Abstract
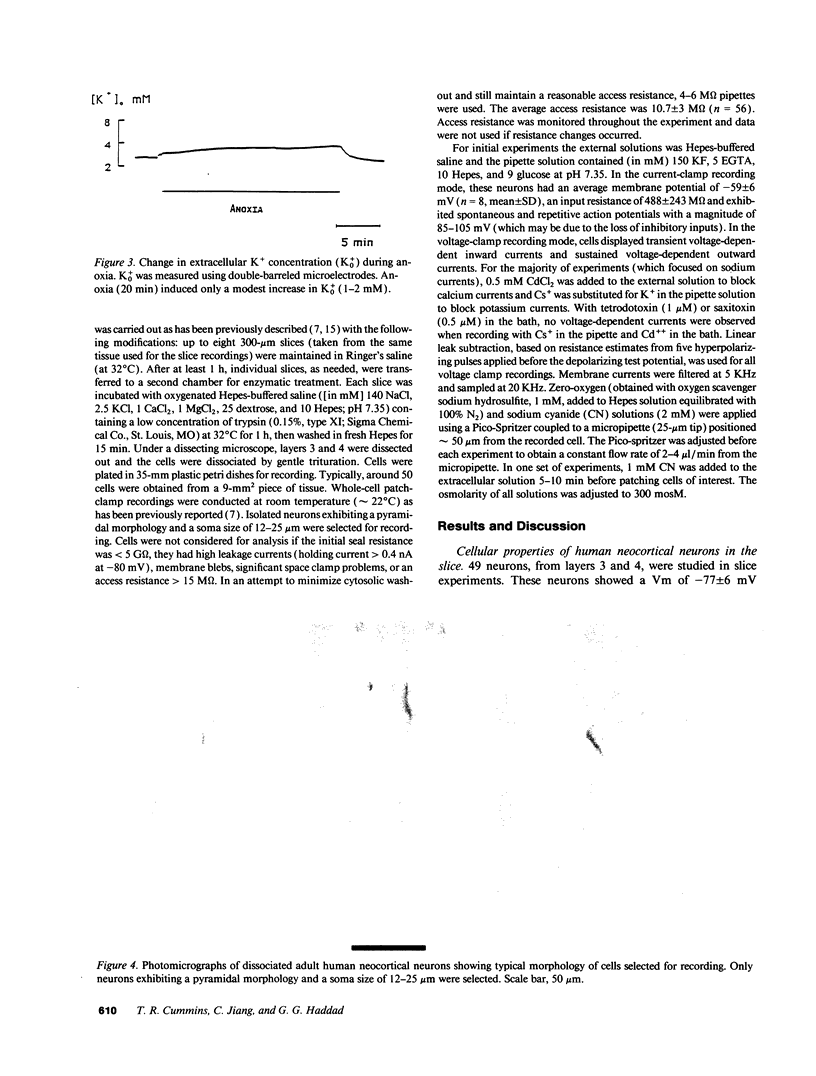
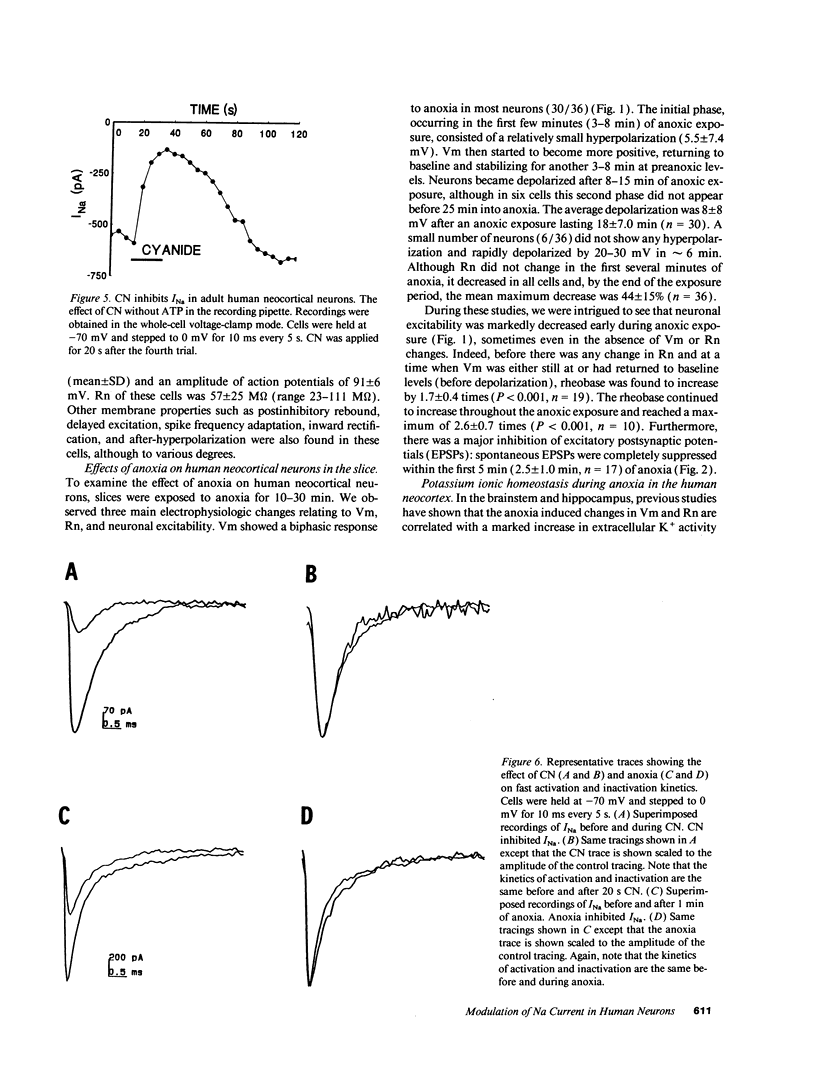
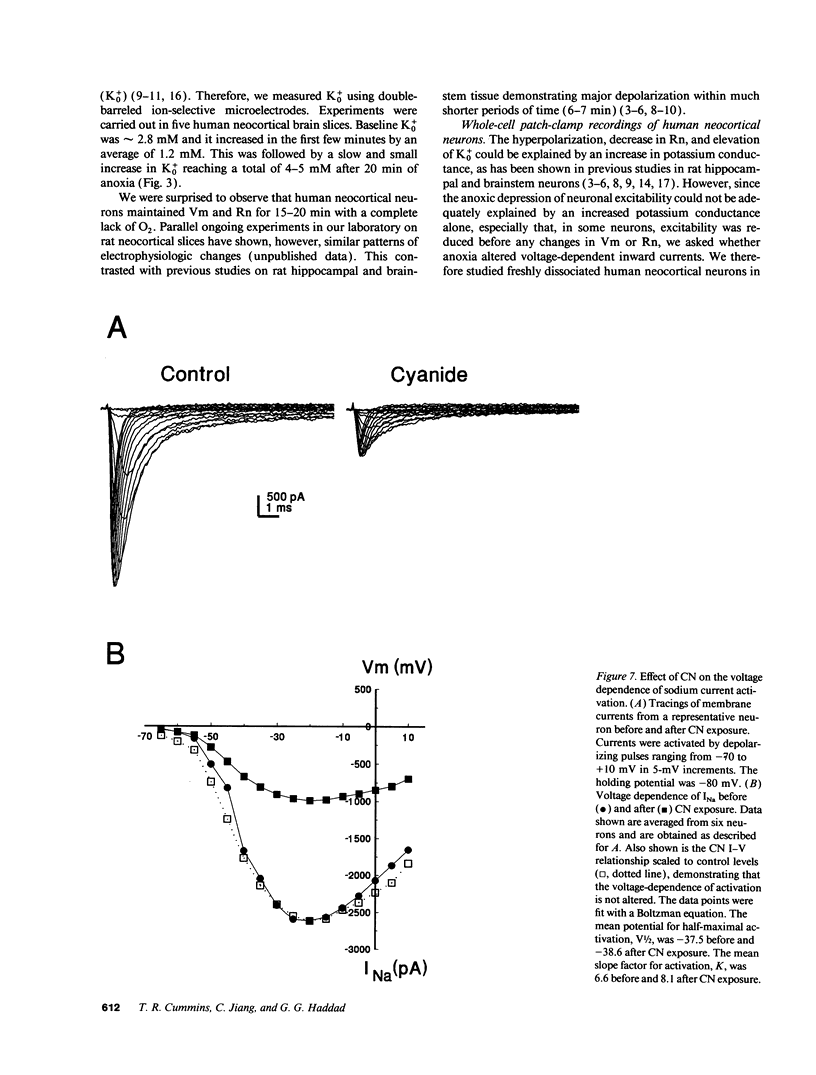
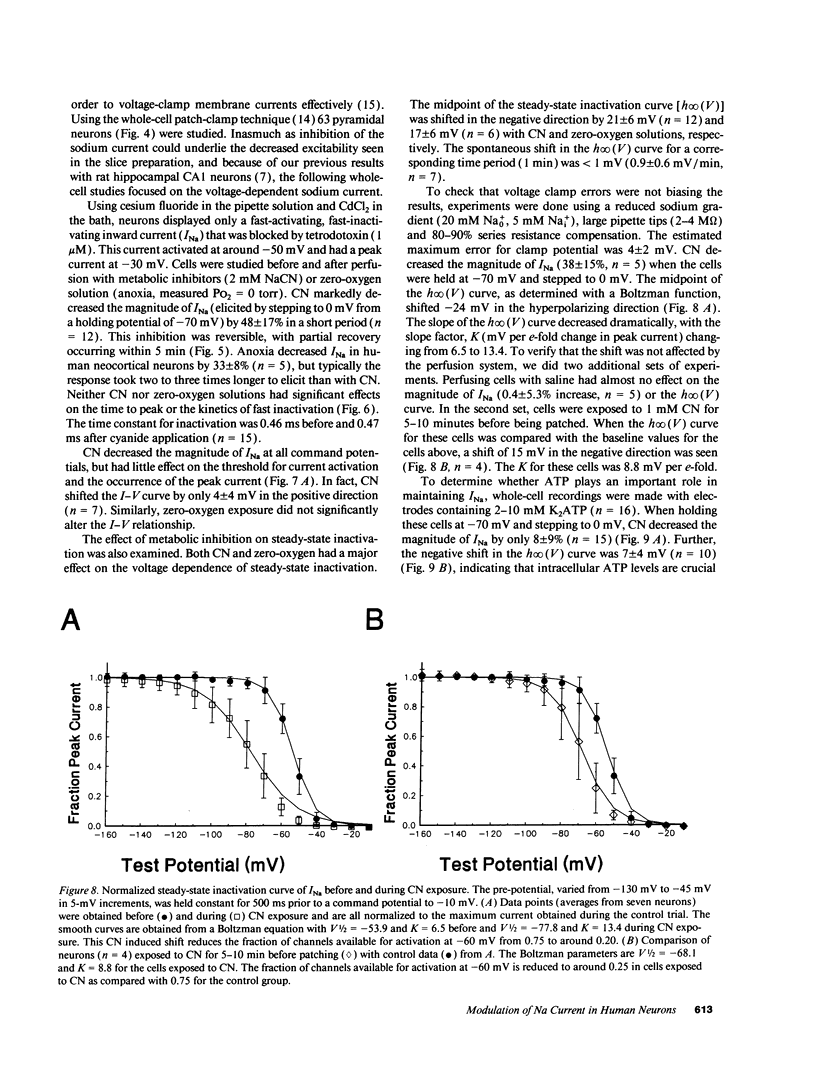
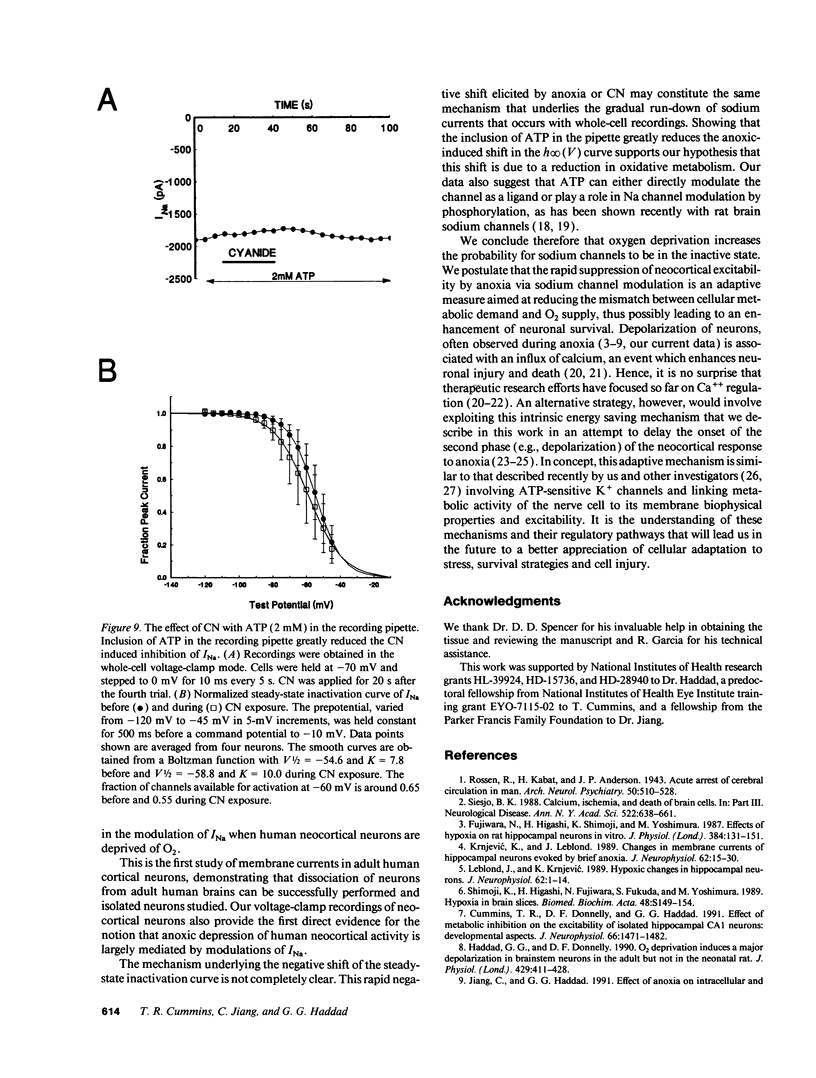
When the central nervous system in humans is deprived of oxygen, the effects are potentially disastrous. Electroencephalographic activity is lost and higher brain function ceases rapidly. Despite the importance of these effects, the mechanisms underlying the loss of cortical activity are poorly understood. Using intracellular recordings of human neocortical neurons in tissue slices, we show that, whereas anoxia produces a relatively small depolarization and modest alterations in passive properties, it causes a major decrease in excitability. Whole-cell voltage-clamp studies of acutely isolated human neocortical pyramidal neurons demonstrate that anoxia and metabolic inhibition produce a large negative shift in the steady-state inactivation [h infinity (V)] curve for the voltage-dependent sodium current (INa). Inclusion of ATP in the patch pipette decreased the shift of the h infinity (V) curve by two-thirds. Because increased inactivation of INa decreases cellular metabolic demand, we postulate that this promotes neuronal survival during periods of oxygen deprivation. These data show a novel mechanism by which anoxia links metabolism to membrane ionic conductances in human cortical neurons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boening J. A., Kass I. S., Cottrell J. E., Chambers G. The effect of blocking sodium influx on anoxic damage in the rat hippocampal slice. Neuroscience. 1989;33(2):263–268. doi: 10.1016/0306-4522(89)90205-4. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990 Aug;10(8):2493–2501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins T. R., Donnelly D. F., Haddad G. G. Effect of metabolic inhibition on the excitability of isolated hippocampal CA1 neurons: developmental aspects. J Neurophysiol. 1991 Nov;66(5):1471–1482. doi: 10.1152/jn.1991.66.5.1471. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F., Jiang C., Haddad G. G. Comparative responses of brain stem and hippocampal neurons to O2 deprivation: in vitro intracellular studies. Am J Physiol. 1992 May;262(5 Pt 1):L549–L554. doi: 10.1152/ajplung.1992.262.5.L549. [DOI] [PubMed] [Google Scholar]
- Edwards R. A., Lutz P. L., Baden D. G. Relationship between energy expenditure and ion channel density in the turtle and rat brain. Am J Physiol. 1989 Dec;257(6 Pt 2):R1354–R1358. doi: 10.1152/ajpregu.1989.257.6.R1354. [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Higashi H., Shimoji K., Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol. 1987 Mar;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G. G., Donnelly D. F. O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. J Physiol. 1990 Oct;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Hounsgaard J., Jahnsen H. Anoxia increases potassium conductance in hippocampal nerve cells. Acta Physiol Scand. 1982 Jul;115(3):301–310. doi: 10.1111/j.1748-1716.1982.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Jiang C., Agulian S., Haddad G. G. O2 tension in adult and neonatal brain slices under several experimental conditions. Brain Res. 1991 Dec 24;568(1-2):159–164. doi: 10.1016/0006-8993(91)91392-e. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Traynelis S. F., Dingledine R. Different responses of CA1 and CA3 regions to hypoxia in rat hippocampal slice. J Neurophysiol. 1990 Mar;63(3):385–394. doi: 10.1152/jn.1990.63.3.385. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Leblond J. Changes in membrane currents of hippocampal neurons evoked by brief anoxia. J Neurophysiol. 1989 Jul;62(1):15–30. doi: 10.1152/jn.1989.62.1.15. [DOI] [PubMed] [Google Scholar]
- Leblond J., Krnjevic K. Hypoxic changes in hippocampal neurons. J Neurophysiol. 1989 Jul;62(1):1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F. B. Calcium, neuronal hyperexcitability and ischemic injury. Brain Res Brain Res Rev. 1989 Jul-Sep;14(3):227–243. doi: 10.1016/0165-0173(89)90002-7. [DOI] [PubMed] [Google Scholar]
- Numann R., Catterall W. A., Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991 Oct 4;254(5028):115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Rothman S. M. Synaptic activity mediates death of hypoxic neurons. Science. 1983 Apr 29;220(4596):536–537. doi: 10.1126/science.6836300. [DOI] [PubMed] [Google Scholar]
- Shimoji K., Higashi H., Fujiwara N., Fukuda S., Yoshimura M. Hypoxia in brain slices. Biomed Biochim Acta. 1989;48(2-3):S149–S154. [PubMed] [Google Scholar]
- Siesjö B. K. Historical overview. Calcium, ischemia, and death of brain cells. Ann N Y Acad Sci. 1988;522:638–661. doi: 10.1111/j.1749-6632.1988.tb33410.x. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R. Activation of protein kinase C differentially modulates neuronal Na+, Ca2+, and gamma-aminobutyrate type A channels. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6192–6196. doi: 10.1073/pnas.85.16.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]