Abstract
Down-regulation of copper transporter 1 (CTR1) reduces uptake and sensitivity, whereas down-regulation of CTR2 enhances both. Cisplatin (DDP) triggers the rapid degradation of CTR1 and thus limits its own accumulation. We sought to determine the effect of DDP and copper on the expression of CTR2. Changes in CTR1 and CTR2 mRNA and protein levels in human ovarian carcinoma 2008 cells and ATOX1(+/+) and ATOX1(−/−) mouse embryo fibroblasts in response to exposure to DDP and copper were measured by quantitative reverse transcriptase-polymerase chain reaction, Western blot analysis, and deconvolution microscopy. DDP triggered rapid degradation of CTR1 in 2008 human ovarian cancer cells. However, it increased the expression of CTR2 mRNA and protein levels. Expression of CTR2 was heavily modulated by changes in intracellular copper concentration; copper depletion produced rapid disappearance of CTR2, whereas excess copper increased the level of CTR2 protein. This increase was associated with an increase in CTR2 mRNA and prolongation of the CTR2 half-life. Consistent with prior observations that short hairpin RNA interference-mediated knockdown of CTR2 enhanced DDP uptake and tumor cell kill, reduction of CTR2 by copper starvation also enhanced DDP uptake and cytotoxicity. Comparison of the ability of copper and DDP to modulate the expression of CTR1 in ATOX1(+/+) and ATOX1(−/−) indicated that ATOX1 participates in the regulation of CTR2 expression. Unlike CTR1, the expression of CTR2 is increased rather than decreased by DDP. Therefore, these two copper transporters have opposite effects on DDP sensitivity. CTR2 expression is regulated by copper availability via the copper-dependent regulator ATOX1.
The copper transporter 1 (CTR1) plays a central role in the transport of the platinum-based chemotherapeutic drug cisplatin (DDP) (Ishida et al., 2002; Lin et al., 2002; Larson et al., 2009). DDP accumulation is markedly reduced in cells in which both alleles of CTR1 have been knocked out, and re-expression of CTR1 in CTR1(−/−) cells restores DDP accumulation (Larson et al., 2009). Consistent with its regulation of cellular accumulation, loss of CTR1 results in resistance to the cytotoxic action of DDP both in vitro and in vivo (Blair et al., 2009; Larson et al., 2009). The second copper transporter found in mammalian cells, copper transporter 2 (CTR2), has been shown to affect the cellular accumulation of DDP in a manner opposite that of CTR1 (Blair et al., 2009). Cells in which the expression of CTR2 has been knocked down (CTR2kd) accumulate 2- to 3-fold more DDP than wild-type parental cells (Blair et al., 2009), and this increase is associated with a large increase in the cytotoxicity of the drug (Blair et al., 2009). Furthermore, the effect of the loss of CTR2 on DDP uptake and sensitivity is independent of the expression of CTR1 (Blair et al., 2009).
Ctr2 in the yeast Saccharomyces cerevisiae, and the Schizosaccharomyces pombe ortholog Ctr6, are associated with vacuoles with the C-terminal tail oriented toward the cytosol (Bellemare et al., 2002; Rees et al., 2004). Ctr2 delivers copper to various chaperones by releasing copper from intercellular vacuolar stores under conditions of copper starvation (Kampfenkel et al., 1995; Portnoy et al., 2001; Rees et al., 2004). When Ctr2 is mutated so that it mislocalizes to the plasma membrane, it can mediate copper influx in a manner similar to Ctr1 (Rees et al., 2004). Less is known about the function of mammalian CTR2. In mammalian cells, CTR2 is reported to be localized to late endosomes and lysosomes, although it also has been found on the plasma membrane in some cells (van den Berghe, 2007). As in yeast, mammalian CTR2 has been shown to increase copper influx in cells in which it localizes to the plasma membrane (Bertinato et al., 2008), although its affinity for copper is less than that of CTR1 (van den Berghe et al., 2007; Bertinato et al., 2008).
ATOX1 is a copper chaperone that accepts copper from CTR1 and delivers it to the copper efflux pumps ATP7A and ATP7B that transfer copper into the secretory pathway (Xiao and Wedd, 2002). ATOX1 expression has been linked to the regulation of several copper proteins such as ATP7B, SOD1, and CCS (Lutsenko et al., 2003; Jeney et al., 2005; Miyayama et al., 2009). ATOX1 has been found to act as a copper-dependent transcription factor that regulates the expression of cyclin D1 SOD1 (Itoh et al., 2008, 2009; Muller and Klomp, 2009).
Copper and DDP can quickly down-regulate the expression of CTR1 (Holzer and Howell, 2006). Within 15 min of DDP exposure, nearly all CTR1 is removed from the plasma membrane in many types of cells in a process that involves macropinocytosis, ubiquitination, and subsequent degradation by the proteosome (Holzer and Howell, 2006; Jandial et al., 2009). However, within ∼30 min after the removal of DDP, the amount of plasma membrane CTR1 returns to normal (Holzer and Howell, 2006). Very little is known about how CTR2 is regulated. Given its similarity to CTR1 with respect to structure and copper transport, it is likely that CTR2 is also regulated by DDP and the availability of copper.
In the current study, we sought to determine how the expression of CTR2 is affected by copper and DDP in both 2008 human ovarian and mouse embryo fibroblast cells. We report here that CTR2 mRNA and protein levels increase when mammalian cells are exposed to either copper or DDP. In addition, we demonstrate that CTR2 protein is rapidly degraded when cells are starved for copper. Similar to the effect of knocking down CTR2 expression using short hairpin RNA interference, the loss of CTR2 caused by depletion of cellular copper leads to increased DDP accumulation and cytotoxicity. We also report that CTR2 is found not only on intracellular membranes but also in the nucleus and that exposure to either copper or DDP increases the level of CTR2 in the nucleus as well as the cytoplasm. Finally, we present evidence that the regulation of CTR2 by copper status is mediated by ATOX1.
Materials and Methods
Drugs and Reagents.
Platinol AQ was a gift from Bristol-Myers Squibb Co. (Stamford, CT); it contains DDP at a concentration of 3.33 mM in 0.9% NaCl. CuSO4 and BCS were purchased from Sigma-Aldrich (St. Louis, MO). The drugs were diluted into Opti-MEM reduced serum media (Invitrogen, Carlsbad, CA) to produce final concentrations. Bradford reagent was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA), sulforhodamine B was obtained from Sigma-Aldrich (St. Louis, MO) and 0.4% sulforhodamine B (w/v) was solubilized in 1% (v/v) acetic acid solution.
Cell Types, Culture, and Engineering.
Parental mouse embryonic fibroblasts containing wild-type alleles of ATOX1 [ATOX1(+/+)] and an isogenic line in which both copies of ATOX1 had been somatically knocked out [ATOX1(−/−)] were a generous gift from Dr. J. D. Gitlin (Washington University, St. Louis, MO) (Hamza et al., 2003). Ovarian carcinoma 2008 cells were obtained from Dr. Phillip DiSaia (DiSaia et al., 1972).
Cell Survival Assay.
Cell survival after exposure to increasing concentrations of drugs was assayed using the sulforhodamine B assay system (Monks et al., 1991). Five thousand cells were seeded into each well of a 96-well tissue culture plate. Cells were incubated overnight at 37°C, 5% CO2, and then exposed to varying drug concentrations in 200 μl of complete medium. Cells were allowed to grow for 5 days, after which the media were removed, and the protein was precipitated with 50% trichloroacetic acid and stained using 100 μl of 0.4% sulforhodamine B in 1% acetic acid at room temperature for 15 min. After washing, the absorbance of each well at 515 nm was recorded using a Versamax tunable microplate reader (Molecular Devices, Sunnyvale, CA). All experiments were repeated at least three times using three cultures for each drug concentration.
Western Blotting.
Whole-cell lysates were dissolved in lysis buffer (150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 10 mM Tris, pH 7.4) and were subjected to electrophoresis on 4 to 15% gels using ∼30 μg of protein per lane. Protein levels were first determined by Bradford assay (Bio-Rad Laboratories). A Bio-Rad trans-blot system was used to transfer the proteins to Immobilin-P membranes (Millipore Corporation, Billerica, MA). Blots were incubated overnight at 4°C in 4% dry, nonfat milk in Tris-buffered saline (150 mM NaCl, 300 mM KCl, and 10 mM Tris, pH 7.4, 0.01% Tween 20). Blots were incubated with primary antibody for 1 h at room temperature. A horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) was dissolved in 4% milk in the Tris-buffered saline buffer and incubated with the blot for 1 h at room temperature. After four 5-min washes, blots were exposed to the Pierce ECL reagent (Thermo Fisher Scientific, Waltham, MA) and detected on X-ray films (HyBlot CL; Denville Scientific, Inc. Metuchen, NJ). The primary antibodies used for Western blot analysis were anti-CTR2 (provided by Dr. Jesse Bertinato, Nutrition Research Division, Health Products and Food Branch, Health Canada, Ottawa, ON, Canada,), anti-CTR2 (Novus Biologicals, Littleton, CO), anti-tubulin, and anti-laminin B1 (both from Santa Cruz Biotechnology, Inc. Santa Cruz, CA).
qRT-PCR.
CTR2 mRNA levels were measured using a qRT-PCR method of detection of relative amounts of first-strand cDNA. cDNA was generated from mRNA isolated using TRIzol (Invitrogen). Purified mRNA was converted to cDNA using oligo(dT)20 priming and the SuperScript III First-Strand kit (Invitrogen). qRT-PCR was performed on an MyIQ qPCR machine (Bio-Rad Laboratories). The forward and reverse primers used were as follows: mCTR2: forward, tccaggtagtcatcagct; reverse, tggcagtgctctgtgatgtc; β-actin: forward, aggtgacagattgcttctg; reverse, gctgcctcaacacctcaac; Gapdh: forward, tcaccaccatggagaaggc; reverse, gctaagcagttggtggtgca; and mATOX1: forward, ctgggaggagtggagttcaa; reverse, gccaaggtaggaaacagcct. Reactions were prepared using iQ SYBR Green Supermix (Bio-Rad Laboratories), according to the manufacturer's recommendations. Samples were prepared in quadruplicate with three independent sample sets being analyzed. Analysis was done using the Bio-Rad iQ5 system software.
Measurement of Drug Accumulation into Whole Cells.
Cells were grown to 90% confluence in T-150 tissue culture flasks. Cells were then harvested using trypsin; 7.5 × 105 cells were placed into each well of six-well tissue culture plates and allowed to grow overnight in 2.5 ml of media at 37°C in 5% CO2. The next day, medium was removed by aspiration, and the cells were pretreated with either CuSO4 or BCS or with untreated control media for 1 h. The media were then removed, and the cells were exposed to 500 μl of cisplatin-containing Opti-MEM medium (Invitrogen) at 37°C for either 0 or 60 min, after which the drug-containing medium was removed, and the plates were washed three times with ice-cold PBS and were then placed on ice. In the case of the time 0 samples, the drug-containing medium was aspirated within 15 s of the start of drug exposure. Concentrated (50–70%) nitric acid (214 μl) was added to each well, and the plate was incubated overnight at room temperature. The following day, the acid was moved into Omni-vials (Wheaton Science Products, Millville, NJ) and incubated at room temperature overnight to thoroughly dissolve all cellular debris. The next day, the nitric acid was diluted with 3 ml of buffer (0.1% Triton X-100, 1.4% nitric acid, and 1 ppb indium in double-distilled H2O). Platinum concentration was measured using an Element 2 ICP-MS (Perkin-Elmer Life and Analytical Sciences, Waltham, MA) located at the Analytical Facility at Scripps Institute of Oceanography at the University of California (San Diego, CA). As a method of normalization, total sulfur was measured using an inductively coupled plasma optical emission spectroscopy (Perkin-Elmer Life and Analytical Sciences), also located at Scripps Institute of Oceanography at the University of California, San Diego. Samples that were prepared previously for the ICP-MS were then introduced into the inductively coupled plasma optical emission spectroscopy, in which the total amount (micrograms of sulfur) was measured. All data presented are the means of at least three independent experiments, each performed with six wells per concentration tested.
Measurement of CTR2 Half-Life.
The 2008 cells were preincubated with CuSO4 and BCS for 1 h as described previously and were then exposed to 100 μg/ml cyclohexamide for 0, 5, 10, 20, 30, or 45 min. Cells were then washed three times with PBS, and lysates were harvested for Western blotting as described previously.
Nuclear Fractionation.
Cells were grown to ∼90% confluence in 10-mm dishes, harvested, and nuclei were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce, Rockford, IL) according to the manufacturer's protocols.
Deconvolution Microscopy.
Cells were grown on eight-well microscope chamber slides (Perkin Elmer Life and Analytical Sciences). Upon reaching ∼60% confluence, the media were removed from each chamber. The chambers were then treated with either 300 μl of Dulbecco's modified Eagle's medium–Reduced Serum alone (Invitrogen) or Dulbecco's modified Eagle's medium–Reduced Serum containing either 200 μM CuSO4, 100 μM BCS, or 30 μM DDP for 1 h. After 1-h drug exposure, the media were removed, and the slide was treated and then washed with PBS in triplicate. Cells were fixed with 3.7% formalin in PBS for 30 min, followed by three 10-min PBS washes. Cells were then permeabilized with 0.3% Triton X-100 in PBS followed by three 1-min PBS washes. The slides were blocked for 1 h with 5% bovine serum albumin in PBS and then treated with 20 μM anti-CTR2 antibody (provided by Dr. Jesse Bertinato) overnight at 4°C followed by three 10-min PBS washes. The slides were then exposed to 1:1000 anti-rabbit Texas Red for 1 h, washed three times in PBS, and viewed using a Deltavision deconvolution microscope (Applied Precision, Inc. Issaquah, WA). Other primary/secondary antibodies used include anti-nuclear pore complex (NPC) proteins 414 (Abcam Inc., Cambridge, MA) and anti-mouse fluorescein isothiocyanate secondary antibody (Invitrogen).
Proteosomal Degradation Assay.
Next, 2008 cells were plated on chamber slides and treated with 50 nM bortezomib for 4 h or were left as untreated controls. During the last hour of bortezomib exposure, selected wells were treated with 100 μM BCS. The cells were then fixed and prepared for microscopy as described previously.
Statistical Analysis.
All data were derived from at least three independent experiments and are presented as the S.E.M. Statistical comparisons were performed using a two-tailed t test with the assumption of unequal variance.
Results
Regulation of CTR2 Expression by Copper and DDP.
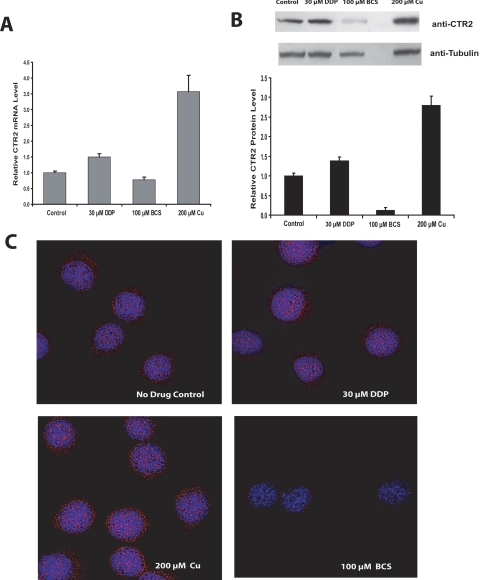
Human ovarian carcinoma 2008 cells were exposed to 200 μM CuSO4 for 1 h, after which mRNA and cellular proteins were isolated to assess the effect of copper on CTR2 expression. At 1 h, the level of CTR2 mRNA had increased by 2.4-fold (p = 0.01), as measured by qRT-PCR (Fig. 1A), and this was accompanied by a 2.4-fold (p = 0.02) increase in CTR2 protein as quantified by Western blot analysis (Fig. 1B). To confirm that the availability of copper affects CTR2 levels, the 2008 cells were treated with 100 μM BCS for 1 h to deplete intracellular copper. As shown in Fig. 1, C and D, this brief period of copper starvation produced a 29% decrease in CTR2 mRNA (p = 0.052) but a more marked 90% decrease in CTR2 protein level (p = 0.0006).
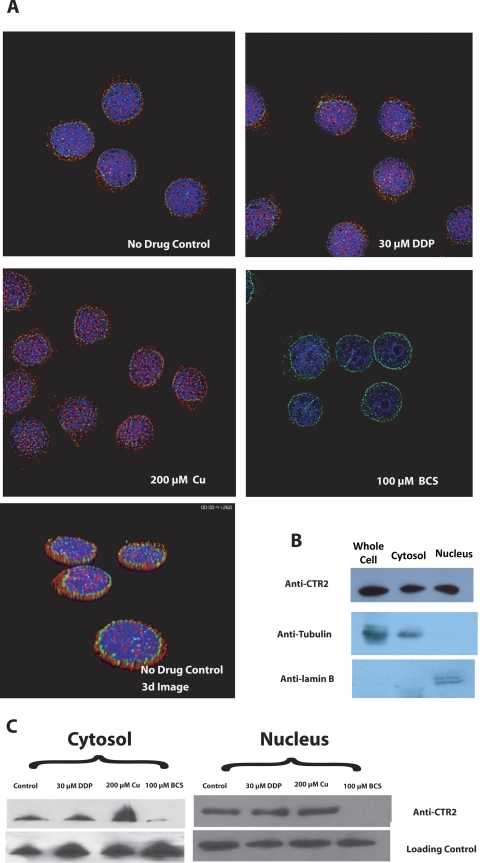
Fig. 1.
Measurement of CTR2 in 2008 cells after 1-h pretreatment with either 200 μM CuSO4, 100 μM BCS, 30 μM DDP, or drug-free media. A, relative mRNA levels measured by qRT-PCR. B, Western blot and densitometric analysis of CTR2 protein levels. C, representative deconvolution micrographs of 2008 cells stained for CTR2 (red).
To determine whether DDP was able to regulate CTR2 expression in a manner similar to that of copper, cellular CTR2 mRNA and protein levels were measured after exposure of the 2008 cells to 30 μM DDP for 1 h. DDP treatment increased the CTR2 mRNA level by 1.4-fold (p = 0.057) (Fig. 1A) and produced a similar 1.4-fold increase in protein level (p = 0.063) (Fig. 1B). Thus, at the concentration tested, DDP caused only modest changes in CTR2 mRNA and protein expression compared with those produced by copper alone when measured at 1 h.
To further document the ability of copper and DDP to regulate the expression of CTR2 at the protein level, 2008 ovarian carcinoma cells were treated for 1 h with either 200 μM CuSO4, 100 μM BCS, 30 μM DDP, or drug-free control media and were then stained with an anti-CTR2 antibody and a fluorescently tagged secondary antibody and visualized by deconvolution microscopy. As shown in Fig. 1C, exposure to 200 μM CuSO4 greatly increased the cellular level of CTR2 protein, and copper starvation produced by a 1-h treatment with 100 μM BCS resulted in a near total loss CTR2 protein. In contrast, exposure to 30 μM DDP for 1 h produced only a modest increase in CTR2 staining.
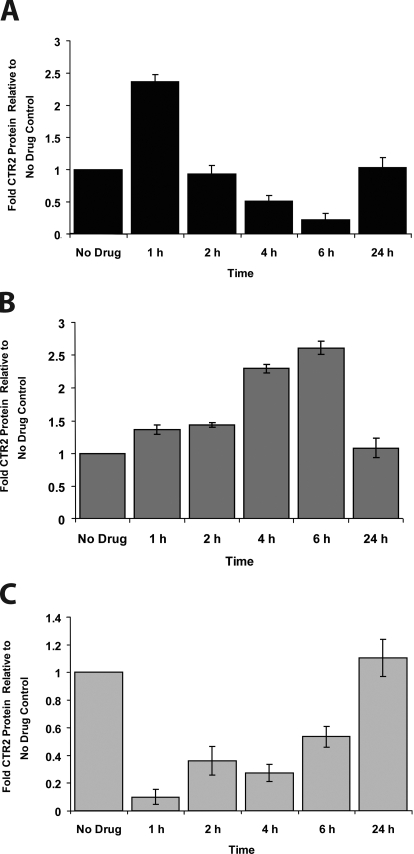
The time course of the changes induced in the level of CTR2 protein by copper, DDP, and BCS were examined after a 1-h exposure to each agent. In response to CuSO4, the increase in CTR2 peaked at the end of the 1-h exposure and then subsequently decreased to a nadir of 22% of control before returning to basal levels at 24 h (Fig. 2A). The marked decrease in CTR2 produced by BCS gradually resolved once the drug was removed at 1 h, and basal levels were attained by 24 h (Fig. 2B). As shown in Fig. 2C, after exposure to DDP, the CTR2 protein level continued to increase over the ensuing 5 h, reaching a peak of 2.6-fold at 6 h from the start of drug exposure but had returned to baseline by 24 h. Thus, a 1-h exposure to each of the three agents produced a perturbation in CTR2 protein level lasting to at least 6 h from the start of drug exposure before resolving by 24 h.
Fig. 2.
Time course of changes produced in CTR2 protein levels by a 1-h exposure to either 200 μM CuSO4 (A), 100 μM BCS (B), or 4.5 μM DDP (C).
Effect of Copper and DDP on CTR2 Half-Life.
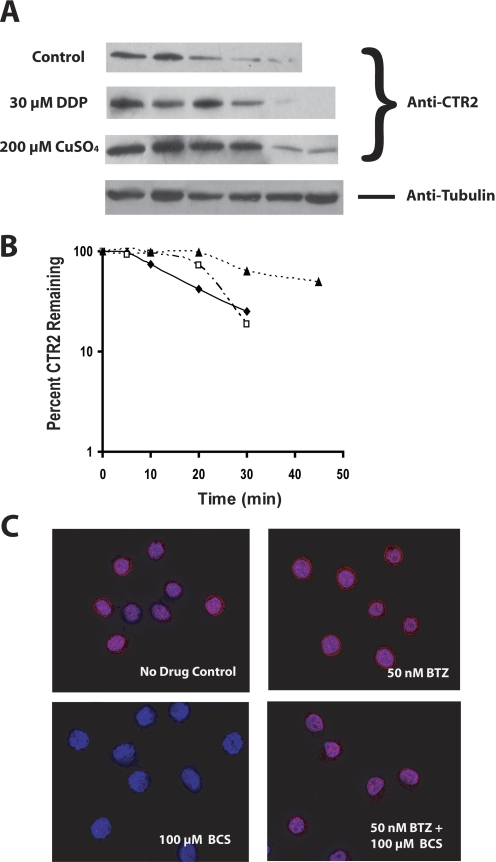
To determine whether the increase in CTR2 level after DDP and copper treatment was due to increased protein stability, 2008 cells were treated with cyclohexamide to block new CTR2 synthesis, and the CTR2 level was determined as a function of time in the presence or absence of either DDP or copper. The data presented in Fig. 3, A and B, demonstrate that both copper and DDP increased CTR2 stability. The half-life of CTR2 was 14.53 ± 2.2 min in the absence of either copper or DDP. The addition of 30 μM DDP for 1 h increased CTR2 half-life to 22.67 ± 0.7 min or by 1.6-fold (p = 0.016). Exposure to copper increased the half-life to 42.56 ± 4.0 min or by 3.1-fold (p = 0.001). Thus, copper and DDP can regulate that expression of CTR2 at the post-transcriptional level.
Fig. 3.
Measurement of CTR2 half-life by Western blot. A, blot showing lysates extracted from cells at 0, 5, 10, 20, 30, or 45 min after the start of exposure to 100 μg/ml cyclohexamide. Cells were pretreated with either drug-free media, 200 μM CuSO4, or 30 μM DDP for 1 h before cyclohexamide exposure; ♦, control; □, DDP pretreatment; ▴, copper pretreatment. B, logarithmic representation of the percentage of remaining CTR2 protein as a function of time after the start of cyclohexamide treatment. C, deconvolution microscopy of 2008 cells after exposure to drug-free control media or 50 nM bortezomib in the presence or absence of 100 μM BCS.
Copper Starvation Enhances DDP Uptake and Sensitivity.
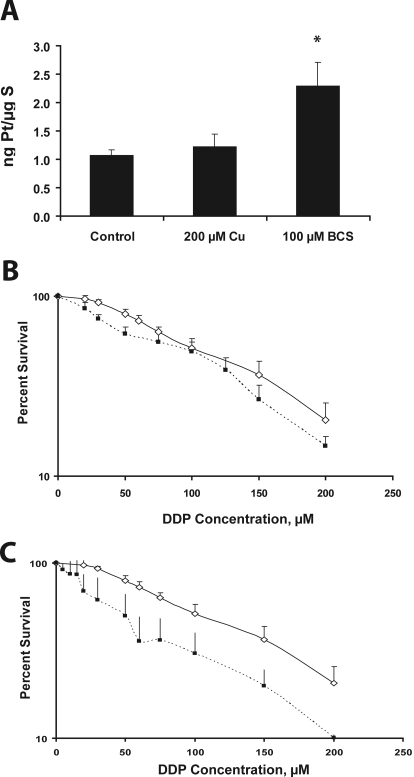
Our previous studies in mouse embryo fibroblasts indicated that knockdown of mCTR2 enhanced the cellular accumulation and cytotoxicity of DDP (Blair et al., 2009). To determine whether reduction of CTR2 expression mediated by copper depletion produced the same effect and whether this occurred in human tumor cells, 2008 ovarian carcinoma cells were treated with either 200 μM CuSO4, 100 μM BCS, or drug-free control media for 1 h and then were exposed to increasing concentrations of DDP for 15 min. The platinum content of the cells was then measured by ICP-MS. The data presented in Fig. 4A indicate that pretreatment with copper did not significantly change the whole-cell accumulation of DDP at 15 min. However, pretreatment with BCS increased whole-cell uptake at 15 min by 2.2-fold (p < 0.0003).
Fig. 4.
A, uptake of DDP after 1 h pretreatment with either 200 μM CuSO4, 100 μM BCS, or drug-free media. Data shown are whole-cell nanograms of platinum accumulation per microgram of sulfur. B, DDP concentration-survival curves for 2008 cells with (◊) and without (■) pretreatment with 200 μM CuSO4 for 1 h. C, DDP concentration-survival curves for 2008 cells with (◊) and without (■) pretreatment with 100 μM BCS for 1 h.
To assess the effect of copper exposure or depletion on the cytotoxicity of DDP, the cells were treated with either 200 μM CuSO4, 100 μM BCS, or drug-free control media for 1 h, and the effect on growth rate over the ensuing 5 days was assessed using a sulforhodamine B assay. Figure 4B shows that pretreatment with copper did not significantly change the sensitivity of the 2008 cells to DDP. The IC50 was 100.4 ± 4.5 μM (mean ± S.E.M.) in the absence of copper pretreatment and 101.8 ± 0.9 μM when the cells were pretreated with copper. However, as shown in Fig. 4C, BCS-mediated copper starvation significantly increased sensitivity to DDP by 2.0-fold. The IC50 value was 100.4 ± 4.5 μM in the absence of BCS pretreatment and 49.1 ± 14.2 μM with BCS pretreatment (p = 0.03). Thus, similar to the effect of knocking down mCTR2 in mouse embryo fibroblasts, depletion of intracellular copper enhanced both the cellular accumulation and cytotoxicity of DDP in 2008 human ovarian carcinoma cells. Although this result is consistent with the concept that the effect of BCS is due to CTR2 depletion, it leaves open the possibility that the effect of BCS is mediated via up-regulation of CTR1 rather than depletion of CTR2. However, previous studies have shown that forced expression of CTR1 greater than its basal level had little effect on DDP cytotoxicity, and BCS only increases CTR1 levels by a modest amount (Holzer et al., 2004b).
Copper Starvation Reduces Nuclear CTR2 Levels.
As shown in Fig. 1C, CTR2 was found abundantly in the nucleus and in the cytoplasm of 2008 cells. To determine whether nuclear and cytoplasmic levels of CTR2 were modulated by copper and DDP, 2008 cells were pretreated with 200 μM CuSO4, 30 μM DDP, 100 μM BCS, or drug-free control media for 1 h and stained with antibodies against CTR2 and the NPC proteins. Figure 5A shows that CTR2 (red) was partially localized within the nucleus, appearing as discrete foci randomly distributed throughout the nucleus and not clearly associated with nucleoli. There seems to be no colocalization of CTR2 with the nuclear envelope (green). Three-dimensional images derived from z-stack sections confirmed that CTR2 is present within the nucleus of these cells (Fig. 5A).
Fig. 5.
A, deconvolution microscopic images of 2008 cells after 1 h of pretreatment with either 200 μM CuSO4, 100 μM BCS, or drug-free media. Red, CTR2; green, NPC proteins. B, Western blot analysis of CTR2, tubulin, and lamin B in the whole cell, cytosolic, and nuclear fractions of 2008 cells. C, Western blot analysis of CTR2 in the cytosolic and nuclear fractions of 2008 cells after 1-h pretreatment with either 200 μM CuSO4, 100 μM BCS, or drug-free media. Loading controls were anti-tubulin (cytosol) and anti-laminin B1 (nucleus).
Exposure of 2008 cells to 30 μM DDP for 1 h produced a small increase in nuclear CTR2 level (Fig. 5A). Exposure to copper produced a more pronounced increase without changing the distribution of CTR2 within the nucleus itself and causing only a small change in the ratio of cytoplasmic to nuclear staining. Depletion of copper with BCS caused almost complete disappearance of CTR2 from the nucleus and from other compartments of the cell. To assess the effect of copper and DDP further, nuclei were isolated and subjected to Western blot analysis. As shown in Fig. 5B, CTR2 was readily detected in the nuclear fraction of 2008 cells. Exposure to 30 μM DDP for 1 h increased the amount of nuclear CTR2 by 25% (p < 0.05). When the cells were treated with 200 μM CuSO4, the total nuclear CTR2 increased by 40% (p < 0.03). Consistent with its effect on the level of CTR2 in the whole cell, exposure to 100 μM BCS completely eliminated CTR2 from the nuclear fraction. Cytosolic CTR2 mimicked the whole-cell response; DDP and copper increased cytosolic CTR2 by 1.3- and 2.4-fold, respectively (Fig. 5B). It is noteworthy that, as assessed by both immunofluorescent staining and Western blot analysis, the effect of DDP and copper on nuclear CTR2 was muted in comparison with their effect on cytosolic CTR2.
ATOX1 Influences the Expression of CTR2.
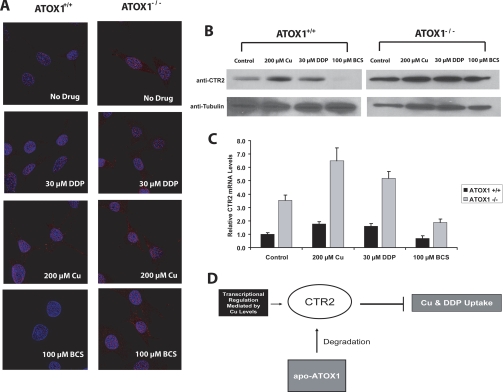
If CTR2 levels are regulated by the availability of intracellular copper, then defects in the network of copper chaperones that control the distribution of copper might be expected to modulate CTR2 levels as well. This question was addressed using a mouse embryo fibroblast subline in which both alleles of ATOX1 had been knocked out [ATOX1(−/−)]. In these cells, there is a failure to deliver copper to the secretory pathway for export from the cell, and their steady-state copper level is 3.2-fold higher than in the parental cells (Hamza et al., 2003). The parental ATOX1(+/+) and ATOX1(−/−) cells were treated with 200 μM CuSO4, 30 μM DDP, 100 μM BCS, or drug-free control media for 1 h and were then fixed and stained with antibody against CTR2. As shown in Fig. 6A, loss of ATOX1 greatly increased the steady-state level of CTR2. However, this change in steady-state level did not seem to alter the subcellular localization of CTR2 protein (red). Copper- and DDP-mediated up-regulation of CTR2 protein in the ATOX1(+/+) cells was similar to that observed in 2008 cells. It is noteworthy that the level of CTR2 protein in ATOX1(−/−) cells did not significantly change when cells were treated with DDP and copper. Furthermore, whereas exposure to 100 μM BCS markedly reduced CTR2 expression in ATOX1(+/+) cells, BCS failed to down-regulate CTR2 expression in the ATOX1(−/−) cells. The requirement for ATOX1 was further examined by Western blot analysis. The data presented in Fig. 6B confirm that the CTR2 protein level in untreated ATOX1(−/−) cells was 3.1-fold higher than in untreated ATOX1(+/+) cells (p = 0.003). Again, whereas DDP or copper exposure affected CTR2 level in ATOX1(+/+) cells in a similar fashion to that in 2008 cells, DDP and copper did not significantly alter CTR2 protein expression in ATOX1(−/−) cells. Exposure to BCS down-regulated the expression of CTR2 in the ATOX1(+/+) cells but failed to do so in the ATOX1(−/−) cells. BCS pretreatment of the ATOX1(+/+) cells caused a near total loss of CTR2 protein (12.5%, p < 0.0001), whereas in the ATOX1(−/−) cells, there was no discernible difference in expression.
Fig. 6.
A, deconvolution microscopic images of ATOX1(+/+) and ATOX1(−/−) cells after 1 h pretreatment with either 200 μM CuSO4, 100 μM BCS, or drug-free media. CTR2 is shown in red. B, Western blot analysis of CTR2 in ATOX1(+/+) and ATOX1(−/−) cells after a 1-h pretreatment with either 200 μM CuSO4, 100 μM BCS, or drug-free media. C, relative CTR2 mRNA levels measured by qRT-PCR of ATOX1(+/+) (black) and ATOX1(−/−) cells (gray). D, model of CTR2 regulation by ATOX1.
To determine how ATOX1 influences CTR2 mRNA levels, CTR2 expression was quantified by qRT-PCR in the ATOX1(+/+) and ATOX1(−/−) cells with and without drug pretreatment. Consistent with the protein expression data, the steady-state level of CTR2 mRNA in ATOX1(−/−) cells was 3.5-fold higher than that in the ATOX1(+/+) cells (Fig. 6C). The magnitude of the change in CTR2 mRNA produced by exposure to 200 μM CuSO4 (1.8-fold increase, p = 0.03), 30 μM DDP (1.6-fold increase, p < 0.04), or 100 μM BCS (32% decrease, p < 0.07) in ATOX1(+/+) cells was similar to that observed in 2008 cells. It is noteworthy that whereas the CTR2 protein level did not significantly change in the ATOX1(−/−) cells in response to DDP or copper exposure, CTR2 mRNA expression in ATOX1(−/−) cells changed in a manner similar to that observed in ATOX1(+/+) and 2008 cells. Exposure to 200 μM CuSO4 led to a 1.9-fold increase in CTR2 mRNA (p < 0.01) and exposure to 30 μM DDP led to a 1.4-fold increase in CTR2 mRNA (p = 0.02). Copper starvation as a result of 100 μM BCS treatment resulted in a 43% decrease in CTR2 mRNA expression.
Discussion
The level of expression of CTR2 is a major determinant of sensitivity to the cytotoxic effect of DDP (Blair et al., 2009). To study the regulation of CTR2, we measured the effect of copper, DDP, and copper starvation on CTR2 mRNA and protein expression using drug concentrations at which CTR2 knockdown is known to produce phenotypic effects (Holzer et al., 2004a; Holzer and Howell, 2006; Blair et al., 2009). Treatment of 2008 human ovarian carcinoma cells with 200 μM CuSO4 for 1 h led to a large 2.4-fold increase in both CTR2 mRNA expression and protein level. On the other hand, starving the 2008 cells of copper by treating with 100 μM BCS for 1 h depleted CTR2 mRNA and reduced the protein to nearly undetectable levels. Similar results were obtained in mouse embryo fibroblast cells, indicating that these phenomena are not artifacts of a single cell line. Thus, copper regulates CTR2 expression, and the close relationship between the changes in mRNA and protein levels support the conclusion that this regulation occurs, at least partially, at the transcriptional level. Having shown previously that the expression of CTR2 has a large effect on both the accumulation and cytotoxicity of DDP (Blair et al., 2009), it was of interest to determine whether DDP also modulates the expression of this protein. Exposure to 30 μM DDP for 1 h increased CTR2 mRNA expression and protein levels by 1.4-fold. This indicates that, at a concentration known to be cytotoxic, DDP also up-regulated CTR2 and thus that DDP induces the expression of a protein that limits its own uptake into tumor cells. It is noteworthy that the two copper transporters, CTR1 and CTR2, have opposite effects on the cellular accumulation of DDP, with CTR1 enhancing uptake and CTR2 limiting uptake (Blair et al., 2009; Larson et al., 2009), and that DDP and copper have opposite effects on the level of expression of these two transporters. DDP causes rapid degradation of CTR1 (Holzer and Howell, 2006; Jandial et al., 2009), whereas at the same concentration, it produces a modest increase in the expression of CTR2.
Although the perturbations in CTR2 protein level produced by all three agents resolved by 24 h, there were differences in the pattern of recovery among them. Copper produced a biphasic response with an initial increase at 1 h followed by a substantial decrease at 6 h indicating a secondary response, perhaps reflecting a compensatory response to the changes in copper homeostasis produced by the initial CTR2 elevation. In contrast, after a 1-h exposure to BCS, there was a gradual return to basal levels without a secondary overshoot. DDP produced a substantially more prolonged increase in CTR2 than copper, perhaps reflecting the fact that a large fraction of DDP that enters the cell resides in compartments from which it is not readily effluxed.
To determine whether copper and DDP additionally regulate CTR2 by altering its degradation, CTR2 half-life and proteosomal degradation was measured after copper exposure or starvation. Cyclohexamide inhibits new protein production, and the disappearance of a given protein during cyclohexamide exposure is commonly used to estimate its half-life. Using this approach, the half-life of CTR2 under basal conditions was found to be ∼14.5 min. After exposure to 200 μM CuSO4 for 1 h, the half-life of CTR2 increased to ∼42.5 min whereas after exposure to 30 μM DDP it increased to ∼22.7 min. Although it is possible that cyclohexamide itself perturbed the half-life of CTR2 in the absence of copper or DDP exposure, it is evident that both copper and DDP increase CTR2 half-life. This indicates that the increase in the level of CTR2 protein after exposure to copper or DDP is in part due to an effect on CTR2 degradation. This was confirmed by showing that the degradation of CTR2 was blocked by the proteosome inhibitor bortezomib. It is of interest that the degradation of CTR1 by DDP and copper is also mediated by proteosomal degradation (Holzer and Howell, 2006; Jandial et al., 2009). Thus, both CTR1 and CTR2 depend on the proteosome for degradation despite the fact that the signals triggering down-regulation differ. CTR1 is known to become polyubiquitinated in response to the signal that triggers its degradation (DDP exposure) (Safaei et al., 2009); whether CTR2 becomes polyubiquitinated in response to its degradation signal (copper starvation) remains to be determined. The results reported here support the conclusion that CTR2 is regulated both transcriptionally and post-transcriptionally by copper availability and exposure to DDP. Caution is required with regard to our data on transcriptional regulation because we have not determined whether there is an effect of copper or DDP on CTR2 mRNA stability. In addition, the results do not permit a quantitative assessment, which is the dominant determinant of CTR2 protein levels.
Knockdown of CTR2 expression using RNA inference results in a large increase in the cellular accumulation and cytotoxicity of DDP (Blair et al., 2009). In addition, increased CTR2 levels have been identified as a potential marker of DDP resistance (Blair et al., 2009). Because copper starvation leads to a rapid and near total loss of CTR2, and copper exposure quickly up-regulates CTR2 levels, it was of interest to determine whether these copper-induced changes in CTR2 also affect DDP accumulation and cytotoxicity. Whole-cell platinum levels were measured by ICP-MS and the extent of cell kill by sulforhodamine B assay after a 15-min exposure to DDP that was preceded by treatment with either copper or BCS. Copper pretreatment did not significantly alter platinum uptake or enhance cytotoxicity; however, BCS pretreatment led to a 2.2-fold increase in whole-cell platinum accumulation and a 2-fold increase in cytotoxicity. These results suggest that DDP accumulation is increased by depletion of CTR2 irrespective of how this was attained, although they do not exclude the possibility that copper starvation also alters the expression of other DDP transporters. These data suggest that copper chelators might be used to enhance tumor sensitivity to the platinum-containing drugs.
Immunocytochemical and deconvolution microscopic examination confirmed the results of the qRT-PCR and Western blot analyses with respect to the ability copper and DDP to increase, and BCS to deplete, CTR2 in the 2008 ovarian cancer cells. Similar effects were also observed in mouse embryo fibroblasts. The immunocytochemical analysis disclosed two additional findings of interest. First, neither copper nor DDP produced a major change in the subcellular localization of CTR2. Second, a substantial amount of CTR2 was present in the nucleus, a novel observation. This observation was confirmed in several cell lines using two independently derived CTR2 antibodies. When costained with an antibody to a nuclear envelope protein, and particularly when the images were reconstructed in three dimensions, it was clear that a fraction of CTR2 resides inside the nucleus. One possible reason why nuclear localization was not detected previously is that prior studies of CTR2 localization relied primary on cells transfected with exogenous CTR2 containing a C-terminal tag that may have interfered with nuclear trafficking (van den Berghe et al., 2007; Bertinato et al., 2008). The nuclear CTR2 was found in discrete randomly distributed sites that yielded a punctate pattern similar to that of processing bodies. Like its effect on the level of CTR2 in the cytosolic and membrane-bound fractions, DDP and copper treatment increased the amount of CTR2 within the nucleus; however, this increase seemed to be less dramatic than in the rest of the cell. These observations were confirmed by Western blot analysis of nuclear fractions. The mechanism by which CTR2 reaches the nucleus and how nuclear levels are regulated is unknown and is the subject of further studies. However, there are now several examples of transmembrane proteins that function at the plasma membrane, including epidermal growth factor receptor (Liao and Carpenter, 2007) and CD44 (Lee et al., 2009), that are also trafficked to the nucleus, in which they participate in transcriptional regulation. Therefore, it is feasible that CTR2 is trafficked to the nucleus in a similar manner and that CTR2 participates in transcriptional activation of copper-responsive genes, either directly or by regulating nuclear copper. The exact role CTR2 plays in the nucleus and the mechanism by which it traffics to the nucleus are important questions for future investigation.
ATOX1 is the copper chaperone that transfers copper to the efflux transporters ATP7A and ATP7B. ATOX1 has also been shown recently to be a copper-dependent transcription factor capable of activating the expression of cyclin D1 and SOD1 (Itoh et al., 2008; Muller and Klomp, 2009). Analysis of the CTR2 promoter region with the GenoMatix software (GenoMatix Software Inc., Ann Arbor, MI) suggested that it contains several potential ATOX1 binding motifs. This raised the possibility that the regulation of CTR2 mRNA expression by copper, DDP, and copper starvation could be due to different transcriptional activation by ATOX1. Under steady-state conditions, the ATOX1(−/−) cells were found to express 3.5-fold more CTR2 mRNA and 3.1-fold more protein than ATOX1(+/+) cells. This suggests that ATOX1 may play a role in inhibiting CTR2 expression under basal conditions either at the transcriptional or post-translational level. It is possible that the observed increase in CTR2 in ATOX1(−/−) cells is due to changes in the copper levels rather than direct ATOX1 transcriptional regulation. Consistent with this concept, ATOX1(−/−) cells have been reported to have higher endogenous copper levels than ATOX1(+/+) cells. Furthermore, the magnitude of the changes in CTR2 mRNA in response to excess copper, DDP, or starvation were similar in the ATOX1(+/+) and ATOX1(−/−) cells, indicating that ATOX1 was not required for these perturbations and therefore is unlikely to be directly regulating CTR2 transcription. ATOX1 has been reported to directly regulate CTR1 proteosomal degradation stimulated by DDP and copper (Safaei et al., 2009). Although not essential for the changes in mRNA level, ATOX1 was essential to the ability of copper starvation to down-regulate CTR2 protein. When cells are starved for copper, CTR2 is quickly degraded by the proteosome. It is likely therefore that the interaction of ATOX1 with copper is necessary for the stabilization of CTR2, and the apo-form of ATOX1 is necessary for CTR2 degradation. Figure 6D presents a model of how CTR2 might be regulated by ATOX1. ATOX1 seems to be necessary for the copper-dependent regulation of CTR2 at the post-transcriptional level, either through direct interaction with CTR2 or a downstream effect on a pathway that controls CTR2 stability. We suggest that ATOX1 in the apo-conformation is necessary for the degradation of CTR2, as evidenced by the fact that both ATOX1 and copper starvation are necessary for post-transcriptional regulation of CTR2. Presence of the unbound form of ATOX1 is needed for CTR2 degradation, because both copper and loss of ATOX1 stabilize CTR2 protein.
In summary, CTR2 has a large effect on DDP accumulation and sensitivity in human ovarian carcinoma cells. As such, the study of its protein regulation remains tremendously important. CTR2 levels are regulated by the availability of copper and by DDP at the transcriptional and post-transcriptional levels. How CTR2 traffics to the nucleus, its role in the nucleus, and how ATOX1 controls CTR2 expression remain worthy of further investigation in an effort to identify strategies by which the effectiveness of DDP can be enhanced.
Acknowledgments
We thank Dr. J. D. Gitlin for generously providing the ATOX1(+/+) and ATOX1(−/−) mouse embryo fibroblasts, Dr. Jessie Bertinato for the anti-CTR2 antibody, and Dr. Martin Hetzer for the NPC antibody.
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA095298]; and the U.S. Department of Defense [Grant W81XWH-08-1-0135].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.062836.
- CTR1
- copper transporter 1, SLC31A1
- CTR2
- copper transporter 2, SLC31A2
- DDP
- cisplatin
- ICP-MS
- inductively coupled plasma mass spectrometry
- BCS
- bathocuproine disulfonate
- PBS
- phosphate-buffered saline
- qRT-PCR
- quantitative reverse transcriptase-polymerase chain reaction
- NPC
- nuclear pore complex.
References
- Bellemare DR, Shaner L, Morano KA, Beaudoin J, Langlois R, Labbe S. (2002) Ctr6, a vacuolar membrane copper transporter in Schizosaccharomyces pombe. J Biol Chem 277:46676–46686 [DOI] [PubMed] [Google Scholar]
- Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'abbé MR. (2008) Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J 409:731–740 [DOI] [PubMed] [Google Scholar]
- Blair BG, Larson CA, Safaei R, Howell SB. (2009) Copper transporter 2 regulates the cellular accumulation and cytotoxicity of Cisplatin and Carboplatin. Clin Cancer Res 15:4312–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSaia PJ, Sinkovics JG, Rutledge FN, Smith JP. (1972) Cell-mediated immunity to human malignant cells. A brief review and further studies with two gynecologic tumors. Am J Obstet Gynecol 114:979–989 [DOI] [PubMed] [Google Scholar]
- Hamza I, Prohaska J, Gitlin JD. (2003) Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci USA 100:1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer AK, Howell SB. (2006) The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res 66:10944–10952 [DOI] [PubMed] [Google Scholar]
- Holzer AK, Katano K, Klomp LW, Howell SB. (2004a) Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res 10:6744–6749 [DOI] [PubMed] [Google Scholar]
- Holzer AK, Samimi G, Katano K, Naerdemann W, Lin X, Safaei R, Howell SB. (2004b) The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol 66:817–823 [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA 99:14298–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T. (2008) Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem 283:9157–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Ozumi K, Kim HW, Nakagawa O, McKinney RD, Folz RJ, Zelko IN, Ushio-Fukai M, Fukai T. (2009) Novel mechanism for regulation of extracellular SOD transcription and activity by copper: role of antioxidant-1. Free Radic Biol Med 46:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB. (2009) Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clinical Cancer Research 15:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeney V, Itoh S, Wendt M, Gradek Q, Ushio-Fukai M, Harrison DG, Fukai T. (2005) Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ Res 96:723–729 [DOI] [PubMed] [Google Scholar]
- Kampfenkel K, Kushnir S, Babiychuk E, Inzé D, Van Montagu M. (1995) Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J Biol Chem 270:28479–28486 [DOI] [PubMed] [Google Scholar]
- Larson CA, Blair BG, Safaei R, Howell SB. (2009) The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 75:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Wang MJ, Chen JY. (2009) Acetylation and activation of STAT3 mediated by nuclear translocation of CD44. J Cell Biol 185:949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HJ, Carpenter G. (2007) Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell 18:1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Okuda T, Holzer A, Howell SB. (2002) The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol 62:1154–1159 [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Tsivkovskii R, Walker JM. (2003) Functional properties of the human copper-transporting ATPase ATP7B (the Wilson's disease protein) and regulation by metallochaperone Atox1. Ann NY Acad Sci 986:204–211 [DOI] [PubMed] [Google Scholar]
- Miyayama T, Suzuki KT, Ogra Y. (2009) Copper accumulation and compartmentalization in mouse fibroblast lacking metallothionein and copper chaperone, Atox1. Toxicol Appl Pharmacol 237:205–213 [DOI] [PubMed] [Google Scholar]
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–765 [DOI] [PubMed] [Google Scholar]
- Muller PA, Klomp LW. (2008) ATOX1: a novel copper-responsive transcription factor in mammals? Int J Biochem Cell Biol 41:1233–1236 [DOI] [PubMed] [Google Scholar]
- Portnoy ME, Schmidt PJ, Rogers RS, Culotta VC. (2001) Metal transporters that contribute copper to metallochaperones in Saccharomyces cerevisiae. Mol Genet Genomics 265:873–882 [DOI] [PubMed] [Google Scholar]
- Rees EM, Lee J, Thiele DJ. (2004) Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem 279:54221–54229 [DOI] [PubMed] [Google Scholar]
- Safaei R, Maktabi MH, Blair BG, Larson CA, Howell SB. (2009) Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J Inorg Biochem 103:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe PV, Folmer DE, Malingré HE, van Beurden E, Klomp AE, van de Sluis B, Merkx M, Berger R, Klomp LW. (2007) Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J 407:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Wedd AG. (2002) A C-terminal domain of the membrane copper pump Ctr1 exchanges copper(I) with the copper chaperone Atx1. Chem Commun (Camb) 6:588–589 [DOI] [PubMed] [Google Scholar]