Abstract
We have screened a chemical library and identified several novel structures of Na/K-ATPase inhibitors. One group of these inhibitors belongs to polyphenolic xanthone derivatives. Functional characterization reveals the following properties of this group of inhibitors. First, like ouabain, they are potent inhibitors of the purified Na/K-ATPase. Second, their effects on the Na/K-ATPase depend on the number and position of phenolic groups. Methylation of these phenolic groups reduces the inhibitory effect. Third, further characterization of the most potent xanthone derivative, MB7 (3,4,5,6-tetrahydroxyxanthone), reveals that it does not change either Na+ or ATP affinity of the enzyme. Finally, unlike that of ouabain, the inhibitory effect of MB7 on Na/K-ATPase is not antagonized by K+. Moreover, MB7 does not activate the receptor Na/K-ATPase/Src complex and fails to stimulate protein kinase cascades in cultured cells. Thus, we have identified a group of novel Na/K-ATPase ligands that can inhibit the pumping function without stimulating the signaling function of Na/K-ATPase.
The Na/K-ATPase, also known as the sodium pump, is a ubiquitous transmembrane enzyme that transports Na+ and K+ across the plasma membrane by hydrolyzing ATP (Skou, 1957; Sweadner, 1989; Lingrel and Kuntzweiler, 1994; Blanco and Mercer, 1998). It belongs to the family of P-type ATPase that transits between E1 and E2 conformational states during pumping cycles. The functional enzyme is composed mainly of α and β subunits. The α subunit is the catalytic component of the holoenzyme because it contains both the nucleotide and cation binding sites (Sweadner, 1989; Lingrel and Kuntzweiler, 1994; Blanco and Mercer, 1998). It is noteworthy that studies during the past few years have uncovered many nonpumping functions of the Na/K-ATPase such as signal transduction (Kometiani et al., 1998; Aizman et al., 2001; Aydemir-Koksoy et al., 2001; Haas et al., 2002; Wang et al., 2004; Yuan et al., 2005; Tian et al., 2006; Nguyen et al., 2007; Cai et al., 2008). Specifically, the signaling Na/K-ATPase resides in caveolae and interacts with a number of signaling proteins such as Src, the inositol 1,4,5-trisphosphate (IP3) receptor, and caveolin-1 (Wang et al., 2004; Yuan et al., 2005; Tian et al., 2006; Cai et al., 2008). Whereas the interaction between the Na/K-ATPase and the IP3 receptor facilitates Ca2+ signaling (Tian and Xie, 2008), the dynamic association between the Na/K-ATPase and Src regulates cellular Src activity and makes it possible for cardiotonic steroids (CTSs) to stimulate protein kinase cascades (Li and Xie, 2009).
CTSs include plant-derived digitalis drugs, such as digoxin and ouabain, and vertebrate-derived aglycones, such as bufalin and marinobufagenin (Akera and Brody, 1976; Schoner and Scheiner-Bobis, 2007). These steroids can be used clinically to treat congestive heart failure because they have well documented inotropic effects on the heart (Akera and Brody, 1976; Repke et al., 1996). Although CTSs have been considered drugs since their discovery, recent studies have identified several of them, including ouabain and marinobufagenin, as endogenous steroids (Hamlyn et al., 1991; Bagrov and Fedorova, 1998). It is known that the Na/K-ATPase serves as a receptor for these steroids. Although binding of CTSs to the Na/K-ATPase inhibits the pumping function, it stimulates the signaling function of Na/K-ATPase (Li and Xie, 2009). For example, the binding of ouabain to the Na/K-ATPase/Src receptor complex stimulates Src kinase. The activated Src, in turn, transactivates receptor tyrosine kinases such as the epidermal growth factor receptor and converts the tyrosine kinase signal to stimulation of serine/threonine kinases, lipid kinases, and lipases and increased production of reactive oxygen species (Liu et al., 2000; Li and Xie, 2009). It is noteworthy that although inhibition of the Na/K-ATPase by CTS is essential for these drugs to increase cardiac contractile function (Reuter et al., 2002; Altamirano et al., 2006), stimulation of protein kinases and subsequent increases in the production of reactive oxygen species by these steroids also cause cardiac hypertrophy and fibrosis in animal studies (Ferrandi et al., 2004; Kennedy et al., 2006).
Because CTSs affect both ion pumping and signal transducing functions of the Na/K-ATPase, we were prompted to search for new Na/K-ATPase ligands that only regulate the ion pumping function of Na/K-ATPase. To achieve this goal, we developed a high-throughput screen assay and tested a chemical library of drug-like small molecules prepared from either Chinese herb medicine or bacterial metabolites. We report here the identification of a novel class of chemicals that are different from CTSs and inhibit the Na/K-ATPase without activating protein kinases in cultured cells.
Materials and Methods
Materials.
ATP and ouabain were obtained from Sigma-Aldrich (St. Louis, MO). Biomol Green was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). The ERK/MAPK (phospho-Thr202/Tyr204) phosphorylation/translocation cell-based assay kit was purchased from Cayman Chemical (Ann Arbor, MI). Purified recombinant Src was obtained from Millipore (Billerica, MA). Polyclonal anti-Tyr(P)418-Src was obtained from Invitrogen (Carlsbad, CA). Anti-c-Src (B-12) monoclonal antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Fresh pig kidneys were purchased from a slaughterhouse and stored at −80°C until used for enzyme preparation.
High-Throughput Screen Assay.
The chemical library used for screening in the present study contained 2600 structurally diverse, drug-like, naturally occurring organic compounds or their semisynthetic derivatives, mainly of plants and bacterial origin. The purity of these compounds was above 95% unless otherwise indicated. Stock compounds were prepared in 96-well plates at 10 mg/ml in dimethyl sulfoxide (DMSO).
The purified Na/K-ATPase was prepared from pig kidney as described previously (Xie et al., 1996). The specific activities of the Na/K-ATPase of various kidney preparations were in the range of 900 to 1200 μmol/mg/h, which was more than 95% of the total ATPase activity. The high-throughput screen was conducted in a 96-well format with the final reaction volume of 100 μl containing the following components: 100 mM NaCl, 20 mM KCl, 1 mM MgCl2, 1 mM EGTA, 20 mM Tris-HCl, pH 7.4, and 0.2 μg of purified Na/K-ATPase. After the compounds were added, the mixtures were incubated at 37°C for 15 min, and reaction was initiated by adding 2 mM ATP·Mg2+ mixture. Reactions were carried out for 15 min and then stopped by the addition of 100 μl of ice-cold 8% trichloroacetic acid. Reaction mixtures were cleared by centrifugation and assayed for released phosphate by using the BIOMOL Green reagent according to the manufacturer's instructions. In addition, control Na/K-ATPase activity was measured in the presence and absence of 1 mM ouabain and taken as 0 and 100%, respectively. Furthermore, 5 μM ouabain and 0.1% DMSO were included in each plate as a positive and vehicle control, respectively. Control experiments showed that ATP hydrolysis catalyzed by the Na/K-ATPase was in linear range within 30 min of incubation under the above experimental conditions.
Cell Culture.
The pig kidney epithelia cells (LLC-PK1 cells) and human nonsmall lung cancer cells (A549 cells) were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium in the presence of 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 humidified incubator. To eliminate the confounding effect of growth factors in the serum, cells were serum-starved for 24 h before experiments unless otherwise indicated.
Western Blot Analysis.
Cells were washed with phosphate-buffered saline and solubilized in ice-old RIPA buffer containing 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 50 mM Tris-HCl, pH 7.4, as described previously (Wang et al., 2004). Cell lysates were then cleared by centrifugation at 14,000 rpm, and supernatants were used for protein assay and subjected to Western blot analysis. Samples were separated on SDS-polyacrylamide gel electrophoresis (50 μg/lane) and transferred to a cellulose membrane. Membranes were blocked with 3% nonfat dried milk for total Src and ERK or 1% bovine serum albumin plus 1% nonfat dried milk for phosphorylated Src and ERK in TBST (10 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween 20, pH 7.5) for 1 h at room temperature and then probed with specific antibodies. Protein signals were detected by using an ECL kit (Thermo Fisher Scientific, Waltham, MA) and quantified with Image J software (http://rsbweb.nih.gov/ij/).
Assay for the Activation of Receptor Na/K-ATPase/Src Complex.
The activity of the receptor Na/K-ATPase/Src complex was assayed as described previously (Tian et al., 2006). In brief, the purified Src (4.5 U) was incubated with 2 μg of the purified Na/K-ATPase in phosphate-buffered saline for 30 min at 37°C. Afterward, the Na/K-ATPase/Src complex was exposed to ouabain or MB7 for 10 min. Reaction was initiated by the addition of 2 mM ATP/Mg2+, continued for 5 min at 37°C, and stopped by addition of the SDS sample buffer. The activation of Src was measured by Western blot using the anti-pY418 antibody. To control for gel loading and transfer, total Src was also probed.
Confocal Imaging and Immunocytochemistry.
LLC-PK1 cells grown on coverslips were serum-starved for 24 h and treated with MB7 or ouabain for various times. Immunostaining of phospho-ERK was performed with the commercial ERK/MAPK (phospho-Thr202/Tyr204) phosphorylation/translocation cell-based assay kit (Cayman Chemical) according to the manufacturer's instructions. The signals were detected by a Leica (Wetzlar, Germany) confocal microscope as described previously (Tian et al., 2006). Leica confocal software was used for data analysis.
Data Analysis.
Data are given as mean ± S.E. Statistics were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc analysis. Student's t test was used for comparisons between two groups. Significance is accepted at p < 0.05.
Results
High-Throughput Screening of Na/K-ATPase Inhibitors.
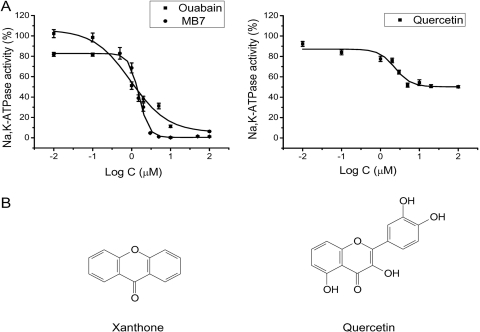
To screen a chemical library for Na/K-ATPase inhibitors, we developed a 96-well format assay. As depicted in Fig. 1A, ouabain, as a positive control, produced a dose-dependent inhibition of the Na/K-ATPase. On the other hand, DMSO, the vehicle, showed no effect on Na/K-ATPase activity when used at a concentration below 0.1% of reaction volume (data not shown). The apparent IC50 for ouabain was approximately 1 μM, comparable with what was reported previously (Böttinger and Habermann, 1984). Thus, we used the same assay and tested a total of 2600 compounds at the final concentration of 10 μg/ml. This concentration was adapted because a majority of compounds has a molecular mass around 200, thus being adopted around 50 μM, 50 times the IC50 of ouabain. Ouabain (5 μM) was used as a positive control, whereas 0.1% DMSO was used as a negative control in each 96-well plate. Assays were conducted in duplicate, and the compound that produced 25% or more inhibition of the Na/K-ATPase was identified as a positive hit. Under these experimental conditions, we found and confirmed a total of 13 positive compounds (Table 1) that include several well known Na/K-ATPase inhibitors such as oligomycin and cardiotonic steroids.
Fig. 1.
A, concentration curves of ouabain, MB7, and quercetin on the Na/K-ATPase. The purified Na/K-ATPase was incubated with different concentrations of compounds for 15 min, then assayed for ouabain-sensitive ATPase activity as described under Materials and Methods. The data are combined from three to five separate experiments and are presented as mean ± S.E. The IC50 for ouabain and MB7 are 1 ± 0.1 and 1.6 ± 0.1 μM, respectively. B, the chemical structures of xanthone (left) and quercetin (right).
TABLE 1.
Na/K-ATPase inhibitors identified by high-throughput screen
These inhibitors produced at least 25% inhibition of Na/K-ATPase at 10 μg/ml.
| Sample | Name | Formula |
|---|---|---|
| 2006BD3 | Metacycline hydrochloride | C22H22N2O8.HCl |
| 2008BB8 | Tyrothricin | Mixture |
| 2018BF7 | Myricetin | C15H10O8 |
| 2021BG2 | Domiphen bromide | C22H40NO.Br |
| 2035BD1 | Resibufogenin | C24H32O4 |
| 2035BA5 | Cinobufagin | C26H34O6 |
| 2036BF1 | Oligomycin | C45H74O11 |
| MB1 | 1,3-Dihydroxyxanthone | C13H8O4 |
| MB2 | 3,4-Dihydroxyxanthone | C13H8O4 |
| MB3 | 1,3,5-Trihydroxyxanthone | C13H8O5 |
| MB5 | 3,4,5-Trihydroxyxanthone | C13H8O5 |
| MB6 | 1,3,5,6-Tetrahydroxyxanthone | C13H8O6 |
| MB7 | 3,4,5,6-Tetrahydroxyxanthone | C13H8O6 |
Identification of Hydroxyxanthones as a New Class of Na/K-ATPase Ligands.
Among the 13 positive hits, many are polyphenolic compounds, including six hydroxyl xanthone derivatives (MB1 to MB7) (Table 1). They are structurally similar to well characterized polyphenolic compounds such as quercetin (Fig. 1B) (Kuriki and Racker, 1976; Robinson et al., 1984). Thus, we further explored the inhibitory properties of these hydroxyxanthones. Because MB7 was the most potent inhibitor of this group (Table 2), it was used in the following studies. In experiments depicted in Fig. 1A, we compared the dose-response curves of MB7, ouabain, and quercetin. Like ouabain, MB7 exhibited dose-dependent inhibition of the Na/K-ATPase. The apparent IC50 (1.6 ± 0.1 μM) is comparable with that of ouabain. In comparison, quercetin is less effective as an inhibitor of Na/K-ATPase. Unlike ouabain and MB7, it produced only a partial inhibition of the Na/K-ATPase. The maximal inhibition was achieved when 5 μM quercetin was applied (Kuriki and Racker, 1976).
TABLE 2.
Structure and activity relationship of xanthone derivatives
The concentration curves of each compound were constructed as in Fig. 1, and the IC50 values were calculated from three independent experiments.
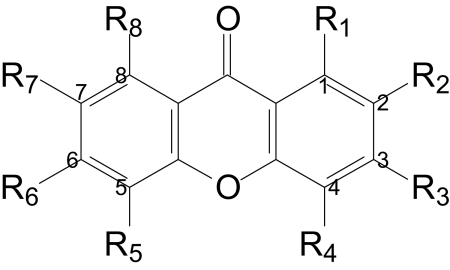
| Sample | Substituent Position |
IC50 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | ||
| μM | |||||||||
| MB1 | OH | H | OH | H | H | H | H | H | >100 |
| MB2 | H | H | OH | OH | H | H | H | H | >100 |
| MB3 | OH | H | OH | H | OH | H | H | H | 65 |
| MB5 | H | H | OH | OH | OH | H | H | H | 10 |
| MB6 | OH | H | OH | H | OH | OH | H | H | 60 |
| MB7 | H | H | OH | OH | OH | OH | H | H | 1.5 |
| MB8 | H | H | OCH3 | OCH3 | H | OCH3 | H | H | >100 |
| MB9 | H | H | H | H | H | H | H | H | >100 |
| 2027BA1 | OH | H | OCH3 | H | H | H | OH | OCH3 | >100 |
| 2027BA2 | OH | H | OCH3 | H | H | H | OCH3 | OH | >100 |
| C-017 | OCH3 | OCH3 | OCH3 | H | H | H | OCH3 | H | >100 |
To determine whether MB7 produces a reversible inhibition of Na,K-ATPase, the purified Na,K-ATPase was treated with 10 μM MB7 at 37°C for 15 min, washed twice by centrifugation, resuspended, then assayed for Na/K-ATPase activity. After washed enzyme was incubated in assay medium for 15 and 60 min, the recovery of the activity reached 67 ± 5 and 94 ± 6%, respectively.
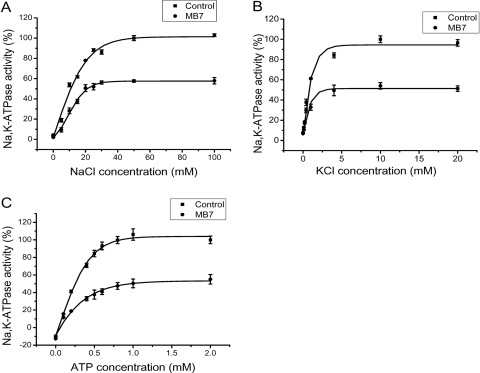
To explore whether MB7 inhibits the Na/K-ATPase by altering substrate affinity, we measured the Na/K-ATPase as a function of substrate concentration in the presence or absence of MB7. As shown in Fig. 2, MB7 had no effect on either Na+ or ATP affinity of the Na/K-ATPase. It is noteworthy that increases in K+ failed to antagonize MB7-induced inhibition of the Na/K-ATPase (Fig. 2B), which is in contrast to ouabain-induced inhibition (Myers et al., 1979). Because MB7 and quercetin both are polyphenolic compounds, we further explored the effect of DMSO on MB7-induced inhibition of Na/K-ATPase. It is known that high concentrations of DMSO inhibit Na/K-ATPase by stabilizing the enzyme at the E2 conformation, which antagonizes the inhibitory effect of quercetin on the Na/K-ATPase (Foster and Ahmed, 1982; Robinson et al., 1984). Indeed, as shown in Table 3, DMSO produced a dose-dependent inhibition of Na/K-ATPase. Under such conditions, the maximal effect of 10 μM quercetin on the Na/K-ATPase was significantly reduced. For example, addition of 5% DMSO abolished the inhibitory effect of quercetin (Table 3). On the other hand, the inhibitory effect of 5 μM MB7 on the Na/K-ATPase was not affected by DMSO.
Fig. 2.
Effects of MB7 on Na+, K+, and ATP dependence. Na/K-ATPase activity was measured as in Fig. 1 as a function of Na+ (A), K+ (B), or ATP (C) concentration. The data are combined from four to six separate experiments and are plotted as mean ± S.E. MB7 was used at 1.5 μM. The apparent Km values for Na+ are 11 ± 1 and 9 ± 1 mM for control and MB7-treated Na/K-ATPase, respectively. The apparent Km values for K+ are 0.6 ± 0.1 and 0.5 ± 0.1 mM for control and MB7, respectively. The Km values for ATP are 0.25 ± 0.02 and 0.22 ± 0.02 mM for control and MB7-treated Na/K-ATPase, respectively.
TABLE 3.
Effects of DMSO on MB7 and quercetin
The data are from three to five separate experiments and are presented as mean ± S.E. The percentage of inhibition of quercetin and MB7 was calculated by using the corresponding DMSO activity as 100%.
| Compound | Inhibition of Na/K-ATPase |
|---|---|
| % | |
| DMSO | |
| 0.1% | 0 |
| 1% | 8 ± 1 |
| 5% | 30 ± 3 |
| Quercetin 10 μM | |
| + 0.1% DMSO | 46 ± 4 |
| + 1% DMSO | 17 ± 3 |
| + 5% DMSO | 3 ± 1 |
| MB7 5 μM | |
| + 0.1% DMSO | 96 ± 2 |
| + 1% DMSO | 96 ± 2 |
| + 5% DMSO | 94 ± 3 |
To explore the structure–activity relationship, we compared the dose-response curves of xanthone, six hydroxyxanthones, and several methylated hydroxyxanthone derivatives. As depicted in Table 2, whereas xanthone failed to inhibit Na/K-ATPase activity, an increase in the number of phenolic groups increased the efficacy and potency of hydroxyxanthones (e.g., comparing MB2 with MB5). Full or partial methylation was consistently able to reduce the inhibitory effect of hydroxyxanthones on the Na/K-ATPase [e.g., comparing MB5 with 3,4,6-trimethoxyxanthone (MB8)]. Finally, when phenolic groups were positioned near the oxygen in the pyrone ring (i.e., at positions 4 and 5), they seemed to have more effect on the potency of these compounds (e.g., comparing MB3 with MB5).
MB7 Fails to Activate the Receptor Na/K-ATPase/Src Complex.
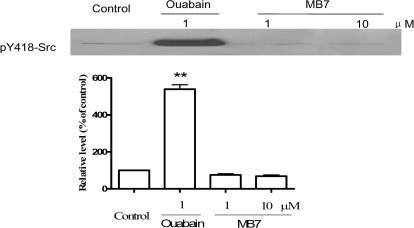
We have demonstrated that the Na/K-ATPase interacts with Src kinase to form a functional receptor complex for ouabain to activate protein kinase cascades (Tian et al., 2006). To test whether MB7 works as ouabain, capable of activating protein kinases, we first measured the effect of MB7 and ouabain on Src activity by using the reconstituted Na/K-ATPase/Src complex. Because 1 μM ouabain, causing 50% inhibition of Na/K-ATPase, was effective in stimulating the Na/K-ATPase-associated Src (Fig. 3), we tested the effect of 1 and 10 μM MB7. At such concentrations, MB7 would produce 31 and 100% inhibition of Na/K-ATPase (Fig. 2). As depicted in Fig. 3, addition of MB7 failed to stimulate Na/K-ATPase-associated Src in the test tube. These findings suggest that MB7 may inhibit ATPase activity without stimulating the receptor function of Na/K-ATPase.
Fig. 3.
Effects of MB7 and ouabain on the receptor Na/K-ATPase/Src complex. The purified Na/K-ATPase and purified Src were incubated in the presence of either ouabain (1 μM) or MB7 (1 and 10 μM) as indicated for 15 min and assayed for Src activation as described under Materials and Methods. Values are mean ± S.E. of three independent experiments. **, p < 0.01 compared with control.
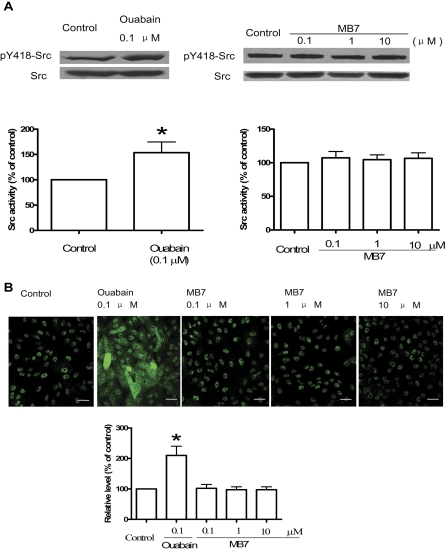
To verify the above findings, we measured the effect of MB7 on Src and ERKs in cultured cells. Ouabain was again used as a positive control. As shown in Fig. 4A, whereas 100 nM ouabain stimulated Src in A549 cells as reported previously (Wang et al., 2009), MB7 from 0.1 to 10 μM failed to do the same. To further confirm these findings, we treated LLC-PK1 cells with either 100 nM ouabain or different concentrations of MB7. We previously showed that ouabain stimulated Src and, subsequently, the ERK cascade in LLC-PK1 cells (Haas et al., 2002; Tian et al., 2006). Indeed, we found that ouabain increased the cellular amount of active ERKs as detected by immunostaining (Fig. 4B). However, under the same experimental conditions, MB7 (from 0.1 to 10 μM) failed to affect cellular ERK activity.
Fig. 4.
Effects of MB7 and ouabain on Src and ERKs. A, A549 cells were treated with ouabain or MB7 for 10 min, and cell lysates (50 μg/lane) were separated by SDS polyacrylamide gel electrophoresis and analyzed for Src activation as in Fig. 3. The values are mean ± S.E. from four to six separate experiments. *, p < 0.05 versus control. B, LLC-PK1 cells were treated with MB7 or ouabain for 10 min and immunostained with the ERK/MAPK (phospho-Thr202/Tyr204) phosphorylation/translocation cell-based assay kit (Cayman Chemical) according to the manufacturer's instructions. The images were collected as described under Materials and Methods. Top, representative images from three experiments are shown. Scale bar, 50 μm. Bottom, the quantitative data of phospho-ERK fluorescence intensity were collected from 40 different fields in three independent experiments and expressed as mean ± S.E. *, p < 0.05 versus control.
Discussion
In this study, we adapted a simple, but effective, high-throughput screen assay and identified 13 Na/K-ATPase inhibitors that represent several structurally divergent classes of compounds. Moreover, we were able to differentiate the newly identified inhibitors from other known inhibitors such as ouabain by assessing their effects on the substrate dependence of the Na/K-ATPase. Finally, we found that the newly identified xanthone derivatives, unlike ouabain, only inhibit ATPase activity, but do not activate the receptor Na/K-ATPase/Src complex. Thus, we have identified a new class of Na/K-ATPase ligands.
Xanthone Derivatives as a New Class of Na/K- ATPase Inhibitors.
According to the Albers-Post reaction scheme, the Na/K-ATPase transits from the E1 to the E2 states via multiple conformational changes. Whereas Na+ favors the E1 state, K+ promotes the conversion of E2P to the E2 state. Over the years, several classes of organic Na/K-ATPase inhibitors have been identified (Fahn et al., 1966; Yoda and Yoda, 1982). They inhibit the Na/K-ATPase by stabilizing the enzyme at different conformational states. For example, ouabain binds and stabilizes the Na/K-ATPase at the E2P. Increases in K+ compete with the binding of ouabain to the Na/K-ATPase and reduce ouabain-induced inhibition. In contrast, K+ fails to antagonize the inhibitory effect of MB7 on the Na/K-ATPase.
Hydroxyxanthones and flavonoids have similar chemical structures because they both contain the benzopyrone structure (Fig. 1B). Moreover, quercetin and myricetin are well known Na/K-ATPase inhibitors (Suolinna et al., 1975). However, unlike MB7, quercetin produces only a partial inhibition of Na/K-ATPase (Fig. 1). In addition, stabilization of Na/K-ATPase by DMSO at E2 conformation blocks quercetin (Robinson et al., 1984), but not MB7-induced inhibition of Na/K-ATPase (Table 3). Thus, the fusion of a benzene ring to benzopyrone may alter the characteristics of interaction between hydroxyxanthones and the Na/K-ATPase.
The data presented in Table 2 point out the importance, from a structural perspective, of phenolic groups in xanthone derivative-induced inhibition of the Na/K-ATPase. The parent compound xanthone has no phenolic group attached to benzene rings and it had no detectable inhibition of the Na/K-ATPase. Although MB7 is the most potent inhibitor, dihydroxyxanthones barely affect ATPase activity. Moreover, the position of phenolic groups also affects the potency of the compounds. For example, although both MB7 and MB6 contain four phenolic groups, MB7 is more potent than MB6.
Because the Na/K-ATPase shares many common features with other P-type ATPases, it is possible that the newly identified xanthone derivatives may also affect other ion pumps. This issue remains to be resolved. In addition, it would be of interest to characterize other positive hits. These compounds have different chemical structures from the known Na/K-ATPase inhibitors and hence may give us another new class of Na/K-ATPase inhibitors working through a different mechanism.
MB7 Does Not Activate the Receptor Na/K-ATPase/Src Complex.
We have demonstrated that the Na/K-ATPase binds Src both in vitro and in vivo (Liang et al., 2006; Chen et al., 2009). This association regulates cellular Src activity by keeping Src in an inactive state (Tian et al., 2006). Moreover, formation of the Na/K-ATPase/Src complex produces a functional receptor for ouabain to stimulate the pump-associated Src, which subsequently assembles and activates multiple downstream protein kinase cascades, including the ERKs. In contrast to ouabain, binding of MB7 to the Na/K-ATPase/Src complex in vitro failed to activate Src (Fig. 3). It consistently had no effect on cellular Src and ERK activity when MB7 was applied to cultured cells (Fig. 4). These findings indicate that MB7, unlike ouabain, fails to alter conformation of the Na/K-ATPase in a way so that the Src kinase domain can be released from the Na/K-ATPase. This is not surprising because MB7 inhibits the Na/K-ATPase in a different way than ouabain. As discussed above, many types of inhibitors have been identified over the years. In view of our new findings, it would be of great interest to test how other Na/K-ATPase inhibitors such as oligomycin and quercetin affect receptor function of the Na/K-ATPase.
Perspectives.
The name xanthone designates a group of secondary metabolites normally found in a small group of plants, fungi, and lichens. The xanthones from plants appear to be associated mainly with the families Polygalaceae, Guttiferae, Moraceae, and Gentianaceae. These plants have been widely used in traditional Chinese medicine. For example, Yuanzhi, the root of Polygala sibirica L., is extensively used for a variety of medical conditions (Yong Jiang, 2001; Morimura, 2003). As phenolic compounds, xanthones have been described for their antioxidant properties (Sato et al., 1992; Minami et al., 1995; Wang et al., 1997). These properties have been implicated in their anti-inflammatory and chemopreventive actions (Pinto et al., 2005). One of the xanthones, dimethylxanthenone-4-acetic acid, is currently undergoing clinical trials as an antitumor agent (McKeage et al., 2009). Based on our new findings, it will be of great interest to test whether MB7 is able to increase cardiac contractile function. If this can be demonstrated, these hydroxyxanthones could be of great value because they do not stimulate the receptor Na/K-ATPase/Src complex. It has been well documented in the literature that stimulation of Na/K-ATPase-mediated signal transduction by either endogenous or exogenous CTSs alters cardiac growth and induces cardiac fibrosis (Ferrandi et al., 2004; Kennedy et al., 2006). Therefore, MB7 and its analogs may improve contractile function without precipitating cardiac hypertrophy and fibrosis seen under clinical conditions of congestive heart failure.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL36573]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM78565]; and the Ministry of Science and Technology of China [International Cooperation Grant 2007DFA31370].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.063974.
- MB7
- 3,4,5,6-tetrahydroxyxanthone
- MB2
- 3,4-dihydroxyxanthone
- MB5
- 3,4,5-trihydroxyxanthone
- MB3
- 1,3,5-trihydroxyxanthone
- MB6
- 1,3,5,6-tetrahydroxyxanthone
- ERK
- extracellular signal-regulated kinase
- MAPK
- mitogen-activated protein kinase
- IP3
- inositol 1,4,5-trisphosphate
- CTS
- cardiotonic steroid
- DMSO
- dimethyl sulfoxide.
References
- Aizman O, Uhlén P, Lal M, Brismar H, Aperia A. (2001) Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci USA 98:13420–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akera T, Brody TM. (1976) Inotropic action of digitalis and ion transport. Life Sci 18:135–144 [DOI] [PubMed] [Google Scholar]
- Altamirano J, Li Y, DeSantiago J, Piacentino V, 3rd, Houser SR, Bers DM. (2006) The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function. J Physiol 575:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir-Koksoy A, Abramowitz J, Allen JC. (2001) Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem 276:46605–46611 [DOI] [PubMed] [Google Scholar]
- Bagrov AY, Fedorova OV. (1998) Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+, K+ pump in human mesenteric arteries. J Hypertens 16:1953–1958 [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol 275:F633–F650 [DOI] [PubMed] [Google Scholar]
- Böttinger H, Habermann E. (1984) Palytoxin binds to and inhibits kidney and erythrocyte Na+, K+-ATPase. Naunyn Schmiedebergs Arch Pharmacol 325:85–87 [DOI] [PubMed] [Google Scholar]
- Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ. (2008) Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol 182:1153–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cai T, Wang H, Li Z, Loreaux E, Lingrel JB, Xie Z. (2009) Regulation of intracellular cholesterol distribution by Na/K-ATPase. J Biol Chem 284:14881–14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Koval GJ, Albers RW. (1966) Sodium potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem 241:1882–1889 [PubMed] [Google Scholar]
- Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. (2004) Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem 279:33306–33314 [DOI] [PubMed] [Google Scholar]
- Foster D, Ahmed K. (1982) Solvent effects on ouabain binding to the (Na+,K+)-ATPase of rat brain. Biochim Biophys Acta 688:123–130 [DOI] [PubMed] [Google Scholar]
- Haas M, Wang H, Tian J, Xie Z. (2002) Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 277:18694–18702 [DOI] [PubMed] [Google Scholar]
- Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. (1991) Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA 88:6259–6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, et al. (2006) Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 47:488–495 [DOI] [PubMed] [Google Scholar]
- Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. (1998) Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 273:15249–15256 [DOI] [PubMed] [Google Scholar]
- Kuriki Y, Racker E. (1976) Inhibition of (Na+, K+)adenosine triphosphatase and its partial reactions by quercetin. Biochemistry 15:4951–4956 [DOI] [PubMed] [Google Scholar]
- Li Z, Xie Z. (2009) The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pfluügers Arch 457:635–644 [DOI] [PubMed] [Google Scholar]
- Liang M, Cai T, Tian J, Qu W, Xie ZJ. (2006) Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Biol Chem 281:19709–19719 [DOI] [PubMed] [Google Scholar]
- Lingrel JB, Kuntzweiler T. (1994) Na+,K+-ATPase. J Biol Chem 269:19659–19662 [PubMed] [Google Scholar]
- Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. (2000) Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem 275:27838–27844 [DOI] [PubMed] [Google Scholar]
- McKeage MJ, Reck M, Jameson MB, Rosenthal MA, Gibbs D, Mainwaring PN, Freitag L, Sullivan R, Von Pawel J. (2009) Phase II study of ASA404 (vadimezan, 5,6-dimethylxanthenone-4-acetic acid/DMXAA) 1800mg/m2 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Lung Cancer 65:192–197 [DOI] [PubMed] [Google Scholar]
- Minami H, Takahashi E, Fukuyama Y, Kodama M, Yoshizawa T, Nakagawa K. (1995) Novel xanthones with superoxide scavenging activity from Garcinia subelliptica. Chem Pharm Bull (Tokyo) 43:347–349 [DOI] [PubMed] [Google Scholar]
- Morimura K. (2003) The role of special group article in ancient Chinese medical prescription. Hist Sci (Tokyo) 13:1–12 [PubMed] [Google Scholar]
- Myers TD, Boerth RC, Post RL. (1979) Effects of vanadate on ouabain binding and inhibition of Na+ + K+-ATPase. Biochim Biophys Acta 558:99–107 [DOI] [PubMed] [Google Scholar]
- Nguyen AN, Wallace DP, Blanco G. (2007) Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol 18:46–57 [DOI] [PubMed] [Google Scholar]
- Pinto MM, Sousa ME, Nascimento MS. (2005) Xanthone derivatives: new insights in biological activities. Curr Med Chem 12:2517–2538 [DOI] [PubMed] [Google Scholar]
- Repke KR, Sweadner KJ, Weiland J, Megges R, Schön R. (1996) In search of ideal inotropic steroids: recent progress. Prog Drug Res 47:9–52 [DOI] [PubMed] [Google Scholar]
- Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD. (2002) The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res 90:305–308 [DOI] [PubMed] [Google Scholar]
- Robinson JD, Robinson LJ, Martin NJ. (1984) Effects of oligomycin and quercetin on the hydrolytic activities of the Na+ +K+-dependent ATPase. Biochim Biophys Acta 772:295–306 [DOI] [PubMed] [Google Scholar]
- Sato T, Kawamoto A, Tamura A, Tatsumi Y, Fujii T. (1992) Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid). Chem Pharm Bull (Tokyo) 40:721–724 [DOI] [PubMed] [Google Scholar]
- Schoner W, Scheiner-Bobis G. (2007) Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol 293:C509–C536 [DOI] [PubMed] [Google Scholar]
- Skou JC. (1957) The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta 23:394–401 [DOI] [PubMed] [Google Scholar]
- Suolinna EM, Buchsbaum RN, Racker E. (1975) The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res 35:1865–1872 [PubMed] [Google Scholar]
- Sweadner KJ. (1989) Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta 988:185–220 [DOI] [PubMed] [Google Scholar]
- Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. (2006) Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell 17:317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Xie ZJ. (2008) The Na-K-ATPase and calcium-signaling microdomains. Physiology (Bethesda) 23:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. (2004) Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 279:17250–17259 [DOI] [PubMed] [Google Scholar]
- Wang JP, Raung SL, Tsao LT, Lin CN. (1997) Evidence for the involvement of protein kinase C inhibition by norathyriol in the reduction of phorbol ester-induced neutrophil superoxide anion generation and aggregation. Eur J Pharmacol 336:81–88 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X, Southall N, Wang S, Xia M, Austin CP, et al. (2009) Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res 69:6556–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Wang Y, Liu G, Zolotarjova N, Periyasamy SM, Askari A. (1996) Similarities and differences between the properties of native and recombinant Na+/K+-ATPases. Arch Biochem Biophys 330:153–162 [DOI] [PubMed] [Google Scholar]
- Yoda A, Yoda S. (1982) Interaction between ouabain and the phosphorylated intermediate of Na,K-ATPase. Mol Pharmacol 22:700–705 [PubMed] [Google Scholar]
- Yong Jiang PT. (2001) Progress in studies on Polygala tenuifolia. Chin Tradit Herbal Drugs 32:759–761 [Google Scholar]
- Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. (2005) Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 16:4034–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]