IgG (100 ng/ml) overnight, and the medium was collected and subjected to T-cell migration assay (top) or enzyme-linked immunosorbent assay (bottom). Data are expressed as the mean ± S.D. of three determinations. *, statistically different migration in the presence of neutralizing Abs (top) or protein production (bottom) at the 5% confidence level. [Reprinted from Pritchard J, Tsui S, Horst N, Cruikshank WW, and Smith TJ (2004) Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves' disease, express high levels of IL-16 when treated with Igs against insulin-like growth factor-1 receptor. J Immunol 173:3564–3569. Copyright © 2004 The American Association of Immunologists, Inc. Used with permission.]
Systemic lupus erythematosus (SLE) is associated with abnormalities of both T- and B-cell function (Kammer et al., 2002; Khan et al., 2003). A hallmark of the disorder is the generation of anti-nuclear antibodies (Tan, 1996). Widespread vascular inflammation in SLE is layered on to organ-specific manifestations and the deposition of immune complexes. Little is currently known about any role that the IGF-I/IGF-IR pathway might play in the pathogenesis of SLE. A single study examining the circulating levels of growth hormone, IGF-I, and somatostatin in age-matched controls and patients with SLE failed to demonstrate any differences (Denko and Malemud, 2004). Although this single report was quite preliminary, its results suggest that abnormally high levels of circulating IGF-I might not be associated with the disease.
Systemic sclerosis represents another vexing chronic process. Interstitial lung disease frequently accompanies systemic sclerosis, but the mechanisms through which pulmonary inflammation and fibrosis occur remain uncertain (Harrison et al., 1991). Although the conventional view embraces inflammation as a precursor of fibroproliferation, recent studies have cast some doubt on the inevitability of that relationship (Krein and Winston, 2002). Most studies have insinuated TGF-β and IGF-I as promoters of fibroblast proliferation and the disordered accumulation of extracellular matrix (Krein and Winston, 2002). Excess collagen is deposited in lung interstitium as a common feature of tissue remodeling. Harrison et al. (1994) have reported that bronchoalveolar lavage fluid from these patients enhances fibroblast proliferation in vitro and that neutralizing antibodies directed against IGF-I can attenuate this effect. Moreover, elevated IGF-I levels were found in this fluid compared with samples from control donors. The findings diverge from an earlier study by Rothe et al. (1988) who reported normal plasma somatomedin C levels in patients with active disease. Reconciling the findings of the two studies suggests that IGF-I may act locally rather than systemically in the disease. Harrison et al. (1994) found that IGF-I levels were elevated only in those patients having abnormal computed tomographic analysis, which was consistent with the disease. Fibroblasts from patients with systemic sclerosis have been shown to respond to growth factors and to produce excessively high levels of glycosaminoglycans (LeRoy et al., 1982; Falanga et al., 1987). Idiopathic pulmonary fibrosis is associated with epithelial cell damage and fibroblast proliferation (Medsger, 1985). In 15 patients with cutaneous disease (morphea), punch biopsies from within lesions were compared with those from normal-appearing skin (Fawzi et al., 2008). Intralesional IGF-I levels were higher than those found in uninvolved skin. Moreover, these patients exhibited elevated serum IGF-I levels. Positive correlations were observed between the intralesional IGF-I levels and Rodnan scores (Fawzi et al., 2008). The authors concluded that IGF-I antagonists, such as octreotide, deserve consideration as therapeutic strategies. The molecular basis for involvement of IGF-I or IGF-IR in systemic sclerosis has yet to be explored, but the particularly unsatisfactory clinical course associated with the disease suggests that further examination is warranted.
4. Experimental Autoimmune Encephalomyelitis.
IGF-I plays an important developmental role in the central nervous system and is synthesized by multiple cell types, including neurons and astrocytes (Bondy, 1991; Lee et al., 1992). Moreover, IGF-I induces oligodendrocyte development (McMorris et al., 1986). Experimental autoimmune encephalomyelitis (EAE) can be induced by immunizing Lewis rats with an emulsion containing guinea pig spinal cord. This animal model resembles that of human multiple sclerosis (Raine, 1984). In rats induced to express EAE, mRNAs encoding IGF-I and glial fibrillary acidic protein were elevated 14 days after disease induction (Liu et al., 1994). Moreover, expression of these transcripts coincided with the appearance of inflammatory infiltrates and demyelination of both white and gray matter (Liu et al., 1994). In contrast, levels of myelin basic protein mRNA were reduced substantially. Experimental animals expressed transiently elevated levels of IGFBP2 mRNA and protein compared with control animals (Liu et al., 1994). Moreover, coexpression of IGF-I and IGFPB2 mRNA was found by in situ hybridization to localize to the same astrocytes. On the other hand, animals treated with exogenous IGF-I (200 μg/day or 1 mg/day) exhibited reduced demyelination, whereas myelin-related protein mRNA levels were induced in oligodendroglial cells (Yao et al., 1995). Larger areas of demyelination were found in spinal cord sections from placebo-treated animals, in addition to substantially more inflammation, and a greater abundance of demyelinated axons compared with those from animals treated with IGF-I (Fig. 14) (Yao et al., 1995). The authors of that study concluded that IGF-I promotes myelination and increases the proliferation of oligodendroglial cells. Their results suggest further that the IGF-I pathway might be manipulated as a strategy for therapeutic intervention in multiple sclerosis and other demyelinating disorders.
Fig. 14.
Thin sections of spinal cord from control rats contain larger demyelinated areas, greater inflammation (A), and a greater abundance of demyelinated axons (arrows in C) than found in IGF-I-treated animals (B and D). Thin, short myelin segments (arrows) surround axons in D. Magnification, 600×. [Reproduced from Yao DL, Liu X, Hudson LD, and Webster HD (1995) Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 92:6190–6194. Copyright © 1995 United States National Academy of Sciences. Used with permission.]
VII. Therapeutic Horizons for Autoimmunity: Focusing on Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-I Receptor
Although not yet completely characterized, abnormalities in the IGF-I/IGF-IR pathway associated with autoimmune diseases suggest that its therapeutic interruption might prove beneficial. This would resemble the approach being undertaken in developing cancer therapy. Despite these findings, none of the agents targeting the pathway has been applied to patients with autoimmunity. Common threads of IGF-I dysfunction seem to tie multiple diseases together. One might therefore predict therapeutic benefit in those diseases where IGF-IR is found to be overexpressed, as is the case in Graves' disease, and particularly in those diseases in which receptor activation leads to abnormal responses in immune cells. Considering the numerous hurdles that have already been overcome in developing these agents for use in cancer, their application in autoimmunity might represent low-hanging fruit to the pharmaceutical industry.
What can we learn from the substantial efforts expended toward targeting the IGF-I/IGF-IR pathway in neoplastic diseases as we formulate new therapies for autoimmune diseases? Are there molecules currently on the shelf that might be used in several of these diseases in which adequate therapies do not exist? A brief review of the strategies employed to treat cancers might orient discussions surrounding similar targets in chronic inflammatory diseases, such as those with an autoimmune basis. These strategies are nicely reviewed in a recently published article by Kim et al. (2009) focusing on pediatric tumors.
A number of membrane-spanning tyrosine kinase receptors have been implicated in the pathogenesis of several common neoplastic diseases. The fundamental concept of interrupting their function as an important means of treating human illness emerged more than two decades ago (Rondon et al., 2007; Frasca et al., 2008). This notion has been fortified by a greater understanding of how growth factors exert their influence on target tissues and relevant populations of cells. Specifically, these receptors and their downstream signaling cascades are involved in promoting enhanced resistance to apoptosis and increased proliferation of cancer cells. They have thus become rational targets for therapy design (Shawver et al., 2002). Among them, HER1, HER2, platelet-derived growth factor receptor, c-kit, colony-stimulating factor-1 receptor, fibroblast growth factor receptor, and IGF-IR have been identified as therapeutic targets of potential clinical importance. Interest in IGF-IR as an antineoplastic target derives from the discovery that the receptor is overexpressed in many tumor types and that its increased presence and activity may constitute the antiapoptotic and proliferation enhancement associated with several cancers.
IGF-I pathway activation alters cancer cell behavior. It has been implicated in tumor genesis, angiogenesis, metastasis, and mitogenesis in preclinical disease models. IGF-IR may undergo divergent post-translational processing in neoplastic cells. In addition, some tumors exhibit down-regulated levels of IGF-IIR, thereby reducing their capacity to decoy IGF-I and limiting their modulation of its actions (Samani et al., 2007). IGF-IR acts as an oncogene when overexpressed (Kaleko et al., 1990). Because it regulates cell proliferation by mediating mitogenic signals, is necessary for maintaining transformed phenotypes, and protects against apoptosis (Resnicoff and Baserga, 1998), interfering with IGF-IR expression/signaling constitutes a currently attractive approach for drug design. Altering the activity of the IGF-I pathway has been proposed in several diseases, including cancer, diabetes, and atherosclerosis (Sachdev et al., 2006, Clemmons, 2007). It would seem that local concentrations of IGF-I and relative levels of IGF-IR and IGF-IIR determine the activity of this pathway (Butler et al., 1998). Thus, the stoichiometric relationship between these components and the IGFBP family may determine the net impact that IGF-I exerts on tumor cells. Local and circulating concentrations of IGF-I also regulate aspects of tumor cell phenotype, including determining their aggressive behavior. For example, a number of studies have suggested an increased risk of prostate cancer in men with elevated plasma IGF-I levels (Woodson et al., 2003; Oliver et al., 2004).
IGF-I and IGF-II, through their activities mediated by IGF-IR, form important autocrine loops in cancer that promote tumor survival advantage and proliferation. Most of these effects are the consequence of IGF-IR autophosphorylation, the conformational changes favoring its association with four IRS docking proteins, and the activation of MAP kinase and PI3 kinase pathways (Samani et al., 2007). The progressive development of synthetic molecules that can alter the biological impact of growth factors, their cognate receptors, and the signaling cascades mediating their actions now allows testing of the overarching hypothesis that clinical benefit accrues from pathway disruption. Targeting the IGF-I pathway, either alone or in aggregate with others, as a broadly based strategy, seems to hold substantial promise for therapy of several forms of cancer. Drug development targeting IGF-IR can be dissected into small molecule inhibitors and monoclonal antibodies (Imai and Takaoka, 2006). In addition, molecular strategies, such as the application of antisense oligodeoxynucleotides to IGF-IR have been shown to inactivate postreceptor signaling in tumor cells (Salatino et al., 2004). IGF-IR gene silencing can be achieved using small interfering RNA, rendering the target cells more susceptible to cytotoxic agents and radiotherapy (Salisbury and Macaulay, 2003). The enormous advantage of targeting the receptor, rather than its downstream signaling, is the ubiquitous involvement of the latter in critical nonpathological metabolic functions. Initial enthusiasm for IGF-IR-centric drugs was tempered by theoretical concerns about the protein's structural similarities to IR and the potential for physiological promiscuity between the two receptors. Subsequently, the successful development of agents targeting receptors for the epidermal growth factor and vascular endothelial growth factor has refocused the potential of IGF-IR interruption. Agents targeting IGF-IR could emerge as attractive agents for treating autoimmune processes. However, given their generally less dire nature, using these agents in autoimmunity places a greater burden on demonstrating safety compared with their use in malignancy.
VIII. Translational Strategies for Modulation of Insulin-Like Growth Factor-I or Insulin-Like Growth Factor-I Receptor
A. Strategies for Generating Insulin-Like Growth Factor-I Receptor Blocking Antibodies
Biological agents have become a major focus in novel therapeutic development for cancer because of their potential for highly specific molecular and cellular targeting combined with their typically low toxicity compared with small-molecule drugs. The rationale for using multiple agents directed at more than a single target stems from efforts to minimize the emergence of drug resistance and to exploit the effectiveness of combined therapies. Development of antibodies capable of altering the signaling characteristics of IGF-IR began more than 20 years ago, when the receptor's potential importance in cancer pathogenesis became clear. Yamashita et al. (1986) reported that the monoclonal antibody αIR3 could block the effects of IGF-I on growth hormone production in human pituitary adenoma cells. Subsequently, Li et al. (1993) described another monoclonal antibody, designated 1H7, that could block the ligation and activation of IGF-IR. In the years that followed, a number of anti-IGF-IR antibodies have been developed. Doern et al. (2009) recently described an extensive panel of antibodies that can inhibit IGF-IR activation and the downstream events associated with its signaling. They screened and categorized these antibodies and divided them into four groups on the basis of their abilities to block receptor ligation by both IGF-I and IGF-II. They found antibodies that could allosterically block either IGF-I or IGF-II, allosterically block both, or competitively block both IGF-I and IGF-II binding to IGF-IR. Their epitope mapping studies used three separate constructs, including human IGF-IR 1–903, mouse 1–904, and human IGF-IR 1–462, which contains the three N-terminal domains, including L1, the cysteine-rich region, and L2. Using a purified IGF-IR library including 64 mutations, they found that the epitopes recognized by antibodies belonging to all four categories bound overlapping surfaces of the cysteine-rich repeat and L2 domains. The surface epitope map analysis of two monoclonal antibodies, BIIB4 and BIIB5, and a summary of the effect of specific IGF-IR mutations on IGF-I and antibody binding are shown in Fig. 15. BIIB4 blocks IGF-I and IGF-II competitively, whereas BIIB5 blocks both ligands through an allosteric mechanism. Furthermore, the authors found that binding of IGF-I and these antibodies resulted in conformation changes in IGF-IR (Doern et al., 2009). These detailed studies may prove invaluable in dissecting the properties of individual antibodies that render optimal results in subsequent clinical trials.
Fig. 15.
Results from epitope map analysis of BIIB4 and BIIB5 on the surface of the X-ray crystal structure of the extracellular domains of IR based on homologous positions determined using a sequence alignment of IR and IGF-IR. [Reproduced from Doern A, Cao X, Sereno A, Reyes CL, Altshuler A, Huang F, Hession C, Flavier A, Favis M, Tran H, et al. (2009) Characterization of inhibitory anti-insulin-like growth factor receptor antibodies with different epitope specificity and ligand-blocking properties: implications for mechanism of action in vivo. J Biol Chem 284:10254–10267. Copyright © 2009 The American Society for Biochemistry and Molecular Biology. Used with permission.]
At least eight different monoclonal antibodies directed at the IGF-IR are currently under development or are being assessed in clinical trials. Each exhibits unique characteristics and has been developed using different strategies. These have been nicely reviewed recently (Feng and Dimitrov, 2008). A spectrum of receptor internalization and blocking activities found among these antibodies should thus put the findings of Doern et al. (2009) into a perspective of clinical efficacy. In brief, figitumumab (CP-751,871) represents a fully human IgG2 exhibiting a binding affinity to IGF-IR of 1.5 nM and an IC50 of 0.4 nM for inhibition of IGF-IR autophosphorylation in NIH 3T3/IGF-IR cells (Cohen et al., 2005). This blockade of IGF-I- and IGF-II-provoked phosphorylation was rapid, as was the inhibition of Akt/Pkb activation. CP-751,871 induced a loss of total cellular IGF-IR and the down-regulation of surface receptor as a consequence of internalization. Although it failed to cross-react with IR, this antibody could recognize IGF-IR/IR heterodimeric complexes in MCF7 cells (Cohen et al., 2005). It is noteworthy that this antibody does not exhibit cellular toxicity while inhibiting the proliferation of NIH 3T3/IGF-IR cells in a concentration-dependent manner. This antiproliferative activity was also found in human colon (Colo-205), lung (H460), and breast (MCF7) xenograph models when administered, either as a single agent or in combination with doxorubicin (Adriamycin) or 5-fluorouracil (Cohen et al., 2005). A phase I clinical trial examining CP-751,871 including 24 patients with refractory solid tumors has been completed, and the compound was found to be safe, with hyperglycemia reported in 4% of patients (Pollak et al., 2007). A phase II study is ongoing and includes 14 subjects with adrenocortical carcinoma and 16 with sarcoma (Feng and Dimitrov, 2008). Nine of the patients with adrenal tumors and three patients with sarcoma exhibited stable disease of a duration greater than 8 weeks (Olmos et al., 2007).
Cixutumumab (IMC-A12) is another fully humanized monoclonal antibody (Burtrum et al., 2003; Rowinsky et al., 2007). It derives from a human naive Fab phage display library. Clones were selected for their ability to block binding of 125I to recombinant IGF-IR. The antibody inhibits the binding of both IGF-I and IGF-II to the receptor on MCF-7 cells but does not alter insulin binding. It also attenuates IGF-I-dependent proliferation of MCF-7, RPMI8226, and BxPC-3 cells in vitro (Burtrum et al., 2003). The latest version of this antibody (A12) binds IGF-IR with an affinity of 40 pM. It blunts IGF-I-dependent IGF-IR phosphorylation and downstream signaling. In human xenograph mouse models, IMC-A12 inhibited the growth of MCF-7, BxPC-3, and Colo205, and it attenuated receptor autophosphorylation, whereas tumor cell apoptosis increased (Burtrum et al., 2003). Multiple phase I trials revealed reduction in absolute lymphocyte counts and hyperglycemia in some subjects. A minority of subjects had stable disease over the course of 4 to 10 months (Higano et al., 2007; Rothenberg et al., 2007; Rowinsky et al., 2007).
BIIB-022 is the product of a human Fab phage display library and represents another fully human antibody with an affinity for IGF-IR of 1.3 nM (Feng and Dimitrov, 2008). It inhibits IGF-I and IGF-II binding and exhibits specificity for IGF-IR in that it fails to bind IR. Early preclinical studies suggest that BIIB-022 will be well tolerated (Hariharan et al., 2007). Determination of its efficacy as an antineoplastic agent awaits the conclusion of ongoing studies. Several characteristics of this antibody suggest that it might prove useful in autoimmune diseases, including its high affinity for the receptor protein and its safety profile.
A humanized monoclonal antibody generated by immunizing mice with IGF-IR, designated h7C10, inhibits the activity of IGF-IR and IGF-IR/IR hybrid proteins (Goetsch et al., 2005). In nude mice harboring either MCF-7 human breast cancer cells or A549 non–small-cell lung cancer xenographs, h7C10 significantly inhibited xenotransplanted tumor growth. The effects were even more dramatic when the antibody was given in combination with another chemotherapeutic agent or with a second antibody targeting EGFR (Goetsch et al., 2005).
The humanized monoclonal antibody R-1507 was generated in human IgG-producing transgenic mice immunized with IGF-IR (Schnitzer et al., 2006). It exhibits antitumor activity in mice harboring 3T3/IGF-IR, NCI-H322M or Colo205 xenotransplants. The drug seems to be well tolerated, although carbohydrate intolerance after oral glucose challenge was detected in some subjects (Rondon et al., 2007). AVE-1642, the human derivative of mouse antibody EM164, has already entered into clinical testing (Feng and Dimitrov, 2008). EM164 blocks IGF-I binding and IGF-I-dependent autophosphorylation of IGF-IR, slows the proliferation of several cancer cell lines in vitro, and results in the regression of BxPC-3 human pancreatic tumors in SCID mice (Maloney et al., 2003). Moreover, it interferes with IGF-IR signaling in CD45− but not in CD45+ human myeloma cells (Descamps et al., 2006). AVE1642 selectively inhibits the proliferation of CD45− myeloma cells but enhances bortezomib-induced apoptosis (Descamps et al., 2009). This effect is apparently absent in CD45+ cells and suggests a degree of target cell specificity that might prove attractive in treating autoimmune diseases.
Besides IGF-IR overexpression, some tumors, including those developing from breast epithelium, can form hybrid IGF-IR/IR receptor proteins. Levels of these chimeric proteins can also increase with tumor dedifferentiation (Siddle et al., 1994; Pandini et al., 1999). Specific antibodies exhibit activity selectively against these chimeric receptors (Pandini et al., 1999) and can also down-regulate IR levels (Sachdev et al., 2006). Whether chimeric IGF-IR/IR receptors play any role in autoimmune diseases or occur naturally in the immune system is uncertain but must be examined if we are to fully understand the IGF-I pathway in this context.
B. Kinase Inhibitors and Other Nonantibody Targeting of IGF-IR
A summary of the approaches currently considered attractive for altering IGF-IR signaling in disease states has been provided (Fig. 16). Monoclonal antibody development has taken priority by many interested in drug development. Another approach to therapeutically target IGF-IR in cancer involves small-molecule kinase inhibitors with variable degrees of selectivity. Many inhibitory small molecules exhibiting activity against IGF-IR are currently undergoing evaluation for their efficacy against neoplastic diseases. This avenue of discovery has been problematic because of the lack of specificity. Specifically, targeting kinases downstream from IGF-IR with small molecules can effectively attenuate receptor-dependent signaling. However, given the substantial overlap exhibited by many relevant signaling pathways, interrupting these downstream kinases may result in unwanted collateral effects. One strategy for overcoming this commonality has been the utilization of sequence-specific nucleic acid probes, such as antisense and small interfering RNAs. Chemical inhibitor development has focused on the tyrosine kinase domain contained in IGF-IRβ, which can be selectively inhibited with agents such as cis-3-[3-(4-methyl-piperazin-l-yl)-cyclobutyl]-1-(2-phenyl-quinolin-7-yl)-imidazo[1,5-a]pyrazin-8-ylamine (Ji et al., 2007). This recently described compound exhibits a high degree of specificity in that 32 other kinases were unaffected or minimally susceptible to its inhibition. In human GEO colon cancer cells, cis-3-[3-(4-methyl-piperazin-l-yl)-cyclobutyl]-1-(2-phenyl-quinolin-7-yl)-imidazo[1,5-a]pyrazin-8-ylamine could block the phosphorylation of Akt and Erk 1/2 and disrupt the IGF-II/IGF-IR autocrine loop (Ji et al., 2007). Nordihydroguaiaretic acid, an inhibitor of the HER2 and IGF-IR tyrosine kinases, blocks the growth of HER2-overexpressing human breast cancer cells (Zavodovskaya et al., 2008). NVP-TAE226 (or TAE226), is a novel dual tyrosine kinase inhibitor of both focal adhesion kinase and IGF-IR that suppresses growth and invasion of glioma cells (Liu et al., 2007). The IGF-IR-specific kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine IGF-I secretion (Tazzari et al., 2007). 1,3-Disubstituted-imidazo[1,5-a]pyrazines might represent important inhibitors of IGF-I action (Mulvihill et al., 2007). Included among them is picropodophyllin, which demonstrates remarkable specificity for IGF-IR and fails to alter tyrosine phosphorylation of the receptors for insulin, fibroblast growth factor, platelet-derived growth factor, or epidermal growth factor (Girnita et al., 2004). This agent induces cell-cycle accumulation and apoptosis in multiple myeloma cells (Strömberg et al., 2006). Cyclolignan picropodophyllin inhibits IGF-IR tyrosine kinase activity through an induction of G2/M-phase accumulation and apoptosis in multiple myeloma cells (Strömberg et al., 2006). A (1H-benzoimidazol-2-yl)-1H-pyridin-2-one inhibitor of IGF-IR kinase, BMS-536924, exhibits antitumor activity in vivo (Vaira et al., 2007). The selective kinase inhibitor NVP-ADW742 has exhibited substantial antitumor activity in multiple myeloma and solid tumors when administered as monotherapy or in combination with cytotoxic agents (Mitsiades et al., 2004). Each of the compounds exhibits potentially acceptable side-effect profiles that may warrant consideration for use in autoimmune diseases.
Fig. 16.
Strategies for drug discovery with a dimeric or dimerizing receptor tyrosine kinase. a, schematic illustration of the mechanism of receptor tyrosine kinase activation by ligand-induced dimerization. b, schematic illustration of the various possible strategies for the search/design of ligand mimetics and antagonists. PTP, protein tyrosine phosphatase; TK, tyrosine-kinase domain. [Reproduced from De Meyts P and Whittaker J (2002) Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov 1:769–783. Copyright © 2002 Nature Publishing Group. Used with permission.]
In addition to using single-target approaches in modifying IGF-IR activity, combining anti-IGF-IR antibody or IGF-IR kinase inhibitors with a second inhibitor directed at one of the signaling pathways downstream from the receptor might enhance both efficacy and specificity. For instance, monoclonal antibodies against IGF-IR in combination with chemical inhibition of the Raf/MEK/ERK and PI3 kinase/AKT/mTOR pathways can effectively suppress IGF-IR-dependent proliferation in the hematopoietic cell line FDC-P1 rendered IL-3-independent (Bertrand et al., 2006). However, the potential for this approach to attenuate necessary signaling initiated by other receptors makes its safety profile uncertain.
IX. Conclusions
Discovery that IGF-I regulates diverse aspects of T-cell, B-cell, and monocyte function through its interactions with IGF-IR opens several potentially exciting avenues for drug development. Its complex role in immune function suggests that abnormalities in the IGF-I pathway might play some part in the pathogenesis of diseases where immunity is altered. Indeed, recent studies suggest that several autoimmune processes, such as Graves' disease, rheumatoid arthritis, EAE, and inflammatory bowel disease might involve derangements of this pathway. Expression of IGF-I and/or IGF-IR seems to be elevated in these diseases, suggesting similarities with certain forms of cancer. Elevated IGF-IR levels and abnormal IGF-IR-dependent signaling in tumors are currently being exploited as therapeutic targets (Pollak, 2008; Kleinberg et al., 2009). These strategies for drug development in cancer should provide valuable lessons for therapy innovation in autoimmunity. However, the roles of the IGF-I/IGF-IR pathway in chronic autoimmunity are likely to prove very complex. On the one hand, IGF-I seems to protect against pancreatic islet tissue damage in some models of type I diabetes and retards demyelination in animals with EAE. On the other, IGF-IR overexpression may play an important role in the development of Graves' disease and RA, in part by orchestrating the abnormal expansion of pathogenic lymphocytes and their trafficking to sites of inflammation and tissue remodeling. Blocking IGF-IR and the abnormal downstream signaling events it initiates could prove effective therapy for those conditions. One of many remaining questions concerns the apparent absence of increased autoimmunity in transgenic mice overexpressing IGF-IR. If overexpression of that receptor were the only requirement for anti-IGF-IR auto-antibody production, shouldn't we expect these animals to manifest Graves' disease or one of the other allied processes? The answer to this question may lie in the timing and duration of receptor overexpression. For instance, if the higher levels of IGF-IR date back to very early in life, these animals might become peripherally tolerant to the protein. Moreover, the tissue distribution of overexpressed IGF-IR, like any other potential self-antigen, might represent a critical determinant for loss of immune tolerance. Lack of sufficient time for disease development might also explain why these animals have not manifested recognizable disease. Other overarching questions remain, even as we learn more about these diseases and the roles that IGF-I might play in their pathogenesis. Not the least of these uncertainties concerns whether down-regulating IGF-I/IGF-IR function as a therapeutic strategy will ultimately prove sufficiently well tolerated to be used in sublethal diseases. This will be answered ultimately with the successful execution of well designed clinical trials. Nevertheless, hints regarding the suitability of these sorts of interventions already exist. Treatment of autoimmune disease has been dramatically transformed with the emergence of monoclonal antibodies directed at various cytokines (Tincani et al., 2007; Guzman Moreno, 2009), whereas other antibodies have been used for B- and T-lymphocyte depletion (Chatenoud et al., 1994; Hasegawa et al., 2006). Those directed against proinflammatory cytokines such as TNF-α and IL-1 have become mainstays of therapy in many autoimmune diseases, including rheumatoid arthritis, psoriasis, SLE, and multiple sclerosis. Moreover, rituximab has become widely and successfully used as a strategy for depleting CD20 B cells in rheumatoid arthritis, lupus, and a number of other autoimmune diseases (Ahuja et al., 2007; Dörner et al., 2009). Anti-CD3 therapy is under evaluation in type I diabetes (Herold et al., 2002). Success in treating several autoimmune diseases with these antibodies might be considered “proof of principle,” because consideration is given to using similar molecules to disrupt IGF-I/IGF-IR signaling. Accumulating additional insight into the abnormal behavior of this pathway in autoimmunity will allow a more thorough assessment of its suitability as a therapeutic target.
Acknowledgments.
This work was supported in part by the National Institutes of Health National Eye Institute [Grants EY008976, EY011708]; by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK063121]; and by the Bell Charitable Trust. I am indebted to Dr. Michael Yeaman (UCLA) and Dr. Raymond S. Douglas (University of Michigan) for their thoughtful suggestions concerning the organization of this manuscript. I am grateful to Linda Polonsky for editorial support. The expert assistance of Debbie Hanaya, Linda Cirenza, and Jen Mironas in the preparation of the manuscript is gratefully acknowledged.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.002469.
- A23187
- calcimycin
- Akt/PKB
- protein kinase B
- AP-2
- adaptor protein-2
- CP-751,871
- figitumumab
- CRP
- C-reactive protein
- DM
- diabetes mellitus
- EAE
- experimental autoimmune encephalomyelitis
- EGFR
- epidermal growth factor receptor
- EH
- Eps homology
- EHD
- Eps homology domain
- Eps
- epidermal growth factor receptor pathway substrate
- Erk
- extracellular signal-regulated kinase
- GD-IgG
- Graves' disease-related immunoglobulin G
- GH
- growth hormone
- Ig
- immunoglobulin
- IGF
- insulin-like growth factor
- IGFBP
- insulin-like growth factors binding protein
- IGF-IR
- insulin-like growth factor-I receptor
- IL
- interleukin
- IMC-A12
- cixutumumab
- IR
- insulin receptor
- IRS
- insulin receptor substrate
- LPS
- lipopolysaccharide
- MAP
- mitogen-activated protein
- MAPK
- mitogen-activated protein kinase
- mTOR
- mammalian target of rapamycin
- PI3
- phosphatidylinositol 3
- PKB
- protein kinase B
- RA
- rheumatoid arthritis
- RANTES
- regulated upon activation, normal T-cell expressed
- RXR
- retinoid X receptor
- SH-2
- Src homology 2
- Shc
- Src homologous and collagen protein
- SHP-2
- SH-2-containing phosphotyrosine phosphatase-2
- SLE
- systemic lupus erythematosus
- STAT
- signal transducer and activator of transcription
- TAO
- thyroid-associated ophthalmopathy
- TCR
- T cell receptor
- TNF
- tumor necrosis factor
- TSHR
- thyrotropin receptor or thyroid-stimulating hormone receptor.
References
- Abroun et al., 2004.Abroun S, Ishikawa H, Tsuyama N, Liu S, Li FJ, Otsuyama K, Zheng X, Obata M, Kawano MM. (2004) Receptor synergy of interleukin-6 (IL-6) and insulin-like growth factor-I in myeloma cells that highly express IL-6 receptor α [published erratum appears in Blood 103:2891, 2004]. Blood 103:2291–2298 [DOI] [PubMed] [Google Scholar]
- Adams et al., 2000.Adams TE, Epa VC, Garrett TP, Ward CW. (2000) Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci 57:1050–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja et al., 2007.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. (2007) Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 179:3351–3361 [DOI] [PubMed] [Google Scholar]
- Alpdogan et al., 2003.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, et al. (2003) Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation 75:1977–1983 [DOI] [PubMed] [Google Scholar]
- Ambrosini et al., 1997.Ambrosini G, Adida C, Altieri DC. (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921 [DOI] [PubMed] [Google Scholar]
- Andress, 1995.Andress DL. (1995) Heparin modulates the binding of insulin-like growth factor (IGF) binding protein-5 to a membrane protein in osteoblastic cells. J Biol Chem 270:28289–28296 [PubMed] [Google Scholar]
- Arkins et al., 1993.Arkins S, Rebeiz N, Biragyn A, Reese DL, Kelley KW. (1993) Murine macrophages express abundant insulin-like growth factor-I class I Ea and Eb transcripts. Endocrinology 133:2334–2343 [DOI] [PubMed] [Google Scholar]
- Arkins et al., 1995a.Arkins S, Rebeiz N, Brunke-Reese DL, Biragyn A, Kelley KW. (1995a) Interferon-γ inhibits macrophage insulin-like growth factor-I synthesis at the transcriptional level. Mol Endocrinol 9:350–360 [DOI] [PubMed] [Google Scholar]
- Arkins et al., 1995b.Arkins S, Rebeiz N, Brunke-Reese DL, Minshall C, Kelley KW. (1995b) The colony-stimulating factors induce expression of insulin-like growth factor-I messenger ribonucleic acid during hematopoiesis. Endocrinology 136:1153–1160 [DOI] [PubMed] [Google Scholar]
- Bagley et al., 1989.Bagley CJ, May BL, Szabo L, McNamara PJ, Ross M, Francis GL, Ballard FJ, Wallace JC. (1989) A key functional role for the insulin-like growth factor 1 N-terminal pentapeptide. Biochem J 259:665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker et al., 2005.Baker G, Mazziotti G, von Ruhland C, Ludgate M. (2005) Reevaluating thyrotropin receptor-induced mouse models of Graves' disease and ophthalmopathy. Endocrinology 146:835–844 [DOI] [PubMed] [Google Scholar]
- Ban and Tomer, 2005.Ban Y, Tomer Y. (2005) Susceptibility genes in thyroid autoimmunity. Clin Dev Immunol 12:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel et al., 1999.Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP, Jr., Roth RA. (1999) Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem 274:20281–20286 [DOI] [PubMed] [Google Scholar]
- Baserga et al., 2003.Baserga R, Peruzzi F, Reiss K. (2003) The IGF-1 receptor in cancer biology. Int J Cancer 107:873–877 [DOI] [PubMed] [Google Scholar]
- Baudler et al., 2005.Baudler S, Baumgartl J, Hampel B, Buch T, Waisman A, Snapper CM, Krone W, Brüning JC. (2005) Insulin-like growth factor-1 controls type 2 T cell-independent B cell response. J Immunol 174:5516–5525 [DOI] [PubMed] [Google Scholar]
- Baxter, 1993.Baxter RC. (1993) IGF binding protein-3 and the acid-labile subunit: formation of the ternary complex in vitro and in vivo. Adv Exp Med Biol 343:237–244 [DOI] [PubMed] [Google Scholar]
- Baxter and Firth, 1995.Baxter RC, Firth SM. (1995) Modulation of human IGF binding protein-3 activity by structural modification. Prog Growth Factor Res 6:215–222 [DOI] [PubMed] [Google Scholar]
- Bayne et al., 1989.Bayne ML, Applebaum J, Underwood D, Chicchi GG, Green BG, Hayes NS, Cascieri MA. (1989) The C region of human insulin-like growth factor (IGF) I is required for high affinity binding to the type 1 IGF receptor. J Biol Chem 264:11004–11008 [PubMed] [Google Scholar]
- Beattie et al., 1998.Beattie RM, Camacho-Hübner C, Wacharasindhu S, Cotterill AM, Walker-Smith JA, Savage MO. (1998) Responsiveness of IGF-I and IGFBP-3 to therapeutic intervention in children and adolescents with Crohn's disease. Clin Endocrinol (Oxf) 49:483–489 [DOI] [PubMed] [Google Scholar]
- Bell et al., 2000.Bell A, Gagnon A, Grunder L, Parikh SJ, Smith TJ, Sorisky A. (2000) Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol Cell Physiol 279:C335–C340 [DOI] [PubMed] [Google Scholar]
- Benmerah et al., 1998.Benmerah A, Lamaze C, Bègue B, Schmid SL, Dautry-Varsat A, Cerf-Bensussan N. (1998) AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol 140:1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berfield et al., 2000.Berfield AK, Andress DL, Abrass CK. (2000) IGFBP-5(201–218) stimulates Cdc42GAP aggregation and filopodia formationin migrating mesangial cells. Kidney Int 57:1991–2003 [DOI] [PubMed] [Google Scholar]
- Bergerot et al., 1995.Bergerot I, Fabien N, Maguer V, Thivolet C. (1995) Insulin-like growth factor-1 (IGF-1) protects NOD mice from insulitis and diabetes. Clin Exp Immunol 102:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman and Center, 1987.Berman JS, Center DM. (1987) Chemotactic activity of porcine insulin for human T lymphocytes in vitro. J Immunol 138:2100–2103 [PubMed] [Google Scholar]
- Bernabei et al., 2003.Bernabei P, Bosticardo M, Losana G, Regis G, Di Paola F, De Angelis S, Giovarelli M, Novelli F. (2003) IGF-1 down-regulates IFN-γR2 chain surface expression and desensitizes IFN-γ/STAT-1 signaling in human T lymphocytes. Blood 102:2933–2939 [DOI] [PubMed] [Google Scholar]
- Bertrand et al., 2006.Bertrand FE, Steelman LS, Chappell WH, Abrams SL, Shelton JG, White ER, Ludwig DL, McCubrey JA. (2006) Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia 20:1254–1260 [DOI] [PubMed] [Google Scholar]
- Beschorner et al., 1991.Beschorner WE, Divic J, Pulido H, Yao X, Kenworthy P, Bruce G. (1991) Enhancement of thymic recovery after cyclosporine by recombinant human growth hormone and insulin-like growth factor-I. Transplantation 52:879–884 [DOI] [PubMed] [Google Scholar]
- Bhaumick and Bala, 1987.Bhaumick B, Bala RM. (1987) Binding and degradation of insulin-like growth factors I and II by rat kidney membrane. Endocrinology 120:1439–1448 [DOI] [PubMed] [Google Scholar]
- Binz et al., 1990.Binz K, Joller P, Froesch P, Binz H, Zapf J, Froesch ER. (1990) Repopulation of the atrophied thymus in diabetic rats by insulin-like growth factor-I. Proc Natl Acad Sci USA 87:3690–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman et al., 1983.Bitterman PB, Adelberg S, Crystal RG. (1983) Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest 72:1801–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke et al., 2001.Blaschke S, Schulz H, Schwarz G, Blaschke V, Müller GA, Reuss-Borst M. (2001) Interleukin 16 expression in relation to disease activity in rheumatoid arthritis. J Rheumatol 28:12–21 [PubMed] [Google Scholar]
- Blat et al., 1994.Blat C, Villaudy J, Binoux M. (1994) In vivo proteolysis of serum insulin-like growth factor (IGF) binding protein-3 results in increased availability of IGF to target cells. J Clin Invest 93:2286–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy, 1991.Bondy CA. (1991) Transient IGF-I gene expression during the maturation of functionally related central projection neurons. J Neurosci 11:3442–3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir and Smith, 1994.Bonner-Weir S, Smith FE. (1994) Islet cell growth and the growth factors involved. Trends Endocrinol Metab 5:60–64 [DOI] [PubMed] [Google Scholar]
- Braulke, 1999.Braulke T. (1999) Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res 31:242–246 [DOI] [PubMed] [Google Scholar]
- Brevetti et al., 2008.Brevetti G, Colao A, Schiano V, Pivonello R, Laurenzano E, Di Somma C, Lombardi G, Chiariello M. (2008) IGF system and peripheral arterial disease: relationship with disease severity and inflammatory status of the affected limb. Clin Endocrinol (Oxf) 69:894–900 [DOI] [PubMed] [Google Scholar]
- Brissenden et al., 1984.Brissenden JE, Ullrich A, Francke U. (1984) Human chromosomal mapping of genes for insulin-like growth factors I and II and epidermal growth factor. Nature 310:781–784 [DOI] [PubMed] [Google Scholar]
- Brix et al., 1998.Brix TH, Christensen K, Holm NV, Harvald B, Hegedüs L. (1998) A population-based study of Graves' disease in Danish twins. Clin Endocrinol (Oxf) 48:397–400 [DOI] [PubMed] [Google Scholar]
- Brown et al., 1983.Brown TJ, Ercolani L, Ginsberg BH. (1983) Properties and regulation of the T lymphocyte insulin receptor. J Recept Res 3:481–494 [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood and Schwabe, 1994.Bryant-Greenwood GD, Schwabe C. (1994) Human relaxins: chemistry and biology. Endocr Rev 15:5–26 [DOI] [PubMed] [Google Scholar]
- Brzozowski et al., 2002.Brzozowski AM, Dodson EJ, Dodson GG, Murshudov GN, Verma C, Turkenburg JP, de Bree FM, Dauter Z. (2002) Structural origins of the functional divergence of human insulin-like growth factor-I and insulin. Biochemistry 41:9389–9397 [DOI] [PubMed] [Google Scholar]
- Buckley et al., 2002.Buckley DA, Loughran G, Murphy G, Fennelly C, O'Connor R. (2002) Identification of an IGF-1R kinase regulatory phosphatase using the fission yeast Schizosaccharomyces pombe and a GFP tagged IGF-1R in mammalian cells. Mol Pathol 55:46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtrum et al., 2003.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, Bassi R, Abdullah R, Hooper AT, Koo H, et al. (2003) A fully human monoclonal antibody to the insulin-like growth factor-I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res 63:8912–8921 [PubMed] [Google Scholar]
- Butler et al., 1998.Butler AA, Blakesley VA, Poulaki V, Tsokos M, Wood TL, LeRoith D, Pouliki V. (1998) Stimulation of tumor growth by recombinant human insulin-like growth factor-I (IGF-I) is dependent on the dose and the level of IGF-I receptor expression. Cancer Res 58:3021–3027 [PubMed] [Google Scholar]
- Buttgereit et al., 2000.Buttgereit F, Burmester GR, Brand MD. (2000) Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol Today 21:192–199 [DOI] [PubMed] [Google Scholar]
- Calderari et al., 2007.Calderari S, Gangnerau MN, Thibault M, Meile MJ, Kassis N, Alvarez C, Portha B, Serradas P. (2007) Defective IGF2 and IGF1R protein production in embryonic pancreas precedes beta cell mass anomaly in the Goto-Kakizaki rat model of type 2 diabetes. Diabetologia 50:1463–1471 [DOI] [PubMed] [Google Scholar]
- Carpenter, 1999.Carpenter G. (1999) Employment of the epidermal growth factor receptor in growth Factor-Independent signaling pathways. J Cell Biol 146:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas et al., 2006.Casellas A, Salavert A, Agudo J, Ayuso E, Jimenez V, Moya M, Muñoz S, Franckhauser S, Bosch F. (2006) Expression of IGF-I in pancreatic islets prevents lymphocytic infiltration and protects mice from type 1 diabetes. Diabetes 55:3246–3255 [DOI] [PubMed] [Google Scholar]
- Cass and Meinkoth, 1998.Cass LA, Meinkoth JL. (1998) Differential effects of cyclic adenosine 3′,5′-monophosphate on p70 ribosomal S6 kinase. Endocrinology 139:1991–1998 [DOI] [PubMed] [Google Scholar]
- Center et al., 1997.Center DM, Kornfeld H, Cruikshank WW. (1997) Interleukin-16. Int J Biochem Cell Biol 29:1231–1234 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran et al., 1999.Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. (1999) Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by α3β1 integrin and regulated by insulin-like growth factor-1 and CD98. J Biol Chem 274:11408–11416 [DOI] [PubMed] [Google Scholar]
- Chatenoud et al., 1994.Chatenoud L, Thervet E, Primo J, Bach JF. (1994) Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA 91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2004.Chen W, Salojin KV, Mi QS, Grattan M, Meagher TC, Zucker P, Delovitch TL. (2004) Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology 145:627–638 [DOI] [PubMed] [Google Scholar]
- Cho, 2008.Cho JH. (2008) The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8:458–466 [DOI] [PubMed] [Google Scholar]
- Choyke et al., 1987.Choyke PL, Zeman RK, Gootenberg JE, Greenberg JN, Hoffer F, Frank JA. (1987) Thymic atrophy and regrowth in response to chemotherapy: CT evaluation. AJR Am J Roentgenol 149:269–272 [DOI] [PubMed] [Google Scholar]
- Christodoulakos et al., 2007.Christodoulakos GE, Lambrinoudaki IV, Economou EV, Papadias C, Vitoratos N, Panoulis CP, Kouskouni EE, Vlachou SA, Creatsas GC. (2007) Circulating chemoattractants RANTES, negatively related to endogenous androgens, and MCP-1 are differentially suppressed by hormone therapy and raloxifene. Atherosclerosis 193:142–150 [DOI] [PubMed] [Google Scholar]
- Clark, 1997.Clark R. (1997) The somatogenic hormones and insulin-like growth factor-1: stimulators of lymphopoiesis and immune function. Endocr Rev 18:157–179 [DOI] [PubMed] [Google Scholar]
- Clark et al., 1993.Clark R, Strasser J, McCabe S, Robbins K, Jardieu P. (1993) Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest 92:540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément et al., 2001.Clément S, Refetoff S, Robaye B, Dumont JE, Schurmans S. (2001) Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-Ir receptor in the thyroid gland. Endocrinology 142:5131–5139 [DOI] [PubMed] [Google Scholar]
- Clemmons, 2001.Clemmons DR. (2001) IGF-1 receptor-mediated signal transduction, in Targets for Growth Hormone and IGF-1 Action (Bouillon R. ed), pp 17–28, Bioscientifica Ltd, Bristol, UK: [Google Scholar]
- Clemmons, 2007.Clemmons DR. (2007) Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov 6:821–833 [DOI] [PubMed] [Google Scholar]
- Clemmons and Maile, 2005.Clemmons DR, Maile LA. (2005) Interaction between insulin-like growth factor-I receptor and αVβ3 integrin linked signaling pathways: cellular responses to changes in multiple signaling inputs. Mol Endocrinol 19:1–11 [DOI] [PubMed] [Google Scholar]
- Clemmons et al., 2000.Clemmons DR, Moses AC, McKay MJ, Sommer A, Rosen DM, Ruckle J. (2000) The combination of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 reduces insulin requirements in insulin-dependent type 1 diabetes: evidence for in vivo biological activity. J Clin Endocrinol Metab 85:1518–1524 [DOI] [PubMed] [Google Scholar]
- Clemmons et al., 2005.Clemmons DR, Moses AC, Sommer A, Jacobson W, Rogol AD, Sleevi MR, Allan G. (2005) Rh/IGF-I/rhIGFBP-3 administration to patients with type 2 diabetes mellitus reduces insulin requirements while also lowering fasting glucose. Growth Horm IGF Res 15:265–274 [DOI] [PubMed] [Google Scholar]
- Cohen et al., 2005.Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, Tengowski MW, Wang F, Gualberto A, Beebe JS, et al. (2005) Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res 11:2063–2073 [DOI] [PubMed] [Google Scholar]
- Colangelo et al., 2009.Colangelo LA, Chiu B, Kopp P, Liu K, Gapstur SM. (2009) Serum IGF-I and C-reactive protein in healthy black and white young men: the CARDIA male hormone study. Growth Horm IGF Res 19:420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez et al., 1996.Cortez AB, Van Dop C, Bailey RC, Bersch N, Scott M, Golde DW, Geffner ME. (1996) IGF-I resistance in virus-transformed B-lymphocytes from African Efe Pygmies. Biochem Mol Med 58:31–36 [DOI] [PubMed] [Google Scholar]
- Craparo et al., 1995.Craparo A, O'Neill TJ, Gustafson TA. (1995) Non-SH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor-I receptor. J Biol Chem 270:15639–15643 [DOI] [PubMed] [Google Scholar]
- Cwyfan Hughes et al., 1992.Cwyfan Hughes SC, Cotterill AM, Molloy AR, Cassell TB, Braude N, Hinds CJ, Wass JA, Holly JM. (1992) The induction of specific proteases for insulin-like growth factor-binding proteins following major heart surgery. J Endocrinol 135:135–145 [DOI] [PubMed] [Google Scholar]
- Davies, 1996.Davies TF. (1996) Graves' disease, in Werner and Ingbar's The Thyroid (Braverman LE, Utiger RD. eds) pp 525–558, Lippincott-Raven, Philadelphia: [Google Scholar]
- Delbé et al., 1991.Delbé J, Blat C, Desauty G, Harel L. (1991) Presence of IDF45 (mlGFBP-3) binding sites on chick embryo fibroblasts. Biochem Biophys Res Commun 179:495–501 [DOI] [PubMed] [Google Scholar]
- de Mello-Coelho et al., 1997.de Mello-Coelho V, Villa-Verde DM, Dardenne M, Savino W. (1997) Pituitary hormones modulate cell-cell interactions between thymocytes and thymic pithelial cells. J Neuroimmunol 76:39–49 [DOI] [PubMed] [Google Scholar]
- de Mello Coelho et al., 2002.de Mello Coelho V, Villa-Verde DM, Farias-de-Oliveira DA, de Brito JM, Dardenne M, Savino W. (2002) Functional insulin-like growth factor-1/insulin-like growth factor-1 receptor-mediated circuit in human and murine thymic epithelial cells. Neuroendocrinology 75:139–150 [DOI] [PubMed] [Google Scholar]
- De Meyts and Whittaker, 2002.De Meyts P, Whittaker J. (2002) Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov 1:769–783 [DOI] [PubMed] [Google Scholar]
- Denko and Malemud, 2004.Denko CW, Malemud CJ. (2004) Age-related changes in serum growth hormone, insulin-like growth factor-1 and somatostatin in system lupus erythematosus. BMC Musculoskelet Disord 5:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps et al., 2009.Descamps G, Gomez-Bougie P, Venot C, Moreau P, Bataille R, Amiot M. (2009) A humanised anti-IGF-1R monoclonal antibody (AVE1642) enhances Bortezomib-induced apoptosis in myeloma cells lacking CD45. Br J Cancer 100:366–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps et al., 2006.Descamps G, Wuillème-Toumi S, Trichet V, Venot C, Debussche L, Hercend T, Collette M, Robillard N, Bataille R, Amiot M. (2006) CD45neg but not CD45pos human myeloma cells are sensitive to the inhibition of IGF-1 signaling by a murine anti-IGF-1R monoclonal antibody, mAVE1642. J Immunol 177:4218–4223 [DOI] [PubMed] [Google Scholar]
- Dey et al., 1996.Dey BR, Frick K, Lopaczynski W, Nissley SP, Furlanetto RW. (1996) Evidence for the direct interaction of the insulin-like growth factor-I receptor with IRS-1, Shc, and Grb10. Mol Endocrinol 10:631–641 [DOI] [PubMed] [Google Scholar]
- DiFedele et al., 2005.DiFedele LM, He J, Bonkowski EL, Han X, Held MA, Bohan A, Menon RK, Denson LA. (2005) Tumor necrosis factor α blockade restores growth hormone signaling in murine colitis. Gastroenterology 128:1278–1291 [DOI] [PubMed] [Google Scholar]
- Doern et al., 2009.Doern A, Cao X, Sereno A, Reyes CL, Altshuler A, Huang F, Hession C, Flavier A, Favis M, Tran H, et al. (2009) Characterization of inhibitory anti-insulin-like growth factor receptor antibodies with different epitope specificity and ligand-blocking properties: implications for mechanism of action in vivo. J Biol Chem 284:10254–10267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman et al., 1995.Dorman JB, Albinder B, Shroyer T, Kenyon C. (1995) The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics 141:1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörner et al., 2009.Dörner T, Radbruch A, Burmester GR. (2009) B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol 5:433–441 [DOI] [PubMed] [Google Scholar]
- Douglas et al., 2007.Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. (2007) Aberrant expression of the IGF-1 receptor by T cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. J Immunol 178:3281–3287 [DOI] [PubMed] [Google Scholar]
- Douglas et al., 2008.Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, Smith TJ. (2008) B cells from patients with Graves' disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol 181:5768–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas et al., 2009.Douglas RS, Brix TH, Hwang CJ, Hegedüs L, Smith TJ. (2009) Divergent frequencies of IGF-1 receptor-expressing blood lymphocytes in monozygotic twin pairs discordant for Graves' disease: evidence for a phenotypic signature ascribable to nongenetic factors. J Clin Endocrinol Metab 94:1797–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drop et al., 1992.Drop SL, Schuller AG, Lindenbergh-Kortleve DJ, Groffen C, Brinkman A, Zwarthoff EC. (1992) Structural aspects of the IGFBP family. Growth Regul 2:69–79 [PubMed] [Google Scholar]
- Du et al., 1999.Du J, Peng T, Scheidegger KJ, Delafontaine P. (1999) Angiotensin II activation of insulin-like growth factor 1 receptor transcription is mediated by a tyrosine kinase-dependent redox-sensitive mechanism. Arterioscler Thromb Vasc Biol 19:2119–2126 [DOI] [PubMed] [Google Scholar]
- Du et al., 2001.Du J, Brink M, Peng T, Mottironi B, Delafontaine P. (2001) Thrombin regulates insulin-like growth factor-1 receptor transcription in vascular smooth muscle: characterization of the signaling pathway. Circ Res 88:1044–1052 [DOI] [PubMed] [Google Scholar]
- Duquesnoy and Pedersen, 1981.Duquesnoy RJ, Pedersen GM. (1981) Immunologic and hematologic deficiencies of the hypopituitary dwarf mouse, in Immunologic Defects in Laboratory Animals (Gershwin ME, Merchant B. eds) vol 1, pp 309–324, Plenum Publishing, New York: [Google Scholar]
- Egan et al., 1993.Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. (1993) Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature 363:45–51 [DOI] [PubMed] [Google Scholar]
- Eisenbarth et al., 1994.Eisenbarth GS, Ziegler AG, Colman PA. (1994) Pathogenesis of insulin-dependent (type I) diabetes mellitus, in Joslin's Diabetes Mellitus (Kahn CR, Weir GC. eds) ed 13, pp 216–239, Lea and Febiger, Philadelphia: [Google Scholar]
- Eivindson et al., 2007.Eivindson M, Grønbaek H, Skogstrand K, Thorsen P, Frystyk J, Flyvbjerg A, Dahlerup JF. (2007) The insulin-like growth factor (IGF) system and its relation to infliximab treatment in adult patients with Crohn's disease. Scand J Gastroenterol 42:464–470 [DOI] [PubMed] [Google Scholar]
- Eizirik et al., 2009.Eizirik DL, Colli ML, Ortis F. (2009) The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 5:219–226 [DOI] [PubMed] [Google Scholar]
- El-Shewy et al., 2004.El-Shewy HM, Kelly FL, Barki-Harrington L, Luttrell LM. (2004) Ectodomain shedding-dependent transactivation of epidermal growth factor receptors in response to insulin-like growth factor type I. Mol Endocrinol 18:2727–2739 [DOI] [PubMed] [Google Scholar]
- El-Shewy et al., 2007.El-Shewy HM, Lee MH, Obeid LM, Jaffa AA, Luttrell LM. (2007) The insulin-like growth factor type 1 and insulin-like growth factor type 2/mannose-6-phosphate receptors independently regulate ERK1/2 activity in HEK293 cells. J Biol Chem 282:26150–26157 [DOI] [PubMed] [Google Scholar]
- El Yafi et al., 2005.El Yafi F, Winkler R, Delvenne P, Boussif N, Belaiche J, Louis E. (2005) Altered expression of type I insulin-like growth factor receptor in Crohn's disease. Clin Exp Immunol 139:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris et al., 1971.Fabris N, Pierpaoli W, Sorkin E. (1971) Hormones and the immunological capacity. 3. The immunodeficiency disease of the hypopituitary Snell-Bagg dwarf mouse. Clin Exp Immunol 9:209–225 [PMC free article] [PubMed] [Google Scholar]
- Fairweather et al., 2008.Fairweather D, Frisancho-Kiss S, Rose NR. (2008) Sex differences in autoimmune disease from a pathological perspective. Am J Pathol 173:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga et al., 1987.Falanga V, Tiegs SL, Alstadt SP, Roberts AB, Sporn MB. (1987) Transforming growth factor-beta: selective increase in glycosaminoglycan synthesis by cultures of fibroblasts from patients with progressive systemic sclerosis. J Invest Dermatol 89:100–104 [DOI] [PubMed] [Google Scholar]
- Fawzi et al., 2008.Fawzi MM, Tawfik SO, Eissa AM, El-Komy MH, Abdel-Halim MR, Shaker OG. (2008) Expression of insulin-like growth factor-I in lesional and non-lesional skin of patients with morphoea. Br J Dermatol 159:86–90 [DOI] [PubMed] [Google Scholar]
- Feliciello et al., 1993.Feliciello A, Porcellini A, Ciullo I, Bonavolontà G, Avvedimento EV, Fenzi G. (1993) Expression of thyrotropin-receptor mRNA in healthy and Graves' disease retro-orbital tissue. Lancet 342:337–338 [DOI] [PubMed] [Google Scholar]
- Feng and Dimitrov, 2008.Feng Y, Dimitrov DS. (2008) Monoclonal antibodies against components of the IGF system for cancer treatment. Curr Opin Drug Discov Devel 11:178–185 [PubMed] [Google Scholar]
- Fernihough et al., 1996.Fernihough JK, Billingham ME, Cwyfan-Hughes S, Holly JM. (1996) Local disruption of the insulin-like growth factor system in the arthritic joint. Arthritis Rheum 39:1556–1565 [DOI] [PubMed] [Google Scholar]
- Firth and Baxter, 2002.Firth SM, Baxter RC. (2002) Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23:824–854 [DOI] [PubMed] [Google Scholar]
- Fisher et al., 2008.Fisher SA, Tremelling M, Anderson CA, Gwilliam R, Bumpstead S, Prescott NJ, Nimmo ER, Massey D, Berzuini C, Johnson C, et al. (2008) Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn's disease. Nat Genet 40:710–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier et al., 1995.Fournier T, Riches DW, Winston BW, Rose DM, Young SK, Noble PW, Lake FR, Henson PM. (1995) Divergence in macrophage insulin-like growth factor-I (IGF-I) synthesis induced by TNF-α and prostaglandin E2. J Immunol 155:2123–2133 [PubMed] [Google Scholar]
- Fowlkes and Serra, 1996.Fowlkes JL, Serra DM. (1996) Characterization of glycosaminoglycan-binding domains present in insulin-like growth factor-binding protein-3. J Biol Chem 271:14676–14679 [DOI] [PubMed] [Google Scholar]
- Fowlkes et al., 1997.Fowlkes JL, Thrailkill KM, George-Nascimento C, Rosenberg CK, Serra DM. (1997) Heparin-binding, highly basic regions within the thyroglobulin type-1 repeat of insulin-like growth factor (IGF)-binding proteins (IGFBPs) -3, -5, and -6 inhibit IGFBP-4 degradation. Endocrinology 138:2280–2285 [DOI] [PubMed] [Google Scholar]
- Franke et al., 2008.Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, Schuldt D, Nikolaus S, Rosenstiel P, Krawczak M, et al. (2008) Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet 40:713–715 [DOI] [PubMed] [Google Scholar]
- Frasca et al., 2008.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. (2008) The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 114:23–37 [DOI] [PubMed] [Google Scholar]
- Frauwirth et al., 2002.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16:769–777 [DOI] [PubMed] [Google Scholar]
- Frauwirth and Thompson, 2004.Frauwirth KA, Thompson CB. (2004) Regulation of T lymphocyte metabolism. J Immunol 172:4661–4665 [DOI] [PubMed] [Google Scholar]
- Freund et al., 1993.Freund GG, Kulas DT, Mooney RA. (1993) Insulin and IGF-1 increase mitogenesis and glucose metabolism in the multiple myeloma cell line, RPMI 8226. J Immunol 151:1811–1820 [PubMed] [Google Scholar]
- Freund et al., 1994.Freund GG, Kulas DT, Way BA, Mooney RA. (1994) Functional insulin and insulin-like growth factor-1 receptors are preferentially expressed in multiple myeloma cell lines as compared to B-lymphoblastoid cell lines. Cancer Res 54:3179–3185 [PubMed] [Google Scholar]
- Geffner et al., 1987.Geffner ME, Kaplan SA, Bersch N, Lippe BM, Smith WG, Nagel RA, Santulli TV, Jr, Li CH, Golde DW. (1987) Leprechaunism: in vitro insulin action despite genetic insulin resistance. Pediatr Res 22:286–291 [DOI] [PubMed] [Google Scholar]
- Geffner et al., 1992.Geffner ME, Bersch N, Golde DW. (1992) Insulin and IGF-I stimulate normal and virally transformed T-lymphocyte cell growth in vitro. Brain Behav Immun 6:377–386 [DOI] [PubMed] [Google Scholar]
- Geffner et al., 1993.Geffner ME, Bailey RC, Bersch N, Vera JC, Golde DW. (1993) Insulin-like growth factor-I unresponsiveness in an Efe Pygmy. Biochem Biophys Res Commun 193:1216–1223 [DOI] [PubMed] [Google Scholar]
- Geffner et al., 1995.Geffner ME, Bersch N, Bailey RC, Golde DW. (1995) Insulin-like growth factor-I resistance in immortalized T cell lines from African Efe Pygmies. J Clin Endocrinol Metab 80:3732–3738 [DOI] [PubMed] [Google Scholar]
- George et al., 2002.George M, Ayuso E, Casellas A, Costa C, Devedjian JC, Bosch F. (2002) β Cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest 109:1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh et al., 2003.Ghosh P, Dahms NM, Kornfeld S. (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4:202–212 [DOI] [PubMed] [Google Scholar]
- Gianoukakis et al., 2006.Gianoukakis AG, Douglas RS, King CS, Cruikshank WW, Smith TJ. (2006) IgG from patients with Graves' disease induces interleukin-16 and RANTES expression in cultured human thyrocytes: a putative mechanism for T-cell infiltration of the thyroid in autoimmune disease. Endocrinology 147:1941–1949 [DOI] [PubMed] [Google Scholar]
- Giannoukakis et al., 2000.Giannoukakis N, Mi Z, Rudert WA, Gambotto A, Trucco M, Robbins P. (2000) Prevention of beta cell dysfunction and apoptosis activation in human islets by adenoviral gene transfer of the insulin-like growth factor-I. Gene Ther 7:2015–2022 [DOI] [PubMed] [Google Scholar]
- Gibson et al., 1993.Gibson LF, Piktel D, Landreth KS. (1993) Insulin-like growth factor-1 potentiates expansion of interleukin-7-dependent pro-B cells. Blood 82:3005–3011 [PubMed] [Google Scholar]
- Girnita et al., 2004.Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. (2004) Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res 64:236–242 [DOI] [PubMed] [Google Scholar]
- Giuliani et al., 2006.Giuliani C, Saji M, Bucci I, Fiore G, Liberatore M, Singer DS, Monaco F, Kohn LD, Napolitano G. (2006) Transcriptional regulation of major histocompatibility complex class I gene by insulin and IGF-I in FRTL-5 thyroid cells. J Endocrinol 189:605–615 [DOI] [PubMed] [Google Scholar]
- Goetsch et al., 2005.Goetsch L, Gonzalez A, Leger O, Beck A, Pauwels PJ, Haeuw JF, Corvaia N. (2005) A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer 113:316–328 [DOI] [PubMed] [Google Scholar]
- Grønborg et al., 1993.Grønborg M, Wulff BS, Rasmussen JS, Kjeldsen T, Gammeltoft S. (1993) Structure-function relationship of the insulin-like growth factor-I receptor tyrosine kinase. J Biol Chem 268:23435–23440 [PubMed] [Google Scholar]
- Grounds, 2002.Grounds MD. (2002) Reasons for the degeneration of ageing skeletal muscle: a central role for IGF-1 signalling. Biogerontology 3:19–24 [DOI] [PubMed] [Google Scholar]
- Grounds et al., 2008.Grounds MD, Radley HG, Gebski BL, Bogoyevitch MA, Shavlakadze T., Radley HG, Gebski BL, Bogoyevitch MA, Shavlakadze T. (2008) Implications of cross-talk between tumour necrosis factor and insulin-like growth factor-1 signalling in skeletal muscle. Clin Exp Pharmacol Physiol 35:846–851 [DOI] [PubMed] [Google Scholar]
- Guzman Moreno, 2009.Guzman Moreno R. (2009) B-cell depletion in autoimmune diseases. Advances in autoimmunity. Autoimmun Rev 8:585–590 [DOI] [PubMed] [Google Scholar]
- Hackel et al., 1999.Hackel PO, Zwick E, Prenzel N, Ullrich A. (1999) Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol 11:184–189 [DOI] [PubMed] [Google Scholar]
- Hadden et al., 1992.Hadden JW, Malec PH, Coto J, Hadden EM. (1992) Thymic involution in aging. Prospects for correction. Ann NY Acad Sci 673:231–239 [DOI] [PubMed] [Google Scholar]
- Han et al., 2005.Han X, Sosnowska D, Bonkowski EL, Denson LA. (2005) Growth hormone inhibits signal transducer and activator of transcription 3 activation and reduces disease activity in murine colitis. Gastroenterology 129:185–203 [DOI] [PubMed] [Google Scholar]
- Hariharan et al., 2007.Hariharan K, Dong J, Demarest S, Joseph I, Chu P, Graff C, Glaser S, Graff C, Kramer-Stickland K, Peach R, Reff M. (2007) BIIB022, a fully human nonglycosylated γ4P antibody targeting IGF-1R for cancer therapy, in 19th Annual AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 22–26 Oct 2007; San Francisco, CA Poster B210 [Google Scholar]
- Harrison et al., 1994.Harrison NK, Cambrey AD, Myers AR, Southcott AM, Black CM, du Bois RM, Laurent GJ, McAnulty RJ. (1994) Insulin-like growth factor-I is partially responsible for fibroblast proliferation induced by bronchoalveolar lavage fluid from patients with systemic sclerosis. Clin Sci (Lond) 86:141–148 [DOI] [PubMed] [Google Scholar]
- Harrison et al., 1991.Harrison NK, Myers AR, Corrin B, Soosay G, Dewar A, Black CM, Du Bois RM, Turner-Warwick M. (1991) Structural features of interstitial lung disease in systemic sclerosis. Am Rev Respir Dis 144:706–713 [DOI] [PubMed] [Google Scholar]
- Hasegawa et al., 2006.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, et al. (2006) B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol 169:954–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, 2003.Hassan AB. (2003) Keys to the hidden treasures of the mannose 6-phosphate/insulin-like growth factor 2 receptor. Am J Pathol 162:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori et al., 1996.Hattori Y, Vera JC, Rivas CI, Bersch N, Bailey RC, Geffner ME, Golde DW. (1996) Decreased insulin-like growth factor-I receptor expression and function in immortalized African Pygmy T cells. J Clin Endocrinol Metab 81:2257–2263 [DOI] [PubMed] [Google Scholar]
- Helderman and Strom, 1977.Helderman JH, Strom TB. (1977) Emergence of insulin receptors upon alloimmune T cells in the rat. J Clin Invest 59:338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman and Strom, 1978.Helderman JH, Strom TB. (1978) Specific insulin binding site on T and B lymphocytes as a marker of cell activation. Nature 274:62–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman, 1981.Helderman JH. (1981) Role of insulin in the intermediary metabolism of the activated thymic-derived lymphocyte. J Clin Invest 67:1636–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold et al., 2002.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, et al. (2002) Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346:1692–1698 [DOI] [PubMed] [Google Scholar]
- Heufelder et al., 1993.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. (1993) Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves' ophthalmopathy and pretibial dermopathy. Thyroid 3:297–300 [DOI] [PubMed] [Google Scholar]
- Heulin et al., 1982.Heulin MH, Artur M, Straczek J, Belleville F, Nabet P, Hermann A, Lebeurre MD, Schimpff RM. (1982) Effect of low molecular weight human serum factors and human somatomedin peptides on human lymphocyte cultures. J Cell Sci 57:129–137 [DOI] [PubMed] [Google Scholar]
- Higano et al., 2007.Higano C, LoRusso P, Gordon M, Yu EY, Whiting SH, Fox F, Katz T, Rowinsky E, Youssoufian H. (2007) A phase I study of the recombinant human IgG1 anti-IGF-IR monoclonal antibody (Mab) IMC-A12, administered on a weekly basis to patients with advanced solid tumors: Interim analysis, in 19th Annual AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 22–26 Oct 2007; San Francisco, CA Poster B19 [Google Scholar]
- Hijikawa et al., 2008.Hijikawa T, Kaibori M, Uchida Y, Yamada M, Matsui K, Ozaki T, Kamiyama Y, Nishizawa M, Okumura T. (2008) Insulin-like growth factor 1 prevents liver injury through the inhibition of TNF-α and iNOS induction in d-galactosamine and LPS-treated rats. Shock 29:740–747 [DOI] [PubMed] [Google Scholar]
- Himpe et al., 2008.Himpe E, Degaillier C, Coppens A, Kooijman R. (2008) Insulin-like growth factor-1 delays Fas-mediated apoptosis in human neutrophils through the phosphatidylinositol-3 kinase pathway. J Endocrinol 199:69–80 [DOI] [PubMed] [Google Scholar]
- Hinton et al., 1998.Hinton PS, Peterson CA, Dahly EM, Ney DM. (1998) IGF-I alters lymphocyte survival and regeneration in thymus and spleen after dexamethasone treatment. Am J Physiol 274:R912–R920 [DOI] [PubMed] [Google Scholar]
- Holzenberger et al., 2003.Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421:182–187 [DOI] [PubMed] [Google Scholar]
- Hunt and Eardley, 1986.Hunt P, Eardley DD. (1986) Suppressive effects of insulin and insulin-like growth factor-1 (IGF1) on immune responses. J Immunol 136:3994–3999 [PubMed] [Google Scholar]
- Hwa et al., 1999.Hwa V, Oh Y, Rosenfeld RG. (1999) The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 20:761–787 [DOI] [PubMed] [Google Scholar]
- Imai and Takaoka, 2006.Imai K, Takaoka A. (2006) Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 6:714–727 [DOI] [PubMed] [Google Scholar]
- Jain et al., 1998.Jain S, Golde DW, Bailey R, Geffner ME. (1998) Insulin-like growth factor-I resistance. Endocr Rev 19:625–646 [DOI] [PubMed] [Google Scholar]
- Jansson et al., 1997.Jansson M, Uhlen M, Nilsson B. (1997) Structural changes in insulin-like growth factor (IGF) I mutant proteins affecting binding kinetic rates to IGF binding protein 1 and IGF-I receptor. Biochemistry 36:4108–4117 [DOI] [PubMed] [Google Scholar]
- Jardieu et al., 1994.Jardieu P, Clark R, Mortensen D, Dorshkind K. (1994) In vivo administration of insulin-like growth factor-I stimulates primary B lymphopoiesis and enhances lymphocyte recovery after bone marrow transplantation. J Immunol 152:4320–4327 [PubMed] [Google Scholar]
- Jefferies et al., 1997.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. (1997) Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J 16:3693–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek et al., 1997.Jelinek DF, Witzig TE, Arendt BK. (1997) A role for insulin-like growth factor in the regulation of IL-6-responsive human myeloma cell line growth. J Immunol 159:487–496 [PubMed] [Google Scholar]
- Jeschke et al., 2000.Jeschke MG, Barrow RE, Herndon DN. (2000) Insulinlike growth factor-I plus insulinlike growth factor binding protein 3 attenuates the proinflammatory acute phase response in severely burned children. Ann Surg 231:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji et al., 2007.Ji QS, Mulvihill MJ, Rosenfeld-Franklin M, Cooke A, Feng L, Mak G, O'Connor M, Yao Y, Pirritt C, Buck E, et al. (2007) A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Mol Cancer Ther 6:2158–2167 [DOI] [PubMed] [Google Scholar]
- Johnson et al., 1992.Johnson EW, Jones LA, Kozak RW. (1992) Expression and function of insulin-like growth factor receptors on anti-CD3-activated human T lymphocytes. J Immunol 148:63–71 [PubMed] [Google Scholar]
- Jones and Clemmons, 1995.Jones JI, Clemmons DR. (1995) Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- Jones et al., 1993.Jones JI, Gockerman A, Busby WH, Jr., Wright G, Clemmons DR. (1993) Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the α5β1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci USA 90:10553–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones et al., 1996.Jones JI, Prevette T, Gockerman A, Clemmons DR. (1996) Ligand occupancy of the α-V-β3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci USA 93:2482–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleko et al., 1990.Kaleko M, Rutter WJ, Miller AD. (1990) Overexpression of the human insulinlike growth factor-I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol 10:464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer et al., 2002.Kammer GM, Perl A, Richardson BC, Tsokos GC. (2002) Abnormal T cell signal transduction in systemic lupus erythematosus. Arthritis Rheum 46:1139–1154 [DOI] [PubMed] [Google Scholar]
- Kant et al., 2007.Kant SG, Kriek M, Walenkamp MJ, Hansson KB, van Rhijn A, Clayton-Smith J, Wit JM, Breuning MH. (2007) Tall stature and duplication of the insulin-like growth factor-I receptor gene. Eur J Med Genet 50:1–10 [DOI] [PubMed] [Google Scholar]
- Kato et al., 1993.Kato H, Faria TN, Stannard B, Roberts CT, Jr., LeRoith D. (1993) Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (αIR-3). J Biol Chem 268:2655–2661 [PubMed] [Google Scholar]
- Kato et al., 1994.Kato H, Faria TN, Stannard B, Roberts CT, Jr., LeRoith D. (1994) Essential role of tyrosine residues 1131, 1135, and 1136 of the insulin-like growth factor-I (IGF-I) receptor in IGF-I action. Mol Endocrinol 8:40–50 [DOI] [PubMed] [Google Scholar]
- Katsanos et al., 2001.Katsanos KH, Tsatsoulis A, Christodoulou D, Challa A, Katsaraki A, Tsianos EV. (2001) Reduced serum insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 levels in adults with inflammatory bowel disease. Growth Horm IGF Res 11:364–367 [DOI] [PubMed] [Google Scholar]
- Kavurma et al., 2007.Kavurma MM, Figg N, Bennett MR, Mercer J, Khachigian LM, Littlewood TD. (2007) Oxidative stress regulates IGF1R expression in vascular smooth-muscle cells via p53 and HDAC recruitment. Biochem J 407:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kecha et al., 2000.Kecha O, Brilot F, Martens H, Franchimont N, Renard C, Greimers R, Defresne MP, Winkler R, Geenen V. (2000) Involvement of insulin-like growth factors in early T cell development: a study using fetal thymic organ cultures. Endocrinology 141:1209–1217 [DOI] [PubMed] [Google Scholar]
- Kelly-Welch et al., 2004.Kelly-Welch AE, Wang HY, Wang LM, Pierce JH, Jay G, Finkelman F, Keegan AD. (2004) Transgenic expression of insulin receptor substrate 2 in murine B cells alters the cell density-dependence of IgE production in vitro and enhances IgE production in vivo. J Immunol 172:2803–2810 [DOI] [PubMed] [Google Scholar]
- Kelley et al., 1996.Kelley KW, Arkins S, Minshall C, Liu Q, Dantzer R. (1996) Growth hormone, growth factors and hematopoiesis. Horm Res 45:38–45 [DOI] [PubMed] [Google Scholar]
- Kennington et al., 2007.Kennington WJ, Hoffmann AA, Partridge L. (2007) Mapping regions within cosmopolitan inversion In(3R)Payne associated with natural variation in body size in Drosophila melanogaster. Genetics 177:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennington et al., 2006.Kennington WJ, Partridge L, Hoffmann AA. (2006) Patterns of diversity and linkage disequilibrium within the cosmopolitan inversion In(3R)Payne in Drosophila melanogaster are indicative of coadaptation. Genetics 172:1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon et al., 1993.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366:461–464 [DOI] [PubMed] [Google Scholar]
- Keyszer et al., 1995.Keyszer GM, Heer AH, Kriegsmann J, Geiler T, Keysser C, Gay RE, Gay S. (1995) Detection of insulin-like growth factor-I and II in synovial tissue specimens of patients with rheumatoid arthritis and osteoarthritis by in situ hybridization. J Rheumatol 22:275–281 [PubMed] [Google Scholar]
- Khan et al., 2003.Khan IU, Tsokos GC, Kammer GM. (2003) Abnormal B cell signal transduction in systemic lupus erythematosus. Curr Dir Autoimmun 6:89–104 [DOI] [PubMed] [Google Scholar]
- Kido et al., 2002.Kido Y, Nakae J, Hribal ML, Xuan S, Efstratiadis A, Accili D. (2002) Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. J Biol Chem 277:36740–36747 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2009.Kim SY, Toretsky JA, Scher D, Helman LJ. (2009) The role of IGF-1R in pediatric malignancies. The Oncologist 14:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata and Fujimoto, 1994.Kimata H, Fujimoto M. (1994) Growth hormone and insulin-like growth factor-I induce immunoglobulin (Ig)E and IgG4 production by human B cells. J Exp Med 180:727–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata and Yoshida, 1994a.Kimata H, Yoshida A. (1994a) Differential effect of growth hormone and insulin-like growth factor-I, insulin-like growth factor-II, and insulin on Ig production and growth in human plasma cells. Blood 83:1569–1574 [PubMed] [Google Scholar]
- Kimata and Yoshida, 1994b.Kimata H, Yoshida A. (1994b) Effect of growth hormone and insulin-like growth factor-I on immunoglobulin production by and growth of human B cells. J Clin Endocrinol Metab 78:635–641 [DOI] [PubMed] [Google Scholar]
- Kincade et al., 1989.Kincade PW, Lee G, Pietrangeli CE, Hayashi S, Gimble JM. (1989) Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol 7:111–143 [DOI] [PubMed] [Google Scholar]
- Kirschner and Sutton, 1986.Kirschner BS, Sutton MM. (1986) Somatomedin-C levels in growth-impaired children and adolescents with chronic inflammatory bowel disease. Gastroenterology 91:830–836 [DOI] [PubMed] [Google Scholar]
- Kirstein et al., 1992.Kirstein M, Aston C, Hintz R, Vlassara H. (1992) Receptor-specific induction of insulin-like growth factor-I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest 90:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, 2003.Klein JR. (2003) Physiological relevance of thyroid stimulating hormone and thyroid stimulating hormone receptor in tissues other than the thyroid. Autoimmunity 36:417–421 [DOI] [PubMed] [Google Scholar]
- Kleinberg et al., 2009.Kleinberg DL, Wood TL, Furth PA, Lee AV. (2009) Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev 30:51–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimiuk et al., 1999.Klimiuk PA, Goronzy JJ, Weyand CM. (1999) IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. J Immunol 162:4293–4299 [PubMed] [Google Scholar]
- Kooijman et al., 1995a.Kooijman R, Scholtens LE, Rijkers GT, Zegers BJ. (1995a) Type I insulin-like growth factor receptor expression in different developmental stages of human thymocytes. J Endocrinol 147:203–209 [DOI] [PubMed] [Google Scholar]
- Kooijman et al., 1995b.Kooijman R, van Buul-Offers SC, Scholtens LE, Schuurman HJ, Van den Brande LJ, Zegers BJ. (1995b) T cell development in insulin-like growth factor-II transgenic mice. J Immunol 154:5736–5745 [PubMed] [Google Scholar]
- Kooijman et al., 1995c.Kooijman RK, Scholtens LE, Rijkers GT, Zegers BJ. (1995c) Differential expression of type I insulin-like growth factor receptors in different stages of human T cells. Eur J Immunol 25:931–935 [DOI] [PubMed] [Google Scholar]
- Kooijman et al., 2002.Kooijman R, Coppens A, Hooghe-Peters E. (2002) Igf-I inhibits spontaneous apoptosis in human granulocytes. Endocrinology 143:1206–1212 [DOI] [PubMed] [Google Scholar]
- Kooijman and Coppens, 2004.Kooijman R, Coppens A. (2004) Insulin-like growth factor-I stimulates IL-10 production in human T cells. J Leukoc Biol 76:862–867 [DOI] [PubMed] [Google Scholar]
- Kornfeld, 1992.Kornfeld S. (1992) Structure and function of the mannose 6-phosphate/insulinlike growth factor-II receptors. Annu Rev Biochem 61:307–330 [DOI] [PubMed] [Google Scholar]
- Krein and Winston, 2002.Krein PM, Winston BW. (2002) Roles for insulin-like growth factor-I and transforming growth factor-beta in fibrotic lung disease. Chest 122 (6 Suppl):289S–293S [DOI] [PubMed] [Google Scholar]
- Kulkarni et al., 2002.Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. (2002) β-Cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat Genet 31:111–115 [DOI] [PubMed] [Google Scholar]
- Kumagai et al., 1981.Kumagai J, Akiyama H, Iwashita S, Iida H, Yahara I. (1981) In vitro regeneration of resting lymphocytes from stimulated lymphocytes and its inhibition by insulin. J Immunol 126:1249–1254 [PubMed] [Google Scholar]
- Kurtz et al., 1982.Kurtz A, Jelkmann W, Bauer C. (1982) A new candidate for the regulation of erythropoiesis. Insulin-like growth factor-I. FEBS Lett 149:105–108 [DOI] [PubMed] [Google Scholar]
- Krug et al., 1972.Krug U, Krug F, Cuatrecasas P. (1972) Emergence of insulin receptors on human lymphocytes during in vitro transformation. Proc Natl Acad Sci USA 69:2604–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande et al., 2007.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, et al. (2007) Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449:564–569 [DOI] [PubMed] [Google Scholar]
- Landreth et al., 1992.Landreth KS, Narayanan R, Dorshkind K. (1992) Insulin-like growth factor-I regulates pro-B cell differentiation. Blood 80:1207–1212 [PubMed] [Google Scholar]
- Lawrence et al., 2007.Lawrence MC, McKern NM, Ward CW. (2007) Insulin receptor structure and its implications for the IGF-1 receptor. Current Opinion in Structural Biology 17:699–705 [DOI] [PubMed] [Google Scholar]
- Leal et al., 1999.Leal SM, Huang SS, Huang JS. (1999) Interactions of high affinity insulin-like growth factor-binding proteins with the type V transforming growth factor-beta receptor in mink lung epithelial cells. J Biol Chem 274:6711–6717 [DOI] [PubMed] [Google Scholar]
- Lebrun et al., 2000.Lebrun P, Baron V, Hauck CR, Schlaepfer DD, Van Obberghen E. (2000) Cell adhesion and focal adhesion kinase regulate insulin receptor substrate-1 expression. J Biol Chem 275:38371–38377 [DOI] [PubMed] [Google Scholar]
- Lebrun et al., 1998.Lebrun P, Mothe-Satney I, Delahaye L, Van Obberghen E, Baron V. (1998) Insulin receptor substrate-1 as a signaling molecule for focal adhesion kinase pp125(FAK) and pp60(src). J Biol Chem 273:32244–32253 [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik et al., 2007.Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ. (2007) Activation of peroxisome proliferator-activated receptor γ (PPARγ) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology 148:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee and Cohen, 2002.Lee KW, Cohen P. (2002) Nuclear effects: unexpected intracellular actions of insulin-like growth factor binding protein-3. J Endocrinol 175:33–40 [DOI] [PubMed] [Google Scholar]
- Lee et al., 1986.Lee PD, Rosenfeld RG, Hintz RL, Smith SD. (1986) Characterization of insulin, insulin-like growth factors I and II, and growth hormone receptors on human leukemic lymphoblasts. J Clin Endocrinol Metab 62:28–35 [DOI] [PubMed] [Google Scholar]
- Lee et al., 1992.Lee WH, Javedan S, Bondy CA. (1992) Coordinate expression of insulin-like growth factor system components by neurons and neuroglia during retinal and cerebellar development. J Neurosci 12:4737–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roith et al., 2002.Le Roith D, Kim H, Fernandez AM, Accili D. (2002) Inactivation of muscle insulin and IGF-I receptors and insulin responsiveness. Curr Opin Clin Nutr Metab Care 5:371–375 [DOI] [PubMed] [Google Scholar]
- LeRoith et al., 1995.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr (1995) Molecular and cellular aspects of the insulin-like growth factor-I receptor. Endocr Rev 16:143–163 [DOI] [PubMed] [Google Scholar]
- LeRoy et al., 1982.LeRoy EC, Mercurio S, Sherer GK. (1982) Replication and phenotypic expression of control and scleroderma human fibroblasts: responses to growth factors. Proc Natl Acad Sci USA 79:1286–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al., 1993.Li SL, Kato J, Paz IB, Kasuya J, Fujita-Yamaguchi Y. (1993) Two new monoclonal antibodies against the α subunit of the human insulin-like growth factor-I receptor. Biochem Biophys Res Commun 196:92–98 [DOI] [PubMed] [Google Scholar]
- Ling et al., 2003.Ling Y, Maile LA, Clemmons DR. (2003) Tyrosine phosphorylation of the β3-subunit of the αVβ3 integrin is required for membrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor-I receptor. Mol Endocrinol 17:1824–1833 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2000.Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, Cohen P. (2000) Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-α regulate transcriptional signaling and apoptosis. J Biol Chem 275:33607–33613 [DOI] [PubMed] [Google Scholar]
- Liu et al., 1993.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor-I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- Liu et al., 2007.Liu TJ, LaFortune T, Honda T, Ohmori O, Hatakeyama S, Meyer T, Jackson D, de Groot J, Yung WK. (2007) Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther 6:1357–1367 [DOI] [PubMed] [Google Scholar]
- Liu et al., 1994.Liu X, Yao DL, Bondy CA, Brenner M, Hudson LD, Zhou J, Webster HD. (1994) Astrocytes express insulin-like growth factor-I (IGF-I) and its binding protein, IGFBP-2, during demyelination induced by experimental autoimmune encephalomyelitis. Mol Cell Neurosci 5:418–430 [DOI] [PubMed] [Google Scholar]
- Lobie et al., 1990.Lobie PE, Breipohl W, Waters MJ. (1990) Growth hormone receptor expression in the rat gastrointestinal tract. Endocrinology 126:299–306 [DOI] [PubMed] [Google Scholar]
- Long et al., 1998.Long E, Huynh HT, Zhao X. (1998) Involvement of insulin-like growth factor-1 and its binding proteins in proliferation and differentiation of murine bone marrow-derived macrophage precursors. Endocrine 9:185–192 [DOI] [PubMed] [Google Scholar]
- Luttrell et al., 1999.Luttrell LM, Daaka Y, Lefkowitz RJ. (1999) Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11:177–183 [DOI] [PubMed] [Google Scholar]
- Maake et al., 1997.Maake C, Yamamoto H, Murphy LJ. (1997) The growth hormone dependent serine protease inhibitor, Spi 2.1 inhibits the des (1–3) insulin-like growth factor-I generating protease. Endocrinology 138:5630–5636 [DOI] [PubMed] [Google Scholar]
- Maile and Clemmons, 2002a.Maile LA, Clemmons DR. (2002a) Regulation of insulin-like growth factor-I receptor dephosphorylation by SHPS-1 and the tyrosine phosphatase SHP-2. J Biol Chem 277:8955–8960 [DOI] [PubMed] [Google Scholar]
- Maile and Clemmons, 2002b.Maile LA, Clemmons DR. (2002b) Regulation of insulin-like growth factor-I receptor dephosphorylation by SHPS-1 and the tyrosine phosphatase SHP-2. J Biol Chem 277:8955–8960 [DOI] [PubMed] [Google Scholar]
- Maile and Clemmons, 2002c.Maile LA, Clemmons DR. (2002c) The αVβ3 integrin regulates insulin-like growth factor-I (IGF-I) receptor phosphorylation by altering the rate of recruitment of the Src-homology 2-containing phosphotyrosine phosphatase-2 to the activated IGF-I receptor. Endocrinology 143:4259–4264 [DOI] [PubMed] [Google Scholar]
- Maile and Clemmons, 2003.Maile LA, Clemmons DR. (2003) Integrin-associated protein binding domain of thrombospondin-1 enhances insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Circ Res 93:925–931 [DOI] [PubMed] [Google Scholar]
- Maiter et al., 1988.Maiter D, Underwood LE, Maes M, Ketelslegers JM. (1988) Acute down-regulation of the somatogenic receptors in rat liver by a single injection of growth hormone. Endocrinology 122:1291–1296 [DOI] [PubMed] [Google Scholar]
- Maloney et al., 2003.Maloney EK, McLaughlin JL, Dagdigian NE, Garrett LM, Connors KM, Zhou XM, Blättler WA, Chittenden T, Singh R. (2003) An anti-insulin-like growth factor-I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res 63:5073–5083 [PubMed] [Google Scholar]
- Mañes et al., 1999.Mañes S, Llorente M, Lacalle RA, Gómez-Moutón C, Kremer L, Mira E, Martínez-A C. (1999) The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem 274:6935–6945 [DOI] [PubMed] [Google Scholar]
- Mañes et al., 1997.Mañes S, Mira E, Barbacid MM, Ciprés A, Fernández-Resa P, Buesa JM, Mérida I, Aracil M, Márquez G, Martínez-A C. (1997) Identification of insulin-like growth factor-binding protein-1 as a potential physiological substrate for human stromelysin-3. J Biol Chem 272:25706–25712 [DOI] [PubMed] [Google Scholar]
- Martin and Baxter, 2007.Martin JL, Baxter RC. (2007) Expression of insulin-like growth factor binding protein-2 by MCF-7 breast cancer cells is regulated through the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway. Endocrinology 148:2532–2541 [DOI] [PubMed] [Google Scholar]
- Matsumoto et al., 1996.Matsumoto T, Gargosky SE, Iwasaki K, Rosenfeld RG. (1996) Identification and characterization of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP proteases in human synovial fluid. J Clin Endocrinol Metab 81:150–155 [DOI] [PubMed] [Google Scholar]
- Matsumoto and Tsurumoto, 2002.Matsumoto T, Tsurumoto T. (2002) Inappropriate serum levels of IGF-I and IGFBP-3 in patients with rheumatoid arthritis. Rheumatology (Oxford) 41:352–353 [DOI] [PubMed] [Google Scholar]
- McCune et al., 1998.McCune JM, Loftus R, Schmidt DK, Carroll P, Webster D, Swor-Yim LB, Francis IR, Gross BH, Grant RM. (1998) High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest 101:2301–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy et al., 2007.McElroy B, Powell JC, McCarthy JV. (2007) The insulin-like growth factor 1 (IGF-1) receptor is a substrate for gamma-secretase-mediated intramembrane proteolysis. Biochem Biophys Res Commun 358:1136–1141 [DOI] [PubMed] [Google Scholar]
- McMorris et al., 1986.McMorris FA, Smith TM, DeSalvo S, Furlanetto RW. (1986) Insulin-like growth factor-I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci USA 83:822–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medsger, 1985.Medsger TA., Jr (1985) Systemic sclerosis, eosinophilic fasciitis and calcinosis, in Arthritis and Allied Conditions (McCarty DJ. ed) pp 966–1036, Lea and Febiger, Philadelphia: [Google Scholar]
- Megyesi et al., 1975.Megyesi K, Kahn CR, Roth J, Neville DM, Jr., Nissley SP, Humbel RE, Froesch ER. (1975) The NSILA-s receptor in liver plasma membranes. Characterization and comparison with the insulin receptor. J Biol Chem 250:8990–8996 [PubMed] [Google Scholar]
- Mellman, 1996.Mellman I. (1996) Endocytosis and molecular sorting. Annu Rev Cell Dev Biol 12:575–625 [DOI] [PubMed] [Google Scholar]
- Merchav et al., 1988.Merchav S, Tatarsky I, Hochberg Z. (1988) Enhancement of human granulopoiesis in vitro by biosynthetic insulin-like growth factor-I/somatomedin C and human growth hormone. J Clin Invest 81:791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, 1996.Miller RA. (1996) The aging immune system: primer and prospectus. Science 273:70–74 [DOI] [PubMed] [Google Scholar]
- Mintz et al., 1999.Mintz L, Galperin E, Pasmanik-Chor M, Tulzinsky S, Bromberg Y, Kozak CA, Joyner A, Fein A, Horowitz M. (1999) EHD1—an EH-domain-containing protein with a specific expression pattern. Genomics 59:66–76 [DOI] [PubMed] [Google Scholar]
- Misbin and Almira, 1989.Misbin RI, Almira EC. (1989) Degradation of insulin and insulin-like growth factors by enzyme purified from human erythrocytes. Comparison of degradation products observed with A14- and B26-[125I] monoiodoinsulin. Diabetes 38:152–158 [DOI] [PubMed] [Google Scholar]
- Mitsiades et al., 2004.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, Hideshima T, Chauhan D, Joseph M, Libermann TA, et al. (2004) Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 5:221–230 [DOI] [PubMed] [Google Scholar]
- Moldawer and Copeland, 1997.Moldawer LL, Copeland EM., 3rd (1997) Proinflammatory cytokines, nutritional support, and the cachexia syndrome: interactions and therapeutic options. Cancer 79:1828–1839 [PubMed] [Google Scholar]
- Mondschein et al., 1990.Mondschein JS, Smith SA, Hammond JM, Smith SA, Hammond JM. (1990) Production of insulin-like growth factor binding proteins (IGFBPs) by porcine granulosa cells: identification of IGFBP-2 and -3 and regulation by hormones and growth factors. Endocrinology 127:2298–2306 [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez and Dorshkind, 1997.Montecino-Rodriguez E, Dorshkind K. (1997) Thymocyte development in vitro: implications for studies of ageing and thymic involution. Mech Ageing Dev 93:47–57 [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez et al., 1998.Montecino-Rodriguez E, Clark R, Dorshkind K. (1998) Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology 139:4120–4126 [DOI] [PubMed] [Google Scholar]
- Moralez et al., 2005.Moralez AM, Maile LA, Clarke J, Busby WH, Jr, Clemmons DR. (2005) Insulin-like growth factor binding protein-5 (IGFBP-5) interacts with thrombospondin-1 to induce negative regulatory effects on IGF-1 actions. J Cell Physiol 203:328–334 [DOI] [PubMed] [Google Scholar]
- Moro et al., 1998.Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. (1998) Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J 17:6622–6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill et al., 2007.Mulvihill MJ, Ji QS, Werner D, Beck P, Cesario C, Cooke A, Cox M, Crew A, Dong H, Feng L, et al. (2007) 1,3-Disubstituted-imidazo[1,5-a]pyrazines as insulin-like growth-factor-I receptor (IGF-IR) inhibitors. Bioorg Med Chem Lett 17:1091–1097 [DOI] [PubMed] [Google Scholar]
- Murphy, 2006.Murphy LJ. (2006) Insulin-like growth factor-I: a treatment for type 2 diabetes revisited. Endocrinology 147:2616–2618 [DOI] [PubMed] [Google Scholar]
- Murphy et al., 1992a.Murphy WJ, Durum SK, Longo DL. (1992a) Human growth hormone promotes engraftment of murine or human T cells in severe combined immunodeficient mice. Proc Natl Acad Sci USA 89:4481–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy et al., 1992b.Murphy WJ, Durum SK, Longo DL. (1992b) Role of neuroendocrine hormones in murine T cell development. Growth hormone exerts thymopoietic effects in vivo. J Immunol 149:3851–3857 [PubMed] [Google Scholar]
- Murphy et al., 1993.Murphy WJ, Durum SK, Longo DL. (1993) Differential effects of growth hormone and prolactin on murine T cell development and function. J Exp Med 178:231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy et al., 1992c.Murphy WJ, Tsarfaty G, Longo DL. (1992c) Growth hormone exerts hematopoietic growth-promoting effects in vivo and partially counteracts the myelosuppressive effects of azidothymidine. Blood 80:1443–1447 [PubMed] [Google Scholar]
- Myers et al., 1993.Myers SE, Cheung PT, Handwerger S, Chernausek SD. (1993) Insulin-like growth factor-I (IGF-I) enhanced proteolysis of IGF-binding protein-4 in conditioned medium from primary cultures of human decidua: independence from IGF receptor binding. Endocrinology 133:1525–1531 [DOI] [PubMed] [Google Scholar]
- Nagaoka et al., 1990.Nagaoka I, Trapnell BC, Crystal RG. (1990) Regulation of insulin-like growth factor-I gene expression in the human macrophage-like cell line U937. J Clin Invest 85:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano et al., 2002.Napolitano LA, Lo JC, Gotway MB, Mulligan K, Barbour JD, Schmidt D, Grant RM, Halvorsen RA, Schambelan M, McCune JM. (2002) Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS 16:1103–1111 [DOI] [PubMed] [Google Scholar]
- Napolitano et al., 2008.Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert EL, Ng MM, Clor JL, Epling L, Sinclair E, Baum PD, et al. (2008) Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest 118:1085–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely et al., 1991.Neely EK, Smith SD, Rosenfeld RG. (1991) Human leukemic T and B lymphoblasts produce insulin-like growth factor binding proteins 2 and 4. Acta Endocrinol (Copenh) 124:707–714 [DOI] [PubMed] [Google Scholar]
- Neidel et al., 1997.Neidel J, Blum WF, Schaeffer HJ, Schulze M, Schönau E, Lindschau J, Föll J. (1997) Elevated levels of insulin-like growth factor (IGF) binding protein-3 in rheumatoid arthritis synovial fluid inhibit stimulation by IGF-I of articular chondrocyte proteoglycan synthesis. Rheumatol Int 17:29–37 [DOI] [PubMed] [Google Scholar]
- Nguyen et al., 1998.Nguyen BY, Clerici M, Venzon DJ, Bauza S, Murphy WJ, Longo DL, Baseler M, Gesundheit N, Broder S, Shearer G, et al. (1998) Pilot study of the immunologic effects of recombinant human growth hormone and recombinant insulin-like growth factor in HIV-infected patients. AIDS 12:895–904 [DOI] [PubMed] [Google Scholar]
- Noble et al., 1993.Noble PW, Lake FR, Henson PM, Riches DW. (1993) Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-α-dependent mechanism in murine macrophages. J Clin Invest 91:2368–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor et al., 2008.O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. (2008) Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 252:91–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh et al., 1993.Oh Y, Müller HL, Lamson G, Rosenfeld RG. (1993) Insulin-like growth factor (IGF)-independent action of IGF-binding protein-3 in Hs578T human breast cancer cells. Cell surface binding and growth inhibition. J Biol Chem 268:14964–14971 [PubMed] [Google Scholar]
- Oliver et al., 2004.Oliver SE, Barrass B, Gunnell DJ, Donovan JL, Peters TJ, Persad RA, Gillatt D, Neal DE, Hamdy FC, Holly JM. (2004) Serum insulin-like growth factor-I is positively associated with serum prostate-specific antigen in middle-aged men without evidence of prostate cancer. Cancer Epidemiol Biomarkers Prev 13:163–165 [DOI] [PubMed] [Google Scholar]
- Olmos et al., 2007.Olmos D, Molife R, Okuno S, Worden F, Hammer G, Yap TA, Shaw H, Schuetze S, Roberts L, Gualberto A, de-Bono J, et al. (2007) Safety tolerability and preliminary efficacy of the anti-IGF-IR monoclonal antibody CP-751,871 in patients with sarcomas and adrenocortical tumors, in 19th Annual AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 22–26 Oct 2007; San Francisco, CA Poster A63 [Google Scholar]
- Oster et al., 1995.Oster MH, Fielder PJ, Levin N, Cronin MJ. (1995) Adaptation of the growth hormone and insulin-like growth factor-I axis to chronic and severe calorie or protein malnutrition. J Clin Invest 95:2258–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini et al., 1999.Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, Belfiore A. (1999) Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res 5:1935–1944 [PubMed] [Google Scholar]
- Park et al., 2000a.Park ES, Kim H, Suh JM, Park SJ, Kwon OY, Kim YK, Ro HK, Cho BY, Chung J, Shong M. (2000a) Thyrotropin induces SOCS-1 (suppressor of cytokine signaling-1) and SOCS-3 in FRTL-5 thyroid cells. Mol Endocrinol 14:440–448 [DOI] [PubMed] [Google Scholar]
- Park et al., 2000b.Park ES, Kim H, Suh JM, Park SJ, You SH, Chung HK, Lee KW, Kwon OY, Cho BY, Kim YK, et al. (2000b) Involvement of JAK/STAT (Janus kinase/signal transducer and activator of transcription) in the thyrotropin signaling pathway. Mol Endocrinol 14:662–670 [DOI] [PubMed] [Google Scholar]
- Park et al., 2005.Park YJ, Kim TY, Lee SH, Kim H, Kim SW, Shong M, Yoon YK, Cho BY, Park DJ. (2005) p66Shc expression in proliferating thyroid cells is regulated by thyrotropin receptor signaling. Endocrinology 146:2473–2480 [DOI] [PubMed] [Google Scholar]
- Parker et al., 1998.Parker A, Rees C, Clarke J, Busby WH, Jr., Clemmons DR. (1998) Binding of insulin-like growth factor (IGF)-binding protein-5 to smooth-muscle cell extracellular matrix is a major determinant of the cellular response to IGF-I. Mol Biol Cell 9:2383–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker et al., 1996.Parker A, Clarke JB, Busby WH, Jr., Clemmons DR. (1996) Identification of the extracellular matrix binding sites for insulin-like growth factor-binding protein 5. J Biol Chem 271:13523–13529 [DOI] [PubMed] [Google Scholar]
- Parmentier et al., 1989.Parmentier M, Libert F, Maenhaut C, Lefort A, Gérard C, Perret J, Van Sande J, Dumont JE, Vassart G. (1989) Molecular cloning of the thyrotropin receptor. Science 246:1620–1622 [DOI] [PubMed] [Google Scholar]
- Parry et al., 1997.Parry RV, Reif K, Smith G, Sansom DM, Hemmings BA, Ward SG. (1997) Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur J Immunol 27:2495–2501 [DOI] [PubMed] [Google Scholar]
- Pennisi et al., 2006.Pennisi P, Gavrilova O, Setser-Portas J, Jou W, Santopietro S, Clemmons D, Yakar S, LeRoith D. (2006) Recombinant human insulin-like growth factor-I treatment inhibits gluconeogenesis in a transgenic mouse model of type 2 diabetes mellitus. Endocrinology 147:2619–2630 [DOI] [PubMed] [Google Scholar]
- Pollak, 2008.Pollak M. (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8:915–928 [DOI] [PubMed] [Google Scholar]
- Pollak et al., 2007.Pollak MN, Lacy MQ, Lipton A, Demers L, Leitzel K, de Bono JS, Yin D, Roberts L, Sharma A, Gualberto A.(2007) Pharmacodynamic properties of the anti-IGF-IR monoclonal antibody CP-751,871 in cancer patients. J Clin Oncol 25 (Suppl 18S):3587 [Google Scholar]
- Pollak et al., 2004.Pollak MN, Schernhammer ES, Hankinson SE. (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4:505–518 [DOI] [PubMed] [Google Scholar]
- Poulin et al., 1999.Poulin JF, Viswanathan MN, Harris JM, Komanduri KV, Wieder E, Ringuette N, Jenkins M, McCune JM, Sékaly RP. (1999) Direct evidence for thymic function in adult humans. J Exp Med 190:479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar et al., 2003.Prabhakar BS, Bahn RS, Smith TJ. (2003) Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev 24:802–835 [DOI] [PubMed] [Google Scholar]
- Pritchard et al., 2002.Pritchard J, Horst N, Cruikshank W, Smith TJ. (2002) Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol 168:942–950 [DOI] [PubMed] [Google Scholar]
- Pritchard et al., 2003.Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. (2003) Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor-I receptor pathway. J Immunol 170:6348–6354 [DOI] [PubMed] [Google Scholar]
- Pritchard et al., 2004.Pritchard J, Tsui S, Horst N, Cruikshank WW, Smith TJ. (2004) Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves' disease, express high levels of IL-16 when treated with Igs against insulin-like growth factor-1 receptor. J Immunol 173:3564–3569 [DOI] [PubMed] [Google Scholar]
- Prummel et al., 2004.Prummel MF, Strieder T, Wiersinga WM. (2004) The environment and autoimmune thyroid diseases. Eur J Endocrinol 150:605–618 [DOI] [PubMed] [Google Scholar]
- Pucilowska et al., 2000.Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, Lund PK. (2000) IGF-I and procollagen α1(I) are coexpressed in a subset of mesenchymal cells in active Crohn's disease. Am J Physiol Gastrointest Liver Physiol 279:G1307–G1322 [DOI] [PubMed] [Google Scholar]
- Raine, 1984.Raine CS. (1984) Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest 50:608–635 [PubMed] [Google Scholar]
- Rajaram et al., 1997.Rajaram S, Baylink DJ, Mohan S. (1997) Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev 18:801–831 [DOI] [PubMed] [Google Scholar]
- Reiss et al., 2001.Reiss K, Tu X, Romano G, Peruzzi F, Wang JY, Baserga R. (2001) Intracellular association of a mutant insulin-like growth factor receptor with endogenous receptors. Clin Cancer Res 7:2134–2144 [PubMed] [Google Scholar]
- Resnicoff and Baserga, 1998.Resnicoff M, Baserga R. (1998) The role of insulin-like growth factor-I receptor in transformation and apoptosis. Ann NY Acad Sci 842:76–81 [DOI] [PubMed] [Google Scholar]
- Ricort et al., 2002.Ricort JM, Lombet A, Lassarre C, Binoux M. (2002) Insulin-like growth factor binding protein-3 increases intracellular calcium concentrations in MCF-7 breast carcinoma cells. FEBS Lett 527:293–297 [DOI] [PubMed] [Google Scholar]
- Ricort and Binoux, 2002.Ricort JM, Binoux M. (2002) Insulin-like growth factor-binding protein-3 activates a phosphotyrosine phosphatase. Effects on the insulin-like growth factor signaling pathway. J Biol Chem 277:19448–19454 [DOI] [PubMed] [Google Scholar]
- Ricort and Binoux, 2001.Ricort JM, Binoux M. (2001) Insulin-like growth factor (IGF) binding protein-3 inhibits type 1 IGF receptor activation independently of its IGF binding affinity. Endocrinology 142:108–113 [DOI] [PubMed] [Google Scholar]
- Rinderknecht and Humbel, 1978.Rinderknecht E, Humbel RE. (1978) The amino acid sequence of human insulin-like growth factor-I and its structural homology with proinsulin. J Biol Chem 253:2769–2776 [PubMed] [Google Scholar]
- Rivas-Santiago et al., 2008.Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, Torres M, Sada E. (2008) Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun 76:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins et al., 1994.Robbins K, McCabe S, Scheiner T, Strasser J, Clark R, Jardieu P. (1994) Immunological effects of insulin-like growth factor-I—enhancement of immunoglobulin synthesis. Clin Exp Immunol 95:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom et al., 1988.Rom WN, Basset P, Fells GA, Nukiwa T, Trapnell BC, Crysal RG. (1988) Alveolar macrophages release an insulin-like growth factor-I-type molecule. J Clin Invest 82:1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom and Pääkkö, 1991.Rom WN, Pääkkö P. (1991) Activated alveolar macrophages express the insulin-like growth factor-I receptor. Am J Respir Cell Mol Biol 4:432–439 [DOI] [PubMed] [Google Scholar]
- Rondon et al., 2007.Rondon J, Patnaik A, Stein M, Tolcher A, Ng C, Dias C, Greig G, Frankel SR, Kurzrock R, Rubin E. (2007) A phase I study of q3W R1507, a human monoclonal antibody IGF-IR (insulin-like growth factor receptor) antagonist in patients with advanced solid tumors, in 19th Annual AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 22–26 Oct 2007; San Francisco, CA Poster A77 [Google Scholar]
- Rosen and Greenberg, 1996.Rosen LB, Greenberg ME. (1996) Stimulation of growth factor receptor signal transduction by activation of voltage-sensitive calcium channels. Proc Natl Acad Sci USA 93:1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld et al., 2000.Rosenfeld RG, Hwa V, Wilson E, Plymate SR, Oh Y. (2000) The insulin-like growth factor-binding protein superfamily. Growth Horm IGF Res 10:S16–S17 [DOI] [PubMed] [Google Scholar]
- Rosette and Karin, 1996.Rosette C, Karin M. (1996) Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274:1194–1197 [DOI] [PubMed] [Google Scholar]
- Ross et al., 1989.Ross M, Francis GL, Szabo L, Wallace JC, Ballard FJ. (1989) Insulin-like growth factor (IGF)-binding proteins inhibit the biological activities of IGF-1 and IGF-2 but not des-(1–3)-IGF-1. Biochem J 258:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem-Yehudar et al., 2001.Rotem-Yehudar R, Galperin E, Horowitz M. (2001) Association of insulin-like growth factor 1 receptor with EHD1 and SNAP29. J Biol Chem 276:33054–33060 [DOI] [PubMed] [Google Scholar]
- Roth et al., 1984.Roth RA, Mesirow ML, Yokono K, Baba S. (1984) Degradation of insulin-like growth factors I and II by a human insulin degrading enzyme. Endocr Res 10:101–112 [DOI] [PubMed] [Google Scholar]
- Rothe et al., 1988.Rothe MJ, Altman RD, Falanga V. (1988) Plasma somatomedin-C levels in systemic sclerosis. Br J Dermatol 119:639–642 [DOI] [PubMed] [Google Scholar]
- Rothenberg et al., 2007.Rothenberg ML, Poplin E, Sander AB, Rubin EH, Fox F, Schwartz J, Vermeulen, Youssoufian H. (2007) Phase I dose-escalation study of the anti-IGF-IR recombinant human IgG1 monoclonal antibody (Mab) IMC-A12, administered every other week to patients with advanced solid tumors, in 19th Annual AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 22–26 Oct 2007; San Francisco, CA p C84 [Google Scholar]
- Roudabush et al., 2000.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. (2000) Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem 275:22583–22589 [DOI] [PubMed] [Google Scholar]
- Rowinsky et al., 2007.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. (2007) IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor-I receptor. Clin Cancer Res 13:5549s–5555s [DOI] [PubMed] [Google Scholar]
- Sachdev et al., 2006.Sachdev D, Singh R, Fujita-Yamaguchi Y, Yee D. (2006) Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: implications for anti-insulin-like growth factor therapy in breast cancer. Cancer Res 66:2391–2402 [DOI] [PubMed] [Google Scholar]
- Salatino et al., 2004.Salatino M, Schillaci R, Proietti CJ, Carnevale R, Frahm I, Molinolo AA, Iribarren A, Charreau EH, Elizalde PV. (2004) Inhibition of in vivo breast cancer growth by antisense oligodeoxynucleotides to type I insulin-like growth factor receptor mRNA involves inactivation of ErbBs, PI-3K/Akt and p42/p44 MAPK signaling pathways but not modulation of progesterone receptor activity. Oncogene 23:5161–5174 [DOI] [PubMed] [Google Scholar]
- Salcini et al., 1997.Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, Di Fiore PP. (1997) Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev 11:2239–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury and Macaulay, 2003.Salisbury AJ, Macaulay VM. (2003) Development of molecular agents for IGF receptor targeting. Horm Metab Res 35:843–849 [DOI] [PubMed] [Google Scholar]
- Samani et al., 2007.Samani AA, Yakar S, LeRoith D, Brodt P. (2007) The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28:20–47 [DOI] [PubMed] [Google Scholar]
- Savino et al., 2002.Savino W, Postel-Vinay MC, Smaniotto S, Dardenne M. (2002) The thymus gland: a target organ for growth hormone. Scand J Immunol 55:442–452 [DOI] [PubMed] [Google Scholar]
- Schalkwijk et al., 1989.Schalkwijk J, Joosten LA, van den Berg WB, van Wyk JJ, van de Putte LB. (1989) Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum 32:66–71 [DOI] [PubMed] [Google Scholar]
- Schall et al., 1990.Schall TJ, Bacon K, Toy KJ, Goeddel DV. (1990) Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 347:669–671 [DOI] [PubMed] [Google Scholar]
- Schillaci et al., 1998.Schillaci R, Brocardo MG, Galeano A, Roldán A. (1998) Downregulation of insulin-like growth factor-1 receptor (IGF-1R) expression in human T lymphocyte activation. Cell Immunol 183:157–161 [DOI] [PubMed] [Google Scholar]
- Schimpff et al., 1983.Schimpff RM, Repellin AM, Salvatoni A, Thieriot-Prevost G, Chatelain P. (1983) Effect of purified somatomedins on thymidine incorporation into lectin-activated human lymphocytes. Acta Endocrinol (Copenh) 102:21–26 [DOI] [PubMed] [Google Scholar]
- Schmid et al., 1998.Schmid SL, McNiven MA, De Camilli P. (1998) Dynamin and its partners: a progress report. Curr Opin Cell Biol 10:504–512 [DOI] [PubMed] [Google Scholar]
- Schnitzer et al., 2006.Schnitzer T, Keuenkele KP, Rebers F, Van Vugt M, Klein C, Lanzendoerfer M, Mundigl O, Parren PW, van de Winkel JG, Schumacher R. (2006) Characterization of a recombinant, fully human monoclonal antibody directed against the human insulin-like growth factor-1 receptor. Eur J Cancer 42 (Suppl):66–67 [Google Scholar]
- Schönland et al., 2003.Schönland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, Weyand CM. (2003) Homeostatic control of T-cell generation in neonates. Blood 102:1428–1434 [DOI] [PubMed] [Google Scholar]
- Sciaky et al., 2000.Sciaky D, Brazer W, Center DM, Cruikshank WW, Smith TJ. (2000) Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J Immunol 164:3806–3814 [DOI] [PubMed] [Google Scholar]
- Segretin et al., 2003.Segretin ME, Galeano A, Roldán A, Schillaci R. (2003) Insulin-like growth factor-1 receptor regulation in activated human T lymphocytes. Horm Res 59:276–280 [DOI] [PubMed] [Google Scholar]
- Shakibaei et al., 1999.Shakibaei M, John T, De Souza P, Rahmanzadeh R, Merker HJ. (1999) Signal transduction by β1 integrin receptors in human chondrocytes in vitro: collaboration with the insulin-like growth factor-I receptor. Biochem J 342:615–623 [PMC free article] [PubMed] [Google Scholar]
- Shawver et al., 2002.Shawver LK, Slamon D, Ullrich A. (2002) Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell 1:117–123 [DOI] [PubMed] [Google Scholar]
- Siddle et al., 1994.Siddle K, Soos MA, Field CE, Navé BT. (1994) Hybrid and atypical insulin/insulin-like growth factor-I receptors. Horm Res 41 (Suppl 2):56–65 [DOI] [PubMed] [Google Scholar]
- Simchen et al., 2000.Simchen C, Lehmann I, Sittig D, Steinert M, Aust G. (2000) Expression and regulation of regulated on activation, normal T cells expressed and secreted in thyroid tissue of patients with Graves' disease and thyroid autonomy and in thyroid-derived cell populations. J Clin Endocrinol Metab 85:4758–4764 [DOI] [PubMed] [Google Scholar]
- Skjaerbaek et al., 1998.Skjaerbaek C, Frystyk J, Orskov H, Kissmeyer-Nielsen P, Jensen MB, Laurberg S, Møller N, Flyvbjerg A. (1998) Differential changes in free and total insulin-like growth factor-I after major, elective abdominal surgery: the possible role of insulin-like growth factor-binding protein-3 proteolysis. J Clin Endocrinol Metab 83:2445–2449 [DOI] [PubMed] [Google Scholar]
- Slonim et al., 2000.Slonim AE, Bulone L, Damore MB, Goldberg T, Wingertzahn MA, McKinley MJ. (2000) A preliminary study of growth hormone therapy for Crohn's disease. N Engl J Med 342:1633–1637 [DOI] [PubMed] [Google Scholar]
- Smith and Hoa, 2004.Smith TJ, Hoa N. (2004) Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab 89:5076–5080 [DOI] [PubMed] [Google Scholar]
- Smith et al., 2002.Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A. (2002) Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 87:385–392 [DOI] [PubMed] [Google Scholar]
- Smith et al., 1995.Smith TJ, Sempowski GD, Wang HS, Del Vecchio PJ, Lippe SD, Phipps RP. (1995) Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab 80:2620–2625 [DOI] [PubMed] [Google Scholar]
- Snow et al., 1980.Snow EC, Feldbush TL, Oaks JA. (1980) The role of insulin in the response of murine T lymphocytes to mitogenic stimulation in vitro. J Immunol 124:739–744 [PubMed] [Google Scholar]
- Soon et al., 1999.Soon L, Flechner L, Gutkind JS, Wang LH, Baserga R, Pierce JH, Li W. (1999) Insulin-like growth factor-I synergizes with interleukin 4 for hematopoietic cell proliferation independent of insulin receptor substrate expression. Mol Cell Biol 19:3816–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen et al., 2004.Sørensen H, Whittaker L, Hinrichsen J, Groth A, Whittaker J. (2004) Mapping of the insulin-like growth factor-II binding site of the Type I insulin-like growth factor receptor by alanine scanning mutagenesis. FEBS Lett 565:19–22 [DOI] [PubMed] [Google Scholar]
- Sørensen et al., 2003.Sørensen OE, Cowland JB, Theilgaard-Mönch K, Liu L, Ganz T, Borregaard N. (2003) Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol 170:5583–5589 [DOI] [PubMed] [Google Scholar]
- Sparrow et al., 1997.Sparrow LG, McKern NM, Gorman JJ, Strike PM, Robinson CP, Bentley JD, Ward CW. (1997) The disulfide bonds in the C-terminal domains of the human insulin receptor ectodomain. J Biol Chem 272:29460–29467 [DOI] [PubMed] [Google Scholar]
- Street et al., 2003.Street ME, de'Angelis G, Camacho-Hübner C, Giovannelli G, Ziveri MA, Bacchini PL, Bernasconi S, Sansebastiano G, Savage MO. (2003) Relationships between serum IGF-1, IGFBP-2, interleukin-1beta and interleukin-6 in inflammatory bowel disease. Horm Res 61:159–164 [DOI] [PubMed] [Google Scholar]
- Strömberg et al., 2006.Strömberg T, Ekman S, Girnita L, Dimberg LY, Larsson O, Axelson M, Lennartsson J, Hellman U, Carlson K, Osterborg A, et al. (2006) IGF-1 receptor tyrosine kinase inhibition by the cyclolignan PPP induces G2/M-phase accumulation and apoptosis in multiple myeloma cells. Blood 107:669–678 [DOI] [PubMed] [Google Scholar]
- Stuart et al., 1991.Stuart CA, Meehan RT, Neale LS, Cintron NM, Furlanetto RW. (1991) Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab 72:1117–1122 [DOI] [PubMed] [Google Scholar]
- Taguchi et al., 2006.Taguchi T, Takenouchi H, Matsui J, Tang WR, Itagaki M, Shiozawa Y, Suzuki K, Sakaguchi S, Ktagiri YU, Takahashi T, et al. (2006) Involvement of insulin-like growth factor-I and insulin-like growth factor binding proteins in pro-B-cell development. Exp Hematol 34:508–518 [DOI] [PubMed] [Google Scholar]
- Tan, 1996.Tan EM. (1996) Pathophysiology of antinuclear antibodies in systemic lupus erythematosus and related diseases. Adv Dent Res 10:44–46 [DOI] [PubMed] [Google Scholar]
- Tapson et al., 1988.Tapson VF, Boni-Schnetzler M, Pilch PF, Center DM, Berman JS. (1988) Structural and functional characterization of the human T lymphocyte receptor for insulin-like growth factor-I in vitro. J Clin Invest 82:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub et al., 1994.Taub DD, Tsarfaty G, Lloyd AR, Durum SK, Longo DL, Murphy WJ. (1994) Growth hormone promotes human T cell adhesion and migration to both human and murine matrix proteins in vitro and directly promotes xenogeneic engraftment. J Clin Invest 94:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzari et al., 2007.Tazzari PL, Tabellini G, Bortul R, Papa V, Evangelisti C, Grafone T, Martinelli G, McCubrey JA, Martelli AM. (2007) The insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin-like growth factor-I secretion. Leukemia 21:886–896 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 1993.Thomas AG, Holly JM, Taylor F, Miller V. (1993) Insulin like growth factor-I, insulin like growth factor binding protein-1, and insulin in childhood Crohn's disease. Gut 34:944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian et al., 1998.Tian Z, Woody MA, Sun R, Welniak LA, Raziuddin A, Funakoshi S, Tsarfaty G, Longo DL, Murphy WJ. (1998) Recombinant human growth hormone promotes hematopoietic reconstitution after syngeneic bone marrow transplantation in mice. Stem Cells 16:193–199 [DOI] [PubMed] [Google Scholar]
- Timmins et al., 1996.Timmins AC, Cotterill AM, Hughes SC, Holly JM, Ross RJ, Blum W, Hinds CJ. (1996) Critical illness is associated with low circulating concentrations of insulin-like growth factors-I and -II, alterations in insulin-like growth factor binding proteins, and induction of an insulin-like growth factor binding protein 3 protease. Crit Care Med 24:1460–1466 [DOI] [PubMed] [Google Scholar]
- Timsit et al., 1992.Timsit J, Savino W, Safieh B, Chanson P, Gagnerault MC, Bach JF, Dardenne M. (1992) Growth hormone and insulin-like growth factor-I stimulate hormonal function and proliferation of thymic epithelial cells. J Clin Endocrinol Metab 75:183–188 [DOI] [PubMed] [Google Scholar]
- Tincani et al., 2007.Tincani A, Andreoli L, Bazzani C, Bosiso D, Sozzani S. (2007) Inflammatory molecules: a target for treatment of systemic autoimmune diseases. Autoimmun Rev 7:1–7 [DOI] [PubMed] [Google Scholar]
- Todd et al., 2007.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, et al. (2007) Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toretsky et al., 1997.Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. (1997) The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem 272:30822–30827 [DOI] [PubMed] [Google Scholar]
- Tramontano et al., 1986.Tramontano D, Cushing GW, Moses AC, Ingbar SH. (1986) Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology 119:940–942 [DOI] [PubMed] [Google Scholar]
- Tramontano et al., 1987.Tramontano D, Moses AC, Picone R, Ingbar SH. (1987) Characterization and regulations of the receptor for insulin-like growth factor-I in the FRTL-5 rat thyroid follicular cell line. Endocrinology 120:785–790 [DOI] [PubMed] [Google Scholar]
- Tramontano et al., 1988a.Tramontano D, Moses AC, Ingbar SH. (1988a) The role of adenosine 3′5′-monophosphate in the regulation of receptors for thyrotropin and insulin-like growth factor-I in the FRTL5 rat thyroid follicular cell. Endocrinology 122:133–136 [DOI] [PubMed] [Google Scholar]
- Tramontano et al., 1988b.Tramontano D, Moses AC, Veneziani BM, Ingbar SH. (1988b) Adenosine 3′5′-monophosphate mediates both the mitogenic effect of thyrotropin and its ability to amplify the response to insulin-like growth factor-I in FRTL5 cells. Endocrinology 122:127–132 [DOI] [PubMed] [Google Scholar]
- Tsarfaty et al., 1994.Tsarfaty G, Longo DL, Murphy WJ. (1994) Human insulin-like growth factor-I exerts hematopoietic growth-promoting effects after in vivo administration. Exp Hematol 22:1273–1277 [PubMed] [Google Scholar]
- Tsui et al., 2008.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. (2008) Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol 181:4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu et al., 2000.Tu W, Cheung PT, Lau YL. (2000) Insulin-like growth factor 1 promotes cord blood T cell maturation and inhibits its spontaneous and phytohemagglutinin-induced apoptosis through different mechanisms. J Immunol 165:1331–1336 [DOI] [PubMed] [Google Scholar]
- Twigg and Baxter, 1998.Twigg SM, Baxter RC. (1998) Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J Biol Chem 273:6074–6079 [DOI] [PubMed] [Google Scholar]
- Uh et al., 1998.Uh ST, Inoue Y, King TE, Jr., Chan ED, Newman LS, Riches DW. (1998) Morphometric analysis of insulin-like growth factor-I localization in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 158:1626–1635 [DOI] [PubMed] [Google Scholar]
- Vaira et al., 2007.Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. (2007) Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene 26:2678–2684 [DOI] [PubMed] [Google Scholar]
- Van Schravendijk et al., 1987.Van Schravendijk CF, Foriers A, Van den Brande JL, Pipeleers DG. (1987) Evidence for the presence of type I insulin-like growth factor receptors on rat pancreatic A and B cells. Endocrinology 121:1784–1788 [DOI] [PubMed] [Google Scholar]
- Vella et al., 2008.Vella A, Bouatia-Naji N, Heude B, Cooper JD, Lowe CE, Petry C, Ring SM, Dunger DB, Todd JA, Ong KK. (2008) Association analysis of the IGF1 gene with childhood growth, IGF-1 concentrations and type 1 diabetes. Diabetologia 51:811–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure et al., 1996.Verschure PJ, Van Noorden CJ, Van Marle J, Van den Berg WB. (1996) Articular cartilage destruction in experimental inflammatory arthritis: insulin-like growth factor-1 regulation of proteoglycan metabolism in chondrocytes. Histochem J 28:835–857 [DOI] [PubMed] [Google Scholar]
- Vladutiu and Rose, 1971.Vladutiu AO, Rose NR. (1971) Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science 174:1137–1139 [DOI] [PubMed] [Google Scholar]
- Walker et al., 2002.Walker JL, Zhang L, Zhou J, Woolkalis MJ, Menko AS. (2002) Role for α6 integrin during lens development: evidence for signaling through IGF-1R and ERK. Dev Dyn 223:273–284 [DOI] [PubMed] [Google Scholar]
- Walsh and O'Connor, 2000.Walsh PT, O'Connor R. (2000) The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J Immunol 30:1010–1018 [DOI] [PubMed] [Google Scholar]
- Walsh et al., 2002.Walsh PT, Smith LM, O'Connor R. (2002) Insulin-like growth factor-1 activates Akt and Jun N-terminal kinases (JNKs) in promoting the survival of T lymphocytes. Immunology 107:461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Klein, 2001.Wang HC, Klein JR. (2001) Immune function of thyroid stimulating hormone and receptor. Crit Rev Immunol 21:323–337 [PubMed] [Google Scholar]
- Wang et al., 1993.Wang LM, Myers MG, Jr., Sun XJ, Aaronson SA, White M, Pierce JH. (1993) IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science 261:1591–1594 [DOI] [PubMed] [Google Scholar]
- Wang et al., 1999.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. (1999) Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 19:4008–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weightman et al., 1993.Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. (1993) Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity 16:251–257 [DOI] [PubMed] [Google Scholar]
- Werner et al., 1995.Werner H, Hernández-Sánchez C, Karnieli E, Leroith D. (1995) The regulation of IGF-I receptor gene expression. Int J Biochem Cell Biol 27:987–994 [DOI] [PubMed] [Google Scholar]
- Wetterau et al., 1999.Wetterau LA, Moore MG, Lee KW, Shim ML, Cohen P. (1999) Novel aspects of the insulin-like growth factor binding proteins. Mol Genet Metab 68:161–181 [DOI] [PubMed] [Google Scholar]
- White, 1997.White MF. (1997) The insulin signalling system and the IRS proteins. Diabetologia 40:S2–S17 [DOI] [PubMed] [Google Scholar]
- Whittaker et al., 2001.Whittaker J, Groth AV, Mynarcik DC, Pluzek L, Gadsbøll VL, Whittaker LJ. (2001) Alanine scanning mutagenesis of a type 1 insulin-like growth factor receptor ligand binding site. J Biol Chem 276:43980–43986 [DOI] [PubMed] [Google Scholar]
- Whitten et al., 2009.Whitten AE, Smith BJ, Menting JG, Margetts MB, McKern NM, Lovrecz GO, Adams TE, Richards K, Bentley JD, Trewhella J, et al. (2009) Solution structure of ectodomains of the insulin receptor family: the ectodomain of the type 1 insulin-like growth factor receptor displays asymmetry of ligand binding accompanied by limited conformational change. J Mol Biol 394:878–892 [DOI] [PubMed] [Google Scholar]
- Withers et al., 1999.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. (1999) Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 23:32–40 [DOI] [PubMed] [Google Scholar]
- Woodson et al., 2003.Woodson K, Tangrea JA, Pollak M, Copeland TD, Taylor PR, Virtamo J, Albanes D. (2003) Serum insulin-like growth factor-I: tumor marker or etiologic factor? A prospective study of prostate cancer among Finnish men. Cancer Res 63:3991–3994 [PubMed] [Google Scholar]
- Wu et al., 2003.Wu J, Haugk K, Plymate SR. (2003) Activation of pro-apoptotic p38-MAPK pathway in the prostate cancer cell line M12 expressing a truncated IGF-IR. Horm Metab Res 35:751–757 [DOI] [PubMed] [Google Scholar]
- Wynes et al., 2004.Wynes MW, Frankel SK, Riches DW. (2004) IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J Leukoc Biol 76:1019–1027 [DOI] [PubMed] [Google Scholar]
- Wynes and Riches, 2003.Wynes MW, Riches DW. (2003) Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. J Immunol 171:3550–3559 [DOI] [PubMed] [Google Scholar]
- Xavier and Podolsky, 2007.Xavier RJ, Podolsky DK. (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434 [DOI] [PubMed] [Google Scholar]
- Xu et al., 1995.Xu X, Mardell C, Xian CJ, Zola H, Read LC. (1995) Expression of functional insulin-like growth factor-1 receptor on lymphoid cell subsets of rats. Immunology 85:394–399 [PMC free article] [PubMed] [Google Scholar]
- Xuan et al., 2002.Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, et al. (2002) Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest 110:1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita et al., 1986.Yamashita S, Weiss M, Melmed S. (1986) Insulin-like growth factor-I regulates growth hormone secretion and messenger ribonucleic acid levels in human pituitary tumor cells. J Clin Endocrinol Metab 63:730–735 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2002.Yang Y, Niu J, Guo L. (2002) The effects of antisense insulin-like growth factor-I receptor oligonucleotide on human cord blood lymphocytes. J Mol Endocrinol 28:207–212 [DOI] [PubMed] [Google Scholar]
- Yao et al., 1995.Yao DL, Liu X, Hudson LD, Webster HD. (1995) Insulin-like growth factor-I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 92:6190–6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto and Murphy, 1995.Yamamoto H, Murphy LJ. (1995) N-terminal truncated insulin-like growth factor-I in human urine. J Clin Endocrinol Metab 80:1179–1183 [DOI] [PubMed] [Google Scholar]
- Zakarija et al., 1988.Zakarija M, Jin S, McKenzie JM. (1988) Evidence supporting the identity in Graves' disease of thyroid-stimulating antibody and thyroid growth-promoting immunoglobulin G as assayed in FRTL5 cells. J Clin Invest 81:879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavodovskaya et al., 2008.Zavodovskaya M, Campbell MJ, Maddux BA, Shiry L, Allan G, Hodges L, Kushner P, Kerner JA, Youngren JF, Goldfine ID. (2008) Nordihydroguaiaretic acid (NDGA), an inhibitor of the HER2 and IGF-1 receptor tyrosine kinases, blocks the growth of HER2-overexpressing human breast cancer cells. J Cell Biochem 103:624–635 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 1998.Zhang Y, Center DM, Wu DM, Cruikshank WW, Yuan J, Andrews DW, Kornfeld H. (1998) Processing and activation of pro-interleukin-16 by caspase-3. J Biol Chem 273:1144–1149 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 1997.Zhang Q, Tally M, Larsson O, Kennedy RT, Huang L, Hall K, Berggren PO. (1997) Insulin-like growth factor-II signaling through the insulin-like growth factor-II/mannose-6-phosphate receptor promotes exocytosis in insulin-secreting cells. Proc Natl Acad Sci USA 94:6232–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al., 2009.Zhao J, Harada N, Sobue K, Katsuya H, Okajima K. (2009) Insulin-like growth factor-I reduces stress-induced gastric mucosal injury by inhibiting neutrophil activation in mice. Growth Horm IGF Res 19:136–145 [DOI] [PubMed] [Google Scholar]
- Zimmermann et al., 2001.Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB. (2001) Insulin-like growth factor-I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol 280:G1022–G1029 [DOI] [PubMed] [Google Scholar]