Abstract
Uric acid (UA) results from xanthine oxidase (XO) catabolism of xanthine and is the final product of purine catabolism in humans. In this species, hyperuricemia is associated with gout, nephropathy, and increased cardiovascular disease risk. Although the effects of hyperuricemia in vascular biology are overall controversial, UA has been described as an antioxidant and as potentially improving endothelial function. Hypertension is associated with endothelial dysfunction. We hypothesized that UA improves the endothelial function of aorta from deoxycorticosterone acetate (DOCA)-salt hypertensive rats. UA (100 μM) in the presence of the uricase inhibitor oxonic acid (10 μM) did not modify relaxation to acetylcholine (ACh) (1 nM–10 μM) in the aorta from nontreated, sham normotensive, and DOCA-salt hypertensive rats [response to 10 μM ACh for UA versus vehicle, respectively: nontreated = 37 ± 7 versus 48 ± 7%, sham = 53 ± 15 versus 57 ± 20%, DOCA = 81 ± 4 versus 85 ± 2% from 20 μM prostaglandin 2α (PGF2α)-induced contraction]. Allopurinol (100 μM), a XO inhibitor, did not significantly alter the ACh-induced relaxation of sham and DOCA aortic rings (response to 10 μM ACh for allopurinol versus vehicle, respectively: sham = 61 ± 5 versus 68 ± 9%, DOCA = 87 ± 6 versus 88 ± 3% from 20 μM PGF2α-induced contraction). Uricemia, ranging from unmeasurable to 547 μM in sham and to 506 μM in DOCA rats, was not significantly different between these two groups. The expression and activity of XO, as well as the expression of uricase, were not different between sham and DOCA rat aorta. We conclude that, at least in vitro, UA does not affect the ACh-induced relaxation of normotensive and DOCA-salt hypertensive rats.
Uric acid or urate (UA) is a small organic molecule formed as a result of xanthine oxidase (XO) metabolism of xanthine. In most species, UA is further catabolized by uricase (or urate oxidase) to allantoin, which is then readily excreted in the urine. Higher primates and humans, as a result of a mutation, do not express uricase (Yeldandi et al., 1991). In these species, UA is the final product of purine catabolism. UA has very low solubility in water and can form crystals of monosodium urate in certain conditions (e.g., high concentrations, acidic pH, and presence of other molecules) (So, 2008).
Therefore, UA was traditionally viewed as metabolically inert, yet capable of exerting harmful effects as a result of crystallization, the most common pathophysiological consequence of which is the development of arthritic gout (So, 2008). Even in the absence of gout, studies have shown hyperuricemia to be a risk factor for several cardiovascular diseases associated with metabolic syndrome and an independent predictor for kidney disease and all-cause mortality (Johnson et al., 2003; Feig et al., 2008; Kim et al., 2008).
Hyperuricemia can be experimentally induced in rodents by chronic treatment with the uricase inhibitor oxonic acid. This leads to development of hypertension and endothelial dysfunction, accompanied by renal fibrosis, increase in renin release, and decrease in renal nitric-oxide synthase expression, in the absence of crystal formation in the kidney (Mazzali et al., 2001; Khosla et al., 2005). In addition, in cultured bovine aortic endothelial cells, UA inhibited both the basal and the vascular endothelial growth factor-stimulated NO production (Khosla et al., 2005). Taken together, these results suggest UA to be a deleterious molecule overall, and on endothelial function in particular.
However, there are several other lines of evidence pointing to the more complicated and potentially beneficial roles of UA. In vitro, UA acts as an antioxidant for superoxide, hydroxyl radical, hydrogen peroxide, and other reactive oxygen species (ROS) (Becker, 1993). Importantly, these reactions occur at UA concentrations that are in the range of low-to-normal human uricemia. The antioxidant effects of UA were confirmed in various biological systems by studies demonstrating attenuation or protection from oxidative stress in the presence of UA (Becker, 1993). These effects might become especially relevant when considering the known correlation between increased ROS and endothelial dysfunction (Cai and Harrison, 2000). Superoxide may negatively affect endothelial function through several mechanisms, including directly reacting with NO and decreasing its bioavailability (Beckman and Koppenol, 1996). In addition, peroxynitrite, the product of this reaction, may have several deleterious effects of its own (Schulz et al., 2008). In this context, it is important to consider that UA may not only react with and inactivate superoxide, but also peroxynitrite. In addition, the latter reaction gave rise to a reaction product with endothelium-independent vasorelaxant properties (Skinner et al., 1998). However, the direct effect of UA on endothelial function in rats has not been reported previously.
In vivo studies performed in humans by Waring et al. (2006, 2007) give further support to the idea of a beneficial role for UA. Acute (1-h) administration of UA to patients with diabetes type I or smokers with endothelial dysfunction leads to an increase in forearm blood flow in response to acetylcholine (ACh), a measure of endothelial function, and a gross measure of NO bioavailability (Waring et al., 2006). Conversely, chronic lowering of uricemia in patients with diabetes type II and associated endothelial dysfunction did not improve their endothelial function (Waring et al., 2007). Unfortunately, performing the same experiments of acute UA administration in rats with endothelial dysfunction would be hampered by the presence of uricase that is active in this species.
Hypertension is commonly associated with increased vascular superoxide production and endothelial dysfunction. Given the aforementioned contradictory reports on the effects of UA on endothelial function, we decided to investigate these effects on the endothelial dysfunction that accompanies deoxycorticosterone acetate (DOCA)-salt hypertension, an experimental model of mineralocorticoid hypertension. This model demonstrates bona fide endothelial dysfunction, as evidenced by impaired ACh-induced relaxation. We hypothesized that UA, perhaps acting as an antioxidant, would improve the endothelial function of arteries from DOCA-salt hypertensive rats in vitro. In addition, we hypothesized that UA levels would be chronically reduced in these rats, contributing to their endothelial dysfunction.
Materials and Methods
Animals.
Male adult Sprague-Dawley rats (225–250 g; Charles River Breeding Laboratories, Portage, MI) were used. For DOCA-salt hypertension, rats were uninephrectomized under isoflurane anesthesia. A subset of rats (DOCA rats) were implanted subcutaneously with a pellet of 200 mg/kg DOCA and were given drinking water supplemented with 1% NaCl and 0.2% KCl. The sham subset did not have a DOCA implant and were given tap water. Systolic blood pressures (SBPs) were measured after 4 weeks using the standard tail cuff method. DOCA rats SBPs were increased by at least 50 mm Hg over sham SBPs. All animal procedures were approved and performed in accordance with regulations of the Institutional Animal Care and Use Committee at Michigan State University.
Isolated Tissue Bath Experiments.
Nontreated, sham uninephrectomized, and DOCA adult male Sprague-Dawley rats were euthanized (60 mg/kg i.p. pentobarbital sodium), and thoracic aortae were removed and cleaned of outer adipose tissue in physiological salt solution containing 130 mM NaCl, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4·7H2O, 14.8 mM NaHCO3, 5.5 mM dextrose, 0.03 mM CaNa2EDTA, and 1.6 mM CaCl2. Endothelial-intact tissue rings were then mounted in warmed (37°C), aerated (95% O2, 5% CO2) physiological salt solution in isolated tissue baths (30 ml) for measurement of isometric contractile force using PowerLab for Macintosh (ADInstruments Ltd., Chalgrove, Oxfordshire, UK). The tissues were placed under optimum resting tension (4 g, previously determined). Contraction to 10−5 M phenylephrine was used to verify their viability. Relaxation from 20 μM PGF2α-induced contraction to a 10−5 M bolus ACh was used to verify endothelium integrity. Cumulative concentration-response curves to UA (10−9–10−4 M) were performed in the presence or absence of oxonic acid (uricase inhibitor, 10 μM). In other experiments, after incubation with UA (100 μM) and oxonic acid (10 μM) (0.5 h), allopurinol (XO inhibitor, 100 μM, 1 h), apocynin (NADPH oxidase inhibitor, 100 μM, 0.5 h), tempol (superoxide dismutase mimetic, 1 mM, 0.5 h), tiron (ROS scavenger, 10 mM, 0.5 h), or their vehicles (NaOH, dimethyl sulfoxide, or distilled H2O), tissues were contracted with 20 μM PGF2α and cumulative concentration-response curves to ACh were constructed. The allopurinol concentration used (100 μM) is inhibitory for XO (IC50 <10 μM; Borges et al., 2002). The contraction induced by PGF2α was not changed by the presence of UA, oxonic acid, allopurinol, or their vehicles. Relaxation curves to ACh were also constructed in tissues contracted with phenylephrine (EC50) for experiments in which apocynin, tempol, or tiron were used, with no qualitative differences observed, compared with PGF2α. For consistency, only results obtained with PGF2α are reported.
Blood Uric Acid Measurement.
Arterial blood was collected by cardiac puncture at the time of sacrifice, and UA was determined using the UAsure meter and test strips (ApexBio, Hsinchu, Taiwan) according to the manufacturer's protocol.
XO Activity.
Assays were performed as reported previously (Szasz et al., 2008). XO urate-producing activity was assayed as follows. In brief, UA was measured spectrophotometrically from aorta tissue homogenates, using a UA standard curve. UA production from samples incubated with 100 μM xanthine (a XO substrate) and 100 μM allopurinol (a XO inhibitor) was subtracted from UA production in the presence of 100 μM xanthine alone and is reported to total protein content of homogenates. For XO H2O2-producing activity assays, whole aorta H2O2 production was measured using the Amplex Red H2O2 kit (Invitrogen, Carlsbad, CA). Similarly, H2O2 produced in the presence of 100 μM xanthine and 100 μM allopurinol was subtracted from H2O2 produced in the presence of 100 μM xanthine alone and reported to the total protein content of tissue samples.
Protein Isolation and Western Blot Analysis for XO and Uricase.
Thoracic aortae were cleaned of outer adipose tissue, pulverized in liquid nitrogen, and solubilized in lysis buffer (0.5 M Tris-HCl, pH 6.8, 10% SDS, and 10% glycerol) supplemented with protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). After a brief sonication, homogenates were centrifuged (11,000g for 10 min at 4°C). The total protein concentration was determined through spectrophotometry using the bicinchoninic acid kit (Sigma-Aldrich, St. Louis, MO). Fifty micrograms of protein from each sample and 10 μl of positive control—rat kidney medulla for XO (XO) and rat liver for uricase (uricase)—were separated on 7% (XO) or 15% (uricase) SDS polyacrylamide gels and transferred to polyvinylidene difluoride (XO) or nitrocellulose (uricase) membranes. The membranes were then blocked with LI-COR blocker (LI-COR Biosciences, Lincoln, NE) (XO) or 5% milk (uricase) for 3 h at 4°C and probed overnight with antibody against XO (1:2000; Rockland Immunochemicals, Gilbertsville, PA) or uricase (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). IRDye 700-conjugated anti-mouse IgG (Invitrogen) (XO) or horseradish peroxidase-conjugated anti-rabbit IgG (Cell Signaling Technology Inc., Danvers, MA) (uricase) was added for 1 h at 4°C, followed by visualization with on a scanner (LI-COR Biosciences) (XO) or with enhanced chemiluminescence (Pierce Chemical, Rockford, IL) (uricase). Smooth muscle α-actin (Calbiochem, EMD Chemicals, Gibbstown, NJ) was used to reprobe each blot to ensure equal loading. Band density was quantified using Image version 1.63 (National Institutes of Health, Bethesda, MD).
Data Analysis.
Data are presented as mean ± S.E.M. for the number of animals (N). Plotting and statistical analysis of data were accomplished using Prism 5 (GraphPad Software Inc., San Diego, CA). To compare groups, the appropriate Student's t test or analysis of variance was performed. For association between uricemia and SBP, a nonparametric correlation (Spearman) analysis was performed. In all cases, a p value of ≤0.05 was considered statistically significant.
Results
UA Did Not Alter Contractile or Relaxant Function of Rat Aorta from Nontreated Rats.
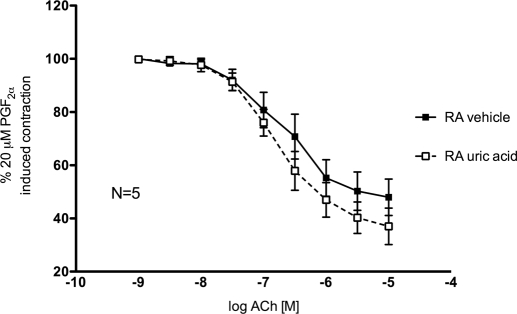
UA (10−9–10−4 M), alone or in combination with a uricase inhibitor (oxonic acid, 10 μM), did not induce contraction of endothelial-intact normal rat aorta rings (data not shown). UA (100 μM) in combination with oxonic acid (10 μM) did not significantly change ACh-induced relaxation curves of endothelial-intact rat aorta rings contracted with 20 μM PGF2α (Fig. 1).
Fig. 1.
Cumulative concentration-response curves to ACh (10−9–10−5 M) of endothelial-intact aorta rings from nontreated rats in the presence of vehicle (closed symbols) or uric acid (100 μM) plus oxonic acid (10 μM) (open symbols). RA, rat aorta. Points represent means ± S.E.M. percentage of contraction induced by 20 μM PGF2α for the respective N.
UA Did Not Alter Endothelial Function of Aorta from Sham Normotensive or DOCA-Salt Hypertensive Rats.
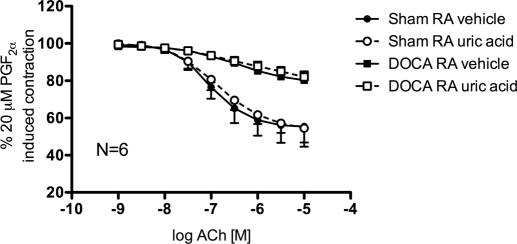
Endothelial function, assessed as relaxation to ACh (10−9–10−5 M) from 20 μM PGF2α-induced contraction, was expectedly impaired in aorta from DOCA-salt hypertensive rats compared with normotensive sham controls, as evidenced by a decreased relaxation response to ACh (Fig. 2; vehicle maximal ACh response: sham = 55.4 ± 10.8%, DOCA = 80.3 ± 4.0% from 20 μM PGF2α-induced contraction). Addition of UA (100 μM) in combination with oxonic acid (10 μM) did not significantly change the ACh-induced relaxation curves of sham and DOCA rat aorta compared with vehicle (Fig. 2; uric acid maximal ACh response sham = 54.5 ± 7.6%, DOCA = 82.0 ± 3.0% from 20 μM PGF2α-induced contraction). Similarly, other compounds that have known antioxidant properties, such as the NADPH oxidase inhibitor apocynin (100 μM) and the ROS scavengers tempol (1 mM) and tiron (10 mM), did not significantly change the ACh-induced relaxation curves of DOCA rat aorta compared with vehicle (data not shown; maximal ACh response DOCA rat aorta vehicle = 77.2 ± 3.3%, apocynin = 81.9 ± 2.2%, tempol = 84.9 ± 1.5%, tiron = 84.9 ± 1.6% from 20 μM PGF2α-induced contraction).
Fig. 2.
Cumulative concentration-response curves to ACh (10−9–10−5 M) of aorta rings from sham normotensive (circles) and DOCA-salt hypertensive rats (squares) in the presence of vehicle (closed symbols) or uric acid (100 μM) plus oxonic acid (10 μM) (open symbols). RA, rat aorta. Points represent means ± S.E.M. percentage of contraction induced by 20 μM PGF2α for the respective N.
XO Inhibition Did Not Alter Endothelial Function of Aorta from DOCA-Salt Hypertensive Rats.
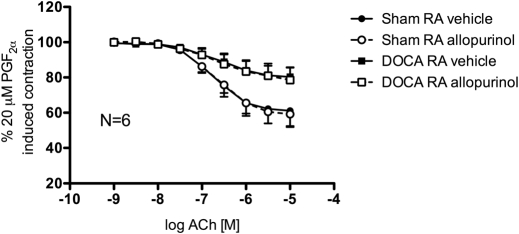
Inhibition of XO enzyme activity with allopurinol (100 μM) did not significantly change ACh-induced relaxation curves of sham and DOCA rat aorta rings contracted with 20 μM PGF2α (Fig. 3).
Fig. 3.
Cumulative concentration-response curves to ACh (10−9–10−5 M) of endothelial-intact aorta rings from sham normotensive (circles) and DOCA-salt hypertensive rats (squares) in the presence of vehicle (closed symbols) or allopurinol (100 μM) (open symbols). RA, rat aorta. Points represent means ± S.E.M. percentage of contraction induced by 20 μM PGF2α for the respective N.
UA Plasma Levels Were Not Changed during DOCA-Salt Hypertension.
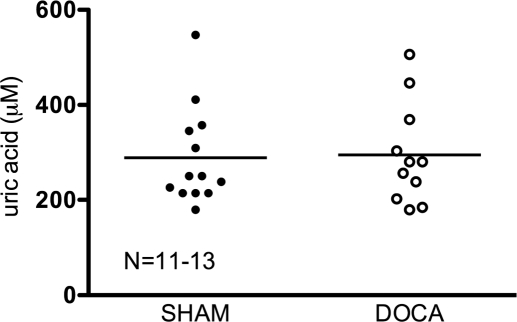
Uricemia was measured in sham normotensive and DOCA-salt hypertensive rats. No significant changes were observed between the two groups (Fig. 4) (sham = 288.8 ± 28.67, DOCA = 294.8 ± 31.98). Moreover, no significant correlation was found between the UA level and blood pressure of individual rats (data not shown).
Fig. 4.
Uric acid levels in arterial blood of individual sham normotensive (closed symbols) and DOCA-salt hypertensive (open symbols) rats. Line represents average uric acid for the respective N.
Aorta XO Expression and Activity Was Not Significantly Changed between Sham Normotensive and DOCA-Salt Hypertensive Rats.
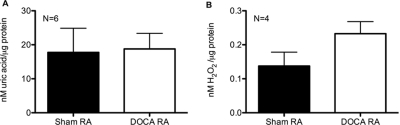
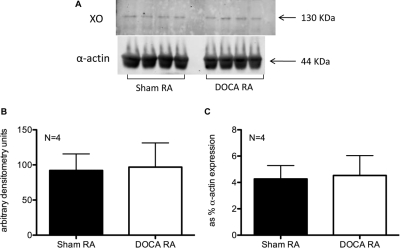
XO activity of aorta was measured in the presence of substrate (100 μM xanthine) and represented as allopurinol (100 μM)-inhibitable production of UA (Fig. 5A) and H2O2 (Fig. 5B), reported to total protein content of samples. Neither of these two activity measures changed in DOCA-salt hypertensive compared with sham normotensive rats (nanomolar uric acid/microgram protein: sham = 17.74 ± 7.12, DOCA = 18.76 ± 4.59; nanomolar H2O2/microgram protein: sham = 0.14 ± 0.04, DOCA = 0.23 ± 0.04). Aortic protein expression of XO was assessed by Western blotting (Fig. 6A). Quantification of the130-kDa band densitometry itself (Fig. 6B) or expressed as percentage of the smooth muscle α-actin band densitometry (Fig. 6C) revealed no significant change between the sham and the DOCA aorta.
Fig. 5.
A, uric acid-producing activity of XO in sham normotensive (closed bars) and DOCA-salt hypertensive (open bars) aorta. B, H2O2-producing activity of XO in sham normotensive (closed bars) and DOCA-salt hypertensive (open bars) aorta. RA, rat aorta. Bars represent means ± S.E.M. for the respective N.
Fig. 6.
A, XO (top) and control α-actin (bottom) protein expression in aorta from sham normotensive and DOCA-salt hypertensive rats. B, band densitometry quantification of XO as the 130-kDa band corresponding to XO. C, band densitometry quantification of XO as percentage of α-actin expression. RA, rat aorta. Bars represent means ± S.E.M. for the respective N.
Aorta Uricase Expression Was Not Altered during DOCA-Salt Hypertension.
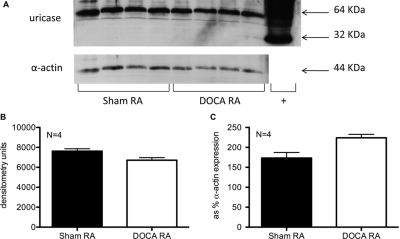
Aortic protein expression of uricase, the UA-degrading enzyme active in rats, was assessed by Western blotting, using a rat liver lysate as positive control. The 32-kDa band corresponding to the uricase monomer was only observed in the positive control. An ∼64-kDa band was observed in all samples (Fig. 7A). Quantification of the 64-kDa band densitometry itself (Fig. 7B) or expressed as percentage of the smooth muscle α-actin band densitometry (Fig. 7C) revealed no significant change between the sham and the DOCA aorta.
Fig. 7.
Uricase (top) and control α-actin (bottom) protein expression in aorta from sham normotensive and DOCA-salt hypertensive rats. Positive control (+) for uricase expression is a rat liver lysate. B, band densitometry quantification of uricase as the 64-kDa band corresponding to the uricase dimer. C, band densitometry quantification of uricase as percentage of α-actin expression. RA, rat aorta atrial. Bars represent means ± S.E.M. for the respective N.
Discussion
In this study, we have tested whether UA would improve the ACh-induced relaxation of aorta from DOCA-salt hypertensive rats in vitro. In our experiments, neither increasing UA by direct addition nor decreasing it via inhibition of XO altered the contractile function of aorta from normotensive or hypertensive rats. The expression and activity of XO, as well as the expression of uricase, were similar in aorta from sham and DOCA rats, suggesting that aortic UA production or destruction was not modified by hypertension. Conversely, uricemia had similar levels in normotensive and hypertensive rats.
Direct Effects of UA on Endothelial Function.
UA may affect endothelial function at least by the following mechanisms: 1) by directly interacting with NO (Gersch et al., 2008) and thereby decreasing its bioavailability; 2) by interacting with superoxide (Becker, 1993) and thereby decreasing the amount of superoxide available to quench NO, thus increasing NO bioavailability; and 3) by interacting with peroxynitrite, the reaction product of superoxide and NO, to form a product shown to have vasorelaxant properties (Skinner et al., 1998) or to decrease peroxynitrite availability. In addition, UA may have antioxidant or other effects intracellularly in vascular smooth muscle cells, where it is taken up by a specific transporter (SLC22A12) (Price et al., 2006).
In our experiments, however, UA did not induce any direct contractile effects on rat aorta and did not alter relaxation to ACh. We used UA at concentrations proven previously to have antioxidant properties in vitro and that were within the range of normal uricemia (Becker, 1993). In addition, we used oxonic acid at a concentration proven to inhibit uricase activity (Fridovich, 1965), thereby preventing degradation of UA. We have not tested whether UA would directly induce relaxation of PGF2α-contracted rat aorta, but the lack of direct vasodilatory effects of UA alone in rat aorta was reported previously (Skinner et al., 1998).
In the study by Waring et al. (2006), UA, administered acutely (over 1 h) at a dose that raised uricemia from normal to the hyperuricemic range, ameliorated the endothelial dysfunction of smokers and patients with diabetes type I. However, it had no impact on the normal endothelial function of control subjects, although the serum antioxidant capacity was equally increased in all groups after the administration of UA. This implies that the beneficial antioxidant effects of UA may only become apparent when there is a pathophysiological excess of oxidants and consequently endothelial dysfunction. However, in vitro, we did not observe such an improvement in the ACh-induced relaxation of rat aorta from DOCA-salt hypertensive rats. Some improvements of endothelial function have been observed previously in certain vascular beds of some hypertensive rat models with single in vitro applications of compounds that act by reducing ROS. Tempol restored the endothelial dysfunction of renal afferent arterioles in Dahl-sensitive rats (Ozawa et al., 2004). Tempol, as well as apocynin, improved the ACh-mediated relaxation of coronary arteries in diabetic mice (Gao et al., 2007). Superoxide dismutase improved the ACh-induced relaxation of aorta in Nω-nitro-l-arginine hypertensive rats (Mitchell et al., 2004). Closest to our study is a report that showed significant improvement of ACh-induced relaxation in the presence of tempol and apocynin of carotid artery from DOCA-salt hypertensive rats (Zheng et al., 2003). However, using the same concentration and exposure times as in the latter report, we have not been able to observe similar improvements in the endothelial function of DOCA aorta, as evidenced by the lack of change in the responses to ACh after apocynin/tempol. This implies that the responses to ACh in the DOCA aorta may not be corrected in vitro by single exposures to antioxidants, because other ROS-independent mechanisms may modulate this particular endothelial dysfunction. However, these results do not exclude a potential effect of antioxidants or of UA on DOCA aorta endothelial dysfunction if administered in vivo or if applied for a longer time in vitro.
We excluded UA degradation by uricase in the rat tissue as a reason for disagreement between our study and the study by Waring et al. (2006), by coadministering oxonic acid, the uricase inhibitor. However, besides the presence of uricase in rat tissue, other species differences cannot be ruled out, such as differences in the presence and/or function of specific urate transporters in rodents compared with humans. In addition, the endothelial dysfunction observed in type I diabetes (the condition where UA had a beneficial effect) may be different from the endothelial dysfunction associated with hypertension (the condition that we modeled), in which pressure-associated shear stress and endothelial injury may be less ROS-dependent. In vivo treatments with various nonspecific and specific antioxidants have been shown experimentally to improve hypertensive endothelial dysfunction (Heitzer et al., 2001; Higashi et al., 2002; Touyz and Schiffrin, 2004); however, it is always difficult to dissociate the direct effects of antioxidants on endothelium from the effects of the accompanying reduction in blood pressure upon antioxidant treatment.
Effects of XO on Endothelial Function.
UA is the product of XO action on xanthine. In addition to UA, superoxide and H2O2 are also formed. There is a large body of evidence supporting the role of XO as a deleterious ROS producer with multiple cardiovascular effects, including effects on endothelial function (Berry and Hare, 2004). Consequently, many reports show improvements in endothelial function in vitro and in vivo by allopurinol or oxypurinol inhibition of XO activity, sometimes independently of other effects (Butler et al., 2000) but mostly in the context of lowering blood pressure or improving end-organ damage (Suzuki et al., 1998; Pacher et al., 2006). These studies would therefore support a harmful effect of UA. Similarly to antioxidant treatments mentioned above, it is however impossible to distinguish between direct effects on endothelial function and the effects of simultaneous lowering of blood pressure. In addition, in these studies, the effects of allopurinol on lowering UA cannot be discerned from effects on lowering ROS and oxidative stress. A convincing attempt was made in this direction in a clinical study that established that ameliorating endothelial dysfunction in chronic heart failure with allopurinol was accomplished by a UA-independent mechanism, because lowering UA to similar levels with the uricosuric agent probenecid did not have any effect on endothelial function (George et al., 2006). The authors therefore concluded that endothelial function was improved by allopurinol because of a reduction in XO-mediated ROS production. In the present study, we observed no effect of in vitro allopurinol on the DOCA-salt rat aorta endothelial dysfunction, as assessed by the response to ACh. In addition, no change was observed in the protein expression and activity of XO in aorta from hypertensive compared with normotensive rats. The lack of change in aortic XO protein expression during DOCA-salt hypertension was reported previously (Viel et al., 2008).
Uricemia.
One of our hypotheses was that during DOCA-salt hypertension uricemia is decreased and that the chronic lack of adequate levels of UA thus contributes to the endothelial dysfunction of hypertension. UA levels in blood however were similar in hypertensive and normotensive rats, and we could not establish any correlation between individual values of uricemia and blood pressure. This is a known association in humans (Krishnan et al., 2007), with hypertensives having increased risk of gout and hyperuricemia increasing the risk for hypertension. As explained previously, rodents have significantly lower uricemia as a result of the presence of functional uricase, the enzyme that degrades UA into allantoin and that is not expressed in humans as a result of a mutation (Yeldandi et al., 1991). We assessed protein expression of uricase as the 64-kDa band observed in our samples. This molecular weight was observed previously and attributed to a uricase dimer (Varela-Echavarría et al., 1988). Similarly to the expression of XO, uricase expression was also not changed in aorta from hypertensive compared with normotensive rats in our study. In the context of the beneficial versus deleterious UA effects controversy, one has to be left wondering whether the evolutionary loss of uricase in higher primates and humans may be explained by hyperuricemia conferring any physiological advantage.
In conclusion, contrary to our original hypothesis, in vitro, UA and inhibition of its main producer XO did not alter the endothelial function of aorta from either normotensive or DOCA-salt hypertensive rats. Similarly, we did not observe any change in the producing and degrading enzymes for UA or in uricemia levels during DOCA-salt hypertension. However, our study was limited in that it did not address the effects of chronic in vivo UA treatment and in that it only used one large artery of one hypertensive animal model. Therefore, our results certainly do not exclude the long-term involvement of UA in the vascular function of humans, other animal models or other vascular beds, through either its antioxidant, mitogenic, or still uncovered properties. Yet, even if proven effective on endothelial function or as a general antioxidant, the desirability of any hyperuricemic treatment has to be questioned, given the controversial evidence and the clear pathophysiological associations of hyperuricemia in humans.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant P01-HL70687] (to S.W.W.) and the American Heart Association [Grant 0715679Z] (to T.S.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160184.
- UA
- uric acid
- XO
- xanthine oxidase
- ROS
- reactive oxygen species
- ACh
- acetylcholine
- DOCA
- deoxycorticosterone acetate
- SBP
- systolic blood pressure
- PGF2α
- prostaglandin 2α.
References
- Becker, 1993.Becker BF. (1993) Towards the physiological function of uric acid. Free Radic Biol Med 14:615–631 [DOI] [PubMed] [Google Scholar]
- Beckman and Koppenol, 1996.Beckman JS, Koppenol WH. (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271:C1424–C1437 [DOI] [PubMed] [Google Scholar]
- Berry and Hare, 2004.Berry CE, Hare JM. (2004) Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges et al., 2002.Borges F, Fernandes E, Roleira F. (2002) Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 9:195–217 [DOI] [PubMed] [Google Scholar]
- Butler et al., 2000.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. (2000) Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35:746–751 [DOI] [PubMed] [Google Scholar]
- Cai and Harrison, 2000.Cai H, Harrison DG. (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844 [DOI] [PubMed] [Google Scholar]
- Feig et al., 2008.Feig DI, Kang DH, Johnson RJ. (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich, 1965.Fridovich I. (1965) The competitive inhibition of uricase by oxonate and by related derivatives of S-triazines. J Biol Chem 240:2491–2494 [PubMed] [Google Scholar]
- Gao et al., 2007.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. (2007) Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115:245–254 [DOI] [PubMed] [Google Scholar]
- George et al., 2006.George J, Carr E, Davies J, Belch JJ, Struthers A. (2006) High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114:2508–2516 [DOI] [PubMed] [Google Scholar]
- Gersch et al., 2008.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. (2008) Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 27:967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer et al., 2001.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104:2673–2678 [DOI] [PubMed] [Google Scholar]
- Higashi et al., 2002.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, Chayama K. (2002) Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens 15:326–332 [DOI] [PubMed] [Google Scholar]
- Johnson et al., 2003.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. (2003) Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41:1183–1190 [DOI] [PubMed] [Google Scholar]
- Khosla et al., 2005.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. (2005) Hyperuricemia induces endothelial dysfunction. Kidney Int 67:1739–1742 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2008.Kim SY, De Vera MA, Choi HK. (2008) Gout and mortality. Clin Exp Rheumatol 26:S115–S119 [PubMed] [Google Scholar]
- Krishnan et al., 2007.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. (2007) Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension 49:298–303 [DOI] [PubMed] [Google Scholar]
- Mazzali et al., 2001.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. (2001) Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38:1101–1106 [DOI] [PubMed] [Google Scholar]
- Mitchell et al., 2004.Mitchell BM, Dorrance AM, Ergul A, Webb RC. (2004) Sepiapterin decreases vasorelaxation in nitric oxide synthase inhibition-induced hypertension. J Cardiovasc Pharmacol 43:93–98 [DOI] [PubMed] [Google Scholar]
- Ozawa et al., 2004.Ozawa Y, Hayashi K, Kanda T, Homma K, Takamatsu I, Tatematsu S, Yoshioka K, Kumagai H, Wakino S, Saruta T. (2004) Impaired nitric oxide- and endothelium-derived hyperpolarizing factor-dependent dilation of renal afferent arteriole in Dahl salt-sensitive rats. Nephrology (Carlton) 9:272–277 [DOI] [PubMed] [Google Scholar]
- Pacher et al., 2006.Pacher P, Nivorozhkin A, Szabó C. (2006) Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58:87–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price et al., 2006.Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, Endou H, Johnson RJ. (2006) Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol 17:1791–1795 [DOI] [PubMed] [Google Scholar]
- Schulz et al., 2008.Schulz E, Jansen T, Wenzel P, Daiber A, Münzel T. (2008) Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10:1115–1126 [DOI] [PubMed] [Google Scholar]
- Skinner et al., 1998.Skinner KA, White CR, Patel R, Tan S, Barnes S, Kirk M, Darley-Usmar V, Parks DA. (1998) Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J Biol Chem 273:24491–24497 [DOI] [PubMed] [Google Scholar]
- So, 2008.So A. (2008) Developments in the scientific and clinical understanding of gout. Arthritis Res Ther 10:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki et al., 1998.Suzuki H, DeLano FA, Parks DA, Jamshidi N, Granger DN, Ishii H, Suematsu M, Zweifach BW, Schmid-Schönbein GW. (1998) Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci USA 95:4754–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz et al., 2008.Szasz T, Thompson JM, Watts SW. (2008) A comparison of reactive oxygen species metabolism in the rat aorta and vena cava: focus on xanthine oxidase. Am J Physiol Heart Circ Physiol 295:H1341–H1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz and Schiffrin, 2004.Touyz RM, Schiffrin EL. (2004) Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122:339–352 [DOI] [PubMed] [Google Scholar]
- Varela-Echavarría et al., 1988.Varela-Echavarría A, Montes de Oca-Luna R, Barrera-Saldaña HA. (1988) Uricase protein sequences: conserved during vertebrate evolution but absent in humans. FASEB J 2:3092–3096 [DOI] [PubMed] [Google Scholar]
- Viel et al., 2008.Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. (2008) Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 295:H281–H288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring et al., 2006.Waring WS, McKnight JA, Webb DJ, Maxwell SR. (2006) Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 55:3127–3132 [DOI] [PubMed] [Google Scholar]
- Waring et al., 2007.Waring WS, McKnight JA, Webb DJ, Maxwell SR. (2007) Lowering serum urate does not improve endothelial function in patients with type 2 diabetes. Diabetologia 50:2572–2579 [DOI] [PubMed] [Google Scholar]
- Yeldandi et al., 1991.Yeldandi AV, Yeldandi V, Kumar S, Murthy CV, Wang XD, Alvares K, Rao MS, Reddy JK. (1991) Molecular evolution of the urate oxidase-encoding gene in hominoid primates: nonsense mutations. Gene 109:281–284 [DOI] [PubMed] [Google Scholar]
- Zheng et al., 2003.Zheng JS, Yang XQ, Lookingland KJ, Fink GD, Hesslinger C, Kapatos G, Kovesdi I, Chen AF. (2003) Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation 108:1238–1245 [DOI] [PubMed] [Google Scholar]