Abstract
Heptahelical G protein-coupled receptors are the most diverse and therapeutically important family of receptors in the human genome. Ligand binding activates heterotrimeric G proteins that transmit intracellular signals by regulating effector enzymes or ion channels. G protein signaling is terminated, in large part, by arrestin binding, which uncouples the receptor and G protein and targets the receptor for internalization. It is clear, however, that heptahelical receptor signaling does not end with desensitization. Arrestins bind a host of catalytically active proteins and serve as ligand-regulated scaffolds that recruit protein and lipid kinase, phosphatase, phosphodiesterase, and ubiquitin ligase activity into the receptor-arrestin complex. Although many of these arrestin-bound effectors serve to modulate G protein signaling, degrading second messengers and regulating endocytosis and trafficking, other signals seem to extend beyond the receptor-arrestin complex to regulate such processes as protein translation and gene transcription. Although these findings have led to a re-envisioning of heptahelical receptor signaling, little is known about the physiological roles of arrestin-dependent signaling. In vivo, the duality of arrestin function makes it difficult to dissociate the consequences of arrestin-dependent desensitization from those that might be ascribed to arrestin-mediated signaling. Nonetheless, recent evidence generated using arrestin knockouts, G protein-uncoupled receptor mutants, and arrestin pathway-selective “biased agonists” is beginning to reveal that arrestin signaling plays important roles in the retina, central nervous system, cardiovascular system, bone remodeling, immune system, and cancer. Understanding the signaling roles of arrestins may foster the development of pathway-selective drugs that exploit these pathways for therapeutic benefit.
I. Introduction
The heptahelical G protein-coupled receptors (GPCRs1) are the largest and most diverse superfamily of cell surface receptors. Approximately 600 distinct genes encoding nonolfactory GPCRs make up greater than 1% of the human genome (Lander et al., 2001; Venter et al., 2001). With alternative splicing, as many as 1000 to 2000 discrete receptor proteins may be expressed. Such evolutionary diversity enables GPCRs to detect an extraordinary array of extracellular stimuli. GPCRs function in neurotransmission, neuroendocrine control of physiologic homeostasis and reproduction, and regulation of hemodynamics and intermediary metabolism, and they control the growth, proliferation, differentiation, and death of multiple cell types. It is estimated that more than half of all drugs in clinical use target GPCRs, acting either to mimic endogenous GPCR ligands, to block ligand access to the receptor, or to modulate ligand production (Flower, 1999).
Nearly all GCPRs function as ligand-activated guanine nucleotide exchange factors (GEFs) for heterotrimeric G proteins. Agonist binding stabilizes the receptor in an “active” conformation, in which it catalyzes GTP-for-GDP exchange on heterotrimeric G protein Gα subunits, promoting dissociation of the GTP-bound Gα subunit from the Gβγ subunit heterodimer. Once dissociated, free Gα-GTP and Gβγ subunits regulate the activity of enzymatic effectors, such as adenylyl cyclases, phospholipase C isoforms, and ion channels, generating small-molecule second messengers that control the activity of key enzymes involved in intermediary metabolism. Although this classic paradigm of GPCR signaling is sufficient to account for most of the rapid cellular responses to receptor activation, it is clear that GPCRs bind numerous other proteins that modify the specificity, selectivity, and time course of signaling by the basic GPCR-G protein-Effector module (Foord and Marshall, 1999; Devi, 2001; Angers et al., 2002; Brady and Limbird, 2002; El Far and Betz, 2002; Bockaert et al., 2003; Maudsley et al., 2005). These protein-protein interactions include the formation of GPCR dimers, the interaction of GPCRs with receptor activity-modifying proteins, and the binding of PDZ domain-containing and non-PDZ domain scaffold proteins to the intracellular loops and C termini of receptors. Such interactions modify GPCR pharmacology and trafficking, localize receptors to specific subcellular domains, limit signaling to predetermined pathways, and poise downstream effectors for efficient activation.
Given their importance as therapeutic targets, two relatively recent discoveries suggesting that GPCR signaling is far more complex than traditionally envisioned warrant particular scrutiny. The first is that GPCRs generate signals that are independent of their intrinsic GEF activity by “coupling” to adapter or scaffold proteins that link the receptor to novel, non-G protein-regulated effectors. The best characterized of these “G protein-independent” signaling networks involves the arrestins, a small family of GPCR-binding proteins originally discovered for their role in receptor desensitization (Ferguson, 2001; Luttrell, 2005). Arrestin-bound GPCRs are incapable of activating G proteins, leading to the concept that arrestin binding “switches” the receptor between two discrete signaling modes, G protein-dependent signals arising from the plasma membrane and arrestin-dependent signals beginning as the receptor desensitizes and enters the endocytic pathway. The second finding is that the G protein-dependent and arrestin-dependent functions of many GPCRs can be dissociated pharmacologically by ligands that exhibit functional selectivity or “bias” favoring one pathway or the other. Thus, differential coupling of receptors to G protein- and arrestin-based pathways is one of several mechanisms underlying the phenomenon of ligand-directed signaling (Kenakin, 2002, 2007; Maudsley et al., 2005; Violin and Lefkowitz, 2007; Gesty-Palmer and Luttrell, 2008). Whereas conventional GPCR agonists and antagonists are believed to activate or inhibit all aspects of signaling equally, “biased agonists” have the potential to change the signal output of a GPCR, thereby controlling not only the quantity but also the quality of efficacy.
Although the twin discoveries of arrestin-dependent GPCR signaling and arrestin pathway-selective biased agonism have fostered the vision of “designer drugs” that exploit ligand bias to maximize clinical effectiveness and minimize unwanted side effects, little is presently known about the scope of arrestin-dependent signaling and even less about its functional significance. Here, we will review the current literature regarding the signaling functions of arrestins and the evidence that arrestin signaling, as opposed to classic arrestin-dependent desensitization, has physiologic relevance in vivo.
II. The Duality of Arrestin Function
A. Arrestin-Dependent Desensitization and Endocytosis
Heterotrimeric G protein signaling is subject to negative regulation at multiple levels. Second messengers are metabolized by cyclic nucleotide phosphodiesterases, phosphatidylinositol phosphatases, diacylglycerol kinases, and the reuptake and extrusion of cytosolic calcium. The half-life of the active Gα-GTP complex is limited by the intrinsic GTPase activity of the Gα subunit and the extrinsic action of regulators of G protein signaling proteins, which function as GTPase activating proteins (Ross and Wilkie, 2000). Receptors undergo phosphorylation-dependent desensitization that limits the efficiency of receptor-G protein coupling (Freedman and Lefkowitz, 1996). Phosphorylation of specific residues within the intracellular domains of the receptor by second messenger-dependent protein kinases like protein kinase (PK) A and PKC causes heterologous desensitization, so called because it is independent of ligand occupancy. By contrast, homologous desensitization is both restricted to ligand-occupied receptors and dependent upon the binding of accessory proteins. G protein-coupled receptor kinases (GRKs) phosphorylate agonist-occupied receptors on serine or threonine residues within the C terminus or third intracellular loop, creating high-affinity binding sites for arrestins, which translocate from the cytosol to the plasma membrane to physically interdict receptor-G protein coupling (Stoffel et al., 1997; Ferguson, 2001).
There are seven known GRKs and four arrestins. GRK1 and -7 are confined to visual sensory tissue, whereas GRK2, -3, -5, and -6 are widely expressed (Stoffel et al., 1997). GRK4 is highly expressed only in the testis. GRK2 and -3 have C-terminal Gβγ subunit binding domains that recruit them to the plasma membrane upon G protein activation (Pitcher et al., 1995b). GRK4, -5, and -6 lack these domains and either localize constitutively to the plasma membrane (GRK4 and -6) or translocate by alternative means (GRK5). Arrestin1 (visual arrestin) and -4 (cone arrestin) have restricted tissue expression similar to GRK1 and -7, localizing primarily to visual sensory tissue. Like GRK2, -3, -5, and -6, the two nonvisual arrestins, arrestin2 (β-arrestin1) and 3 (β-arrestin2), are ubiquitously expressed and interact with the vast majority of G protein-coupled receptors (Ferguson, 2001). Arrestins bind directly to GRK-phosphorylated GPCRs, forming a stoichiometric complex that is precluded from further G protein coupling. A polar core located in the hinge region between the two globular domains of the arrestin interacts with GRK-phosphorylated residues on the receptor tail, displacing the arrestin C terminus and exposing the concave surface of the globular domains to interact with the receptor (Gurevich and Gurevich, 2006). Receptor binding produces significant conformational changes in the arrestin (Shukla et al., 2008), whereas, conversely, arrestin binding stabilizes a high-agonist affinity state of the receptor, prompting some authors to characterize the receptor-arrestin complex as an “alternative ternary complex” analogous to the ternary complex existing between agonist-receptor-G protein in the absence of GTP (Gurevich et al., 1997).
The two nonvisual arrestins, arrestin2 and -3, further dampen G protein signaling by linking receptors to the clathrin-dependent endocytic machinery (Stoffel et al., 1997; Ferguson, 2001). The arrestin C terminus that is displaced upon engagement of the receptor directly binds clathrin heavy chain and the β2 adaptin subunit of the AP-2 complex (Goodman et al., 1996; Krupnick et al., 1997; Laporte et al., 1999, 2000). Clathrin/AP-2 binding causes arrestin-bound receptors to cluster in clathrin-coated pits, which are pinched off the plasma membrane by the motor protein, dynamin. This arrestin-dependent endocytosis, or sequestration, removes receptors from the cell surface, rendering it less responsive to subsequent stimuli. Most GPCRs fall into one of two classes based on their affinity for the two nonvisual arrestin isoforms, and the longevity of the receptor-arrestin interaction (Oakley et al., 2000). One class exhibits higher affinity for arrestin3 than arrestin2 and forms transient receptor-arrestin complexes that dissociate soon after the receptor internalizes. These receptors (e.g., the β2 and α1B adrenergic) tend to be rapidly resensitized and recycled back to the plasma membrane. The other class exhibits equivalent affinities for arrestin2 and -3 and forms more stable receptor-arrestin complexes that remain intact as the receptor undergoes endosomal sorting. These receptors (e.g., angiotensin AT1A and vasopressin V2) are sequestered in endosomes and tend to recycle slowly or undergo degradation.
Arrestins have also been shown to be involved in the endocytosis of certain non-GPCRs, suggesting that they play generalized roles as adapters in clathrin-mediated endocytosis. Wnts are secreted glycoproteins involved in embryologic patterning and development. They bind to seven membrane-spanning receptors called Frizzleds, which cluster within the GPCR superfamily but do not signal via heterotrimeric G proteins (Fredriksson et al., 2003). Frizzleds recruit cytosolic proteins called dishevelleds, and in Drosophila melanogaster, the complex mediates the endocytosis and lysosomal degradation of Wnt protein, Wingless, a key step in establishing morphogen gradients during D. melanogaster development (Dubois et al., 2001). Arrestin3 binds to disheveled 2, and in a heterologous expression system in HEK293 cells, Wnt5A-stimulated endocytosis of Frizzled4 is dependent upon both arrestin2 and disheveled 2 (Chen et al., 2003). The insulin and insulin-like growth factor type 1 receptors also interact with arrestins (Hupfeld and Olefsky, 2007). Clathrin-mediated endocytosis of insulin-like growth factor type 1 receptors involves both GRKs and arrestin2, and both Gβγ subunits and arrestin2 play roles in insulin-like growth factor type 1 stimulated extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation and mitogenesis (Luttrell et al., 1995; Lin et al., 1998; Dalle et al., 2001).
B. Arrestins as Signal Transducers
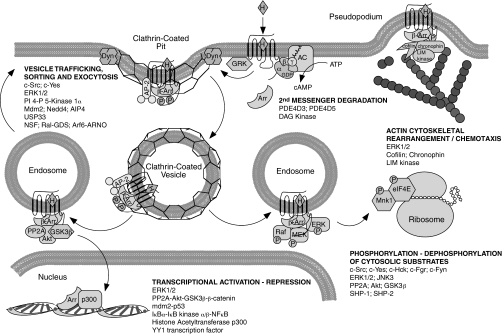
Unlike the catalytic GPCR-G protein interaction, arrestin-bound GPCRs exist in a relatively stable complex that persists on a time scale of minutes to hours (Charest et al., 2005; Pfleger et al., 2006). It was the discovery that arrestins serve as adapters not only in the context of GPCR sequestration but also in linking activated GPCRs to other enzymatic effectors that prompted a re-envisioning of GPCR signal transduction (Miller and Lefkowitz, 2001; Perry and Lefkowitz, 2002; Maudsley et al., 2005; Shenoy and Lefkowitz, 2005a; Gesty-Palmer and Luttrell, 2008). It is now clear that a number of catalytically active proteins bind arrestins and are recruited to agonist-occupied GPCRs, among them Src family tyrosine kinases (Luttrell et al., 1999; Barlic et al., 2000; DeFea et al., 2000a), components of the ERK1/2 and c-Jun N-terminal kinase 3 (JNK3) mitogen-activated protein (MAP) kinase cascades (DeFea et al., 2000b; McDonald et al., 2000; Luttrell et al., 2001), the E3 ubiquitin ligase, Mdm2 (Shenoy et al., 2001), the cAMP phosphodiesterases (PDE), PDE4D3/5 (Perry et al., 2002), diacylglycerol kinase (Nelson et al., 2007), the inhibitor of nuclear factor (NF)κB, IκBα (Witherow et al., 2004), the Ral-GDP dissociation stimulator (GDS), Ral-GDS (Bhattacharya et al., 2002), and the Ser/Thr protein phosphatase (PP)2A (Beaulieu et al., 2005). It is via these interactions that arrestin-binding confers unique signaling properties upon agonist-occupied GPCRs, opening up a broad realm of previously unappreciated GPCR signal transduction (Fig. 1).
Fig. 1.
The pleuridimensionality of G protein-coupled receptor signaling. A, classic GPCR signaling arises from heterotrimeric G protein-dependent activation of membrane-delimited effectors [e.g., adenylyl cyclase (AC), phospholipase Cβ isoforms (PLCβ), and ion channels] that generate intracellular second messengers. In the current model, arrestins function as ligand-regulated scaffolds, linking GPCRs to nontraditional effector pathways [e.g., nonreceptor tyrosine kinases (TK), MAP kinases (MAPK), and E3 ubiquitin ligases]. Because arrestin binding precludes further heterotrimeric G protein coupling, these two signaling “states” of the receptor are mutually exclusive. B, G protein-dependent signaling is characterized by rapid onset followed by waning intensity, reflecting progressive desensitization as a result of receptor phosphorylation by second messenger-dependent protein kinases and GRK-dependent arrestin binding. In contrast, arrestin-mediated signals are of somewhat slower onset and often sustained in duration. Unlike G protein signaling, arrestin-dependent signals originate within stoichiometric complexes of receptors, arrestins, and effectors, often termed “signalsomes.”
Because arrestin binding uncouples receptor and G protein, the transmission of G protein-dependent and arrestin-dependent signals should be mutually exclusive, at least at the level of the individual receptor. In effect, arrestin binding should “switch” the GPCR between two temporally discrete signaling modes. Indeed, comparison of the time course of ERK1/2 activation resulting from G protein signaling and from the arrestin-dependent formation of an ERK1/2 activation complex on the angiotensin AT1A, lysophosphatidic acid (LPA), type I parathyroid hormone/PTH-related peptide (PTH1), and β2 adrenergic receptors demonstrate that the onset of arrestin-dependent ERK1/2 activation coincides with the waning of G protein signaling and persists as receptors internalize (Ahn et al., 2004; Gesty-Palmer et al., 2005, 2006; Shenoy et al., 2006). Likewise, because the receptor-arrestin complex can be envisioned as nucleating a membrane-delimited “signalsome” complex, arrestin-dependent signaling should be spatially discrete. Again, using the well studied ERK1/2 cascade, it is clear that receptors that form stable receptor-arrestin complexes, such as protease-activated receptor (PAR)-2, angiotensin AT1A, vasopressin V2, and neurokinin NK-1 receptors, activate a pool of ERK1/2 that accumulates in early endosomes along with the receptor (DeFea et al., 2000a,b; Luttrell et al., 2001, Tohgo et al., 2002). Unlike ERK1/2 activated by heterotrimeric G protein-mediated pathways, signalsome-associated ERK1/2 does not translocate to the cell nucleus and fails to elicit a transcriptional response or stimulate cell proliferation (DeFea et al., 2000b; Tohgo et al., 2002). This contrasts with receptors, such as the β2 adrenergic and LPA receptors, that form transient receptor-arrestin complexes that dissociate upon internalization of the receptor. These receptors do not constrain ERK1/2 activity within endosomes and may be able to use arrestins to generate a mobile ERK1/2 pool that is transcriptionally competent (Gesty-Palmer et al., 2005; Shenoy et al., 2006). Indeed, when the C terminus of the V2 receptor is exchanged for that of the β2 adrenergic receptor, converting the arrestin interaction from stable to transient, vasopressin activated ERK1/2 enters the cell nucleus and promotes cell proliferation, consistent with the hypothesis that the stability of the receptor-arrestin interaction is a major determinant of ERK1/2 action (Tohgo et al., 2003).
Although, under physiologic conditions, arrestin-mediated signaling commences in the setting of concurrent G protein activation, it is also clear that at least some arrestin-mediated signals do not require heterotrimeric G protein activity. Complementary data obtained using G protein-uncoupled receptor mutants and arrestin pathway-selective biased ligands have shown that arrestin-dependent activation of ERK1/2 by the angiotensin AT1A, β2 adrenergic, and PTH1 receptors is G protein-independent (Azzi et al., 2003; Wei et al., 2003; Gesty-Palmer et al., 2006; Shenoy et al., 2006; Lee et al., 2008). Even Gβγ subunit-dependent recruitment of GRK2/3 is apparently not required. Data obtained using isoform-selective silencing of GRKs suggests that GRK2 and GRK3 phosphorylation of the angiotensin AT1A and vasopressin V2 receptors promotes arrestin-dependent desensitization, whereas GRK5 and GRK6 seem to be exclusively responsible for initiating arrestin-dependent ERK1/2 activation (Kim et al., 2005; Ren et al., 2005).
It is unclear how arrestin-recruitment, in the absence of G protein activation, promotes signaling. Some data suggest that the arrestin simply acts as a scaffold, using the ligand-occupied GPCR on the plasma membrane as a nothing more than a docking site. Overexpression of a G protein-uncoupled neurokinin NK-1 receptor-arrestin2 chimera in HEK cells results in constitutive activation of a pool of ERK1/2 that remains bound, along with its upstream kinases, c-Raf1 and MAP kinase/ERK kinase 1/2, to the internalized receptor-arrestin chimera (Jafri et al., 2006). Because membrane targeting of c-Raf1 is itself sufficient to activate ERK1/2 (Stokoe et al., 1994), one possibility is that the arrestin works simply by assembling the pathway components on a membrane surface. The finding that plasma membrane recruitment of arrestin3 independent of GPCR binding is sufficient to activate ERK1/2 is consistent with this model (Terrillon and Bouvier, 2004). Alternatively, arrestin signaling may involve the recruitment of additional upstream pathway activators. For example, another putative arrestin binding partner, PP2A, promotes ERK1/2 activation by acting on c-Raf1 Ser259, an inhibitory site that must be dephosphorylated for Raf activation (Abraham et al., 2000; Adams et al., 2005).
III. Arrestin Signaling in Vitro
A. Diversity in Arrestin Signaling
Although there is ample in vitro evidence that arrestin-dependent signaling occurs at endogenous levels of receptor and arrestin expression, relatively little is known about its true scope and even less about its physiologic roles. Efforts to identify arrestin binding partners using yeast two-hybrid or proteomic approaches have uncovered a plethora of potential interactions, most of which remain to be functionally validated. For example, a recent proteomic screen identified 337 distinct proteins that coprecipitated with epitope-tagged arrestin2 or -3 under varying conditions (Xiao et al., 2007), implying that arrestins might link GPCRs to a wide range of signaling pathways. Such diversity is conceptually difficult to reconcile with the universality of arrestin function. In contrast to heterotrimeric G proteins, where there are 16 mammalian Gα subunits, five Gβ subunits, and 12 Gγ subunit genes (Downes and Gautam, 1999), there are only two nonvisual arrestins, they are ubiquitously expressed outside the retina, and they bind to the vast majority of GPCRs, any of which, presumably, could use them for signaling. In addition, although simultaneous deletion of arrestin2 and -3 results in embryonic lethality, deletion of either alone produces relatively mild phenotypes (Conner et al., 1997; Bohn et al., 1999), suggesting that either isoform can fulfill the obligate arrestin functions in vivo.
Table 1 summarizes many of the experimentally validated arrestin-dependent GPCR signaling pathways reported to date (Kendall and Luttrell, 2009). The evidence supporting their existence ranges from coprecipitation studies using epitope-tagged, overexpressed pathway components to isolation of endogeous arrestin-effector complexes from native tissues, and from loss of function studies using arrestin dominant-negative mutants and isoform-selective RNA interference to rescue studies performed using arrestin2/3-null murine embryo fibroblasts (MEFs). Viewed as a whole, arrestin signaling seems to encompass a fairly discrete set of functions, linking GPCRs to non-receptor tyrosine kinases, MAP kinases, lipid kinases, protein phosphatases, ubiquitin ligases and deubiquitinating enzymes, enzymes involved in second messenger degradation, and a number of exchange factors that regulate the activity of Ras-family small GTPases. Many of these putative effectors are not known to be regulated by heterotrimeric G protein subunits, suggesting that GPCR-arrestin-effector pathways function in parallel with GPCR-G protein-effector pathways to add previously unrecognized dimensions to GPCR signaling.
TABLE 1.
Arrestin-mediated signaling pathways
| Effector | Arrestin | Reported Functions | References |
|---|---|---|---|
| Src family tyrosine kinases | Arr1 | ERK1/2 activation | Luttrell et al., 1999; DeFea et al., 2000a |
| c-Src; c-Yes; c-Hck; c-Fgr; c-Fyn | Arr2 | Dynamin 1 phosphorylation | Miller et al., 2000 |
| Arr3 | Exocytosis/granule release | Barlic et al., 2000 | |
| Phosphorylation/destabilization of GRK2 | Penela et al., 2001 | ||
| FAK phosphorylation | Galet and Ascoli, 2008 | ||
| EGF receptor transactivation | Noma et al., 2007 | ||
| Phosphorylation of β2 adaptin subunit of AP-2 complex | Zimmerman et al., 2009 | ||
| c-Raf1-MEK1/2-ERK1/2 | Arr2 | Activation of cytosolic ERK1/2 | DeFea et al., 2000b; Luttrell et al., 2001 |
| Arr3 | p90RSK phosphorylation | Seta et al., 2002 | |
| Actin cytoskeletal reorganization/chemotaxis | Ge et al., 2003 | ||
| ERK1/2 dependent transcription | Gesty-Palmer et al., 2005 | ||
| Mnk1/eIF4E phosphorylation/protein translation | DeWire et al., 2008 | ||
| ASK1-MKK4-JNK3 | Arr1 | Activation of cytosolic JNK3 | McDonald et al., 2000 |
| Arr3 | Song et al., 2007 | ||
| Arr4 | |||
| PP2A-Akt-GSK3β | Arr3 | Activation of Akt | Goel et al., 2002 |
| Inactivation of Akt/GSK3β | Beaulieu et al., 2005 | ||
| Activation of β-catenin signaling | |||
| SHP-1; SHP-2 | Arr3 | Inhibition of NK cell cytotoxicity | Yu et al., 2008 |
| cAMP phosphodiesterases | Arr2 | Attenuation of cAMP signaling | Perry et al., 2002 |
| PDE4D3; PDE4D5 | Arr3 | ||
| Diacylglycerol kinase | Arr2 | Attenuation of PKC signaling | Nelson et al., 2007 |
| Arr3 | |||
| PI 4-Phosphate 5-kinase Iα | Arr2 | Control of GPCR internalization | Nelson et al., 2008 |
| Arr3 | |||
| Phospholipase A2 | Arr2 | Vasodilation and flushing | Walters et al., 2009 |
| E3 ubiquitin ligases | Arr1 | Ubiquitination of β-arrestin2 | Shenoy et al., 2001 |
| Mdm2; Nedd4; AIP4; TRAF6 | Arr2 | Stabilization of GPCR-arrestin complex | Shenoy and Lefkowitz, 2003 |
| Arr3 | Increased p53-mediated apoptosis | Wang et al., 2003 | |
| Inhibition of Toll-like receptor signaling | Wang et al., 2006 | ||
| Stabilization of GPCR-arresti-ERK1/2 signalsome | Shenoy et al., 2007 | ||
| GPCR ubiquitination and downregulation | Bhandari et al., 2007; Shenoy, et al., 2008 | ||
| Ubiquitin-specific protease 33 | Arr3 | Deubiquitination of β-arrestin2 | Shenoy et al., 2009 |
| Control of GPCR internalization | |||
| N-Ethylmaleimide-sensitive factor | Arr2 | Control of GPCR internalization | McDonald et al., 1999 |
| Ral-GDS | Arr2 | Cytoskeletal reorganization/granule exocytosis | Bhattacharya et al., 2002 |
| Arr3 | |||
| ARF6-ARNO | Arr2 | GPCR endocytosis | Claing et al., 2001 |
| Arr3 | |||
| Cofilin; Chronophin; LIM kinase | Arr3 | Actin cytoskeletal reorganization/chemotaxis | Zoudilova et al., 2007 |
| Dishevelled 2 | Arr2 | Frizzled endocytosis and Wnt signaling | Chen et al., 2004 |
| IκBα-IκB kinase α/β | Arr2 | Attenuation of NFκB signaling | Gao et al., 2004; Witherow et al., 2004 |
| Arr3 | Fan et al., 2007 | ||
| Kif3A kinesin motor protein | Arr2 | Targeting and internalization of Smoothened | Chen et al., 2004 |
| Arr3 | Gli-dependent transcription | Kovacs et al., 2008 | |
| Histone acetyltransferase p300 | Arr2 | Transcription of p27 and c-Fos | Kang et al., 2005 |
| YY1 transcription factor | Arr2 | Repression of cdx4-hox transcription | Yue et al., 2009 |
FAK, focal adhesion kinase; eIF, eukaryotic initiation factor.
Even with this more limited repertoire of arrestin-mediated signals, the question arises as to whether and how specificity in arrestin-effector coupling might be achieved. Although a few binding partners seem to show selective activation by one arrestin isoform (e.g., JNK3) (Miller et al., 2001; Song et al., 2009), others clearly interact with both (e.g., ERK1/2) (DeFea et al., 2000b; Luttrell et al., 2001), suggesting that primary structure is not a major determinant of arrestin binding selectivity. On the other hand, arrestins seem to adopt different conformations depending on which GPCR they bind and which GRK phosphorylated the receptor. Evidence of the former comes from characterization of arrestin ubiquitination. Ubiquitination of lysines 11 and 12 of arrestin3 is necessary for it to remain stably bound to the angiotensin AT1A receptor, yet an arrestin3 (K11,12R) mutant is still ubiquitinated and fully functional when recruited to the vasopressin V2 receptor (Shenoy and Lefkowitz, 2005b). All 31 lysines must be mutated before arrestin3 ubiquitination is lost upon β2 adrenergic receptor binding (Shenoy et al., 2007). This variability suggests either that the conformation or the accessibility of surface epitopes on the arrestin differs depending on the GPCR binding partner. Within a single receptor, the finding that conventional and “biased” ligands elicit qualitatively different changes in the intramolecular bioluminescence resonance energy transfer signal produced when a luciferase-arrestin3-yellow fluorescent protein chimera binds to the angiotensin AT1A, PTH1, or β2 adrenergic receptors likewise argues in favor of conformational flexibility that could specify effector coupling (Shukla et al., 2008). Additional support for different arrestin conformations comes from the finding that although vasopressin V2 receptors phosphorylated by GRK2 and GRK3 undergo arrestin-dependent desensitization, arrestin-dependent ERK1/2 activation occurs only if the receptor is phosphorylated by GRK5 and GRK6 (Ren et al., 2005). The physical basis for this specialization remains unclear, but it may indicate that phosphorylation of specific GRK sites on the receptor affects arrestin conformation and thus its ability to engage particular binding partners.
What then, are the functions that can be ascribed to arrestin-dependent GPCR signaling? Current evidence suggests arrestin signaling can be grouped into those regulating basic GPCR functions, such as second messenger production, receptor endocytosis, and vesicle trafficking, and a more limited number of signals extending beyond the signalsome to affect cellular processes such as gene transcription and protein translation. Considering the lack of molecular diversity in the arrestin family and their relative promiscuity in receptor binding, such a focus is inherently appealing in that it suggests that arrestin signaling, like arrestin-mediated desensitization and sequestration, is biased toward the control of processes that are fundamental to GPCR regulation. Nonetheless, a small but growing literature suggests that arrestin signaling has much broader ramifications at the cellular and organismal levels.
B. Receptor Desensitization and Second Messenger Production
The first described and best known function of arrestins is in homologous desensitization, the physical interdiction of receptor-G protein coupling that occurs upon arrestin binding. A number of arrestin binding partners, including Src family tyrosine kinases, ERK1/2, Mdm2, cAMP phosphodiesterases, and diacylglycerol kinase, have been implicated in the control of desensitization and second-messenger degradation, consistent with a complementary role for arrestin-regulated effectors in the negative regulation of G protein signaling pathways.
Arrestin-bound Src kinases and ERK1/2 are involved in the regulation of GRK and arrestin function. c-Src was the first catalytically active signalsome component to be described (Luttrell et al., 1999; DeFea et al., 2000a), and it seems to perform several GPCR housekeeping functions. GRK2 undergoes rapid proteosome-dependent turnover, a process that is accelerated by GPCR activation and dependent upon both GRK2 catalytic activity and arrestins. Upon β2 adrenergic receptor stimulation, arrestin-scaffolded c-Src phosphorylates GRK2, promoting its entry into the proteosome pathway and providing negative feedback on receptor desensitization (Penela et al., 2001).
Within the signalsome, activated ERK1/2 also influences receptor desensitization by phosphorylating GRKs and arrestins. Arrestin-dependent phosphorylation of GRK2 Ser670 by ERK1/2 is agonist-dependent, enhanced by prior c-Src phosphorylation, and further accelerates GRK2 turnover (Elorza et al., 2003). ERK1/2 also phosphorylates Ser412 in the C terminus of arrestin2 (Lin et al., 1997, 1999). In the cytosol, arrestin 2 is almost stoichiometrically phosphorylated on Ser412, and it must be dephosphorylated upon receptor binding to engage clathrin and support receptor internalization. Rephosphorylation of Ser412 by ERK1/2 either provides negative feedback regulation of receptor endocytosis or facilitates receptor internalization by promoting the dissociation of arrestin and clathrin, allowing the receptor to exit clathrin-coated vesicles. Ser412 phosphorylation also seems to disrupt the arrestin-Src interaction, possibly regulating Src-dependent signals emanating from the signalsome (Luttrell et al., 1999). Arrestin2-bound PP2A reportedly catalyzes the reverse reaction, dephosphorylating Ser412 (Hupfeld et al., 2005). Dephosphorylation of GRK-phosphorylated GPCRs, a prerequisite for receptor resensitization, also involves PP2A (Pitcher et al., 1995a; Krueger et al., 1997), but it is not known whether arrestins target the PP2A pool involved in receptor dephosphorylation.
E3 ubiquitin ligases catalyze the transfer of ubiquitin to the ε-amino group of lysine residues in substrate proteins. Arrestins interact with at least four different E3 ubiquitin ligases: Mdm2, atrophin-interacting protein 4, Nedd4, and TRAF6 (Shenoy et al., 2001; Wang et al., 2006; Bhandari et al., 2007; Shenoy et al., 2008). Arrestin-dependent recruitment of E3 ligases into the signalsome complex regulates ubiquitination of both receptors and arrestins. Mdm2 ubiquitinates arrestin3, whereas another arrestin binding partner, ubiquitin-specific protease 33, catalyzes the reverse reaction (Shenoy et al., 2001, 2009). The reversible ubiquitination of arrestin3 has profound effects on the stability of the GPCR-arrestin complex and the characteristics of receptor desensitization, internalization, and subsequent trafficking. Stimulation of β2 adrenergic receptors leads to transient Mdm2-dependent ubiquitination of arrestin3. Arrestin3 ubiquitination stabilizes the receptor-arrestin interaction, because a lysine-less arrestin3 mutant is unable to remain associated with the receptor, and genetic ablation of Mdm2 blocks β2 receptor endocytosis (Shenoy et al., 2001, 2007). Conversely, deubiquitination releases arrestin from the receptor after internalization, because an arrestin3-ubiquitin fusion protein remains bound to the β2 receptor, traffics with it into early endosomes, and enhances receptor degradation (Shenoy and Lefkowitz, 2003). Studies with the angiotensin AT1A receptor, which characteristically remains arrestin-bound after internalization, supports the model (Shenoy and Lefkowitz, 2005b). Unlike the β2 adrenergic receptor, arrestin3 becomes stably ubiquitinated on lysines 11 and 12 when bound to the AT1A receptor. Expression of a K11/12R arrestin3 mutant reverses the pattern of arrestin binding, such that the AT1A receptor adopts the β2 receptor pattern of transient arrestin binding at the plasma membrane followed by dissociation upon internalization. Using bioluminescence resonance energy transfer, it has been possible to confirm that the kinetics of arrestin ubiquitination in living cells parallel those of the receptor-arrestin complex (Perroy et al., 2004). Arrestin ubiquitination is detectable within 2 min of stimulation of either β2 adrenergic or vasopressin V2 receptors, but within 5 min, the β2 receptor-bound arrestin is deubiquitinated, whereas V2 receptor-bound arrestin remains stably ubiquitinated beyond 10 min.
Arrestin-bound effectors also complement desensitization by localizing enzymes that catalyze second messenger degradation. Arrestins 2 and -3 interact with all five type 4D isoforms of cAMP phosphodiesterase, PDE4D1–5. The Gs-coupled β2 adrenergic receptor forms a complex with arrestin3 and PDE4D3/5, leading to accelerated cAMP degradation (Perry et al., 2002). Recruitment of PDE4D3/5 into the signalsome seems to be highly receptor-specific, because the closely related β1 adrenergic receptor was recently shown to recruit a different isoform, PDE4D8, and to do so without the aid of arrestin (Richter et al., 2008). In an analogous manner, arrestin-dependent recruitment of diacylglycerol kinase dampens M1 muscarinic receptor-mediated PKC activity (Nelson et al., 2007). Diacylglycerol kinase inhibits PKC by converting diacylglycerol produced by phospholipase Cβ to phosphatidic acid.
C. Receptor Endocytosis and Vesicle Trafficking
Besides GRK2 and arrestins, several proteins involved in the endocytosis and intracellular trafficking of GPCRs are regulated by arrestins, reinforcing the theme that arrestin-bound effectors perform many GPCR signaling and housekeeping functions. Arrestin-dependent recruitment of Src kinases controls the function of dynamin1 and the β2 adaptin subunit of AP-2, two proteins that are essential for clathrin-dependent GPCR endocytosis. Src phosphorylation of dynamin1 on Tyr497 promotes dynamin self-assembly, and expression of a dominant-negative c-Src fragment that disrupts arrestin-Src binding inhibits both Tyr497 phosphorylation and receptor endocytosis (Ahn et al., 1999; Miller et al., 2000). Expression of a Y497F mutant of dynamin1 impairs the internalization of both the β2 adrenergic and the M2 muscarinic receptors (Ahn et al., 1999; Werbonat et al., 2000). The β2 adaptin subunit of AP-2 is also regulated by arrestin-dependent Src phosphorylation (Fessart et al., 2005, 2007; Zimmerman et al., 2009). c-Src stabilizes the constitutive association of arrestin3 and β2 adaptin independent of its kinase activity. Phosphorylation of β2 adaptin Tyr737 occurs in clathrin-coated pits on the plasma membrane in response to AT1A, β2 adrenergic, V2 vasopressin, or B2 bradykinin receptor activation, prompting AP-2 to dissociate from the complex. If β2 adaptin phosphorylation is blocked, receptor-arrestin complexes are retained at the membrane in clathrin-coated pits. Some evidence suggests that arrestin-Src complexes also regulate GPCR-mediated exocytosis. Arrestin-dependent activation of the Src family kinases c-Hck and c-Fgr by the interleukin (IL)-8 receptor (CXCR1) seems to control granule release, because expression of a P91G/P121G arrestin2 mutant with impaired Src binding antagonizes CXCR1-induced exocytosis in granulocyte cells (Barlic et al., 2000). Likewise, the endothelin type A (ET-A) receptor assembles an arrestin2-dependent complex with c-Yes that positively regulates endothelin-1-stimulated translocation of vesicles containing the glucose transporter Glut4 to the plasma membrane (Imamura et al., 2001).
Another arrestin3 binding partner, phosphatidylinositol 4-phosphate 5-kinase 1α, regulates clathrin and AP2 function during clathrin-dependent GPCR endocytosis (Nelson et al., 2008). Phosphatidylinositol 4-phosphate 5-kinase 1α produces phosphatidylinositol 4,5-bisphosphate on the inner leaflet of the clathrin-coated pit, promoting polymerization of clathrin and AP-2 and assembly of the clathrin coat. Arrestin3 recruits phosphatidylinositol 4-phosphate 5-kinase 1α to activated β2 adrenergic receptors, increasing phosphatidylinositol 4,5-bisphosphate formation and enhancing receptor endocytosis.
Arrestin-bound E3 ubiquitin ligases also affect receptor fate after endocytosis. GPCRs that form stable receptor-arrestin complexes are retained inside the cell after endocytosis and either recycle slowly or are degraded (Oakley et al., 2000, 2001; Barak et al., 2001). By controlling whether the receptor-arrestin complex dissociates, arrestin-bound Mdm2 dictates the rate of receptor recycling (Shenoy and Lefkowitz, 2003). Other arrestin-bound E3 ligases catalyze receptor ubiquitination. The β2 adrenergic receptor is ubiquitinated by the E3 ligase Nedd4, which is recruited to the receptor by arrestin3. Nedd4 promotes β2 receptor down-regulation by accelerating its proteosomal degradation (Shenoy et al., 2008). The chemokine receptor CXCR4 is ubiquitinated by the E3 ligase atrophin-interacting protein 4, which binds to the amino terminal half of arrestin2 (Bhandari et al., 2007).
Several other proteins that regulate the trafficking of membrane-bound vesicles reportedly interact with arrestins to regulate internalized receptor-arrestin complexes. The ATPase N-ethylmaleimide-sensitive factor interacts with both arrestin2 and -3 and is important for β2 adrenergic receptor internalization (McDonald et al., 1999). ADP-ribosylation factor (ARF) 6 and ARF nucleotide binding site opener also bind arrestins and contribute to β2 adrenergic receptor endocytosis (Claing et al., 2001). ARF6 is a monomeric small G protein that regulates vesicular trafficking, and ARF nucleotide binding site opener acts as an ARF6 GEF. Like ARF6, Ral is a small Ras family G protein that regulates cytoskeletal dynamics. Ral is activated by the GEF activity of Ral-GDP dissociation simulator (Ral-GDS). Ral-GDS constitutively interacts with cytoplasmic arrestin2 and -3. Upon activation of the formyl-Met-Leu-Phe receptor and arrestin recruitment, Ral-GDS is released from the arrestin complex, whereupon it regulates cytoskeleton rearrangement and exocytic granule release in polymorphonuclear neutrophilic leukocytes (Bhattacharya et al., 2002).
Thus, arrestin-dependent recruitment of a number of enzymatic effectors augments the classic desensitization-related role of the protein (i.e., steric hindrance of receptor-G protein coupling), indicating that a major role of the signalsome is to regulate second messenger production, receptor desensitization, internalization, and trafficking. However, signalsome formation has effects on cellular processes extending from the plasma membrane to the cell nucleus, suggesting that the distinction commonly drawn between the desensitization-related and “signaling” functions of arrestins is more semantic than functional (Fig. 2).
Fig. 2.
The formation of arrestin-dependent signalsomes affects diverse cellular processes. Given their nearly ubiquitous binding to agonist-occupied GPCRs, it is not surprising that arrestins recruit effector enzymes that promote the degradation of second messengers and regulate GPCR endocytosis and intracellular trafficking, complementing their classic roles in receptor desensitization. Arrestin-based signaling complexes also contribute to membrane and cytosolic processes in which spatially constrained activation of effectors is required, such as vesicle exocytosis, chemotaxis, and phosphorylation-dephosphorylation of cytosolic proteins. Emerging data are also implicating arrestins in transcriptional regulation, where they affect gene expression by attenuating the activity of some pathways and enhancing the activity of others.
D. Arrestin Signaling beyond the Receptor
Because arrestins interface with a number of pleiotropic signaling pathways, it seems probable that arrestin signaling would extend beyond the signalsome to affect broader aspects of cellular function. Features common to GPCR-arrestin complexes, such as their longevity and restricted localization, suggest they could act as signaling platforms for processes in which signaling must be spatially constrained. One setting in which these properties are paramount is in cell migration, and a significant body of literature supports a role for arrestin scaffolds in the control of cell motility and chemotaxis.
Chemotaxis is the process whereby migrating cells follow a concentration gradient to its source. Chemoattractant receptor activation induces actin cytoskeletal rearrangement forming leading and trailing edges. A dominant pseudopodium forms at the leading edge that protrudes forward driven by F-actin polymerization and actin-myosin contraction forces (Machesky, 1997; Brahmbhatt and Klemke, 2003). Splenocytes derived from arrestin3-null mice exhibit strikingly impaired chemotactic responses to stromal cell-derived factor-1, CXCL12 (Fong et al., 2002). Although impaired gradient sensing caused by the loss of arrestin-mediated desensitization might be a contributing factor (Aragay et al., 1998), evidence suggests that arrestin-dependent regulation of ERK1/2 and cortical actin assembly at the leading edge is required for GPCR-mediated chemotaxis (Ge et al., 2003, 2004; Barnes et al., 2005; Hunton et al., 2005; Zoudilova et al., 2007). PAR-2 induced chemotaxis in MDA breast cancer cells requires both arrestin2 and -3 (Ge et al., 2004). During chemotaxis, a PAR-2-arrestin-ERK1/2 complex localizes to the leading edge and activates actin cytoskeleton reorganization (Ge et al., 2003). In addition, arrestins scaffold a complex containing the actin filament-severing protein cofilin, LIM kinase, and the cofilin-specific phosphatase chronophin, which is required for the dephosphorylation and activation of cofilin (Zoudilova et al., 2007). Arrestin-bound cofilin generates the free barbed ends on actin filaments that permit filament extension. In AT1A receptor-expressing HEK293 cells, angiotensin II and the arrestin pathway-selective ligand Sar1-Ile4-Ile8 activate AT1A receptor chemotaxis using an arrestin3-dependent mechanism that is independent of G protein activity (Hunton et al., 2005). Arrestins also affect cell shape change by interacting with the actin bundling protein, filamin A. Assembly of an AT1A receptor-arrestin-ERK1/2-Filamin A complex is required for the formation of membrane ruffles in Hep2 cells (Scott et al., 2006).
The constraint imposed by arrestin binding seems to preferentially target ERK1/2 to membrane or cytosolic substrates. Cytosolic ERK1/2 substrates include the ribosomal S6 kinase p90RSK (Aplin et al., 2007) and MAP kinase-interacting kinase 1, a regulator of the ribosomal protein translation initiation complex. ERK1/2 phosphorylation of p90RSK is activated by a mutant angiotensin AT1A receptor with a deletion in its second intracellular loop that inhibits G protein coupling (Seta et al., 2002). Using RNA interference to down-regulate arrestin3, it has been possible to show that arrestin-dependent ERK1/2 activation by the AT1A receptor mediates phosphorylation of MAP kinase-interacting kinase 1 and eukaryotic translation initiation factor 4E, increasing rates of mRNA translation (DeWire et al., 2008).
Arrestin signaling has been implicated in other cellular responses to GPCR activation. One well characterized mechanism whereby GPCRs affect cell proliferation and survival is stimulation of the metalloprotease-dependent shedding of preformed epidermal growth factor (EGF)-family growth factors, leading to paracrine transactivation of EGF receptors (Luttrell, 2003). In β1 adrenergic receptor-expressing HEK293 cells, EGF receptor transactivation and ERK1/2 activation are inhibited by siRNA-mediated down-regulation of arrestin2 or -3, or GRK5 or -6, inhibiting Src kinase or matrix metalloprotease activity or exposure to a heparin-binding-EGF neutralizing antibody, suggesting that β1 receptor-mediated EGF receptor transactivation is arrestin-dependent (Noma et al., 2007). Likewise, arrestin3-dependent activation of the Src family tyrosine kinase c-Fyn is involved in luteinizing hormone receptor-mediated phosphorylation of the antiapoptotic focal adhesion kinase and the release of EGF-like growth factors (Galet and Ascoli, 2008). On the other hand, EGF receptor transactivation is the primary mechanism of ERK1/2 activation by endogenous LPA receptors in arrestin2/3-null MEFs, demonstrating convincingly that not all GPCRs use arrestins to mediate EGF receptor cross-talk (Gesty-Palmer et al., 2005).
Also unsettled is the role of arrestins in regulation of the JNK cascade. JNK1–3 are stress-activated kinases that regulate apoptosis by stimulating cytochrome c release from the mitochondria during cellular stress and control transcription by phosphorylating the transcription factor c-Jun (Tournier et al., 2000). There are three JNK isoforms, of which JNK1 and JNK2 are widely expressed, whereas JNK3 is highly expressed only in brain, heart, and testes, where it is known to play an important role in neuronal apoptosis caused by cerebral ischemia (Kuan et al., 2003). JNK2 and JNK3 were found to interact with arrestin3 in yeast two-hybrid screens, but only the JNK3 interaction has been observed in mammalian cells (McDonald et al., 2000). JNK3 also interacts with rod and cone arrestins (Song et al., 2007). In overexpression systems, arrestin3 binds the components of the JNK pathway, Ask1, MKK4, and JNK3, forming a complex that dramatically potentiates JNK3 phosphorylation. As with ERK1/2, arrestin3-bound JNK3 is restricted to the cytoplasmic compartment (McDonald et al., 2000; Scott et al., 2002; Wang et al., 2003), and it is unclear whether JNK3 activated in this manner can phosphorylate c-Jun. Unlike ERK1/2, however, there is thus far no evidence that GPCRs use arrestin scaffolds to regulate JNK3, so the physiologic relevance of this scaffolding function remains unknown.
Although it is likely that many cytosolic and membrane functions remain to be described, available evidence suggests that GPCR-arrestin signalsomes function in the regulation of processes that require spatial localization and sustained activation of signaling proteins, such as cytoskeletal rearrangement during chemotaxis. By imposing spatial constraint, arrestins limit the access of pleiotropic kinases such as ERK1/2 and JNK3 to cytosolic substrates.
E. Genomic Effects of Arrestin Signaling
An area of growing interest is the possibility that GPCRs use arrestin-dependent mechanisms to control gene expression. Although it is clear that arrestin binding negatively regulates nuclear signaling by the ERK1/2 and NFκB pathways by sequestering key pathway components, emerging evidence also suggests that arrestins affect transcription by regulating β-catenin and p53 signaling, and that in some cases, arrestins themselves translocate to the cell nucleus and interact with transcription factors.
It has been shown repeatedly that overexpression of arrestins inhibits ERK1/2 mediated transcription (Luttrell et al., 2001; Tohgo et al., 2002). Such results are predictable in that arrestins are predominantly cytosolic proteins that bind and sequester ERK1/2. Nonetheless, the arrestin content of a cell has profound effects on nuclear signaling by ERK1/2 at endogenous levels of expression. In arrestin2/3-null MEFs, ERK1/2 activation by LPA receptors arises primarily from G protein-dependent transactivation of EGF receptors (Gesty-Palmer et al., 2005). Because LPA receptor desensitization is impaired, the EGF receptor-dependent ERK1/2 signal is persistent, lasting for several hours in the continued presence of LPA. Reintroducing arrestin3, which restores desensitization, makes the transactivation-dependent signal transient, such that it contributes significantly to ERK1/2 activation only during the first few minutes of stimulation. At the same time, arrestin3 confers a long-lasting EGF receptor-independent ERK1/2 signal that presumably reflects activation of the arrestin pathway. Whereas most of the early LPA-stimulated transcriptional responses in arrestin2/3-null MEFs are EGF receptor-dependent, expression of arrestin3 enables LPA to elicit ERK1/2-dependent responses that do not require the EGF receptor, suggesting that the arrestin-ERK1/2 pathway is transcriptionally competent. Conversely, a G protein-uncoupled mutant of the angiotensin AT1A receptor that activates ERK1/2 only via the arrestin pathway does not elicit a transcriptional response in HEK293 cells, whereas silencing endogenous arrestin3 expression enhances the transcriptional activity of the wild-type AT1A receptor (Lee et al., 2008). Comparable results suggesting that the G protein-independent arrestin-ERK1/2 pathway cannot signal to the cell nucleus have been obtained using AT1A receptor mutants with impaired arrestin-binding (Wei et al., 2004). The basis for the apparent difference between AT1A and LPA receptors is unclear, but it is noteworthy that LPA receptors, unlike AT1A receptors, form transient receptor-arrestin complexes, so it is possible that ERK1/2 activated by the arrestin pathway can reach the cell nucleus upon dissociation of the receptor-arrestin complex (Gesty-Palmer et al., 2005).
Arrestins 2 and 3 also seem to act as negative regulators of NFκB-dependent transcription. Phosphorylation of IκBα by IκBα kinases accelerates its degradation by the proteosome, resulting in increased NFκB activity. IκBα binds the arrestin3 N terminus, and arrestins 2 and 3 interact with IκB kinase α/β and NFκB-inducing kinase. β2-Adrenergic receptor stimulation increases arrestin3 binding to IκBα, preventing its phosphorylation-dependent degradation and inhibiting IL-8 stimulated NFκB activity (Gao et al., 2004; Luan et al., 2005). Down-regulating arrestin2 increases NFκB activation by tumor necrosis factor α consistent with the hypothesis that arrestins tonically inhibit NFκB signaling by protecting IκBα from degradation (Witherow et al., 2004). In HEK293 cells, down-regulating arrestin expression attenuates Toll-like receptor 4-mediated ERK1/2 activation while at the same time enhancing NFκB reporter activity, suggesting that arrestins exert opposing effects on the ERK1/2 and NFκB pathways (Fan et al., 2007). Arrestin binding to another E3 ubiquitin ligase, TRAF6, probably augments the inhibition of Toll-like receptor signaling (Wang et al., 2006). TRAF6 is normally recruited to Toll-like receptor/IL-1 family receptors, where it facilitates IκB kinase and NFκB activation. Binding of TRAF6 to arrestin2 and -3 in response to lipopolysaccharide or IL-1 stimulation prevents TRAF6 oligomerization and autoubiquitination, negatively regulating lipopolysaccharide and IL-1 signaling.
β-Catenin and Akt signaling in the D2 dopamine receptor-rich striatum of mice is regulated by an arrestin3 signalsome complex composed of the catalytic subunit of PP2A, Akt, and glycogen synthase kinase 3β (GSK3β) (Beaulieu et al., 2005). As with NFκB, the dominant effect of arrestin binding seems to be dampening β-catenin signaling. Within the D2 receptor-arrestin3 signalsome, PP2A dephosphorylates Akt Thr308, rendering it inactive. Because Akt phosphorylation of GSK3α/β inhibits its activity, signalsome formation releases this inhibition, increasing signalsome-associated GSK3α/β activity. GSK3β, in turn, phosphorylates β catenin, accelerating its degradation. Predictably, striatal extracts from arrestin3-null mice show higher levels of β catenin expression, presumably resulting from the loss of signalsome-mediated Akt inhibition and GSK3β activation (Beaulieu et al., 2008).
Besides controlling arrestin ubiquitination, arrestin3 regulates other functions of Mdm2. Mdm2 is a major negative regulator of the p53 tumor suppressor, because ubiquitination of p53 promotes its degradation by the proteosome. Similar to the TRAF6 story, arrestin binding prevents Mdm2 self-ubiquitination and p53 ubiquitination. In this case, however, decreasing p53 ubiquitination increases its abundance, leading to enhanced p53 signaling. As a result, overexpressing arrestin3 enhances p53-mediated apoptosis and down-regulating arrestin3 expression attenuates it (Wang et al., 2003a).
Hedgehog signaling plays a key role in cell fate determination during embryonic patterning. Smoothened, a seven-membrane-spanning receptor that is not G protein-coupled, is a component the Hedgehog signaling pathway. Smoothened activity is normally repressed by binding to a 12 membrane-spanning receptor, Patched. Binding of the extracellular glycoprotein Sonic hedgehog (Shh) to Patched relieves this inhibition, allowing Smoothened to activate the Gli family of transcription factors by uncoupling them from their negative regulator, Su(fu). Activation of Smoothened reportedly promotes its GRK2-dependent association with arrestin3 and internalization, in a manner analogous to most conventional GPCRs (Chen et al., 2004). In addition, arrestins mediate the interaction of Smoothened with the kinesin motor protein, Kif3A, in a multimeric complex localized within the primary cilium (Kovacs et al., 2008). Down-regulating arrestin expression leads to mislocalization of Smoothened and loss of Smoothened-dependent activation of Gli1.
Finally, some evidence suggests that arrestins engage directly in nucleocytoplasmic shuttling as a means of conveying information between GPCRs on the plasma membrane and transcriptional regulatory elements in the nucleus. Activation of the δ-opioid receptor reportedly causes arrestin2 to move into the nucleus, where it interacts with the p27 and c-Fos promoters and stimulates transcription by recruiting histone acetyltransferase p300 and enhancing local histone H4 acetylation (Kang et al., 2005; Ma and Pei, 2007). It is noteworthy that arrestin3, but not arrestin2, has a discrete nuclear export signal (Scott et al., 2002; Wang et al., 2003). When the nuclear export signal is mutated or changed to the corresponding sequence in arrestin2, nuclear arrestin3 actively accumulates, suggesting that it is transported both in and out of the nucleus. However arrestin3 lacks a classic nuclear localization sequence, raising the possibility that one or more of its binding partners confers nuclear targeting of the complex.
The arrestin content of a cell has significant effects on the flow of information between receptors on the plasma membrane and transcriptional regulatory elements within the nucleus. Arrestins seem to exert predominantly negative regulatory effects on ERK1/2 and NFκB signaling. In the case of ERK1/2, signalsome formation enhances its activity toward membrane and cytosolic substrates but restricts its direct access to the nuclear compartment. On the other hand, arrestins seem to enhance p53 and Hedghog signaling, and arrestin2 may even function as a nuclear chaperone for p300 histone acetyltransferase. Thus, arrestins seem to regulate the balance between several different transcriptional regulatory pathways.
IV. Dissociating Arrestin Signaling from Desensitization in Vivo
Based on the evidence to date, it seems clear that many of the functions performed by arrestin-bound effectors complement their known roles in GPCR desensitization, sequestration, and trafficking. At the same time, arrestins seem to interface with transcriptional and translational regulatory pathways that allow them to transmit signals independent of G protein activity. What then is the evidence for physiologically relevant G protein-independent signaling in vivo? The question is surprisingly difficult to answer, principally due to limitations of the available tools for experimentation. Efforts to define the role of arrestin signaling in vivo have primarily employed three strategies: arrestin knockouts, mutated GPCRs impaired in either G protein or arrestin coupling, and “biased” ligands that couple receptors to arrestins without activating G protein pathways.
A. Arrestin Knockouts
Although simple eukaryotes such as Saccharomyces cerevisiae possess GPCRs and heterotrimeric G proteins, they do not contain arrestins. Arrestins are expressed in many invertebrates, however, including the genetically tractable model organisms Caenorhabditis elegans and D. melanogaster (Hyde et al., 1990; Smith et al., 1990; Yamada et al., 1990; Jansen et al., 1999; Palmitessa et al., 2005). Mutation of the two photoreceptor-specific arrestin genes in D. melanogaster, Arr1 and Arr2, provided the first demonstration that arrestins mediate rhodopsin inactivation in vivo and are essential for termination of the phototransduction cascade (Dolph et al., 1993). Deletion of kurtz, the only nonvisual arrestin in D. melanogaster, produces lethality linked to loss of arrestin function in the central nervous system (Roman et al., 2000). Deletion of ARR-1, the sole arrestin in C. elegans, leads to defects in olfactory adaptation and recovery that are consistent with its known roles in GPCR desensitization, endocytosis, and resensitization (Palmitessa et al., 2005).
Vertebrates, including amphibians, birds, and mammals, possess both visual and nonvisual arrestins (Ferguson, 2001). As in mammals, the zebrafish genome contains four arrestin genes, two visual and two nonvisual (Wilbanks et al., 2004). In mice, individual arrestin2 and arrestin3 knockouts have been generated (Conner et al., 1997; Bohn et al., 1999), although to date neither isoform has been floxed to enable tissue-specific ablation. Simultaneous ablation of both nonvisual arrestins results in early embryonic death, so only MEFs are available as a true mammalian arrestin2/3-null background (Kohout et al., 2001). Studies of mice lacking a single nonvisual arrestin isoform suggest considerable functional redundancy. Arrestin2- and arrestin3-null mice are grossly normal, with phenotypes that become apparent only upon challenge with pharmacologic doses of GPCR agonists. Arrestin2-null mice exhibit exaggerated cardiac sensitivity to β adrenergic agonists (Conner et al., 1997), whereas arrestin3-null mice demonstrate enhanced morphine-induced analgesia and attenuation of opiate tolerance (Bohn et al., 1999, 2002). In each case, the phenotypes are consistent with impaired GPCR desensitization rather than the loss of arrestin-mediated signaling. Although the early embryonic lethality of arrestin2/3-null mice suggests a developmental role, it is difficult to attribute this to the loss of any specific arrestin function.
Apart from lethality, the duality of arrestin function itself imposes limitations upon the utility of arrestin knockouts for studying the in vivo relevance of arrestin signaling. In most cases, loss of arrestin functions that limit G protein signaling (e.g., regulation of cAMP phosphodiesterase or diacylglycerol kinase activity) cannot be discriminated from its roles in GPCR desensitization and sequestration. Moreover, an observed phenotype might reflect either impaired GPCR desensitization (i.e., excessive G protein signaling) or the loss of arrestin-dependent signaling. Evidence that the loss of arrestin signaling accounts for a phenotype must be interpreted in light of the fact that GPCR desensitization is simultaneously affected. This is critical in vivo, where stimulations are carried out in the presence of endogenous ligands, and is a significant confounder in several studies of arrestin signaling.
B. Transgenic Expression of G Protein-Uncoupled G Protein-Coupled Receptor Mutants
A number of mutated GPCRs have been characterized that are selectively impaired either in G protein activation or in arrestin recruitment and desensitization, including G protein “uncoupled” and nondesensitizing variants of the angiotensin AT1A (Seta et al., 2002; Gáborik et al., 2003; Wei et al., 2004) and β1/2 adrenergic receptors (Rapacciuolo et al., 2003; Shenoy et al., 2006). In transfected cell systems, G protein-uncoupled AT1A and β2 adrenergic receptor mutants, combined with isoform-selective down-regulation of arrestins by RNA interference, have been used to characterize the spatial, temporal, and transcriptional regulatory properties of arrestin signaling (Wei et al., 2003; Ahn et al., 2004; Shenoy et al., 2006; Lee et al., 2008). In a few cases, these mutated receptors have been used to create transgenic mice for the purpose of studying G protein-independent signaling in vivo (Zhai et al., 2005; Noma et al., 2007). Although a potentially informative strategy, especially in an arrestin-only gain-of-function model, results must still be interpreted cautiously. Receptor mutants are rarely completely deficient in one function or another and are often overexpressed to high levels in transgenic models, creating the potential that an apparently G protein-independent response might still arise from G protein activation. In addition, these models are typically constructed on a wild-type background, where endogenous receptors and arrestins are present to activate the full GPCR response profile in response to endogenous or pharmacologic stimuli. Confirmatory loss-of-function experiments that might implicate arrestin signaling in a response (e.g., recreating a transgenic on an arrestin-null background strain) are time consuming and difficult to perform.
C. Arrestin Pathway-Selective Biased Agonists
Biased agonists are GPCR ligands that elicit only a subset of the response profile of their cognate receptor. The classic model of GPCR efficacy envisions the receptor as existing in spontaneous equilibrium between two states, inactive (R) and active (R*), that differ in their ability to engage effectors (De Lean et al., 1980; Samama et al., 1993). This model effectively describes the behavior of “inverse agonists,” ligands that suppress the basal activity of constitutively active receptors, and encompasses a full spectrum of ligands from inverse agonists through neutral antagonists to partial and full agonists, based on the concept that the intrinsic efficacy of a ligand is a reflection of its ability to alter the R-R* equilibrium. What the models do not address is the possibility that GPCRs might be able to adopt more than one “active” conformation. In a simple two-state model, the agonist pharmacology of a receptor would be the same regardless of the signaling output being measured. Nonetheless, extensive pharmacological and biophysiological evidence supports the existence of multiple active GPCR conformations (Kenakin, 2002, 2007).
Most significantly, a number of ligands have been characterized that exhibit paradoxical reversal of efficacy (e.g., acting as antagonists or inverse agonists of G protein signaling but behaving as agonists with respect to arrestin recruitment and arrestin-dependent signaling). For example, Sar1-Ile4-Ile8, an angiotensin AT1A receptor antagonist, promotes arrestin recruitment and receptor sequestration without detectable G protein activation (Holloway et al., 2002). Likewise, the PTH analog (d-Trp12,Tyr34)PTH(7–34) acts as an inverse agonist for PTH1 receptor-Gs coupling and promotes arrestin-dependent sequestration (Gardella et al., 1996; Sneddon et al., 2004). As a result, each is able to elicit arrestin-dependent ERK1/2 activation under conditions in which G protein activation is either absent or actively reversed (Wei et al., 2003; Gesty-Palmer et al., 2006). Likewise, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol (ICI118551), propranolol, and carvedilol, β2-adrenergic receptor ligands that act as partial inverse agonists with respect to Gs activation, function as partial agonists for the ERK1/2 pathway by engaging arrestins (Azzi et al., 2003; Wisler et al., 2007; Drake et al., 2008). Such behavior cannot be accommodated by a two-state model and hints that most, if not all, GPCRs can assume multiple active states.
The ability to engage endogenous GPCRs and initiate G protein-independent signals while antagonizing G protein activation makes arrestin pathway-selective biased agonists attractive tools for studying arrestin-dependent signaling in vivo. Nonetheless, they have limitations. When administered in vivo, biased agonists will block heterotrimeric G protein signaling in response to endogenous hormones or neurotransmitters. This creates interpretive problems, especially in disease models such as heart failure, where inhibiting G protein signaling is known to confer benefit. In such cases, it is difficult to convincingly demonstrate that a physiologic response is due to the activation of G protein-independent pathways. Given the ever-expanding scope of GPCR signaling events, it is likewise difficult to attribute a G protein-independent response to arrestin-mediated signaling unless the experiment is repeated in an appropriately arrestin-null background.
V. Arrestin Signaling in Vivo
Recent studies have begun to reveal some of the physiologic functions of arrestin that extend beyond its role in GPCR desensitization. As summarized in Table 2, current evidence suggests that arrestin signaling plays important roles in some aspects of embryologic development, retinal physiology, central nervous system and cardiovascular function, bone remodeling, immune regulation, cancer, and energy metabolism.
TABLE 2.
Reported in vivo roles of arrestin-dependent signaling
| Physiologic Process | Receptor | Arrestin | Proposed/Possible Effector Pathway(s) | References |
|---|---|---|---|---|
| Embryologic development | ||||
| Hedgehog signaling | ||||
| Hematopoiesis | Smootheneda | z-Arr2 | YY1 transcription factor | Yu et al., 2008 |
| Embryo patterning | Smootheneda | z-Arr3 | Gli family transcription factors | Wilbanks et al., 2004 |
| Retinal function | ||||
| Autosomal dominant retinitis pigmentosa | Rhodopsin K296E | Arr1 | N.D. | Li et al., 1995 |
| Photoreceptor cell apoptosis | Rhodopsin | d-Arr1 | RdgC-inhibition of caspase-dependent cell death | Alloway et al., 2000; Kiselev et al., 2000 |
| Dopamine-dependent behaviors | ||||
| Attenuation of dopamine-dependent hyperactivity | Dopamine D2 | Arr3 | Activation of GSK3β by arrestin3-PP2A-Akt complex | Beaulieu et al., 2004, 2005 |
| Lithium and neuroleptic drug action | Dopamine D2 | Arr3 | Blockade of arrestin-dependent GSK3β activation | Beaulieu et al., 2008; Masri et al., 2008 |
| Cardiovascular regulation | ||||
| Cardiomyocyte hypertrophy; bradycardia | Angiotensin AT1a | N.D. | Src; ERK1/2; p90RSK | Zhai et al., 2005 |
| Aldosterone secretion and volume retention | Angiotensin AT1a | Arr2 | ERK1/2; upregulated StAR expression | Lymperopoulos et al., 2009 |
| Cardiomyocyte survival in heart failure | β1 adrenergic | Arr3 | Src-dependent EGF receptor transactivation | Noma et al., 2007 |
| Vascular smooth muscle neointimal hyperplasia | N.D. | Arr3 | ERK1/2; EGF receptor transactivation; p90RSK-Akt | Ahn et al., 2009; Kim et al., 2008 |
| Cutaneous vasodilation and flushing | GPR109A | Arr2 | cPLA2-prostaglandin D2 pathway activation | Walters et al., 2009 |
| Skeletal remodelling | ||||
| Stimulation of osteoblastic bone formation | PTH1 | Arr3 | N.D. | Gesty-Palmer et al., 2009 |
| Immune system function | ||||
| Allergen-induced Th2 lymphocyte chemotaxis | N.D. | Arr3 | N.D. | Walker et al., 2003 |
| Inhibition of neutrophil chemotaxis | CXCR2 | Arr3 | N.D. | Su et al., 2005 |
| Inhibition of chemotaxis and cytokine production | TLR4a | Arr3 | ERK1/2 activation; IκB-NFκB inhibition | Basher et al., 2008 |
| Inhibition of NK cell cytotoxicity | KIR2DL1a | Arr3 | SHP-1, SHP-2 dependent signaling | Yu et al., 2008 |
| Tumor growth and metastasis | ||||
| Bladder cancer proliferation; migration; metastasis | Thromboxane TP-β | Arr3 | ERK1/2; FAK | Moussa et al., 2008 |
| Breast cancer invasion; metastasis | LPA1; LPA2 | Arr2 | Ral, Ral-GDS mediated cytoskeletal rearrangement | Li et al., 2009 |
| Ovarian cancer progression; metastasis | Endothelin ET-A | Arr2 | Src-EGF receptor-ERK1/2-Akt; GSK3β−β-catenin | Rosanò et al., 2009 |
| Phorbol ester-induced papilloma formation | Prostaglandin EP2 | Arr2 | Src-EGF receptor-Ras-ERK1/2-Akt | Chun et al., 2009 |
| Inhibition of tumor cell migration | TGFβ receptor IIIa | Arr3 | Activation of cdc42; Inhibition of NFκB | Mythreye and Blobe, 2009; You et al., 2009 |
| Androgen-independent prostate cancer growth | Androgen receptora | Arr3 | Mdm2-dependent AR ubiquitination/degradation | Lakshmikanthan et al., 2009 |
| Metabolic regulation | ||||
| Increased insulin sensitivity | Insulin receptora | Arr3 | IR-Akt-Src complex assembly | Luan et al., 2009 |
z-, Zebrafish arrestin; d-, D. melanogaster arrestin; N.D., not determined.
Non-GPCR.
A. Arrestins in Development
The early embryonic lethality associated with complete loss of nonvisual arrestin expression in D. melanogaster and mice implies a role in development, and in vitro data supporting the idea that arrestins interface with a wide range of transcriptional regulatory pathways provide a number of plausible mechanisms. Recent work in zebrafish using morpholinos to silence arrestin expression offers dramatic evidence of the roles of arrestins in development and is starting to unravel the underlying signaling mechanisms.
One role of arrestins in development derives from its involvement in Hedgehog signaling. In developing zebrafish embryos, knockdown of arrestin3 phenocopies many of the defects observed in Smoothened loss-of-function mutants, including increased ventral body curvature, underdeveloped heads, U-shaped somites with fewer slow muscle fibers, partial cyclopia, lack of the optic nerve at the midline, loss of craniofacial muscle and pectoral fin development, and reduction of floor plate development (Wilbanks et al., 2004). In situ hybridization performed in arrestin3 morpholinos shows loss of expression of Shh-regulated genes, including nkx2.2 and ptc, consistent with loss of Smoothened signaling. The phenotypes resulting from arrestin3 deficiency can be rescued by expression of wild-type arrestin3 or by constitutive activation of the Hedgehog pathway downstream of Smoothened but not by overexpression of Shh, indicating that without arrestin3, the Hedgehog pathway is blocked downstream of the Shh signal but upstream of Su(fu) and Gli1/2. This is consistent with the proposed role of arrestins in Smoothened-dependent of Gli-dependent transcription (Kovacs et al., 2008) and strongly supportive of a signaling role for arrestins that is independent of their role as negative regulators of G protein signaling.
Comparison of the function of arrestin2 and arrestin3 in zebrafish development provides additional evidence of their role as transcriptional regulators (Yue et al., 2009). Whereas expression of ptc1, a downstream marker of Hedgehog signaling, is disrupted in arrestin3-deficient embryos, loss of arrestin2 has no effect on the Hedgehog pathway. Instead, arrestin2-deficient embryos show decreased expression of early hematopoietic and vascular progenitor markers, including lmo2 and fli1. Arrestin2-deficient embryos exhibit severe posterior defects and fail to undergo hematopoiesis, corresponding to down-regulation of cdx4, a homeobox transcription factor that specifies the hematopoietic lineage by modulating hox gene expression. The hematopoietic defects can be rescued by reintroducing arrestin2 or by injecting cdx4, hoxa9a, or hoxb4a mRNA, demonstrating the linkage to arrestin2 signaling. The underlying mechanism seems to involve arrestin2 binding to the polycomb group recruiter YY1, a ubiquitously expressed transcription factor essential for embryonic development. Arrestin2 binding sequesters YY1, relieving polycomb group-mediated repression of cdx4-hox pathway.
B. Photoreceptor Function and Retinal Degeneration
Oguchi disease, a hereditary from of stationary night blindness in humans, results from inactivating mutations in the genes encoding arrestin1 or GRK1 that impair photoreceptor desensitization (Baylor and Burns, 1998). Among the causes of retinitis pigmentosa are mutations in rhodopsin that result either in constitutive receptor signaling or constitutive receptor-arrestin interactions. Arrestin-mediated signaling may underlie an autosomal-dominant form of retinitis pigmentosa associated with a K296E mutation in the opsin binding site of rhodopsin that precludes chromophore binding. The mutant receptor cannot be activated by light but is capable of constitutively activating transducin in vitro. In vivo, however, it is constitutively phosphorylated and bound to arrestin and elicits no detectable transducin activity, suggesting that the retinal degeneration does not arise from continuous activation of the phototransduction cascade (Li et al., 1995). Given that both arrestin 1 and 4 bind JNK3 and Mdm2, it is tempting to speculate that an arrestin-mediated proapoptotic signal is involved (Song et al., 2006, 2007).
The best evidence of a role for arrestin signaling in the retina comes from D. melanogaster; studies suggest that excess stimulation of transducin-dependent phototransduction pathways that are inhibited by arrestin lead to photoreceptor necrosis, whereas arrestin-mediated G protein-independent signals mediate photoreceptor apoptosis (Ranganathan, 2003). Unlike vertebrates, which use a transducin-activated cGMP phosphodiesterase for phototransduction, D. melanogaster uses a Gq-coupled pathway that is inactivated by arrestins. Along with arrestin, the diacylglycerol kinase RdgA is required for rhodopsin desensitization in the invertebrate eye. RdgA catalyzes the conversion of diacylglycerol to phosphatidic acid, reducing diacylglycerol levels and dampening the PKC pathway. A loss-of-function mutation in RdgA permits constitutive activity of light-sensitive TRP channels, leading to photoreceptor cell degeneration and blindness (Raghu et al., 2000). Similar to the RdgA mutant, rhodopsin activation will induce necrosis if arrestin is lost (Dolph et al., 1993). Retinal degeneration in arrestin-null flies cannot be rescued by eye-specific expression of the baculoviral p35 Caspase inhibitor protein (Alloway et al., 2000) but can be rescued by disruption of Gq function (Kiselev et al., 2000), indicating that the protection from necrosis arises from arrestin-mediated desensitization of a Gq-dependent pathway.
In contrast, the RdgC loss-of-function mutation in D. melanogaster produces light-dependent photoreceptor cell apoptosis (Davidson and Steller, 1998). RdgC is a calcium-dependent kinase that promotes dissociation of the rhodopsin-arrestin complex. This form of retinal degeneration is enhanced by a loss-of-function Gq mutation or by deleting the arrestin phosphorylation domain (Kiselev et al., 2000), both of which stabilize the rhodopsin-arrestin interaction. Triple inactivation of Gαq, arrestin, and RdgC rescues the phenotype, as does expression of the p35 caspase inhibitor, implying an arrestin-dependent apoptotic signal transmitted by a stable rhodopsin-arrestin complex. Likewise, deletion of the eye-specific phospholipase C gene in D. melanogaster results in the constitutive formation of rhodopsin-arrestin complexes and retinal degeneration by apoptosis. The degeneration is rescued by blocking rhodopsin endocytosis with an inactivating mutation of the D. melanogaster dynamin homolog or by simultaneously deleting arrestin (Alloway et al., 2000).
C. Dopamine Receptor Signaling and Behavior
Dopaminergic neurotransmission in the central nervous system regulates behavioral responses such as locomotor activity and neural reward mechanisms. Loss of dopaminergic cells in the substantia nigra leads to a loss of locomotor control in Parkinson's disease. Conversely, D2 dopamine receptor antagonists are effective neuroleptic drugs used in the treatment of schizophrenia and attention deficit hyperactivity disorder, which are thought to result from excess dopaminergic neurotransmission. Several lines of evidence suggest that arrestin signaling complexes regulate dopamine-dependent behaviors. Locomotor hyperactivity induced by the dopaminergic drug apomorphine, a D2 receptor agonist, is reduced in arrestin3 knockout mice (Beaulieu et al., 2008). Likewise, the hyperactivity displayed by dopamine transporter knockout mice, which results from increased synaptic dopamine concentration, is reduced when dopamine transporter knockout mice are crossbred with arrestin3 knockouts, a paradoxical result, because G protein-mediated responses would be enhanced by the loss of arrestin-dependent desensitization. Likewise, GRK6 knockout mice have augmented locomotor responses to dopaminergic drugs (Gainetdinov et al., 2003). GRK6 is the most abundant GRK in the striatum and has been implicated in arrestin signaling via the AT1A receptor (Kim et al., 2005).
The molecular basis of these effects may lie in the scaffolding function of arrestins. D2 receptors have been reported to positively (Brami-Cherrier et al., 2002) and negatively regulate the prosurvival kinase Akt (Beaulieu et al., 2004). D2 receptor-mediated phosphatidylinositol-3 kinase-independent Akt activation is not fully understood, but D2 receptor-mediated inhibition of Akt involves the formation of a D2 receptor-arrestin3-PP2A-Akt complex. Arrestin3-bound PP2A dephosphorylates Akt Thr308, inactivating it. Because Akt inhibits GSK3β by phosphorylating it on Ser9, D2 receptor-mediated Akt inhibition favors GSK3β activation (Beaulieu et al., 2004, 2005, 2008). Amphetamine treatment, which increases synaptic dopamine release, increases the PP2A-Akt association in wild-type, but not arrestin3 knockout mice, suggesting that arrestins mediate the interaction (Beaulieu et al., 2008). Directly inhibiting PP2A or GSK3β attenuates hyperactive locomotor activity in dopamine transporter knockout mice, suggesting that dopamine-mediated activation of GSK3β results from PP2A-dependent Akt inhibition that is scaffolded by arrestin 3 (Beaulieu et al., 2005).
Modulation of arrestin scaffolding may play a role in the mechanism of action of many antipsychotic drugs. Lithium, a mood stabilizer used in the treatment of schizophrenia, modulates dopamine-dependent behavior (such as horizontal activity) in mice. GSK3β haploinsufficient mice exhibit augmented lithium-induced antidepressant and anxiolytic effects compared with wild-type animals, suggesting that lithium acts by inhibiting GSK3β. In addition to directly inhibiting GSK3β, therapeutic concentrations of lithium disrupt the interaction between arrestin, Akt, and PP2A, relieving PP2A-mediated negative regulation of Akt, allowing it to phosphorylate and inactivate GSK3β (Beaulieu et al., 2008). The arrestin3-PP2A-Akt complex requires magnesium, and lithium is thought to destabilize the complex by competing for magnesium binding. The clinical efficacy of essentially all other classes of antipsychotic drug correlates directly to their dopamine D2 receptor binding affinity and ability to antagonize the receptor. In a recent in vitro screen using fluorescence-based reporters, it was found that although different classes of antipsychotics exhibit complex efficacy profiles with respect to D2 receptor-G protein coupling, they share the property of antagonizing the D2 receptor-arrestin3 interaction (Masri et al., 2008).
D. Cardiovascular Roles of Arrestins
GPCRs play important roles throughout the cardiovascular system. Blood pressure is regulated by changes in heart rate, vascular resistance, and fluid/electrolyte balance, each of which is regulated to a large degree by GPCRs. Heart rate is regulated by adrenergic and muscarinic receptor activation by norepinephrine and acetylcholine, respectively. Vascular tone is regulated by adrenergic, angiotensin II, and bradykinin receptor activation, and renal function is influenced by angiotensin II, adrenergic, vasopressin, and dopamine receptors. In addition to arrestin2- and arrestin3-null mice, efforts to define a physiologic role for arrestin signaling in the cardiovascular system has been facilitated by the availability of G protein-uncoupled mutant receptors and pathway-selective biased agonists. Angiotensin AT1A receptor signaling has been studied using receptors mutated in the conserved DRYXX(V/I)XXPL region of the amino terminal portion of the second intracellular loop: D125G/R126G/Y127A/M134A (AT1-i2m) and D125A/R126A (DRY/AAY), which fail to activate G protein signaling but nonetheless support arrestin recruitment (Seta et al., 2002; Gáborik et al., 2003), and using the angiotensin II analog Sar1-Ile4-Ile8-Ang II, which antagonizes Gq/11 coupling but promotes GRK phosphorylation, arrestin recruitment, and receptor endocytosis (Holloway et al., 2002). Analogous reagents for the β2 adrenergic receptor include the T68F/Y132G/Y219A mutant [β2AR(TYY)] (Shenoy et al., 2006) and a number of arrestin pathway-selective β2 receptor inverse agonists, including ICI118,551 and the clinically used drugs propranolol and carvedilol (Gong et al., 2002; Azzi et al., 2003; Wisler et al., 2007; Drake et al., 2008).
1. Cardiac Hypertrophy and Failure.
In vitro, the AT1-i2m mutant angiotensin receptor lacks demonstrable G protein coupling but activates a Src-Ras-ERK1/2 pathway leading to cytosolic ERK1/2 and p90RSK activation (Seta et al., 2002). In vivo, cardiomyocyte-specific overexpression of AT1-i2m leads to greater cardiomyocyte hypertrophy, bradycardia, and fetal cardiac gene expression than comparable overexpression of the wild-type receptor. Conversely, overexpressed wild-type AT1A receptors produce greater cardiomyocyte apoptosis and interstitial fibrosis than the G protein-uncoupled mutant, suggesting that G protein-dependent and -independent AT1A receptor signals mediate different aspects of the hypertrophic response (Zhai et al., 2005).
Although these studies do not directly implicate arrestin signaling, because they were not repeated in arrestin-null mice, studies using Sar1-Ile4-Ile8 provide insights into the possible role of arrestins in cardiac AT1A receptor signaling. In primary cardiomyocytes, Sar1-Ile4-Ile8 activates cytosolic but not nuclear ERK1/2, causing activation of p90RSK but not the nuclear transcription factor Elk1, which requires nuclear translocation of ERK1/2. In the absence of G protein activity, Sar1-Ile4-Ile8 stimulates cardiomyocyte proliferation but not hypertrophy, which requires Gq/11 (Aplin et al., 2007). Sar1-Ile4-Ile8 also produces positive inotropic and lusitropic effects on isolated murine cardiomyocytes (Rajagopal et al., 2006). These effects require GRK6 and arrestin 3, whereas GRK2 seems to oppose them, consistent with the specialized role of GRK isoforms described in a transfected system (Kim et al., 2005). On the other hand, Sar1-Ile4-Ile8 does not produce inotropic or chronotropic effects in isolated Langendorff-perfused cardiac preparations despite its ability to activate ERK1/2 (Aplin et al., 2007). Sar1-Ile4-Ile8 has also been studied in vivo, where its principal effect seems to be to promote aldosterone production via arrestin2- and ERK1/2-dependent up-regulation of steroidogenic acute regulatory protein, a cholesterol transport protein regulating aldosterone biosynthesis. The resultant marked elevation in circulating aldosterone levels contributes to adverse cardiac remodeling and heart failure progression (Lymperopoulos et al., 2009).
In contrast to the apparently harmful effects of arrestin-dependent signaling downstream of the AT1A receptor, arrestins reportedly mediate beneficial effects in the heart when engaged by the β1 adrenergic receptor. The β1 adrenergic receptor regulates inotropy and chronotropy in the heart by coupling to the Gs-adenylyl cyclase-PKA pathway. In heart failure, chronic catecholamine stimulation promotes selective β1 receptor down-regulation and also promotes cardiac hypertrophy, decreased contractility, and increased myocyte apoptosis (Ungerer et al., 1993; Xiang and Kobilka, 2003). As a result, administration of β-adrenergic receptor blockers is currently part of standard care in the clinical management of congestive heart failure. In transfected HEK293 cells, β1 receptor-mediated mitogenic signaling via EGF receptor transactivation is arrestin-dependent. Consistent with this, a mutant β1 receptor lacking 14 GRK phosphorylation sites in its C-terminal tail (−GRKβ1), which cannot undergo arrestin-dependent desensitization, fails to transactivate the EGF receptors despite exaggerated G protein activation. In response to chronic isoproterenol stimulation, transgenic mice expressing the −GRKβ1 receptor develop more severe dilated cardiomyopathy with significantly increased LV end-diastolic dimension, decreased fractional shortening, and increased myocardial apoptosis compared with wild-type β1 receptor transgenic mice. In this model, inhibiting EGF receptors worsens the dilated cardiomyopathy, suggesting a protective rather than deleterious role for transactivated EGF receptors in the heart (Noma et al., 2007). Although the authors cannot exclude a role for exaggerated cAMP production by the nondesensitizing −GRKβ1 receptor in causing the more severe cardiac phenotype, they speculate that arrestin-dependent EGF receptor transactivation exerts a cardioprotective effect and that activating β1 receptor-mediated arrestin signaling may be of therapeutic benefit in heart failure.
Several inverse agonists for β adrenergic receptor - Gs coupling, including carvedilol and alprenolol, have been shown to promote arrestin-dependent EGF receptor transactivation and ERK1/2 activation in vitro (Wisler et al., 2007; Kim et al., 2008a) Unfortunately, arrestin pathway-selective agonists are of limited value in determining whether β1 receptor-mediated arrestin signaling produces salutary effects in heart failure models; like nonselective β-adrenergic receptor blockers, they antagonize activation of Gs-cAMP signaling by endogenous catecholamines, making it difficult to attribute changes to the activation of arrestin pathways as opposed to the inhibition of G protein signaling. Likewise, it is unclear what role, if any, arrestin signaling plays in the demonstrated survival benefit of carvedilol in human heart failure, where the positive outcome might be attributable to its actions as a nonselective β1/2 and α1 adrenergic receptor antagonist (Packer et al., 1996; Packer et al., 2002).
2. Vascular Smooth Muscle Hypertrophy and Hyperplasia.
Arrestin-dependent signaling appears to promote the development and progression of atherosclerotic vascular disease. Neointimal hyperplasia after carotid endothelial injury is enhanced in arrestin2-null mice but diminished in arrestin3-null mice. Loss of arrestin3 is associated with decreased GPCR-stimulated vascular smooth muscle cell proliferation and ERK1/2 activation/migration, consistent with a role for arrestin3-signaling in the injury response. When the low-density lipoprotein receptor-null mouse, a genetic model of enhanced atherogenesis, is crossed onto an arrestin3-null background, both the prevalence of atheromas and the involved area are significantly reduced (Kim et al., 2008b). These in vivo findings are supported by in vitro studies in vascular smooth muscle demonstrating arrestin-dependent mitogenic and antiapoptotic effects of the angiotensin AT1A receptor. Both G protein- and arrestin-dependent pathways converge on the EGF receptor in primary vascular smooth muscle cells to stimulate proliferation. In vascular smooth muscle, Sar1-Ile4-Ile8 induces ERK1/2 activation and proliferation by promoting AT1A receptor-dependent EGF receptor transactivation without stimulating G protein activity (Miura et al., 2004). Angiotensin II and Sar1-Ile4-Ile8 both stimulate Src-dependent EGF receptor phosphorylation on Tyr845, an effect that is lost when arrestin3 is down-regulated by RNA interference (Kim et al., 2009). In addition, arrestin-dependent activation of the ERK1/2 substrate p90RSK acts in concert with phosphatidylinositol-3 kinase-Akt to down-regulate phospho-BAD, inducing antiapoptotic cytoprotective effects in rat vascular smooth muscle (Ahn et al., 2009).
3. Vascular Tone and Reactivity.
Through its actions on GPR109A, nicotinic acid has been shown to decrease serum free fatty acids by activating Gi/Go signaling and to increase cutaneous blood flow/flushing in humans and in mice by stimulating cytosolic phospholipase A2, leading to the production and secretion of prostaglandin D2 (Kather et al., 1983; Pike, 2005). Comparison of the responses of wild-type C57BL/6, arrestin2-null, and arrestin3-null mice to nicotinic acid demonstrates that neither arrestin2 nor arrestin3 is required for nicotinic acid-induced changes in free fatty acid levels. In contrast, flushing, measured by perfusion of the ventral ear, is stimulated by nicotinic acid treatment in wild-type and arrestin3-null mice, but not in arrestin2-null mice. In vitro, nicotinic acid promotes arrestin2 binding and activation of phospholipase A2, stimulating the release of arachidonate, the precursor of prostaglandin D2, the vasodilator responsible for the flushing response (Walters et al., 2009). These data suggest that a GPR109A ligand capable of activating G protein signaling without arrestin recruitment would mimic the effects of niacin on lipid metabolism without the side effect of flushing, a major limitation to its clinical use. Indeed, 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopentapyrazole (MK-0354), a recently developed partial agonist of the nicotinic acid receptor GPR109A that decreases serum free fatty acids without producing cutaneous flushing (Semple et al., 2008), does not promote arrestin binding in vitro.
E. Skeletal Remodeling
PTH is an 84-amino acid peptide hormone that serves as the principal regulator of calcium and phosphate homeostasis. It acts via PTH1 receptors that are highly expressed in kidney and bone. PTH directly stimulates bone-forming osteoblasts, promoting the deposition of new bone matrix and accelerating the rate of mineralization by increasing both osteoblast number and activity. At the same time, PTH stimulates bone resorption by increasing the recruitment, differentiation, and activity of bone-resorbing osteoclasts. Lacking PTH receptors, osteoclasts respond to factors such as receptor activator of NFκB ligand and osteoprotegerin that are secreted by osteoblasts in response to PTH. Because the anabolic and catabolic effects of PTH are coupled, the net effect of PTH on bone depends on the kinetics of receptor activation, with intermittent exposure leading to a net increase in bone formation, whereas continuous exposure produces net bone loss (Hock and Gera, 1992; Dobnig and Turner, 1995; Qin et al., 2004). N-terminal analogs of PTH [e.g., PTH(1–34)] can elicit the full range of PTH1 receptor signaling. PTH(1–34) activates G protein signaling, including the Gs-adenylyl cyclase-PKA and Gq/11-phospholipase Cβ-PKC pathways. In addition, PTH(1–34) promotes translocation of both arrestin2 and arrestin3 to the plasma membrane and arrestin-dependent internalization of PTH1 receptor (Ferrari et al., 1999; Vilardaga et al., 2002). PTH(1–34) stimulates ERK1/2 by two temporally distinct mechanisms: a conventional G protein-dependent pathway that involves PKA or PKC in a cell-type-specific manner, and a G protein-independent pathway transmitted by arrestins (Verheijen and Defize, 1997; Cole 1999; Gesty-Palmer et al., 2006).
The signaling output of PTH1 receptors is sensitive to changes in ligand structure, and pathway-selective PTH analogs have proven to be valuable tools for determining the role of different PTH1 receptor signaling pathways both in vitro and in vivo. Whereas PTH(1–34) activates both the PKA and PKC pathways, PTH(1–31) stimulates only cAMP production (Azarani et al., 1996; Takasu et al., 1999; Mohan et al., 2000). Daily administration of either PTH(1–34) or PTH(1–31) produces an anabolic skeletal effect in rats. However, PTH(3–38), which activates PKC, but not PKA, does not (Mohan et al., 2000). The G protein-dependent and arrestin-dependent actions of PTH are also dissociable using PTH analogs that induce Gs-coupled (Trp1)PTHrp-(1–36) or arrestin-coupled (d-Trp12,Tyr34)-PTH(7–34) conformations of the receptor (Gesty-Palmer et al., 2006).
Recent data obtained using this arrestin pathway-selective PTH1 receptor agonist have provided surprising insights into the role of arrestin signaling in bone (Gesty-Palmer et al., 2009). In vivo, arrestin3-null mice have normal serum calcium levels and grossly normal skeletal structure. Circulating levels of endogenous PTH are suppressed, presumably in compensation for impaired desensitization of PTH-stimulated G protein activation (Pi et al., 2005). However the loss of arrestin3 does alter underlying bone metabolism. Basal rates of bone turnover are higher. Osteoid surface and osteocalcin mRNA levels are increased, consistent with an increase in the rate of bone formation. At the same time bone, resorption is accelerated, as evidenced by increased osteoclast surface, marrow osteoclast precursors, and bone turnover markers, such as receptor activator of NFκB ligand and osteoprotegerin mRNA and urine deoxypyridinoline (Gesty-Palmer et al., 2009; Pierroz et al., 2009). Arrestin3-null mice have an impaired anabolic response to exogenous PTH(1–34), with blunted increases in bone volume and trabecular thickness and no change in trabecular number, periosteal circumference, or cortical thickness compared with control mice. The attenuated response is associated with exaggerated increases in osteoclast number and urine deoxypyridinoline, indicative of accelerated bone resorption (Bouxsein et al., 2005; Ferrari et al., 2005; Gesty-Palmer et al., 2009). Collectively, these data indicate that bone turnover, reflective of osteoblast-dependent osteoclast activation, is primarily a G protein-mediated response to PTH that is restrained by arrestin-dependent desensitization. However, wild-type mice treated with the arrestin pathway-selective PTH analog (d-Trp12,Tyr34)-PTH(7–34) show a paradoxical increase in bone formation, characterized by increased trabecular number and thickness, increased osteoblast number, osteoid surface, and mineral apposition rate but no concomitant increase in osteoclast number or markers of accelerated bone turnover (Gesty-Palmer et al., 2009). The response is absent in arrestin3-null mice, consistent with its dependence on arrestin-mediated signaling. Thus, arrestin signaling in vivo seems to contribute to the anabolic response to PTH and is sufficient to stimulate osteoblastic bone formation but not to stimulate osteoblast-dependent osteoclast activation. The ability of an arrestin pathway-selective PTH agonist to uncouple bone formation from bone resorption provides the first compelling example of an arrestin pathway-selective GPCR ligand that may possess clinically useful properties distinct from those of a conventional agonist.
F. Chemotaxis and the Immune Response
The immune response in vivo is a complex process, wherein tissue injury triggers an initial release of chemoattractants that prompt circulating leukocytes to adhere to the vascular endothelium, penetrate the vessel wall, migrate to the site of injury, and degranulate. The process is amplified as more inflammatory cells accumulate and additional cytokines are synthesized and released. Arrestins may function in both positive and negative regulation of the immune response, and the net effect in vivo seems to be highly context dependent.
Arrestin-mediated receptor desensitization and recycling are likely to be critical for migrating cells to maintain the ability to sense and follow a chemoattractant gradient (Tomhave et al., 1994; Aragay et al., 1998). At the same time, arrestin scaffolding seems to be required for the establishment of cell polarity and to facilitate degranulation at the site of injury. There is no doubt that splenocytes from arrestin3-deficient mice show impaired CCR4-mediated chemotaxis in transwell and transendothelial migration assays despite amplified G protein signaling (Fong et al., 2002). Similar data have been obtained for other GPCRs, including PAR-2 receptors in MDA-MB-231 cells (Ge et al., 2004), angiotensin AT1A receptors in HEK293 cells (Hunton et al., 2005), CXCR1 (IL-8) receptors in RBL cells (Barlic et al., 1999), CXCR2 (IL-8) receptors in neutrophils (Richardson et al., 2003), and CXCR3 (CCL-9, -10), CXCR4 (stromal cell-derived factor 1α), and CCR5 [RANTES (regulated on activation normal T cell-expressed and secreted)] receptors in HEK293 cells (Sun et al., 2002; Colvin et al., 2004; Lagane et al., 2005). Of these, the involvement of G protein-independent signaling is most convincing for the AT1A receptor, because cells will follow a gradient of the arrestin pathway-selective AT1A receptor agonist Sar1-Ile4-Ile8, a response that is lost when arrestins are down-regulated. In addition to regulating cortical actin assembly through arrestin-dependent activation of ERK1/2 and a cofilin-LIM kinase-chronophin complex (DeFea, 2007), recent evidence suggests that other arrestin-regulated effectors, including the Src family kinase, Lyn (Cheung et al., 2009), PP2A (Basu et al., 2007), and the small GTPase, Ral (de Gorter et al., 2008; Li et al., 2009) are involved in chemokine-induced chemotaxis in vitro.
On the other hand, the finding that arrestins function as negative regulators of transcriptional pathways involved in cytokine signaling, e.g., NFκB, suggests that they may dampen the immune response by inhibiting the production of pro-inflammatory cytokines, thereby limiting the intensity of the chemotactic stimulus itself. Consistent with such an inhibitory role, isolated peritoneal neutrophils from arrestin3-null animals show increased basal and lipopolysaccharide-stimulated tumor necrosis factor α and IL-6 production (Basher et al., 2008). A novel arrestin3-dependent mechanism also seems to negatively regulate the activity of natural killer (NK) cells, a key component of the innate immune response. Arrestin3 mediates recruitment of the tyrosine phosphatases SHP-1 and SHP-2 to KIR2DL1, an inhibitory receptor of NK cells, facilitating downstream inhibitory signaling (Yu et al., 2008).
Which of the potentially opposing functions of arrestins dominates in vivo is unclear. A study of allergic asthma found that in allergen-sensitized arrestin3-null mice, Th2 cells did not accumulate in the airways; levels of the Th-derived cytokines IL-4, IL-5, and IL-13 in bronchoalveolar lavage fluid were markedly reduced; and Th2 cell chemotaxis into the lungs was blunted, prompting the authors to speculate that novel therapies targeting arrestin function might be of therapeutic value in asthma (Walker et al., 2003). In contrast, an in vivo study of CXCR2-mediated chemotaxis found that neutrophil recruitment to sites of inflammation was increased in arrestin3-null mice despite impaired receptor desensitization and endocytosis (Su et al., 2005). These authors found that neutrophil recruitment in both a dorsal air pouch and an excisional wound model was enhanced in the absence of arrestin3, as was wound re-epithelialization, prompting them to conclude that arrestin3 is an overall negative regulator of CXCR2 signaling in vivo. Similar results were reported using intraperitoneal injection of oyster glycogen and cecal ligation and puncture models of neutrophil infiltration, where arrestin3-null mice showed significantly higher peritoneal and pulmonary neutrophil accumulation than wild-type control mice (Basher et al., 2008). Supportive of the negative role of arrestin3, NK cell cytotoxicity is higher in arrestin3-null mice and attenuated in arrestin3 transgenics. Consistent with this, arrestin3-null mice are less susceptible than wild-type mice to murine cytomegalovirus infection, protection that is lost upon depletion of NK cells (Yu et al., 2008). Whether these conflicting results indicate that arrestins perform different functions in different leukocyte populations, or that some functions of arrestins (e.g., negative regulation of pro-inflammatory NFκB signaling) have greater impact on the immune response in vivo that its role in chemotaxis, it is clear that additional work is required to understand the relevance of arrestin signaling in the immune system.
G. Tumor Growth and Migration
In some respects, tumor growth and migration may be a simpler model in which to understand the roles of arrestin signaling in cell growth, survival, and migration than the immune system, simply because tumor cells are not subject to as much negative regulation. Recent evidence from a number of in vivo tumor models supports a role for arrestin signaling in cancer development or progression. One of the more striking, because it is supported by human outcomes data, involves invasive transitional cell carcinoma of the bladder. Unlike normal bladder epithelium, human bladder cancer cells express high levels of both the thromboxane (TP)-β receptor isoform and arrestins, and the degree of TP-β up-regulation correlates with poorer prognosis (Moussa et al., 2008). TP-α and TP-β are splice variants that share the first 328 residues but differ in the C terminus, TP-β carrying a longer tail. This allows TP-β, but not TP-α, to engage arrestin3 and undergo agonist-dependent internalization (Parent et al., 1999). Expressing TP-β in nontransformed SV-HUV urothelial cells, which normally express only TP-α, confers agonist-dependent ERK1/2 and focal adhesion kinase phosphorylation, and enhances cell proliferation, migration, and invasion in vitro, responses that are lost when arrestin3, but not arrestin2, is down-regulated by RNA inference. Treatment with a TP receptor antagonist delays tumor onset and prolongs survival in a murine xenograft model using human TCC-SUP bladder cancer cells, which, like native tumors, expresses high levels of TP-β (Moussa et al., 2008).
Several arrestin-dependent signals may promote more aggressive cancer phenotypes. LPA1 and LPA2 receptors, arrestins2 and -3, Ral and Ral-GDS are up-regulated in more advanced stages of human breast cancer. In vitro, LPA stimulates the migration and invasion of MDA-MB-231 breast cancer cells, which overexpress arrestin2 relative to nontumorigenic MCF-10A cells, and down-regulating arrestin or Ral expression inhibits the response (Li et al., 2009). Endothelin ET-A receptors are expressed in a majority of human ovarian cancers, and its coexpression with arrestin2 increases with advancing tumor grade. In ovarian cancer cells in culture, arrestins promote the assembly of ET-A receptor complexes with c-Src, leading to EGF receptor transactivation and β-catenin phosphorylation, and with axin, promoting GSK3β inactivation and β-catenin stabilization. Silencing arrestin expression inhibits ET-A receptor activation of c-Src, ERK1/2, Akt, and EGF receptor and completely blocks β-catenin-dependent activation of a TCF/Lef reporter. In a tumor xenograft model, ovarian cancer cells expressing the arrestin2 S412D mutant, which impairs arrestin-dependent ET-A receptor signaling, develop fewer metastases, suggesting that arrestin signaling contributes to ovarian tumorigenesis and progression (Rosanò et al., 2009). Arrestin2-dependent activation of c-Src and EGF receptor also seems to contribute to the tumor-promoting effects of prostaglandin E2 receptors in papilloma formation. In vivo, phorbol esters promote papilloma formation in part by stimulating prostaglandin E2 production. Prostaglandin E2 receptors stimulate cAMP production and EGF receptor-dependent activation of h-Ras, ERK1/2, and Akt, and prostaglandin E2 receptor-null mice show reduced activity in these pathways and develop far fewer papillomas upon exposure to phorbol ester. EGF receptor transactivation is associated with formation of a prostaglandin E2 receptor-arrestin2-Src complex, suggesting a role for arrestin-dependent EGF receptor transactivation in the development of cutaneous tumors (Chun et al., 2009).
Arrestin effects in cancer seem to extend beyond the regulation of GPCR signaling networks. Loss of expression of the type III transforming growth factor-β (TβRIII) occurs in a variety of human malignancies, including breast, lung, pancreatic, renal cell, ovarian, and prostate cancer. TβRIII is thought to function as a tumor suppressor by reducing cell motility. Clathrin-dependent endocytosis of TβRIII (albeit not a GPCR) and down-regulation of TGFβ signaling depends on its interaction with arrestin3 (Finger et al., 2008). Recent in vitro evidence indicates that the scaffolding functions of arrestin3 play a key role in the ability of TβRIII to inhibit cell migration. In breast and ovarian cancer cell lines, activation of the small GTPase Cdc42 by TβRIII alters actin cytoskeletal rearrangement and reduces random cell migration (Mythreye and Blobe, 2009). A TβRIII mutant unable to interact with arrestins fails to inhibit migration, and the wild-type receptor effect is blocked by down-regulating arrestin3. In addition, the interaction between TβRIII and arrestin3 negatively regulates NFκB transcriptional activity, further inhibiting cell migration (You et al., 2009). Arrestins even seem to modulate androgen receptor function in prostate cancer. Arrestin3 binds to the androgen receptor and acts as an androgen receptor corepressor in androgen-dependent prostate cancer cells. The arrestin functions as an adapter, bringing Mdm2 into proximity with the androgen receptor and promoting its ubiquitination and degradation (Lakshmikanthan et al., 2009). As a result, overexpression of arrestin3 attenuates, and down-regulation of arrestin3 increases, androgen-induced expression of prostate-specific antigen. In this model, loss of arrestin3 expression would lead to increased androgen receptor expression and the dysregulation of androgen receptor signaling network that promotes androgen-independent growth and tumor progression. Consistent with this, human prostate tissue exhibits an inverse relationship between arrestin2 expression and prostate-specific antigen level.
H. Metabolic Regulation
The maintenance of glucose homeostasis is achieved by the opposing actions of insulin (the major hormone promoting peripheral glucose uptake, lipogenesis, and glycogen synthesis), and a number of counterregulatory hormones, such as epinephrine and glucagon, which promote gluconeogenesis, lipolysis, and glycogenolysis. Arrestin-dependent desensitization of these receptors would dampen counter-regulation, leading to the prediction that loss of arrestins should promote insulin resistance by sensitizing tissues to the actions of counter-regulatory GPCRs. In addition, arrestin-dependent signals reportedly affect insulin signaling in ways that might contribute to insulin resistance and promote hyperglycemia. In cultured pancreatic β cells, arrestin2 plays a positive role in signaling by the glucagon-like peptide-1 receptor, a physiologically important enhancer of insulin secretion. Silencing arrestin2 expression attenuates glucagon-like peptide-1 signaling, leading to decreased cAMP levels and decreased ERK1/2 and CREB activation, and impaired insulin secretion (Sonoda et al., 2008). In cultured fibroblasts, arrestin2 increases expression of insulin receptor substrate-1, a key adapter protein in the insulin receptor signaling cascade, by inhibiting its insulin-induced ubiquitination by Mdm2 and protecting it from degradation (Usui et al., 2004).
Not surprisingly, then, arrestin2-null mice are insulin-resistant, as demonstrated by oral glucose and insulin tolerance testing, whereas transgenic mice with hepatic overexpression of human arrestin3 exhibit enhanced insulin sensitivity. Likewise, insulin sensitivity in spontaneously diabetic db/db mice improves when hepatic arrestin3 expression is increased by adenoviral overexpression. Arrestin3-null mice have decreased insulin-stimulated phosphorylation of Akt, GSK-3β, and FOXO1, a finding that correlates with the loss of an arrestin3 scaffolding complex containing the insulin receptor, Akt and Src. Likewise, wild-type mice and db/db mice develop insulin resistance when an arrestin3(1–185) truncation mutant that disrupts the arrestin-mediated Src-Akt interaction is introduced into the liver, prompting the authors to propose that arrestin-dependent insulin receptor-Src-Akt signaling is an important determinant of insulin sensitivity in vivo (Luan et al., 2009). Although these experiments do not exclude a role for impaired GPCR desensitization and enhanced counter-regulatory hormone action in the development of insulin resistance, they raise the intriguing possibility that arrestin scaffolding plays a direct role in insulin receptor signaling in vivo.
VI. Summary and Future Directions
Since the initial discovery more than a decade ago that arrestins recruit Src family kinases to activated GPCRs, we have come to appreciate that arrestins function as versatile scaffolds that modulate signaling by several types of receptor. Arrestin signaling brings a new dimension to GPCR biology; like the heterotrimeric G proteins, arrestins should be viewed as signal transducers linking GPCRs to a unique set of effector pathways. As our understanding of arrestin signaling grows, the finding that G protein- and arrestin-dependent signals can be dissociated using pathway-selective “biased” agonists takes on significance in the arena of drug discovery. It is now apparent that functional selectivity can be exploited to qualitatively change GPCR signal output. Although our understanding is far from complete, one can easily envision the clinical utility of biased ligands that preferentially activate or oppose G protein- and arrestin-mediated pathways to achieve desired therapeutic effects. For example, G protein pathway-selective compounds such as MK-0354, which circumvents arrestin-mediated vasodilation, may produce desirable lipid-lowering effects without the unwanted side effect of cutaneous flushing (Walters et al., 2009), whereas arrestin pathway-selective PTH1 receptor agonists may have utility in osteoporosis treatment (Gesty-Palmer et al., 2009). At the same time, it is clear that the potential for unwanted activation of arrestin-mediated pathways should not be overlooked during the drug discovery process. Although it is clear in hindsight that some current therapeutics, such as propranolol and carvedilol, possess arrestin pathway bias, one might imagine that true “neutral” antagonists of the angiotensin receptor intended for use as antihypertensives and in congestive heart failure may be preferable, because activation of arrestin pathways by AT1a receptors may promote cardiac hypertrophy and increase volume retention (Lymperopoulos et al., 2009). The situation is similar in cancer, where antagonizing arrestin binding to the TP-β receptor reduces bladder cancer invasiveness and metastatic potential (Moussa et al., 2008). What has emerged thus far from the study of arrestin signaling in vivo is proof of concept that selective pathway activation/inhibition by biased GPCR agonists has the potential to modify physiology in ways that cannot be duplicated by conventional drugs. The key for the future will be to determine what efficacy profile will produce the optimal treatment response for any given disease.
Acknowledgments.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK64353, DK55524 (to L.M.L.)]; the National Institutes of Health National Institute of Child Health & Human Development [Grant HD043446 (to D.G.-P.)]; the Arthritis Foundation (to D.G.-P.); and the Research Services of the Charleston, SC, and Durham, NC, Veterans Affairs Medical Centers. We offer our sincere apologies to the many outstanding researchers whose work has been omitted because of space and topic constraints. There is no intent to diminish or downplay the significance of their contributions to this rapidly expanding field.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.002436.
- AP-2
- activator protein-2
- ARF
- ADP-ribosylation factor
- AT1-i2m
- D125G/R126G/Y127A/M134A angiotensin II type 1a receptor
- EGF
- epidermal growth factor
- ERK
- extracellular signal-regulated kinase
- ET-A
- endothelin receptor type A
- GDS
- GDP dissociation stimulator
- GEF
- guanine nucleotide exchange factor
- GPCR
- G protein-coupled receptor
- GRK
- G protein-coupled receptor kinase
- GSK3β
- glycogen synthase kinase 3β
- HEK
- human embryonic kidney
- IκB
- inhibitor of nuclear factor-κB
- ICI 118551
- (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol
- IL
- interleukin
- JNK
- c-Jun NH2-terminal kinase
- LPA
- lysophosphatidic acid
- MAP
- mitogen-activated protein
- MEF
- murine embryonic fibroblast
- MK-0354
- 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopentapyrazole
- NFκB
- nuclear factor κB
- NK
- natural killer
- PAR
- protease-activated receptor
- PDE
- phosphodiesterase
- PKA
- protein kinase A
- PKC
- protein kinase C
- PP2A
- protein phosphatase 2A
- PTH
- parathyroid hormone
- Shh
- Sonic hedgehog
- TβRIII
- type III transforming growth factor-β
- TP
- thromboxane.
References
- Abraham et al., 2000.Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings BA, Dilworth SM, Mischak H, Kolch W, Baccarini M. (2000) Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J Biol Chem 275:22300–22304 [DOI] [PubMed] [Google Scholar]
- Adams et al., 2005.Adams DG, Coffee RL, Jr, Zhang H, Pelech S, Strack S, Wadzinski BE. (2005) Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem 280:42644–42654 [DOI] [PubMed] [Google Scholar]
- Ahn et al., 2009.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. (2009) Beta-arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem 284:8855–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn et al., 1999.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. (1999) Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 274:1185–1188 [DOI] [PubMed] [Google Scholar]
- Ahn et al., 2004.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. (2004) Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279:35518–35525 [DOI] [PubMed] [Google Scholar]
- Alloway et al., 2000.Alloway PG, Howard L, Dolph PJ. (2000) The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron 28:129–138 [DOI] [PubMed] [Google Scholar]
- Angers et al., 2002.Angers S, Salahpour A, Bouvier M. (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42:409–435 [DOI] [PubMed] [Google Scholar]
- Aplin et al., 2007.Aplin M, Christensen GL, Schneider M, Heydorn A, Gammeltoft S, Kjølbye AL, Sheikh SP, Hansen JL. (2007) Differential extracellular signal-regulated kinases 1 and 2 activation by the angiotensin type 1 receptor supports distinct phenotypes of cardiac myocytes. Basic Clin Pharmacol Toxicol 100:296–301 [DOI] [PubMed] [Google Scholar]
- Aragay et al., 1998.Aragay AM, Mellado M, Frade JM, Martin AM, Jimenez-Sainz MC, Martinez-A C, Mayor F., Jr (1998) Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proc Natl Acad Sci USA 95:2985–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarani et al., 1996.Azarani A, Goltzman D, Orlowski J. (1996) Structurally diverse N-terminal peptides of parathyroid hormone (PTH) and PTH-related peptide (PTHrp) inhibit the Na+/H+ exchanger NHE3 isoform by binding to the PTH/PTHrp receptor type I and activating distinct signaling pathways. J Biol Chem 271:14931–14936 [DOI] [PubMed] [Google Scholar]
- Azzi et al., 2003.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G. (2003) Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100:11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak et al., 2001.Barak LS, Oakley RH, Laporte SA, Caron MG. (2001) Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 98:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlic et al., 2000.Barlic J, Andrews JD, Kelvin AA, Bosinger SE, DeVries ME, Xu L, Dobransky T, Feldman RD, Ferguson SS, Kelvin DJ. (2000) Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol 1:227–233 [DOI] [PubMed] [Google Scholar]
- Barlic et al., 1999.Barlic J, Khandaker MH, Mahon E, Andrews J, DeVries ME, Mitchell GB, Rahimpour R, Tan CM, Ferguson SS, Kelvin DJ. (1999) Beta-arrestins regulate interleukin-8-induced CXCR1 internalization. J Biol Chem 274:16287–16294 [DOI] [PubMed] [Google Scholar]
- Barnes et al., 2005.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. (2005) beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem 280:8041–8050 [DOI] [PubMed] [Google Scholar]
- Basher et al., 2008.Basher F, Fan H, Zingarelli B, Borg KT, Luttrell LM, Tempel GE, Halushka PV, Cook JA. (2008) beta-Arrestin 2: a Negative Regulator of Inflammatory Responses in Polymorphonuclear Leukocytes. Int J Clin Exp Med. 1:32–41 [PMC free article] [PubMed] [Google Scholar]
- Basu et al., 2007.Basu S, Ray NT, Atkinson SJ, Broxmeyer HE. (2007) Protein phosphatase 2A plays an important role in stromal cell-derived factor-1/CXC chemokine ligand 12-mediated migration and adhesion of CD34+ cells. J Immunol 179:3075–3085 [DOI] [PubMed] [Google Scholar]
- Baylor and Burns, 1998.Baylor DA, Burns ME. (1998) Control of rhodopsin activity in vision. Eye 12:521–525 [DOI] [PubMed] [Google Scholar]
- Beaulieu et al., 2008.Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. (2008) A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132:125–136 [DOI] [PubMed] [Google Scholar]
- Beaulieu et al., 2005.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. (2005) An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261–273 [DOI] [PubMed] [Google Scholar]
- Beaulieu et al., 2004.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. (2004) Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA 101:5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari et al., 2007.Bhandari D, Trejo J, Benovic JL, Marchese A. (2007) Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem 282:36971–36979 [DOI] [PubMed] [Google Scholar]
- Bhattacharya et al., 2002.Bhattacharya M, Anborgh PH, Babwah AV, Dale LB, Dobransky T, Benovic JL, Feldman RD, Verdi JM, Rylett RJ, Ferguson SS. (2002) Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat Cell Biol 4:547–555 [DOI] [PubMed] [Google Scholar]
- Bockaert et al., 2003.Bockaert J, Marin P, Dumuis A, Fagni L. (2003) The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett 546:65–72 [DOI] [PubMed] [Google Scholar]
- Bohn et al., 2002.Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci 22:10494–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn et al., 1999.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498 [DOI] [PubMed] [Google Scholar]
- Bouxsein et al., 2005.Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, Ferrari SL. (2005) Beta-arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res 20:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady and Limbird, 2002.Brady AE, Limbird LE. (2002) G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal 14:297–309 [DOI] [PubMed] [Google Scholar]
- Brahmbhatt and Klemke, 2003.Brahmbhatt AA, Klemke RL. (2003) ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J Biol Chem 278:13016–13025 [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier et al., 2002.Brami-Cherrier K, Valjent E, Garcia M, Pagès C, Hipskind RA, Caboche J. (2002) Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci 22:8911–8921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest et al., 2005.Charest PG, Terrillon S, Bouvier M. (2005) Monitoring agonist-promoted conformational changes of beta-arrestin in living cells by intramolecular BRET. EMBO Rep 6:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2003.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. (2003) Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301:1391–1394 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2004.Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. (2004) Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science 306:2257–2260 [DOI] [PubMed] [Google Scholar]
- Cheung et al., 2009.Cheung R, Malik M, Ravyn V, Tomkowicz B, Ptasznik A, Collman RG. (2009) An arrestin-dependent multi-kinase signaling complex mediates MIP-1beta/CCL4 signaling and chemotaxis of primary human macrophages. J Leukoc Biol 86:833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun et al., 2009.Chun KS, Lao HC, Trempus CS, Okada M, Langenbach R. (2009) The prostaglandin receptor EP2 activates multiple signaling pathways and beta-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis 30:1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing et al., 2001.Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ. (2001) beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J Biol Chem 276:42509–42513 [DOI] [PubMed] [Google Scholar]
- Cole, 1999.Cole JA. (1999) Parathyroid hormone activates mitogen-activated protein kinase in opossum kidney cells. Endocrinology 140:5771–5779 [DOI] [PubMed] [Google Scholar]
- Colvin et al., 2004.Colvin RA, Campanella GS, Sun J, Luster AD. (2004) Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem 279:30219–30227 [DOI] [PubMed] [Google Scholar]
- Conner et al., 1997.Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. (1997) Beta-arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 81:1021–1026 [DOI] [PubMed] [Google Scholar]
- Dalle et al., 2001.Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM. (2001) Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem 276:15688–15695 [DOI] [PubMed] [Google Scholar]
- Davidson and Steller, 1998.Davidson FF, Steller H. (1998) Blocking apoptosis prevents blindness in Drosophila retinal degeneration mutants. Nature 391:587–591 [DOI] [PubMed] [Google Scholar]
- DeFea, 2007.DeFea KA. (2007) Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol 69:535–560 [DOI] [PubMed] [Google Scholar]
- DeFea et al., 2000a.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Déry O, Bunnett NW. (2000a) The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA 97:11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea et al., 2000b.DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett NW. (2000b) Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148:1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gorter et al., 2008.de Gorter DJ, Reijmers RM, Beuling EA, Naber HP, Kuil A, Kersten MJ, Pals ST, Spaargaren M. (2008) The small GTPase Ral mediates SDF-1-induced migration of B cells and multiple myeloma cells. Blood 111:3364–3372 [DOI] [PubMed] [Google Scholar]
- De Lean et al., 1980.De Lean A, Stadel JM, Lefkowitz RJ. (1980) A ternary complex model explains the agonist specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem 255:7108–7117 [PubMed] [Google Scholar]
- Devi, 2001.Devi LA. (2001) Heterodimerization of G-protein-coupled receptors: Pharmacology, signaling and trafficking. Trends Pharmacol Sci 22:532–537 [DOI] [PubMed] [Google Scholar]
- DeWire et al., 2008.DeWire SM, Kim J, Whalen EJ, Ahn S, Chen M, Lefkowitz RJ. (2008) Beta-arrestin-mediated signaling regulates protein synthesis. J Biol Chem 283:10611–10620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobnig and Turner, 1995.Dobnig H, Turner RT. (1995) Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136:3632–3638 [DOI] [PubMed] [Google Scholar]
- Dolph et al., 1993.Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS. (1993) Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science 260:1910–1916 [DOI] [PubMed] [Google Scholar]
- Downes and Gautam, 1999.Downes GB, Gautam N. (1999) The G protein subunit gene families. Genomics 62:544–552 [DOI] [PubMed] [Google Scholar]
- Drake et al., 2008.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. (2008) beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem 283:5669–5676 [DOI] [PubMed] [Google Scholar]
- Dubois et al., 2001.Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. (2001) Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell 105:613–624 [DOI] [PubMed] [Google Scholar]
- El Far and Betz, 2002.El Far O, Betz H. (2002) G-protein-coupled receptors for neurotransmitter amino acids: C-terminal tails, crowded signalosomes. Biochem J 365:329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorza et al., 2003.Elorza A, Penela P, Sarnago S, Mayor F., Jr (2003) MAPK-dependent degradation of G protein-coupled receptor kinase 2. J Biol Chem 278:29164–29173 [DOI] [PubMed] [Google Scholar]
- Fan et al., 2007.Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA. (2007) β-Arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol 44:3092–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, 2001.Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharm Rev 53:1–24 [PubMed] [Google Scholar]
- Ferrari et al., 1999.Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. (1999) Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J Biol Chem 274:29968–29975 [DOI] [PubMed] [Google Scholar]
- Ferrari et al., 2005.Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. (2005) Bone response to intermittent parathyroid hormone is altered in mice null for beta-arrestin2. Endocrinology 146:1854–1862 [DOI] [PubMed] [Google Scholar]
- Fessart et al., 2005.Fessart D, Simaan M, Laporte SA. (2005) c-Src regulates clathrin adapter protein 2 interaction with beta-arrestin and the angiotensin II type 1 receptor during clathrin- mediated internalization. Mol Endocrinol 19:491–503 [DOI] [PubMed] [Google Scholar]
- Fessart et al., 2007.Fessart D, Simaan M, Zimmerman B, Comeau J, Hamdan FF, Wiseman PW, Bouvier M, Laporte SA. (2007) Src-dependent phosphorylation of beta2-adaptin dissociates the beta-arrestin-AP-2 complex. J Cell Sci 120:1723–1732 [DOI] [PubMed] [Google Scholar]
- Finger et al., 2008.Finger EC, Lee NY, You HJ, Blobe GC. (2008) Endocytosis of the type III transforming growth factor-beta (TGF-beta) receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor down-regulation. J Biol Chem 283:34808–34818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower, 1999.Flower DR. (1999) Modelling G-protein-coupled receptors for drug design. Biochim Biophys Acta 1422:207–234 [DOI] [PubMed] [Google Scholar]
- Fong et al., 2002.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. (2002) Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA 99:7478–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord and Marshall, 1999.Foord SM, Marshall FH. (1999) RAMPs: accessory proteins for seven transmembrane domain receptors. Trends Pharmacol Sci 20:184–187 [DOI] [PubMed] [Google Scholar]
- Fredriksson et al., 2003.Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272 [DOI] [PubMed] [Google Scholar]
- Freedman and Lefkowitz, 1996.Freedman NJ, Lefkowitz RJ. (1996) Desensitization of G protein-coupled receptors. Recent Prog Horm Res 51:319–351 [PubMed] [Google Scholar]
- Gáborik et al., 2003.Gáborik Z, Jagadeesh G, Zhang M, Spät A, Catt KJ, Hunyady L. (2003) The role of a conserved region of the second intracellular loop in AT1 angiotensin receptor activation and signaling. Endocrinology 144:2220–2228 [DOI] [PubMed] [Google Scholar]
- Gainetdinov et al., 2003.Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, et al. (2003) Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38:291–303 [DOI] [PubMed] [Google Scholar]
- Galet and Ascoli, 2008.Galet C, Ascoli M. (2008) Arrestin-3 is essential for the activation of Fyn by the luteinizing hormone receptor (LHR) in MA-10 cells. Cell Signal 20:1822–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al., 2004.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. (2004) Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell 14:303–317 [DOI] [PubMed] [Google Scholar]
- Gardella et al., 1996.Gardella TJ, Luck MD, Jensen GS, Schipani E, Potts JT, Jr, Jüppner H. (1996) Inverse agonism of amino-terminally truncated parathyroid hormone (PTH) and PTH-related peptide (PTHrP) analogs revealed with constitutively active mutant PTH/PTHrP receptors. Endocrinology 137:3936–3941 [DOI] [PubMed] [Google Scholar]
- Ge et al., 2003.Ge L, Ly Y, Hollenberg M, DeFea K. (2003) A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem 278:34418–34426 [DOI] [PubMed] [Google Scholar]
- Ge et al., 2004.Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. (2004) Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem 279:55419–55424 [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer et al., 2006.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, et al. (2006) Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281:10856–10864 [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer et al., 2009.Gesty-Palmer D, Flannery P, Yuan L, Spurney R, Lefkowitz RJ, Luttrell LM. (2009) A beta-arrestin biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med 1:1ra1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesty-Palmer et al., 2005.Gesty-Palmer D, El Shewy H, Kohout TA, Luttrell LM. (2005) beta-Arrestin 2 expression determines the transcriptional response to lysophosphatidic acid stimulation in murine embryo fibroblasts. J Biol Chem 280:32157–32167 [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer and Luttrell, 2008.Gesty-Palmer D, Luttrell LM. (2008) Heptahelical terpsichory. Who calls the tune? J Recept Signal Transduct Res 28:39–58 [DOI] [PubMed] [Google Scholar]
- Goel et al., 2002.Goel R, Phillips-Mason PJ, Raben DM, Baldassare JJ. (2002) alpha-Thrombin induces rapid and sustained Akt phosphorylation by beta-arrestin1-dependent and -independent mechanisms, and only the sustained Akt phosphorylation is essential for G1 phase progression. J Biol Chem 277:18640–18648 [DOI] [PubMed] [Google Scholar]
- Gong et al., 2002.Gong H, Sun H, Koch WJ, Rau T, Eschenhagen T, Ravens U, Heubach JF, Adamson DL, Harding SE. (2002) Specific beta(2)AR blocker ICI 118,551 actively decreases contraction through a G(i)-coupled form of the beta(2)AR in myocytes from failing human heart. Circulation 105:2497–2503 [DOI] [PubMed] [Google Scholar]
- Goodman et al., 1996.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. (1996) Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383:447–450 [DOI] [PubMed] [Google Scholar]
- Gurevich and Gurevich, 2006.Gurevich VV, Gurevich EV. (2006) The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther 110:465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich et al., 1997.Gurevich VV, Pals-Rylaarsdam R, Benovic JL, Hosey MM, Onorato JJ. (1997) Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem 272:28849–28852 [DOI] [PubMed] [Google Scholar]
- Hock and Gera, 1992.Hock JM, Gera I. (1992) Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res 7:65–72 [DOI] [PubMed] [Google Scholar]
- Holloway et al., 2002.Holloway AC, Qian H, Pipolo L, Ziogas J, Miura S, Karnik S, Southwell BR, Lew MJ, Thomas WG. (2002) Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol Pharmacol 61:768–777 [DOI] [PubMed] [Google Scholar]
- Hunton et al., 2005.Hunton DL, Barnes WG, Kim J, Ren XR, Violin JD, Reiter E, Milligan G, Patel DD, Lefkowitz RJ. (2005) Beta-arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol Pharmacol 67:1229–1236 [DOI] [PubMed] [Google Scholar]
- Hupfeld and Olefsky, 2007.Hupfeld CJ, Olefsky JM. (2007) Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. Annu Rev Physiol 69:561–577 [DOI] [PubMed] [Google Scholar]
- Hupfeld et al., 2005.Hupfeld CJ, Resnik JL, Ugi S, Olefsky JM. (2005) Insulin-induced beta-arrestin1 Ser-412 phosphorylation is a mechanism for desensitization of ERK activation by Galphai-coupled receptors. J Biol Chem 280:1016–1023 [DOI] [PubMed] [Google Scholar]
- Hyde et al., 1990.Hyde DR, Mecklenburg KL, Pollock JA, Vihtelic TS, Benzer S. (1990) Twenty Drosophila visual system clones: one is a homolog of human arrestin. Proc Natl Acad Sci USA 87:1008–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura et al., 2001.Imamura T, Huang J, Dalle S, Ugi S, Usui I, Luttrell LM, Miller WE, Lefkowitz RJ, Olefsky JM. (2001) Beta-arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J Biol Chem 276:43663–43667 [DOI] [PubMed] [Google Scholar]
- Jafri et al., 2006.Jafri F, El-Shewy HM, Lee MH, Kelly M, Luttrell DK, Luttrell LM. (2006) Expression of a chimeric neurokinin NK-1 receptor-beta-arrestin 1 fusion protein produces constitutive ERK1/2 activation in HEK-293 cells. Probing the composition and function of the G protein-coupled receptor “signalsome”. J Biol Chem 281:19346–19357 [DOI] [PubMed] [Google Scholar]
- Jansen et al., 1999.Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH. (1999) The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet 21:414–419 [DOI] [PubMed] [Google Scholar]
- Kang et al., 2005.Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, et al. (2005) A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell 123:833–847 [DOI] [PubMed] [Google Scholar]
- Kather et al., 1983.Kather H, Aktories K, Schulz G, Jakobs KH. (1983) Islet-activating protein discriminates the antilipolytic mechanism of insulin from that of other antilipolytic compounds. FEBS Lett 161:149–152 [DOI] [PubMed] [Google Scholar]
- Kenakin, 2002.Kenakin T. (2002) Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol 42:349–379 [DOI] [PubMed] [Google Scholar]
- Kenakin, 2007.Kenakin T. (2007) Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol 72:1393–1401 [DOI] [PubMed] [Google Scholar]
- Kendall and Luttrell, 2009.Kendall RT, Luttrell LM. (2009) Diversity in arrestin function. Cell Mol Life Sci 66:2953–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2008a.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. (2008a) Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA 105:14555–14560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2009.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. (2009) Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem 284:11953–11962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2005.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. (2005) Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA 102:1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2008b.Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, DeWire SM, Exum ST, Lefkowitz RJ, Freedman NJ. (2008b) Beta-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res 103:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev et al., 2000.Kiselev A, Socolich M, Vinós J, Hardy RW, Zuker CS, Ranganathan R. (2000) A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron 28:139–152 [DOI] [PubMed] [Google Scholar]
- Kohout et al., 2001.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. (2001) beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA 98:1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs et al., 2008.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. (2008) Beta-arrestin-mediated localization of smoothened to the primary cilium. Science 320:1777–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger et al., 1997.Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. (1997) The role of sequestration in G protein-coupled receptor resensitization. Regulation of beta2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem 272:5–8 [DOI] [PubMed] [Google Scholar]
- Krupnick et al., 1997.Krupnick JG, Goodman OB, Jr, Keen JH, Benovic JL. (1997) Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem 272:15011–15016 [DOI] [PubMed] [Google Scholar]
- Kuan et al., 2003.Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, et al. (2003) A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci USA 100:15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagane et al., 2005.Lagane B, Ballet S, Planchenault T, Balabanian K, Le Poul E, Blanpain C, Percherancier Y, Staropoli I, Vassart G, Oppermann M, et al. (2005) Mutation of the DRY motif reveals different structural requirements for the CC chemokine receptor 5-mediated signaling and receptor endocytosis. Mol Pharmacol 67:1966–1976 [DOI] [PubMed] [Google Scholar]
- Lakshmikanthan et al., 2009.Lakshmikanthan V, Zou L, Kim JI, Michal A, Nie Z, Messias NC, Benovic JL, Daaka Y. (2009) Identification of betaArrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc Natl Acad Sci USA 106:9379–9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander et al., 2001.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409:860–921 [DOI] [PubMed] [Google Scholar]
- Laporte et al., 2000.Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. (2000) The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem 275:23120–23126 [DOI] [PubMed] [Google Scholar]
- Laporte et al., 1999.Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. (1999) The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA 96:3712–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2008.Lee MH, El-Shewy HM, Luttrell DK, Luttrell LM. (2008) Role of beta-arrestin-mediated desensitization and signaling in the control of angiotensin AT1a receptor-stimulated transcription. J Biol Chem 283:2088–2097 [DOI] [PubMed] [Google Scholar]
- Li et al., 1995.Li T, Franson WK, Gordon JW, Berson EL, Dryja TP. (1995) Constitutive activation of phototransduction by K296E opsin is not a cause of photoreceptor degeneration. Proc Natl Acad Sci USA 92:3551–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al., 2009.Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV, Bhattacharya M. (2009) Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol Cancer Res 7:1064–1077 [DOI] [PubMed] [Google Scholar]
- Lin et al., 1997.Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, Lefkowitz RJ. (1997) Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem 272:31051–31057 [DOI] [PubMed] [Google Scholar]
- Lin et al., 1998.Lin FT, Daaka Y, Lefkowitz RJ. (1998) Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem 273:31640–31643 [DOI] [PubMed] [Google Scholar]
- Lin et al., 1999.Lin FT, Miller WE, Luttrell LM, Lefkowitz RJ. (1999) Feedback regulation of beta-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem 274:15971–15974 [DOI] [PubMed] [Google Scholar]
- Luan et al., 2005.Luan B, Zhang Z, Wu Y, Kang J, Pei G. (2005) Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. EMBO J 24:4237–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan et al., 2009.Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. (2009) Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature 457:1146–1149 [DOI] [PubMed] [Google Scholar]
- Luttrell, 2003.Luttrell LM. (2003) 'Location, location, location': spatial and temporal regulation of MAP kinases by G protein-coupled receptors. J Mol Endocrinol 30:117–126 [DOI] [PubMed] [Google Scholar]
- Luttrell, 2005.Luttrell LM. (2005) Regulators of GPCR activity: the Arrestins, in Contemporary Clinical Neuroscience: The G Protein-Coupled Receptors Handbook (Devi L. ed) pp 159–198, Humana Press, Inc, Totowa, NJ: [Google Scholar]
- Luttrell et al., 1999.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. (1999) Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283:655–661 [DOI] [PubMed] [Google Scholar]
- Luttrell et al., 2001.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. (2001) Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA 98:2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell et al., 1995.Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Touhara K, Lefkowitz RJ. (1995) G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem 270:16495–16498 [DOI] [PubMed] [Google Scholar]
- Lymperopoulos et al., 2009.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Soltys S, Koch WJ. (2009) An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci USA 106:5825–5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma and Pei, 2007.Ma L, Pei G. (2007) Beta-arrestin signaling and regulation of transcription. J Cell Sci 120:213–218 [DOI] [PubMed] [Google Scholar]
- Machesky, 1997.Machesky LM. (1997) Cell motility: complex dynamics at the leading edge. Curr Biol 7:R164–167 [DOI] [PubMed] [Google Scholar]
- Masri et al., 2008.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, Caron MG. (2008) Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA 105:13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley et al., 2005.Maudsley S, Martin B, Luttrell LM. (2005) The origins of diversity and specificity in G protein-coupled receptor signaling. J Pharmacol Exp Ther 314:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald et al., 1999.McDonald PH, Cote NL, Lin FT, Premont RT, Pitcher JA, Lefkowitz RJ. (1999) Identification of NSF as a beta-arrestin1-binding protein. Implications for beta2-adrenergic receptor regulation. J Biol Chem 274:10677–10680 [DOI] [PubMed] [Google Scholar]
- McDonald et al., 2000.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. (2000) Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290:1574–1577 [DOI] [PubMed] [Google Scholar]
- Miller and Lefkowitz, 2001.Miller WE, Lefkowitz RJ. (2001) Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol 13:139–145 [DOI] [PubMed] [Google Scholar]
- Miller et al., 2000.Miller WE, Maudsley S, Ahn S, Khan KD, Luttrell LM, Lefkowitz RJ. (2000) Beta-arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC. Role of beta-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J Biol Chem 275:11312–11319 [DOI] [PubMed] [Google Scholar]
- Miller et al., 2001.Miller WE, McDonald PH, Cai SF, Field ME, Davis RJ, Lefkowitz RJ. (2001) Identification of a motif in the carboxyl terminus of beta-arrestin2 responsible for activation of JNK3. J Biol Chem 276:27770–27777 [DOI] [PubMed] [Google Scholar]
- Miura et al., 2004.Miura S, Zhang J, Matsuo Y, Saku K, Karnik SS. (2004) Activation of extracellular signal-activated kinase by angiotensin II-induced Gq-independent epidermal growth factor receptor transactivation. Hypertens Res 27:765–777 [DOI] [PubMed] [Google Scholar]
- Mohan et al., 2000.Mohan S, Kutilek S, Zhang C, Shen HG, Kodama Y, Srivastava AK, Wergedal JE, Beamer WG, Baylink DJ. (2000) Comparison of bone formation responses to parathyroid hormone(1–34), (1–31), and (2–34) in mice. Bone 27:471–478 [DOI] [PubMed] [Google Scholar]
- Moussa et al., 2008.Moussa O, Ashton AW, Fraig M, Garrett-Mayer E, Ghoneim MA, Halushka PV, Watson DK. (2008) Novel role of thromboxane receptors beta isoform in bladder cancer pathogenesis. Cancer Res 68:4097–4104 [DOI] [PubMed] [Google Scholar]
- Mythreye and Blobe, 2009.Mythreye K, Blobe GC. (2009) The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc Natl Acad Sci USA 106:8221–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson et al., 2007.Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. (2007) Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science 315:663–666 [DOI] [PubMed] [Google Scholar]
- Nelson et al., 2008.Nelson CD, Kovacs JJ, Nobles KN, Whalen EJ, Lefkowitz RJ. (2008) Beta-arrestin scaffolding of phosphatidylinositol 4-phosphate 5-kinase Ialpha promotes agonist-stimulated sequestration of the beta2-adrenergic receptor. J Biol Chem 283:21093–21101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma et al., 2007.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, et al. (2007) Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 117:2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley et al., 2001.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. (2001) Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis. J Biol Chem 276:19452–19460 [DOI] [PubMed] [Google Scholar]
- Oakley et al., 2000.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210 [DOI] [PubMed] [Google Scholar]
- Packer et al., 1996.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 334:1349–1355 [DOI] [PubMed] [Google Scholar]
- Packer et al., 2002.Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, et al. (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106:2194–2199 [DOI] [PubMed] [Google Scholar]
- Palmitessa et al., 2005.Palmitessa A, Hess HA, Bany IA, Kim YM, Koelle MR, Benovic JL. (2005) Caenorhabditus elegans arrestin regulates neural G protein signaling and olfactory adaptation and recovery. J Biol Chem 280:24649–24662 [DOI] [PubMed] [Google Scholar]
- Parent et al., 1999.Parent JL, Labrecque P, Orsini MJ, Benovic JL. (1999) Internalization of the TXA2 receptor α and β isoforms. Role of the differentially spliced cooh terminus in agonist-promoted receptor internalization. J Biol Chem 274:8941–8948 [DOI] [PubMed] [Google Scholar]
- Penela et al., 2001.Penela P, Elorza A, Sarnago S, Mayor F., Jr (2001) Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J 20:5129–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy et al., 2004.Perroy J, Pontier S, Charest PG, Aubry M, Bouvier M. (2004) Real-time monitoring of ubiquitination in living cells by BRET. Nat Methods 1:203–208 [DOI] [PubMed] [Google Scholar]
- Perry et al., 2002.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, et al. (2002) Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298:834–836 [DOI] [PubMed] [Google Scholar]
- Perry and Lefkowitz, 2002.Perry SJ, Lefkowitz RJ. (2002) Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol 12:130–138 [DOI] [PubMed] [Google Scholar]
- Pfleger et al., 2006.Pfleger KD, Dromey JR, Dalrymple MB, Lim EM, Thomas WG, Eidne KA. (2006) Extended bioluminescence resonance energy transfer (eBRET) for monitoring prolonged protein-protein interactions in live cells. Cell Signal 18:1664–1670 [DOI] [PubMed] [Google Scholar]
- Pi et al., 2005.Pi M, Oakley RH, Gesty-Palmer D, Cruickshank RD, Spurney RF, Luttrell LM, Quarles LD. (2005) Beta-arrestin- and G protein receptor kinase-mediated calcium-sensing receptor desensitization. Mol Endocrinol 19:1078–1087 [DOI] [PubMed] [Google Scholar]
- Pike, 2005.Pike NB. (2005) Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J Clin Invest 115:3400–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz et al., 2009.Pierroz DD, Rufo A, Bianchi EN, Glatt V, Capulli M, Rucci N, Cavat F, Rizzoli R, Teti A, Bouxsein ML, et al. (2009) Beta-arrestin2 regulates RANKL and ephrins gene expression in response to bone remodeling in mice. J Bone Miner Res 24:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher et al., 1995a.Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ. (1995a) The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc Natl Acad Sci USA 92:8343–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher et al., 1995b.Pitcher JA, Touhara K, Payne ES, Lefkowitz RJ. (1995b) Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J Biol Chem 270:11707–11710 [DOI] [PubMed] [Google Scholar]
- Qin et al., 2004.Qin L, Raggatt LJ, Partridge NC. (2004) Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab 15:60–65 [DOI] [PubMed] [Google Scholar]
- Raghu et al., 2000.Raghu P, Usher K, Jonas S, Chyb S, Polyanovsky A, Hardie RC. (2000) Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron 26:169–179 [DOI] [PubMed] [Google Scholar]
- Rajagopal et al., 2006.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. (2006) Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA 103:16284–16289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan, 2003.Ranganathan R. (2003) Cell biology. A matter of life or death. Science 299:1677–1679 [DOI] [PubMed] [Google Scholar]
- Rapacciuolo et al., 2003.Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA. (2003) Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J Biol Chem 278:35403–35411 [DOI] [PubMed] [Google Scholar]
- Ren et al., 2005.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. (2005) Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 102:1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson et al., 2003.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. (2003) Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol 170:2904–2911 [DOI] [PubMed] [Google Scholar]
- Richter et al., 2008.Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, et al. (2008) Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J 27:384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman et al., 2000.Roman G, He J, Davis RL. (2000) kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics 155:1281–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanò et al., 2009.Rosanò L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. (2009) Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA 106:2806–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross and Wilkie, 2000.Ross EM, Wilkie TM. (2000) GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem 69:795–827 [DOI] [PubMed] [Google Scholar]
- Samama et al., 1993.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. (1993) A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem 268:4625–4636 [PubMed] [Google Scholar]
- Scott et al., 2002.Scott MG, Le Rouzic E, Périanin A, Pierotti V, Enslen H, Benichou S, Marullo S, Benmerah A. (2002) Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem 277:37693–37701 [DOI] [PubMed] [Google Scholar]
- Scott et al., 2006.Scott MG, Pierotti V, Storez H, Lindberg E, Thuret A, Muntaner O, Labbé-Jullié C, Pitcher JA, Marullo S. (2006) Cooperative regulation of extracellular signal-regulated kinase activation and cell shape change by filamin A and beta-arrestins. Mol Cell Biol 26:3432–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple et al., 2008.Semple G, Skinner PJ, Gharbaoui T, Shin YJ, Jung JK, Cherrier MC, Webb PJ, Tamura SY, Boatman PD, Sage CR, et al. (2008) 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydro-cyclopentapyrazole (MK-0354): a partial agonist of the nicotinic acid receptor, G-protein coupled receptor 109a, with antilipolytic but no vasodilatory activity in mice. J Med Chem 51:5101–5108 [DOI] [PubMed] [Google Scholar]
- Seta et al., 2002.Seta K, Nanamori M, Modrall JG, Neubig RR, Sadoshima J. (2002) AT1 receptor mutant lacking heterotrimeric G protein coupling activates the Src-Ras-ERK pathway without nuclear translocation of ERKs. J Biol Chem 277:9268–9277 [DOI] [PubMed] [Google Scholar]
- Shenoy et al., 2007.Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, Luttrell LM, Lefkowitz RJ. (2007) Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem 282:29549–29562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy et al., 2006.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. (2006) Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281:1261–1273 [DOI] [PubMed] [Google Scholar]
- Shenoy and Lefkowitz, 2003.Shenoy SK, Lefkowitz RJ. (2003) Trafficking pattern of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem 278:14498–14506 [DOI] [PubMed] [Google Scholar]
- Shenoy and Lefkowitz, 2005a.Shenoy SK, Lefkowitz RJ. (2005a) Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE 2005:cm10 [DOI] [PubMed] [Google Scholar]
- Shenoy and Lefkowitz, 2005b.Shenoy SK, Lefkowitz RJ. (2005b) Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem 280:15315–15324 [DOI] [PubMed] [Google Scholar]
- Shenoy et al., 2001.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. (2001) Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294:1307–1313 [DOI] [PubMed] [Google Scholar]
- Shenoy et al., 2009.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. (2009) Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci USA 106:6650–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy et al., 2008.Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. (2008) Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem 283:22166–22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla et al., 2008.Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. (2008) Distinct conformational changes in beta-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA 105:9988–9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 1990.Smith DP, Shieh BH, Zuker CS. (1990) Isolation and structure of an arrestin gene from Drosophila. Proc Natl Acad Sci USA 87:1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon et al., 2004.Sneddon WB, Magyar CE, Willick GE, Syme CA, Galbiati F, Bisello A, Friedman PA. (2004) Ligand-selective dissociation of activation and internalization of the parathyroid hormone (PTH) receptor: conditional efficacy of PTH peptide fragments. Endocrinology 145:2815–2823 [DOI] [PubMed] [Google Scholar]
- Sonoda et al., 2008.Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. (2008) Beta-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc Natl Acad Sci USA 105:6614–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al., 2009.Song X, Coffa S, Fu H, Gurevich VV. (2009) How does arrestin assemble MAPKs into a signaling complex? J Biol Chem 284:685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al., 2007.Song X, Gurevich EV, Gurevich VV. (2007) Cone arrestin binding to JNK3 and Mdm2: conformational preference and localization of interaction sites. J Neurochem 103:1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al., 2006.Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV. (2006) Visual and both non-visual arrestins in their “inactive” conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem 281:21491–21499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel et al., 1997.Stoffel RH, 3rd, Pitcher JA, Lefkowitz RJ. (1997) Targeting G protein-coupled receptor kinases to their receptor substrates. J Membr Biol 157:1–8 [DOI] [PubMed] [Google Scholar]
- Stokoe et al., 1994.Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. (1994) Activation of Raf as a result of recruitment to the plasma membrane. Science 264:1463–1467 [DOI] [PubMed] [Google Scholar]
- Su et al., 2005.Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. (2005) Altered CXCR2 signaling in β-arrestin-2-deficient mouse models. J Immunol 175:5396–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al., 2002.Sun Y, Cheng Z, Ma L, Pei G. (2002) Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem 277:49212–49219 [DOI] [PubMed] [Google Scholar]
- Takasu et al., 1999.Takasu H, Gardella TJ, Luck MD, Potts JT, Jr, Bringhurst FR. (1999) Amino-terminal modifications of human parathyroid hormone (PTH) selectively alter phospholipase C signaling via the type 1 PTH receptor: implications for design of signal-specific PTH ligands. Biochemistry 38:13453–13460 [DOI] [PubMed] [Google Scholar]
- Terrillon and Bouvier, 2004.Terrillon S, Bouvier M. (2004) Receptor activity-independent recruitment of betaarrestin2 reveals specific signalling modes. EMBO J 23:3950–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgo et al., 2003.Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. (2003) The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem 278:6258–6267 [DOI] [PubMed] [Google Scholar]
- Tohgo et al., 2002.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. (2002) Beta-arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem 277:9429–9436 [DOI] [PubMed] [Google Scholar]
- Tomhave et al., 1994.Tomhave ED, Richardson RM, Didsbury JR, Menard L, Snyderman R, Ali H. (1994) Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J Immunol 153:3267–3275 [PubMed] [Google Scholar]
- Tournier et al., 2000.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870–874 [DOI] [PubMed] [Google Scholar]
- Ungerer et al., 1993.Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ. (1993) Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 87:454–463 [DOI] [PubMed] [Google Scholar]
- Usui et al., 2004.Usui I, Imamura T, Huang J, Satoh H, Shenoy SK, Lefkowitz RJ, Hupfeld CJ, Olefsky JM. (2004) beta-arrestin-1 competitively inhibits insulin-induced ubiquitination and degradation of insulin receptor substrate 1. Mol Cell Biol 24:8929–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga et al., 2002.Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. (2002) Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J Biol Chem 277:8121–8129 [DOI] [PubMed] [Google Scholar]
- Venter et al., 2001.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. (2001) The sequence of the human genome. Science 291:1304–1351 [DOI] [PubMed] [Google Scholar]
- Verheijen and Defize, 1997.Verheijen MH, Defize LH. (1997) Parathyroid hormone activates mitogen-activated protein kinase via a cAMP-mediated pathway independent of Ras. J Biol Chem 272:3423–3429 [DOI] [PubMed] [Google Scholar]
- Violin and Lefkowitz, 2007.Violin JD, Lefkowitz RJ. (2007) Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28:416–422 [DOI] [PubMed] [Google Scholar]
- Walker et al., 2003.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. (2003) Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 112:566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters et al., 2009.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. (2009) beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 119:1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2003a.Wang P, Gao H, Ni Y, Wang B, Wu Y, Ji L, Qin L, Ma L, Pei G. (2003a) Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem 278:6363–6370 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2006.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. (2006) Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 7:139–147 [DOI] [PubMed] [Google Scholar]
- Wei et al., 2004.Wei H, Ahn S, Barnes WG, Lefkowitz RJ. (2004) Stable interaction between beta-arrestin 2 and angiotensin type 1A receptor is required for beta-arrestin 2-mediated activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem 279:48255–48261 [DOI] [PubMed] [Google Scholar]
- Wei et al., 2003.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100:10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbonat et al., 2000.Werbonat Y, Kleutges N, Jakobs KH, van Koppen CJ. (2000) Essential role of dynamin in internalization of M2 muscarinic acetylcholine and angiotensin AT1A receptors. J Biol Chem 275:21969–21974 [DOI] [PubMed] [Google Scholar]
- Wilbanks et al., 2004.Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, Caron MG. (2004) Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science 306:2264–2267 [DOI] [PubMed] [Google Scholar]
- Wisler et al., 2007.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. (2007) A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA 104:16657–16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherow et al., 2004.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. (2004) beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci USA 101:8603–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang and Kobilka, 2003.Xiang Y, Kobilka BK. (2003) Myocyte adrenoceptor signaling pathways. Science 300:1530–1532 [DOI] [PubMed] [Google Scholar]
- Xiao et al., 2007.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, 3rd, Lefkowitz RJ. (2007) Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA 104:12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada et al., 1990.Yamada T, Takeuchi Y, Komori N, Kobayashi H, Sakai Y, Hotta Y, Matsumoto H. (1990) A 49-kilodalton phosphoprotein in the Drosophila photoreceptor is an arrestin homolog. Science 248:483–486 [DOI] [PubMed] [Google Scholar]
- You et al., 2009.You HJ, How T, Blobe GC. (2009) The type III transforming growth factor-beta receptor negatively regulates nuclear factor kappa B signaling through its interaction with beta-arrestin2. Carcinogenesis 30:1281–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al., 2008.Yu MC, Su LL, Zou L, Liu Y, Wu N, Kong L, Zhuang ZH, Sun L, Liu HP, Hu JH, et al. (2008) An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol 9:898–907 [DOI] [PubMed] [Google Scholar]
- Yue et al., 2009.Yue R, Kang J, Zhao C, Hu W, Tang Y, Liu X, Pei G. (2009) Beta-arrestin1 regulates zebrafish hematopoiesis through binding to YY1 and relieving polycomb group repression. Cell 139:535–546 [DOI] [PubMed] [Google Scholar]
- Zhai et al., 2005.Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, Irie K, Holle E, Yu X, Kupershmidt S, et al. (2005) Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest 115:3045–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman et al., 2009.Zimmerman B, Simaan M, Lee MH, Luttrell LM, Laporte SA. (2009) c-Src-mediated phosphorylation of AP-2 reveals a general mechanism for receptors internalizing through the clathrin pathway. Cell Signal 21:103–110 [DOI] [PubMed] [Google Scholar]
- Zoudilova et al., 2007.Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. (2007) Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem 282:20634–20646 [DOI] [PubMed] [Google Scholar]