Abstract
Abnormal growth of glomerular mesangial cells (GMCs) contributes to the pathophysiology of many types of nephropathy. Because adenosine is an autocrine/paracrine factor that potentially could regulate GMC proliferation and because the extracellular 3′,5′-cAMP-adenosine pathway (i.e., the conversion of extracellular 3′,5′-cAMP to 5′-AMP and adenosine on the cell surface) could generate adenosine in the biophase of GMC receptors, we investigated the role of the 3′,5′-cAMP-adenosine pathway in modulating growth [cell proliferation, DNA synthesis ([3H]thymidine incorporation), collagen synthesis ([3H]proline incorporation), and mitogen-activated protein kinase activity] of GMCs. The addition of exogenous 3′,5′-cAMP to human GMCs increased extracellular levels of 5′-AMP, adenosine, and inosine, and 3-isobutyl-1-methylxanthine (phosphodiesterase inhibitor), 1,3-dipropyl-8-p-sulfophenylxanthine (ecto-phosphodiesterase inhibitor), and α,β-methylene-adenosine-5′-diphosphate (ecto-5′-nucleotidase inhibitor) attenuated the increases in adenosine and inosine. Forskolin augmented extracellular 3′,5′-cAMP and adenosine concentrations, and 2′,5′-dideoxyadenosine (adenylyl cyclase inhibitor) blocked these increases. Exogenous 3′,5′-cAMP and forskolin inhibited all indices of cell growth, and antagonism of A2 [(E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine, KF17837] or A1/A2 (1,3-dipropyl-8-p-sulfophenylxanthine, DPSPX), but not A1 (8-cyclopentyl-1,3-dipropylxanthine), or A3{N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea, VUF5574}, adenosine receptors blocked the growth-inhibitory actions of exogenous 3′,5′-cAMP, but not the effects of 8-bromo-3′,5′-cAMP (stable 3′,5′-cAMP analog). Erythro-9-(2-hydroxy-3-nonyl)adenine (adenosine deaminase inhibitor) plus 5-iodotubercidin (adenosine kinase inhibitor) enhanced the growth inhibition by exogenous 3′,5′-cAMP and forskolin, and A2 receptor antagonism blocked this effect. In rat GMCs, down-regulation of A2B receptors with antisense, but not sense or scrambled, oligonucleotides abrogated the inhibitory effects of 3′,5′-cAMP and forskolin on cell growth. The extracellular 3′,5′-cAMP-adenosine pathway exists in GMCs and attenuates cell growth via A2B receptors. Pharmacological augmentation of this pathway could abate pathological glomerular remodeling.
There is an increasing awareness and consensus that abnormal growth and activity of glomerular mesangial cells (GMCs) importantly participates in the pathophysiology of a wide range of renal diseases (Schlondörff and Banas, 2009). Therefore, there is intense interest in investigating the pharmacology and biochemistry of autocrine and paracrine factors that regulate the growth of GMCs. In this regard, adenosine, an endogenous nucleoside, may be of considerable importance. Adenosine is involved in regulating multiple physiological systems in the kidney, and these actions of adenosine are mediated by specific subtypes of adenosine receptors (A1, A2A, A2B, and A3 receptors) (Vallon et al., 2006). For this reason and because adenosine is a potent growth inhibitor in both cardiac fibroblasts (Dubey et al., 1997) and vascular smooth muscle cells (Dubey et al., 2000a), it is conceivable that adenosine also regulates the growth of GMCs. Indeed, experiments with specific adenosine receptor agonists and antagonists, and with antisense oligonucleotides, demonstrate that adenosine inhibits mitogen-induced growth of GMCs via activation of A2B receptors (Dubey et al., 2005). Therefore, it is important to investigate the mechanisms that are critical for physiological modulation of adenosine synthesis by GMCs.
It is possible that a mechanism called the 3′,5′-cAMP-adenosine pathway may be involved in adenosine production by GMCs. The 3′,5′-cAMP-adenosine pathway involves the conversion of 3′,5′-cAMP to 5′-AMP and hence to adenosine by the enzymes phosphodiesterase (PDE) and 5′-nucleotidase (5′-NT), respectively (Jackson and Dubey, 2001). The 3′,5′-cAMP-adenosine pathway may have both intracellular and extracellular arms, i.e., adenosine may be formed within the cell and transported to the extracellular space or it may be formed directly in the extracellular space from extracellular 3′,5′-cAMP that is transported to the cell surface by multidrug-resistance proteins (Cheng et al., 2010).
In support of the concept of an extracellular 3′,5′-cAMP-adenosine pathway, treatment of vascular smooth muscle cells and cardiac fibroblasts with exogenous (extracellular) 3′,5′-cAMP increases 5′-AMP, adenosine, and inosine levels, and inhibition of PDE with 3-isobutyl-1-methylxanthine (IBMX) and ecto-PDE with 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX) blocks these increases (Dubey et al., 1996b, 2001a). In addition, in these two cell types, α,β-methylene-adenosine-5′-diphosphate [AMPCP; ecto-5′-NT inhibitor (Zimmermann, 1992)] blocks the metabolism of exogenous 3′,5′-cAMP to adenosine and inosine, but not to 5′-AMP (Dubey et al., 1996b, 2001a). Together, these findings are consistent with the hypothesis that the extracellular limb of the putative 3′,5′-cAMP-adenosine pathway is a viable metabolic mechanism for the production of adenosine, at least in vascular smooth muscle cells and cardiac fibroblasts. More recent studies demonstrate the presence of the 3′,5′-cAMP-adenosine pathway in rat ileum (Giron et al., 2008), in skeletal muscle (Chiavegatti et al., 2008), and on the intracellular membrane surface of adipocytes (Müller et al., 2008).
Given the fact that GMCs robustly produce 3′,5′-cAMP (Yao et al., 2006, 2007; Zhu et al., 2006) and adenosine inhibits GMC growth, we decided to test the hypothesis that the extracellular 3′,5′-cAMP-adenosine pathway regulates GMC growth. To achieve this goal, we examined the effects of exogenous and endogenous 3′,5′-cAMP on growth in GMCs and investigated whether adenosine biosynthesis was involved in the antimitogenic effects of 3′,5′-cAMP.
Materials and Methods
Materials.
3′,5′-cAMP, 2′,5′-dideoxyadenosine (DDA), α,β-methylene-adenosine-5′-diphosphate (AMPCP), 8-bromo-3′,5′-cyclic monophosphate (Br-cAMP), erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA), platelet-derived growth factor-BB (PDGF-BB), 5-iodotubercidin (ITUB), 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 3-isobutyl-1-methylxanthine (IBMX), forskolin (FOR), and N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea (VUF5574) were purchased from Sigma-Aldrich (St. Louis, MO). (E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine (KF17837) was procured from Kyowa Hakko Kogyo Co. Ltd., (Sunto, Shizuoka, Japan). [3H]thymidine (specific activity, 11.8 Ci/mmol) and l-[3H]proline (23 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). 2′-Amino-3′-methoxyflavone (PD98059) was obtained from Tocris Bioscience (Ellisville, MO). Table 1 summarizes the pharmacological activity of the pharmacological agents used in this study.
TABLE 1.
Summary of pharmacological agents
| Pharmacological Agent | Abbreviation | Activity |
|---|---|---|
| α,β-Methylene-adenosine-5′-diphosphate | AMPCP | Inhibitor of ecto-5′-nucleotidase (CD73) |
| 2′,5′-Dideoxyadenosine | DDA | Inhibitor of adenylyl cyclase |
| 8-Bromo-3′,5′-cAMP | Br-cAMP | Membrane-permeable analogue of 3′,5′-cAMP that is not metabolized to adenosine |
| 1,3-Dipropyl-8-p-sulfophenylxanthine | DPSPX | At low concentrations is a nonselective adenosine receptor angatonist; at high concentrations inhibits ecto-phosphodiesterase |
| 8-Cyclopentyl-1,3-dipropylxanthine | DPCPX | Selective A1 adenosine receptor antagonist |
| Erythro-9-(2-hydroxy-3-nonyl)adenine | EHNA | Inhibitor of adenosine deaminase |
| Forskolin | FOR | Direct activator of adenylyl cyclase |
| 3-Isobutyl-1-methylxanthine | IBMX | Broad spectrum inhibitor of phosphodiesterases |
| 5-Iodotubercidin | ITUB | Inhibitor of adenosine kinase |
| (E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine | KF17837 (KF) | Selective A2 adenosine receptor antagonist |
| 2′-Amino-3′-methoxyflavone | PD98059 | Blocks MAPK pathway by inhibiting MAPK kinase (MEK) |
| Platelet-derived growth factor-BB | PDGF-BB | Growth factor that activates the MAPK pathway |
| N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea | VUF5574 (VUF) | Selective A3 adenosine receptor antagonist |
Glomerular Mesangial Cell Cultures.
Human GMCs in the 3rd passage were obtained from Lonza Walkersville, Inc. (Walkersville, MD), and human GMCs in the 5th to 6th passages were used for all experiments. Rat GMCs were isolated and cultured as described (Inoue et al., 1998), and rat GMCs in the 4th passage were used for the experiments with antisense oligonucleotides.
3′,5′-cAMP Metabolism and Synthesis Studies.
Human GMCs were washed twice with HEPES-buffered Hanks' balanced salt solution and treated with 0.5 ml of Dulbecco's phosphate-buffered saline (PBS) with 25 mM HEPES and 13 mM NaHCO3 in the presence and absence of various treatments. Extracellular (supernatant) levels of purines were analyzed by high-performance liquid chromatography (HPLC) according to our previously described method (Jackson et al., 1996). The concentrations of purines in the samples were calculated from a standard curve and normalized to cell number. To ensure that the various treatments caused no toxic effects or cell death, trypan blue exclusion assays were used to evaluate the viability of cells treated in parallel.
Antisense Oligonucleotides for A2B Receptors.
GMCs cultured from rat kidneys, rather than human GMCs, were treated with an antisense oligonucleotide. In a previous study, we showed that the antisense oligonucleotide 5′-CTCGTGTTCCAGTGACCAA-3′ against the rat A2B receptor effectively down-regulates A2B receptors in rat GMCs (Dubey et al., 2005). Therefore, in the experiments with antisense oligonucleotides we used this construct in rat GMCs. Sense (5′-TTGGTCACTGGAACACGAG-3′) and scrambled (5′-GCACGCTCTATACTGCATG-3′) oligonucleotides were used as controls.
Growth Studies.
GMCs were plated at a density of 5 × 103 cells/well in 24-well tissue culture dishes and allowed to grow to subconfluence. Cells were then growth-arrested with DMEM containing 0.25% albumin for 48 h in the presence or absence of 0.5 μM antisense, sense, or scrambled oligonucleotides. For cells treated with oligonucleotides, fresh oligonucleotides were added upon initiation of the various pharmacological treatments. [3H]Thymidine incorporation (index of DNA synthesis), [3H]proline incorporation (index of collagen synthesis), and cell number studies were conducted as described previously (Dubey et al., 2000a) in the absence or presence of the indicated pharmacological treatments. The growth stimulus was either fetal calf serum (FCS; 2.5%) or PDGF-BB (25 ng/ml), and the treatments were coadministered with the growth stimulus daily. Treatment durations were 24 h, 48 h, and 4 days for DNA synthesis, collagen synthesis, and cell number, respectively.
MAPK Activity.
PDGF-BB-induced MAPK activity in the cytosolic extracts of human GMCs was quantified by our previously described radioactive method with myelin basic protein as the substrate and [γ32P]ATP (Dubey et al., 2000b). GMCs were treated with PDGF-BB (25 ng/ml) and the various pharmacological treatments for 24 h before MAPK activity was measured. PDGF-BB was used in these studies, rather than 2.5% FCS, because antisense oligonucleotides are degraded by FCS and the increase in MAPK activity is less variable with PDGF-BB.
Statistics.
All growth experiments were performed in triplicates or quadruplicates with three to four separate cultures. Data are presented as mean ± S.E.M. Statistical analysis was performed by using analysis of variance, paired Student's t test, or Fisher's least significant difference test as appropriate. A value of p < 0.05 was considered statistically significant.
Results
Metabolism of 3′,5′-cAMP by Human GMCs.
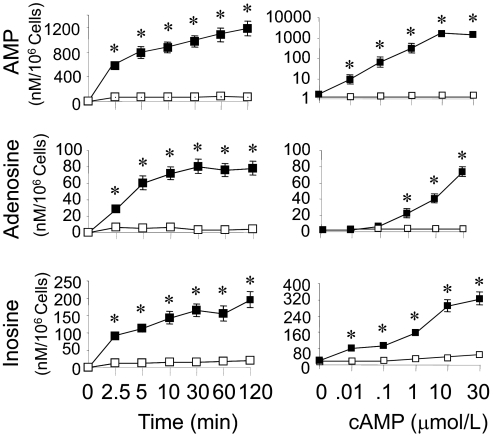
As shown in Fig. 1, left, addition of 3′,5′-cAMP (30 μM) to human GMCs caused a time-related increase in the extracellular levels of 5′-AMP, adenosine, and inosine. Compared with GMCs not treated with 3′,5′-cAMP, the levels of 5′-AMP, adenosine, and inosine increased significantly (p < 0.05 versus vehicle-treated controls) in samples incubated for 1 to 120 min. The metabolism of 3′,5′-cAMP to 5′-AMP, adenosine, and inosine was also concentration-dependent (Fig. 1, right). Compared with the untreated controls, 5′-AMP, adenosine, and inosine levels were significantly different in GMCs incubated for 60 min with concentrations of 3′,5′-cAMP equal to or more than 1 μM. Compared with adenosine, formation of inosine was observed at lower concentrations (0.01 μM) of 3′,5′-cAMP. The greater increase in inosine compared with adenosine at any concentration of 3′,5′-cAMP was likely because in the GMC culture system adenosine was converted readily to inosine, whereas inosine was not further metabolized and therefore accumulated. The levels of 5′-AMP, adenosine, and inosine were near detection limit in cells not treated with 3′,5′-cAMP.
Fig. 1.
Line graphs showing the time-dependent (left) and concentration-dependent (right) metabolism of 3′,5′-cAMP to 5′-AMP (top), adenosine (middle), and inosine (bottom) by cultured human GMCs. For the time course study, cells were treated for 1 to 120 min under standard tissue culture conditions with 30 μM 3′,5′-cAMP (n = 6). For the concentration-dependence study, cells were treated with different concentrations (0.01–30 μM) of 3′,5′-cAMP (n = 6) for 60 min. 5′-AMP, adenosine, and inosine in the medium were analyzed by HPLC. Values are means ± S.E.M. of number of human GMCs cultures. *, p < 0.05 compared with levels at time 0 or levels in medium of GMCs not treated with 3′,5′-cAMP.
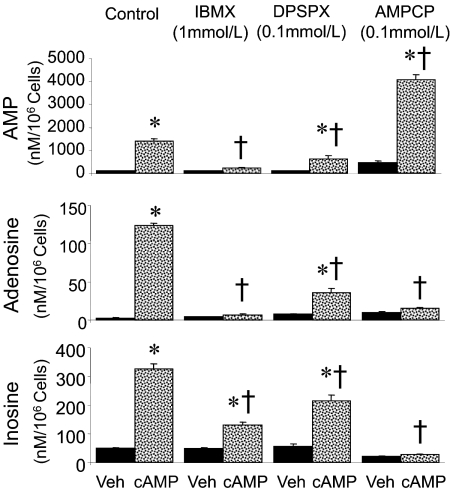
The effects of various inhibitors on the metabolism of 3′,5′-cAMP to purines is shown in Fig. 2. Compared with human GMCs treated with PBS alone (vehicle), the extracellular (medium) levels of 5′-AMP, adenosine, and inosine increased significantly in GMCs treated with 30 μM 3′,5′-cAMP. In vehicle-treated GMCs, the levels (nM/106cells) of 5′-AMP and adenosine were near the assay detection limit, whereas the levels of inosine were 42 ± 1.3. In 3′,5′-cAMP-treated cells, the levels (nM/106 cells) of 5′-AMP, adenosine, and inosine were 1410 ± 111, 124 ± 3.6, and 328 ± 21, respectively (p < 0.05 versus vehicle-treated GMCs).
Fig. 2.
Bar graphs showing the metabolism of 3′,5′-cAMP to 5′-AMP (top), adenosine (middle), and inosine (bottom) by human GMCs in the presence and absence of various inhibitors. Cells were treated for 60 min under standard tissue culture conditions with Dulbecco's PBS (Veh; n = 6) or 3′,5′-cAMP (30 μM; n = 6) in the absence or presence of IBMX (1 mM; n = 6), AMPCP (0.1 mM; n = 6), or DPSPX (0.1 mM; n = 6). 5′-AMP, adenosine, and inosine in the medium were analyzed by HPLC. Values are means ± S.E.M. of number of GMC cultures. *, p < 0.05 compared with corresponding vehicle group in pair; †, p < 0.05 compared with control GMCs treated with 3′,5′-cAMP.
Metabolism of 3′,5′-cAMP into 5′-AMP, adenosine, and inosine was significantly inhibited by IBMX (1 mM; Fig. 2), a PDE inhibitor that crosses cell membranes (Beavo and Reifsnyder, 1990). The levels of adenosine and 5′-AMP were near detection limit in the medium of GMCs treated with IBMX alone and were not significantly increased by 3′,5′-cAMP (30 μM) in GMCs treated with IBMX. Inosine levels were detectable in the media of IBMX-treated cells, and compared with GMCs treated with IBMX alone, the levels were marginally, but significantly, increased in GMCs treated with IBMX plus 3′,5′-cAMP. However, the increase in inosine levels induced by 3′,5′-cAMP was markedly attenuated in IBMX-treated cells compared with control cells.
Metabolism of 3′,5′-cAMP to 5′-AMP, adenosine, and inosine was also attenuated by a high concentration of DPSPX (0.1 mM; Fig. 2). DPSPX is a xanthine that is a nonselective adenosine receptor antagonist at low concentrations (nM) but that inhibits ecto-PDE at high concentrations (micromolar to millimolar) and is restricted to the extracellular compartment so as not to inhibit intracellular PDE (Tofovic et al., 1991). In GMCs treated with a high concentration of DPSPX alone, the levels of 5′-AMP, adenosine, and inosine were near detection limit. Compared with control GMCs treated with 3′,5′-cAMP (30 μM) in the absence of a high concentration of DPSPX, the levels of 5′-AMP, adenosine, and inosine were decreased by ≥50% in GMCs treated with 3′,5′-cAMP plus DPSPX (p < 0.05), indicating that DPSPX attenuated the metabolism of 3′,5′-cAMP to 5′-AMP, adenosine, and inosine.
Treatment of GMCs with 3′,5′-cAMP in the presence of the ecto-5′-NT inhibitor AMPCP (0.1 mM) prevented the metabolism of 3′,5′-cAMP to adenosine and inosine, but not to 5′-AMP (Fig. 2). The levels of adenosine and inosine in GMCs treated with AMPCP alone or AMPCP plus 3′,5′-cAMP were near detection limit, whereas the levels of 5′-AMP (nM/106 cells) were 471 ± 52 and 4137 ± 253, respectively (p < 0.05).
No loss in cell viability was observed in cells treated with IBMX, AMPCP, or DPSPX in the absence or presence of 3′,5′-cAMP, suggesting that the changes in 3′,5′-cAMP metabolism were caused by inhibition of biochemical pathways and not cell toxicity.
Effects of Exogenous and Endogenous 3′,5′-cAMP on DNA Synthesis, Cell Proliferation, and Collagen Synthesis in Human GMCs in the Absence and Presence of Adenosine Receptor Antagonists (KF17837, DPSPX, VUF5574, and DPCPX) and Adenylyl Cyclase Inhibitor (DDA).
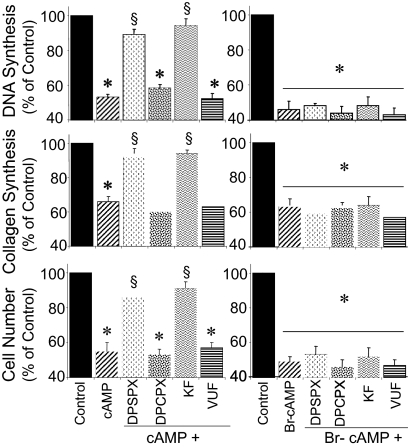
FCS (2.5%) significantly induced DNA synthesis (from 2771 ± 307 to 32,228 ± 1740 dpm/well), collagen synthesis (from 8506 ± 485 to 96,258 ± 1529 dpm/well), and cell proliferation (from 7168 ± 408 to 80,386 ± 1538 cells/well) in growth-arrested human GMCs. Treatment of growth-arrested GMCs with exogenous 3′,5′-cAMP significantly inhibited FCS-induced growth of GMCs (Fig. 3). To confirm that the effects of 3′,5′-cAMP on DNA synthesis, cell proliferation, and collagen synthesis were in part mediated via generation of adenosine, the effects of exogenous 3′,5′-cAMP were evaluated in the presence and absence of KF17837 [a selective A2 receptor antagonist (Jacobson and Knutsen, 2001)], a low concentration of DPSPX [a nonselective adenosine receptor antagonist at low concentrations (Jacobson and Knutsen, 2001)], VUF5574 [a selective A3 receptor antagonist (van Muijlwijk-Koezen et al., 2000)], and DPCPX [a selective A1 receptor antagonist (Jacobson and Knutsen, 2001)]. The inhibitory effects of 3′,5′-cAMP on FCS-induced DNA synthesis, cell proliferation, and proline incorporation were significantly reduced in the presence of both KF17837 (10 nM) and DPSPX (10 nM), but not in the presence of DPCPX (10 nM) or VUF5574 (10 nM) (Fig. 3). Similar to 3′,5′-cAMP, treatment of GMCs with Br-cAMP (10 μM), a membrane-permeant, nonmetabolizable analog of 3′,5′-cAMP, inhibited FCS-induced DNA synthesis, collagen synthesis, and cell proliferation. However, in contrast to 3′,5′-cAMP, the inhibitory effect of Br-cAMP on FCS-induced GMC growth was not reduced by KF17837 or DPSPX (Fig. 3).
Fig. 3.
Bar graphs comparing the inhibitory effects of 3′,5′-cAMP (10 μM) or Br-cAMP (10 μM) on FCS-induced DNA synthesis ([3H]thymidine incorporation) (top), collagen synthesis ([3H]proline incorporation) (middle), and cell number (bottom) in cultured human GMCs in the presence and absence of KF17837 (KF; 10 nM), DPSPX (10 nM), DPCPX (10 nM), or VUF5574 (VUF; 10 nM). Values for each bar represent means ± S.E.M. from three to four separate experiments, conducted in quadruplicate, using separate cultures. *, p < 0.01 compared with control (2.5% FCS); §, p < 0.01 compared with 3′,5′-cAMP.
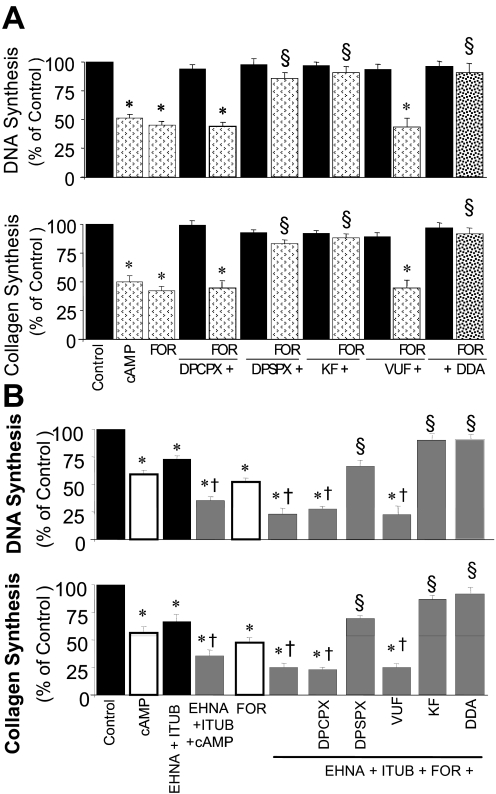
To investigate the potential physiological role of endogenous 3′,5′-cAMP in regulating GMC growth, the effects of forskolin [agent well known to increase cellular 3′,5′-cAMP levels (Insel and Ostrom, 2003)] on FCS-induced DNA synthesis and collagen synthesis were investigated. Similar to 3′,5′-cAMP, forskolin inhibited FCS-induced DNA and collagen synthesis, and the inhibitory effects of forskolin were significantly attenuated by a low concentration of DPSPX (10 nM) and KF17837 (10 nM), but not DPCPX (10 nM) or VUF5574 (10 nM), suggesting that the inhibitory effects were mediated via A2 adenosine receptors (Fig. 4A). To confirm that the inhibitory effects of forskolin were 3′,5′-cAMP-mediated, the inhibitory effects of these agents were assayed in presence of DDA [adenylyl cyclase inhibitor (Johnson et al., 1989)]. In the presence of DDA, the inhibitory effects of forskolin on DNA and collagen synthesis were significantly attenuated (Fig. 4A).
Fig. 4.
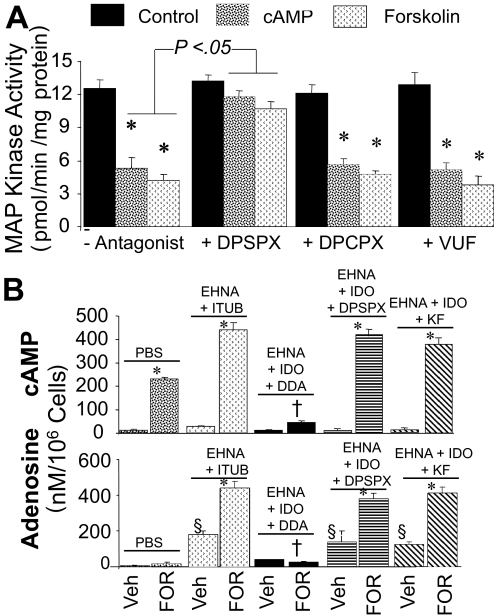
A, bar graphs showing the inhibitory effects of FOR on 2.5% FCS-induced DNA synthesis ([3H]thymidine incorporation) (top) and collagen synthesis ([3H]proline incorporation) (bottom) in cultured human GMCs, in the presence and absence of DPSPX (10 nM), KF17837 (10 nM; KF), DPCPX (10 nM), VUF5574 (10 nM; VUF), or DDA (10 μM). Data are presented as percentage of control. Values for each bar represent mean ± S.E.M. from three to four separate experiments, conducted in quadruplicate. *, p < 0.01 compared with control (2.5% FCS); §, p < 0.01 compared with 3′,5′-cAMP or forskolin alone. B, bar graphs showing the inhibitory effects of 3′,5′-cAMP (10 μM) and FOR (10 μM) on FCS (2.5%)-induced DNA synthesis (top) and collagen synthesis (bottom) in human GMCs in the presence of EHNA (10 μM) plus ITUB (0.1 μM). Also shown are the effects of DPSPX (10 nM), KF17837 (10 nM; KF), DPCPX (10 nM), VUF5574 (10 nM; VUF), and DDA (10 μM) on responses to EHNA + ITUB + FOR. *, p < 0.05 versus cells treated with vehicle; §, p < 0.01 versus cells treated with FOR + EHNA + ITUB in PBS; †, p < 0.01 compared with 3′,5′-cAMP or forskolin alone. Data are presented as percentage of control. Values for each bar represent mean ± S.E.M. from three to four separate experiments, conducted in quadruplicate.
Effects of GMC-Derived 3′,5′-cAMP on DNA Synthesis in the Absence and Presence of Modulators of Adenosine Levels.
Treatment of human GMCs with 3′,5′-cAMP, forskolin, or EHNA [10 μM; elevates endogenous levels of adenosine by inhibiting adenosine deaminase (Ijzerman and Van Der Wenden, 1997)] plus ITUB [0.1 μM; increases endogenous adenosine by inhibiting adenosine kinase (Ijzerman and Van Der Wenden, 1997)], inhibited FCS-induced DNA synthesis. When added together with EHNA and ITUB, the inhibitory effects of 3′,5′-cAMP and forskolin were significantly enhanced. Furthermore, a low concentration of DPSPX (10 nM) and KF17837 (10 nM), but not DPCPX (10 nM) or VUF5574 (10 nM), significantly reversed the inhibitory effects of forskolin in the presence of EHNA plus ITUB (Fig. 4B).
Effects of 3′,5′-cAMP and Forskolin on MAPK Activity.
In human GMCs, PDGF-BB increased MAPK activity from 0.7 ± 0.08 to 12.83 ± 0.8 pmol/min/mg protein. The MAPK pathway inhibitor PD98059 (10 μM) attenuated the stimulatory effects of PDGF-BB to 1.4 ± 0.07 pmol/min/mg protein. PDGF-BB-mediated stimulation of MAPK activity was reduced in GMCs pretreated for 24 h with 3′,5′-cAMP or forskolin (Fig. 5, top). A low concentration of DPSPX (10 nM), but not DPCPX (10 nM) or VUF5574 (10 nM), attenuated the inhibitory effects of 3′,5′-cAMP and forskolin on MAPK activity.
Fig. 5.
A, bar graph depicting the inhibitory effects of 3′,5′-cAMP (10 μM) and forskolin (10 μM) on PDGF-BB (25 ng/ml)-induced MAPK activity in human GMCs in the presence and absence of DPSPX (10 nM), DPCPX (10 nM), or VUF5574 (10 nM; VUF). *, p < 0.01 compared with control (PDGF-BB). B, bar graph showing the synthesis of 3′,5′-cAMP (cAMP) (top) and adenosine (bottom) by human GMCs in response to FOR (10 μM) in the presence and absence of EHNA (10 μM) plus ITUB (0.1 μM), EHNA + ITUB + low-dose-DPSPX (10 nM), EHNA + ITUB + KF17837 (10 nM), or EHNA + ITUB + DDA (10 μM). Results for 3′,5′-cAMP and adenosine are expressed as nM/106 cells, and each value represents mean ± S.E.M. from six experiments conducted with separate cultures. *, p < 0.01 compared with respective vehicle; †, p < 0.01 versus cells treated with FOR + EHNA + ITUB; §, p < 0.01 compared with vehicle (PBS) only.
Synthesis of 3′,5′-cAMP and Adenosine in Response to Forskolin in the Presence and Absence of EHNA + ITUB, DDA, DPSPX, DPCPX, and KF17837.
To ascertain whether the modulation of the growth effects by forskolin were mediated by 3′,5′-cAMP-derived adenosine, we assayed the levels of 3′,5′-cAMP and adenosine in human GMCs treated with the various treatments (Fig. 5, bottom). Compared with GMCs treated with PBS, the levels of 3′,5′-cAMP, but not adenosine, increased significantly in the medium of GMCs treated with forskolin and were (nM/106 cells) 11.5 ± 6.4 and 224 ± 9.6, respectively. Because extracellular adenosine is rapidly metabolized, we assayed the effects of forskolin in the presence of EHNA + ITUB (blockers of adenosine catabolism). Treatment of GMCs with EHNA + ITUB increased the levels of adenosine and 3′,5′-cAMP in the medium from 5.9 ± 3.36 to 178 ± 20 nM/106 cells and 11.5 ± 6.4 to 32 ± 2.41 nM/106 cells, respectively. In addition, in the presence of EHNA + ITUB, forskolin increased both 3′,5′-cAMP and adenosine levels in the medium, and these effects were significantly inhibited in the presence of the adenylyl cyclase inhibitor DDA. The stimulatory effects of forskolin on 3′,5′-cAMP and adenosine synthesis were not inhibited by a low concentration of DPSPX (10 nM) that antagonizes A2 receptors but does not block ecto-PDE or by KF17837 (10 nM) (Fig. 5, bottom), nor by the A1 or A3 receptor blockers DPCPX (10 nM) and VUF5574 (10 nM) (not shown).
Modulatory Effects of A2B Receptor Down-Regulation by Antisense Oligonucleotide on the Inhibitory Effects of Exogenous and GMC-Derived 3′,5′-cAMP on Growth.
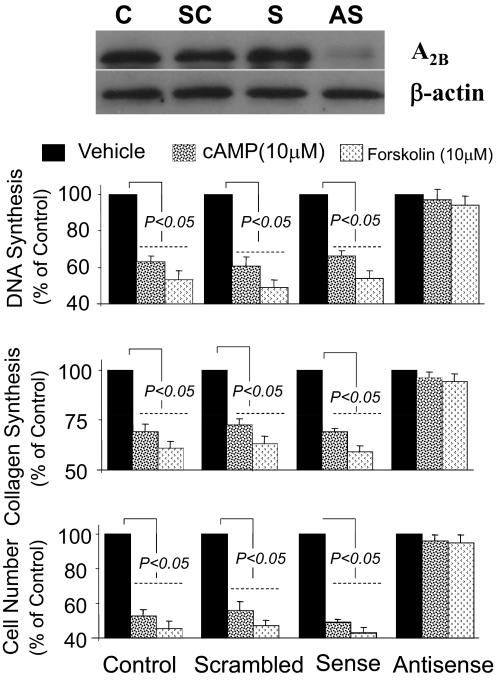
Treatment of rat GMCs (in three separate experiments) with 0.5 μM antisense, but not sense or scrambled, oligonucleotides to A2B receptors down-regulated the A2B receptor by 88 ± 6% (typical Western blot shown in Fig. 6). Treatment of rat GMCs with 3′,5′-cAMP (10 μM) or forskolin (10 μM) under identical conditions inhibited PDGF-BB-induced growth in GMCs treated with scrambled or sense oligonucleotides, but not in GMCs treated with antisense (Fig. 6).
Fig. 6.
Bar graphs show the inhibitory effects of 3′,5′-cAMP (10 μM) and FOR (10 μM) on PDGF-BB (25 ng/ml)-induced DNA synthesis ([3H]thymidine incorporation) (top), collagen synthesis ([3H]proline incorporation) (middle), and cell number (bottom) in rat GMCs treated with 0.5 μM antisense, sense, or scrambled oligonucleotides to adenosine A2B receptor. Representative Western blot depicts the down-regulation of A2B receptor expression by antisense (AS), but not by sense (S) and scrambled (SC), oligonucleotides. Treatment with AS down-regulated A2B by 88 ± 6% (mean ± S.E.M.; n = 3). Values for growth studies represent mean ± S.E.M. from four separate experiments, each conducted in quadruplicate.
Discussion
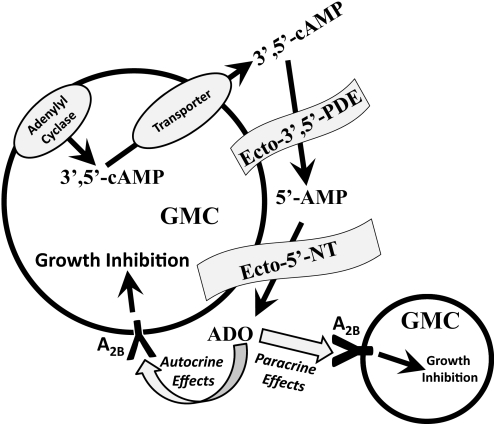
Stimulation of adenylyl cyclase increases 3′,5′-cAMP levels, which could theoretically increase adenosine biosynthesis both intracellularly and extracellularly. Intracellular metabolism of 3′,5′-cAMP to 5′-AMP and 5′-AMP to adenosine could be catalyzed by cytosolic PDE and cytosolic 5′-NT, respectively, and the adenosine thus formed could reach the extracellular space by way of facilitated transport. However, intracellular formation of adenosine may be diminished by the competition of cytosolic 5′-NT and adenylate kinase for 5′-AMP. Moreover, any intracellular adenosine formed would not necessarily reach cell surface receptors because transport mechanisms must compete with intracellular adenosine kinase and adenosine deaminase for intracellular adenosine. Therefore, the extracellular limb of the 3′,5′-cAMP-adenosine pathway may be quantitatively more important because stimulation of adenylyl cyclase causes egress of 3′,5′-cAMP into the highly localized extracellular space via multidrug resistance protein 4 (Cheng et al., 2010), and the 3′,5′-cAMP released onto the cell surface could be efficiently converted to adenosine by ecto-PDE and ecto-5′-NT (CD73), an enzyme tethered to the extracellular face of the plasma membrane (Zimmermann, 2006). Hence, production of adenosine by GMCs via the extracellular 3′,5′-cAMP potentially could give rise to significant concentrations of adenosine at the cell surface, which would act, in an autocrine/paracrine fashion, on cell-surface A2B adenosine receptors expressed by GMCs to inhibit GMC growth (Fig. 7).
Fig. 7.
Schematic representation of GMC growth regulation by the extracellular 3′,5′-cAMP-adenosine pathway. ADO, adenosine.
The presence of the extracellular 3′,5′-cAMP-adenosine pathway in GMCs is supported by the observations that exogenous 3′,5′-cAMP increases extracellular levels of 5′-AMP, adenosine, and inosine (metabolite of adenosine) in a time- and concentration-dependent fashion. Because 3′,5′-cAMP is highly hydrophilic and does not penetrate lipid bilayers, the conversion of exogenous 3′,5′-cAMP to extracellular adenosine most likely is caused by extracellular metabolism of 3′,5′-cAMP. This conclusion is supported by the results with AMPCP and DPSPX because AMPCP inhibits ecto-5′-NT but not cytosolic 5′-NT (Zimmermann, 2006), and DPSPX does not penetrate cell membranes (Tofovic et al., 1991). The ability of these inhibitors to attenuate the conversion of exogenous 3′,5′-cAMP to extracellular adenosine and its metabolites is strong evidence for an extracellular site of 3′,5′-cAMP metabolism. Moreover, the ability of AMPCP to increase extracellular 5′-AMP levels is consistent with an extracellular site of 3′,5′-cAMP conversion to 5′-AMP. In this regard, if 3′,5′-cAMP were metabolized to 5′-AMP within the cell, inhibition of ecto-5′-NT with AMPCP would not prevent the further metabolism of 5′-AMP to adenosine and would not cause the accumulation of extracellular 5′-AMP. The existence of the extracellular 3′,5′-cAMP-adenosine pathway in GMCs is also supported by the observation that activation of adenylyl cyclase with forskolin increases extracellular levels of 3′,5′-cAMP and adenosine and that this effect is inhibited by DDA, a drug that blocks adenylyl cyclase.
The current findings also support the hypothesis that in GMCs the extracellular 3′,5′-cAMP pathway inhibits growth via adenosine activating on A2 receptors. In this regard, EHNA, which prevents the metabolism of adenosine to inosine, plus ITUB, which inhibits the metabolism of adenosine to 5′-AMP, enhance the inhibitory effects of 3′,5′-cAMP on FCS-induced GMC growth, indicating a role for adenosine. This conclusion is further supported by the findings that the inhibitory effects of exogenous and endogenous 3′,5′-cAMP on GMC growth are prevented by two different adenosine receptor antagonists, namely KF17837 and DPSPX. The fact that KF17837 and DPSPX (A2 receptor blockers) antagonize the growth inhibitory effects of 3′,5′-cAMP, whereas DPCPX and VUF5574 (A1 and A3 receptor antagonists, respectively) do not, suggests that the growth inhibitory effects of 3′,5′-cAMP are mediated via adenosine acting on A2 receptors. Importantly, neither KF17837, DPSPX, DPCPX, nor VUF5574 interfere with the inhibitory effects of 8-bromo-3′,5′-cAMP, which is a metabolically stable 3′,5′-cAMP analog, suggesting that the affects of KF17837 and DPSPX are specifically mediated via antagonism of A2 receptors and are not caused by nonspecific effects. The findings that forskolin inhibits FCS-induced GMC growth and that these effects are blocked by the adenylyl cyclase inhibitor DDA and the A2 adenosine receptor antagonists KF17837 and DPSPX further supports the concept that the extracellular 3′,5′-cAMP-adenosine pathway regulates GMC growth via adenosine acting on A2 receptors.
There are two subtypes of A2 receptors, namely A2A and A2B. The results of the current study show that down-regulation of A2B receptors by antisense oligonucleotides prevents the ability of both 3′,5′-cAMP and forskolin to inhibit GMC growth. Thus, it appears that A2B receptors are responsible for inhibition of GMC growth by adenosine formed from the extracellular 3′,5′-cAMP-adenosine pathway. These findings are in full accord with previous work in vascular smooth muscle cells, cardiac fibroblasts, and GMCs showing that inhibition of growth by adenosine is mediated by A2B receptor (Dubey et al., 1996a, 1997, 1998a,b, 2000a, 2001b, 2005).
The significance of the present study is that the results demonstrate the concept that sufficient quantities of adenosine are formed by GMCs from the metabolism of exogenous or endogenous 3′,5′-cAMP to engage A2B receptors and thereby regulate GMC biology. These experiments provide, therefore, the first evidence that human GMCs express the extracellular 3′,5′-cAMP-adenosine pathway and that adenosine generated via this pathway inhibits mitogen-induced growth and MAPK activity in GMCs via the A2B receptor. Although previous studies support the existence of the 3′,5′-cAMP-adenosine pathway in the renal preglomerular microcirculation (Jackson and Mi, 2000), the identification of this pathway in human GMCs is of key importance because of the pivotal role of GMCs in the pathophysiology of glomerulopathies such as diabetic nephropathy and hypertension-induced glomerulosclerosis (Kwoh et al., 2006; LeHir and Kriz, 2007) and many other glomerulopathies (Schlondörff and Banas, 2009).
The work was supported by the Swiss National Science Foundation [Grants 3200B0-106098/1, 320000-117998/1]; Oncosuisse [Grant OCS-01551-08-2004], EMDO Stiftung; and the National Institutes of Health [Grants HL069846, DK068575, DK079307].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.166371.
- GMC
- glomerular mesangial cell
- PDE
- phosphodiesterase
- 5′-NT
- 5′-nucleotidase
- MAPK
- mitogen-activated protein kinase
- AMPCP
- α,β-methylene-adenosine-5′-diphosphate
- Br-cAMP
- 8-bromo-3′,5′-cyclic monophosphate
- DDA
- 2′,5′-dideoxyadenosine
- DPSPX
- 1,3-dipropyl-8-p-sulfophenylxanthine
- DPCPX
- 8-cyclopentyl-1,3-dipropylxanthine
- EHNA
- erythro-9-(2-hydroxy-3-nonyl)adenine
- IBMX
- 3-isobutyl-1-methylxanthine
- VUF5574
- N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea
- FOR
- forskolin
- KF17837
- (E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine
- ITUB
- 5-iodotubercidin
- PD98059
- 2′-amino-3′-methoxyflavone
- FCS
- fetal calf serum
- PDGF-BB
- platelet-derived growth factor-BB
- PBS
- phosphate-buffered saline
- HPLC
- high-performance liquid chromatography.
References
- Beavo and Reifsnyder, 1990.Beavo JA, Reifsnyder DH. (1990) Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci 11:150–155 [DOI] [PubMed] [Google Scholar]
- Cheng et al., 2010.Cheng D, Ren J, Jackson EK. (2010) Multidrug resistance protein 4 mediates cAMP efflux from rat preglomerular vascular smooth muscle cells. Clin Exp Pharmacol Physiol 37:205–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavegatti et al., 2008.Chiavegatti T, Costa VL, Jr, Araújo MS, Godinho RO. (2008) Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol 153:1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey et al., 1996a.Dubey RK, Gillespie DG, Osaka K, Suzuki F, Jackson EK. (1996a) Adenosine inhibits growth of rat aortic smooth muscle cells: possible role of A2b receptor. Hypertension 27:786–793 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 1998a.Dubey RK, Gillespie DG, Jackson EK. (1998a) Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: potential role of A2B receptors. Hypertension 31:943–948 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 1997.Dubey RK, Gillespie DG, Mi Z, Jackson EK. (1997) Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation 96:2656–2666 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 1998b.Dubey RK, Gillespie DG, Mi Z, Jackson EK. (1998b) Adenosine inhibits growth of human aortic smooth muscle cells via A2B receptors. Hypertension 31:516–521 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 2001a.Dubey RK, Gillespie DG, Mi Z, Jackson EK. (2001a) Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension 37:1095–1100 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 2005.Dubey RK, Gillespie DG, Mi Z, Jackson EK. (2005) Adenosine inhibits PDGF-induced growth of human glomerular mesangial cells via A2B receptors. Hypertension 46:628–634 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 2000a.Dubey RK, Gillespie DG, Shue H, Jackson EK. (2000a) A2B receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension 35:267–272 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 2001b.Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK. (2001b) A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension 37:716–721 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 2000b.Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B, Keller PJ. (2000b) Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and mitogen-activated protein kinase activity. Arterioscler Thromb Vasc Biol 20:964–972 [DOI] [PubMed] [Google Scholar]
- Dubey et al., 1996b.Dubey RK, Mi Z, Gillespie DG, Jackson EK. (1996b) Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension 28:765–771 [DOI] [PubMed] [Google Scholar]
- Giron et al., 2008.Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. (2008) Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology 134:1116–1126 [DOI] [PubMed] [Google Scholar]
- Ijzerman and Van Der Wenden, 1997.Ijzerman AP, Van Der Wenden NM. (1997) Modulators of adenosine uptake, release, and inactivation, in Purinergic Approaches in Experimental Therapeutics (Jacobson KA, Jarvis MF. eds) pp 129–148, Willey-Liss, Inc., New York [Google Scholar]
- Inoue et al., 1998.Inoue T, Mi Z, Gillespie DG, Jackson EK. (1998) Cyclooxygenase inhibition reveals synergistic action of vasoconstrictors on mesangial cell growth. Eur J Pharmacol 361:285–291 [DOI] [PubMed] [Google Scholar]
- Insel and Ostrom, 2003.Insel PA, Ostrom RS. (2003) Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson and Dubey, 2001.Jackson EK, Dubey RK. (2001) Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal Physiol 281:F597–F612 [DOI] [PubMed] [Google Scholar]
- Jackson and Mi, 2000.Jackson EK, Mi Z. (2000) Preglomerular microcirculation expresses the cAMP-adenosine pathway. J Pharmacol Exp Ther 295:23–28 [PubMed] [Google Scholar]
- Jackson et al., 1996.Jackson EK, Mi Z, Koehler MT, Carcillo JA, Jr, Herzer WA. (1996) Injured erythrocytes release adenosine deaminase into the circulation. J Pharmacol Exp Ther 279:1250–1260 [PubMed] [Google Scholar]
- Jacobson and Knutsen, 2001.Jacobson KA, Knutsen LJS. (2001) P1 and P2 purine and pyrimidine receptor ligands, in Purinergic and Pyrmidinergic Signaling I (Abbracchio MP, Williams M. eds) pp 129–175, Springer-Verlag, Berlin, Germany [Google Scholar]
- Johnson et al., 1989.Johnson RA, Yeung SM, Stübner D, Bushfield M, Shoshani I. (1989) Cation and structural requirements for P site-mediated inhibition of adenylate cyclase. Mol Pharmacol 35:681–688 [PubMed] [Google Scholar]
- Kwoh et al., 2006.Kwoh C, Shannon MB, Miner JH, Shaw A. (2006) Pathogenesis of nonimmune glomerulopathies. Annu Rev Pathol 1:349–374 [DOI] [PubMed] [Google Scholar]
- LeHir and Kriz, 2007.LeHir M, Kriz W. (2007) New insights into structural patterns encountered in glomerulosclerosis. Curr Opin Nephrol Hypertens 16:184–191 [DOI] [PubMed] [Google Scholar]
- Müller et al., 2008.Müller G, Wied S, Over S, Frick W. (2008) Inhibition of lipolysis by palmitate, H2O2, and the sulfonylurea drug, glimepiride, in rat adipocytes depends on cAMP degradation by lipid droplets. Biochemistry 47:1259–1273 [DOI] [PubMed] [Google Scholar]
- Schlöndorff and Banas, 2009.Schlöndorff D, Banas B. (2009) The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20:1179–1187 [DOI] [PubMed] [Google Scholar]
- Tofovic et al., 1991.Tofovic SP, Branch KR, Oliver RD, Magee WD, Jackson EK. (1991) Caffeine potentiates vasodilator-induced renin release. J Pharmacol Exp Ther 256:850–860 [PubMed] [Google Scholar]
- Vallon et al., 2006.Vallon V, Mühlbauer B, Osswald H. (2006) Adenosine and kidney function. Physiol Rev 86:901–940 [DOI] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen et al., 2000.van Muijlwijk-Koezen JE, Timmerman H, van der Goot H, Menge WM, Frijtag Von Drabbe Künzel J, de Groote M, IJzerman AP. (2000) Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A(3) receptor. J Med Chem 43:2227–2238 [DOI] [PubMed] [Google Scholar]
- Yao et al., 2006.Yao J, Kitamura M, Zhu Y, Meng Y, Kasai A, Hiramatsu N, Morioka T, Takeda M, Oite T. (2006) Synergistic effects of PDGF-BB and cAMP-elevating agents on expression of connexin43 in mesangial cells. Am J Physiol Renal Physiol 290:F1083–F1093 [DOI] [PubMed] [Google Scholar]
- Yao et al., 2007.Yao J, Zhu Y, Sun W, Sawada N, Hiramatsu N, Takeda M, Kitamura M. (2007) Irsogladine maleate potentiates the effects of nitric oxide on activation of cAMP signaling pathways and suppression of mesangial cell mitogenesis. Br J Pharmacol 151:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al., 2006.Zhu Y, Yao J, Meng Y, Kasai A, Hiramatsu N, Hayakawa K, Miida T, Takeda M, Okada M, Kitamura M. (2006) Profiling of functional phosphodiesterase in mesangial cells using a CRE-SEAP-based reporting system. Br J Pharmacol 148:833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, 1992.Zimmermann H. (1992) 5′-Nucleotidase: molecular structure and functional aspects. Biochem J 285:345–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, 2006.Zimmermann H. (2006) Ectonucleotidases in the nervous system. Novartis Found Symp 276:113–128 [PubMed] [Google Scholar]