Abstract
Parkinson's disease (PD) is a progressive neurological disorder characterized by a selective loss of dopamine (DA) neurons in the substantia nigra (SN). Although current therapy can control symptoms of this disorder, there is no effective therapy available to halt its progression. Recently, neuroinflammation has been recognized as an important contributor to the pathogenesis of PD, and nuclear factor-κB (NF-κB) plays a key role in regulating neuroinflammation. Hence, the modulation of NF-κB pathway may have therapeutic potential for PD. Activation of NF-κB depends on the phosphorylation of its inhibitor, IκB, by the specific IκB kinase (IKK) subunit IKK-β. Compound A (7-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-5-[(3S)-3-piperidinyl]-1, 4-dihydro-2H-pyrido[2,3-d][1,3]oxazin-2-one hydrochloride), a potent and selective inhibitor of IKK-β, has recently been reported to provide cardioprotection through specific suppression of NF-κB signaling. The present study, for the first time, elucidates neuroprotective effects of compound A. Daily subcutaneous injection of compound A (1 mg/kg) for 7 days inhibited the activation of microglia induced by nigral stereotaxic injection of lipopolysaccharide (LPS) and significantly attenuated LPS-induced loss of DA neurons in the SN. In vitro mechanistic studies revealed that neuroprotective effects of compound A were mediated by 1) suppressing the activity of microglial NADPH oxidase and decreasing the production of reactive oxygen species, and 2) inhibiting NF-κB-mediated gene transcription of various proinflammatory mediators in microglia via IKK-β suppression. These findings indicate that compound A afforded potent neuroprotection against LPS-induced neurodegeneration through selective inhibition of NF-κB activation and may be of potential benefit in the treatment of PD.
Parkinson's disease (PD) is the second most common neurodegenerative disorder, and it is characterized by a slow and progressive loss of dopamine (DA) neurons in the substantia nigra (SN) pars compacta of the ventral midbrain and the subsequent loss of DA content in the striatum. The cardinal clinical symptoms of PD include dyskinesia, resting tremor, rigidity, and gait disturbance. Current therapeutic interventions are limited to alleviating the symptoms of PD, and they fail to halt the progression of neurodegeneration (Gao et al., 2003a).
Although oxidative stress, mitochondrial dysfunction, excitotoxicity, and apoptotic processes have been identified as being involved in neuronal degeneration (Nagatsu and Sawada, 2006), the mechanisms responsible for the progressive feature of neurodegeneration are not fully understood. In recent years, increasing evidence has strongly suggested that neuroinflammation is an important contributor to the pathogenesis of neurodegenerative diseases, such as PD, Alzheimer's disease, and multiple sclerosis. The hallmark of brain neuroinflammation is the activation of microglia (Hirsch and Hunot, 2009). Microglia are the resident immune cells in the brain and serve critical roles in immune surveillance under normal conditions. Once activated after brain damage or exposure to inflammogen, microglia can release many proinflammatory and cytotoxic factors, including prostaglandins, cytokines, reactive oxygen species (ROS), and reactive nitrogen species. The accumulation of these factors is thought to contribute to the loss of DA neurons. Subsequently, the continuing processes involved in the dying/dead DA neurons, in turn, lead to the secondary activation of microglia and further DA neuronal loss. Thus, a vicious “self-propelling” cycle is created, which causes progressive neurodegeneration (Gao and Hong, 2008). Taken together, strong evidence shows a pivotal role of neuroinflammation in the manifestation of PD, and thus the inhibition of microglia-mediated inflammatory processes is becoming a promising therapeutic potential for the treatment of PD.
It is well known that nuclear factor-κB (NF-κB) is an important regulator of brain inflammation. For example, NF-κB plays a critical role in the regulation of microglial production of several proinflammatory factors including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, cyclooxygenase-2, and nitric oxide (NO) (Jana et al., 2001; Lee et al., 2004; Moriyama et al., 2006; Werner et al., 2008). NF-κB activation is detected within the SN of PD patients and a PD animal model created by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Hunot et al., 1997; Ghosh et al., 2007). Of particular interest is marked colocalization of p65 with CD11b-positive activated microglia in the SN of postmortem PD brains (Ghosh et al., 2007). Moreover, selective inhibition of NF-κB activation suppressed microglial activation and prevented DA neuronal loss against MPTP-intoxicated PD mice (Ghosh et al., 2007). Similarly, inhibition of NF-κB and suppression of cyclooxygenase-2 activity are involved in the neuroprotection of pioglitazone against lipopolysaccharide (LPS) insult on DA neurons (Xing et al., 2007).
In resting conditions, NF-κB is sequestered in the cytosol by binding to its inhibitor, IκB. Activation of NF-κB requires the activity of IκB kinase (IKK) complex, in a manner dependent mainly on the IKK-β catalytic subunit (Karin, 1999). A variety of inflammatory stimuli, including endotoxin (e.g., LPS) and cytokines, induce activation and nuclear translocation of NF-κB after rapid phosphorylation, degradation, and release of IκB (Zhang and Ghosh, 2001). Compound A (7-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-5-[(3S)-3-piperidinyl]-1,4-dihydro-2H-pyrido[2,3-d][1,3]oxazin-2-one hydrochloride) is a selective inhibitor of IKK-β (Ziegelbauer et al., 2005). It prevents pulmonary inflammation (Moss et al., 2008) and attenuates myocardial injury and dysfunction after ischemia–reperfusion injury, in which specific suppression of NF-κB signaling and consequent decrease in serum levels of TNFα and IL-6 underlie cardioprotection (Ziegelbauer et al., 2005; Moss et al., 2007). In the present study, we report that compound A exerted neuroprotective effects against LPS-induced neurotoxicity both in vitro and in vivo. Mechanistic studies showed that the neuroprotective effects of compound A were mediated by suppressing the activity of NADPH oxidase [phagocytic oxidase (PHOX)] and the activation of NF-κB cascade signaling pathway in activated microglia through specific IKK-β inhibition.
Materials and Methods
Reagents.
Compound A (Bay 65-1942) was purchased from TheraLogics Inc. (Chapel Hill, NC). All cell culture materials were purchased from Invitrogen (Carlsbad, CA). LPS (Escherichia coli strain O111:B4) and the fluorescence probe dichlorodihydrofluorescein diacetate (DCFH-DA) were obtained from Calbiochem (San Diego, CA). 1-Methyl-4-phenylpyridinium (MPP+), cytosine β-d-arabinofuranoside, leu-leu methyl ester, and superoxide dismutase (SOD) were purchased from Sigma-Aldrich (St. Louis, MO). [3H]DA (30 Ci/mM) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). The polyclonal antityrosine hydroxylase (TH) antibody was a gift from Dr. John Reinhard (GlaxoSmithKline, Research Triangle Park, NC). Monoclonal antibody against the CR3 complement receptor (OX-42) was obtained from BD Pharmingen (San Diego, CA). A vectastain avidin–biotin complex kit and biotinylated secondary antibody were obtained from Vector Laboratories (Burlingame, CA). Also used were: WST-1 (Dojindo Laboratories, Gaithersburg, MD), TRIzol reagent (Invitrogen), RNeasy Kit (QIAGEN, Valencia, CA), SYBR green polymerase chain reaction (PCR) master mix (Applied Biosystems, Foster City, CA), and an enhanced chemiluminescence kit (GE Healthcare, Chalfont St. Giles, UK).
Animals and Treatment.
Male Fisher rats (200–225 g) and timed-pregnant Fisher F344 rats were obtained from Charles River Laboratories, Inc. (Wilmington, MA). Experimental use of the animals was performed in strict accordance with National Institutes of Health Guidelines. To investigate the effect of compound A on LPS-induced neurotoxicity, male rats received a single LPS injection (5 μg in 2 μl of saline) into the SN pars compacta on one side of the brain, followed by the coordinates 4.8 mm posterior to bregma, 1.7 mm lateral to the midline, and 8.2 mm ventral to the surface of the skull (Liu et al., 2000b). Compound A (1 mg/kg/day s.c.) was administrated once a day for 7 consecutive days beginning 30 min before LPS injection. Animals were sacrificed 1 day after the last compound A treatment.
Primary Rat Mesencephalic Neuron-Glia and Neuron-Astrocyte Cultures.
Primary neuron-glia cultures were prepared from the ventral mesencephalic tissues of embryonic day 14 and 15 rats as described previously (Zhang et al., 2006). In brief, dissociated cells were seeded at 5 × 105/well and 1 × 105/well in poly-d-lysine-coated 24- and 96-well plates, respectively. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air in maintenance medium that was made up of minimum essential medium containing 10% heat-inactivated fetal bovine serum, 10% heat-inactivated horse serum, 1 g/liter glucose, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin. Seven-day-old cultures were used for drug treatments. At the time of treatment, immunocytochemical analysis indicated that the rat neuron-glia cultures consisted of 10% microglia, 50% astrocytes, 40% neurons, and 1% TH-immunoreactive neurons. For treatment, cultures were changed to treatment medium composed of minimum essential medium, 2% fetal bovine serum, 2% horse serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 U/ml penicillin, and 50 μg/ml streptomycin. Primary neuron-astrocyte cultures were obtained by suppressing microglia proliferation with 1.5 mM leu-leu methyl ester added to neuron-glia cultures 1 day after seeding the cells as described previously (Liu et al., 2000a). Three days later, cultures were changed back to maintenance medium and used for treatment 7 days after initial seeding. The percentage of microglia in the cultures was <1%.
Primary Microglia-Enriched Cultures.
Primary microglia-enriched cultures were prepared from the whole brains of 1-day-old rat pups as described previously (Zhang et al., 2006). After a confluent monolayer of glia cells had been obtained, microglia were shaken off and immunocytochemical analysis indicated that the cultures were 95 to 98% pure for microglia. Cells were seeded at 5 × 105/well and 1 × 105/well in 24- and 96-well plates, respectively, and used for treatment the next day.
Primary Midbrain Neuron-Enriched and Reconstituted Neuron-Microglia Cultures.
Midbrain neuron-enriched cultures were established as described previously. One day after seeding, cytosine β-d-arabinofuranoside was added to a final concentration of 6 to 8 μM to suppress glia proliferation. The 7-day-old neuron-enriched cultures were composed of 90% neurons, 10% astrocytes, and <0.1% microglia. The reconstituted cultures were established by adding 10% (5 × 104/well) of primary microglia back to neuron-enriched cultures as described previously (Liu et al., 2000a).
[3H]DA Uptake Assay.
[3H]DA uptake assay was performed as described previously (Zhang et al., 2006). Cultures were incubated for 20 min at 37°C with 1 μM [3H]DA in Krebs-Ringer buffer. Cells were washed with ice-cold Krebs-Ringer buffer, and then collected in 1 N NaOH. Radioactivity was determined by liquid scintillation counting. Nonspecific DA uptake observed in the presence of mazindol (10 μM) was subtracted.
Immunocytochemical Staining.
Immunostaining was performed as described previously (Liu et al., 2000a). In brief, 3.7% formaldehyde-fixed cells were treated with 1% hydrogen peroxide followed by sequential incubation with blocking solution, after which the cultures were incubated overnight at 4°C with primary anti-TH antibody (1:5000). Cells were incubated with biotinylated secondary antibody for 1 h followed by incubation with Vectastain avidin–biotin complex reagents for 40 min, and then color was developed with 3,3′-diaminobenzidine. For morphological analysis, the images were recorded with a charge-coupled device camera (Dage-MTI, Michigan City, IN) and operated with MetaMorph software (Molecular Devices, Sunnyvale, CA). For visual counting of TH-positive neurons, four representative areas per well of the 24-well plate were counted. In each condition, three wells were used for cell counting.
Nitrite, TNF-α, and IL-1β Assay.
The production of NO was accessed by measuring the accumulated levels of nitrite in the culture supernatants with Griess reagent. The release of TNFα and IL-1β was measured with immunosorbent assay kits from R&D Systems (Minneapolis, MN).
Real-Time Reverse Transcription-PCR.
Total RNA was isolated by using TRIzol reagent and purified with a RNeasy Kit. The primers were designed with ABI Primer Express software (Applied Biosystems, Foster City, CA). The sequences of the primers were: β-actin, GTATGACTCCACTCACGGCAAA (F), GGTCTCGCTCCTGGAAGATG (R); inducible NO synthase (iNOS), ACATCAGGTCGGCCATCACT (F), CGTACCGGATGAGCTGTGAATT (R); TNFα, GACCCTCACACTCAGATCATC-TTCT (F), CCTCCACTTGGTGGTTTGCT (R); and IL-1β, CTGGTGTGTGACGTTCCC-ATTA (F), CCGACAGCACGAGGCTTT (R). Total RNA was reverse-transcribed with murine leukemia virus reverse transcriptase and oligo(dT) primers. The SYBR Green PCR Master Mix was used for real-time PCR analysis. The relative differences in the expression of these inflammatory factors among groups were expressed by using cycle time values as follows: the cycle time values of the interested genes were first normalized with β-actin of the same sample, and then the relative difference between control and each treatment group was calculated and expressed as a relative reduction, setting the LPS at 100%.
Superoxide Assay.
The production of superoxide was accessed by measuring the SOD-inhibitable reduction of the tetrazolium salt WST-1. Primary microglia-enriched cultures in 96-well plates were washed twice with Hanks' balanced salt solution (HBSS) without phenol red. Cells were then incubated at 37°C for 30 min with vehicle control or compound A in HBSS (50 μl/well). Subsequently, 50 μl of HBSS with and without SOD (50 U/ml) was added to each well along with 50 μl of WST-1 (1 mM) in HBSS and 50 μl of vehicle or LPS (10 ng/ml). The absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices) every 5 min for 1 h. The different absorbance observed in the presence and absence of SOD was considered to be the amount of produced superoxide.
Intracellular ROS Assay.
Intracellular ROS were determined by using the DCFH-DA assay. Primary microglia-enriched cultures were seeded in a 96-well plate and then exposed to DCFH-DA for 1 h, followed by pretreatment with compound A for 30 min and then treatment with HBSS containing LPS. After incubation at 37°C for 30 min, the fluorescence was read at 485 nm for excitation and 530 nm for emission by using a SpectraMax Gemini XS fluorescence microplate reader (Molecular Devices).
Western Blot Analysis.
For subcellular fractions, primary microglia-enriched cultures were lysed in hypotonic lysis buffer incubated on ice for 30 min, and then subjected to homogenization. The lysates were loaded onto a sucrose gradient in lysis buffer and centrifuged at 1600g for 15 min. The supernatant above the sucrose gradient was used as the cytosolic fraction after centrifugation at 150,000g for 1 h. The pellet was solubilized in hypotonic lysis buffer and used as the membranous fraction. For extracting the whole cell lysates, microglia-enriched cultures were washed with cold phosphate-buffered saline and lysed with cell lysis buffer. The lysates were incubated on ice for 30 min and then centrifuged at 12,000g for 25 min. Protein levels were quantified by using BCA assay. Membranes were blocked with 5% nonfat milk and then incubated with the following primary antibodies: anti-β-actin, anti-iNOS, anti-phospho-p65, anti-p65, anti-phospho-IκBα, anti-IκBα, anti-phospho-IKK–β, anti-IKK-β, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-p38, anti-p38, anti-phospho-JNK, and anti-JNK at 1:1000 dilution (Cell Signaling Technology Inc., Danvers, MA); anti-p47PHOX at 1:1000 dilution (Millipore, Billerica, MA); anti-gp91PHOX at 1:1000 dilution (BD Biosciences, San Jose, CA); anti-Iba-1 at 1:1000 dilution (Wako Pure Chemicals, Tokyo, Japan), and horseradish peroxidase-conjugated secondary antibodies at 1:2500 dilution (GE Healthcare). The blots were developed with enhanced chemiluminescence reagent.
Immunohistochemistry and Cell Counting in the SN of Rat.
Rat brains were cut on a horizontal sliding microtome into 35-μm transverse free-floating sections. A total of 36 consecutive brain slices throughout the entire SN was collected, and every 6th section was processed for the immunocytochemical detection. DA neurons were recognized with an anti-TH antibody and microglia with OX-42 by using the avidin–biotin complex peroxidase method described previously. Digital images of TH-positive neurons and microglia in the SN were acquired on an Olympus microscope (Olympus, Tokyo, Japan) using an attached Polaroid digital microscope camera (Polaroid, Cambridge, MA). Quantification of TH-positive neurons was performed through visually counting the number of TH-positive neuronal cell bodies blindly by two investigators, and the results were obtained from the average. The mean value for the number of SN TH-positive neurons was then deduced by averaging the counts of six sections for each animal.
Statistical Analysis.
Data were expressed as mean ± S.E. Statistical significance was analyzed by one-way analysis of variance, followed by Bonferroni's post hoc t test. A value of p < 0.05 was considered statistically significant.
Results
Compound A Protected DA Neurons Against LPS-Induced Neurotoxicity.
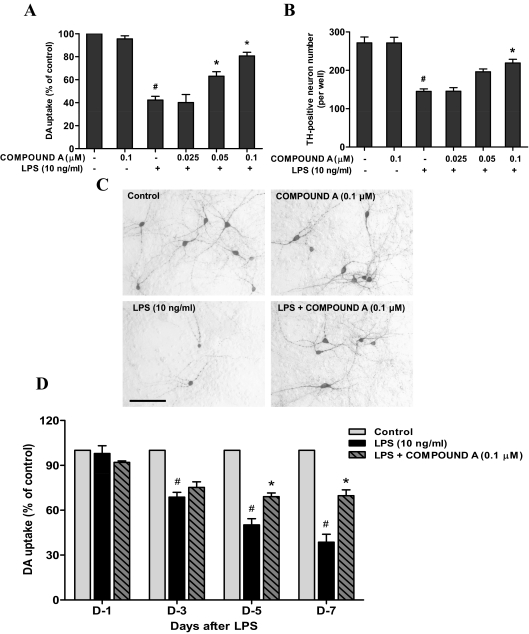
Mesencephalic neuron-glia cultures were used to investigate whether LPS-induced neurotoxicity was inhibited by compound A. The cultures were pretreated with compound A (0.025–0.1 μM) for 30 min followed by the application of LPS (10 ng/ml). Seven days later, degeneration of DA neurons was determined by [3H]DA uptake assay, immunocytochemical staining, and counting TH-positive neurons. The [3H]DA uptake assay indicated that LPS treatment reduced the capacity of the cultures to take up DA by approximately 60% compared with the vehicle-treated control cultures, and this reduction was prevented by compound A in a concentration-dependent manner (Fig. 1A). Consistent with results from the [3H]DA uptake assay, the neuroprotective effects of compound A on LPS-induced neurotoxicity were further confirmed by counting TH-positive neurons (Fig. 1B). From the morphological analysis, LPS treatment caused apparent damage in the neuritis of the remaining DA neurons. Pretreatment of compound A (0.1 μM), which by itself showed no effect, reversed LPS-induced neurotoxicity (Fig. 1C).
Fig. 1.
Compound A protected DA neurons against LPS-induced neurotoxicity. Rat primary mesencephalic neuron-glia cultures seeded in 24-well culture plates at 5 × 105/well were pretreated with various concentrations of compound A for 30 min before the addition of 10 ng/ml LPS. A and B, 7 days later, the LPS-induced DA neurotoxicity was quantified by the [3H]DA uptake assay (A) and TH-positive neuron counting using immunocytochemical analysis (B). C, representative images of immunostaining from three experiments are shown. Scale bar, 200 μm. For the time course study, the DA neurotoxicity was measured by [3H]DA uptake assay at 1, 3, 5 and 7 days after LPS treatment. Results are expressed as a percentage of the vehicle control cultures and are the mean ± S.E. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
To study the time course of compound A-elicited neuroprotrection, [3H]DA uptake assay was performed 1, 3, 5 and 7 days after LPS treatment. A clear time-dependent decrease of [3H]DA uptake was observed in LPS-treated cultures: 25, 50, and 60% at 3, 5, and 7 days after treatment, respectively. The progressive decrease in [3H]DA uptake was prevented by compound A pretreatment. Although the initial 20% decrease in [3H]DA uptake 3 days after LPS treatment was not restored by compound A, there was no further loss of [3H]DA uptake at days 5 and 7 in the compound A-pretreated group (Fig. 1D).
Microglia Were Indispensable to the Neuroprotective Effects of Compound A.
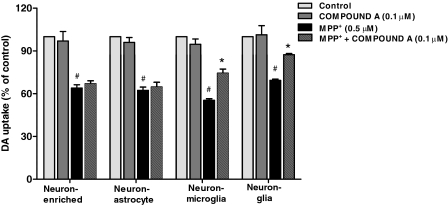
To determine which cell type mediated the neuroprotective effects of compound A, four types of cultures including neuron-glia, neuron-microglia, neuron-enriched, and neuron-astrocyte were prepared. These cultures were pretreated with compound A (0.1 μM) 30 min before 0.5 μM MPP+ application. MPP+, the active metabolite of MPTP, kills DA neurons directly; toxic factors such as α-synuclein released by these dying/dead neurons in turn contribute to reactive microgliosis and produce further neurotoxicity (Jackson-Lewis and Smeyne, 2005). In these four culture systems, MPP+ treatment reduced DA uptake by approximately 40% compared with the vehicle control cultures; the neurotoxicity was differentially attenuated by compound A. In neuron-glia cultures and neuron-microglia cultures, but not in neuron-enriched or neuron-astrocyte cultures, neuroprotection was detected. These results demonstrated that compound A-mediated neuroprotective effects on DA neurons were microglia-dependent (Fig. 2).
Fig. 2.
Microglia were indispensable to compound A-mediated neuroprotection. Four types of cultures prepared as described in Materials and Methods were pretreated with compound A (0.1 μM) for 30 min followed by the administration of MPP+ (0.5 μM). Seven days after MPP+ treatment, DA neurotoxicity was quantified by [3H]DA uptake assay. Results are expressed as a percentage of vehicle control cultures and are the mean ± S.E. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with MPP+-treated cultures.
Compound A Inhibited LPS-Induced Release of Proinflammatory Factors in Neuron-Glia Cultures.
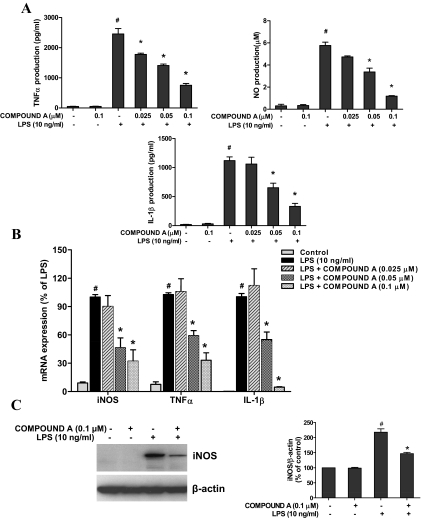
Neuroinflammation has been found to play an important role in the loss of DA neurons in PD animal models and patients. To investigate whether compound A was able to reduce the microglia-mediated neuroinflammation, levels of several major proinflammatory factors in supernatant and cells of neuron-glia cultures were determined. As various proinflammatory factors released from microglia vary in terms of time and quantities, different time points were adjusted for their measurement, such as TNFα at 3 to 6 h and NO and IL-1β at 1 day after LPS treatment. The results demonstrated that compound A significantly suppressed LPS-induced production of proinflammatory factors measured from the supernatant of neuron-glia cultures (Fig. 3A). Likewise, as shown by real-time RT-PCR (Fig. 3B), LPS (10 ng/ml) led to a notable increase in mRNA expression of iNOS, TNFα, and IL-1β in neuron-glia cultures, which was significantly inhibited by compound A. Western blot analysis for iNOS also showed that compound A (0.1 μM) attenuated LPS-induced increase of iNOS protein expression (Fig. 3C).
Fig. 3.
Compound A inhibited the production of proinflammatory factors by LPS-activated microglia. Neuron-glia cultures were pretreated with compound A (0.025–0.1 μM) for 30 min and then stimulated with 10 ng/ml LPS. A, after 3 h (TNFα) or 1 day (NO and IL-1β), an aliquot of the supernatant was collected for enzyme-linked immunosorbent assay analysis of TNFα and IL-1β and Griess reaction analysis of nitrite levels. For mRNA assessment, total RNA was harvested 1 h (TNFα) or 3 h (iNOS and IL-1β) after LPS treatment. B, the gene expressions were determined by real-time reverse transcription–PCR. C, Western blot analysis revealed iNOS protein expression 1 day after LPS treatment. Results are expressed as a percentage of the vehicle control cultures and are the mean ± S.E. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
Compound A Inhibited the Activation of NF-κB Signaling Pathway in LPS-Activated Microglia.
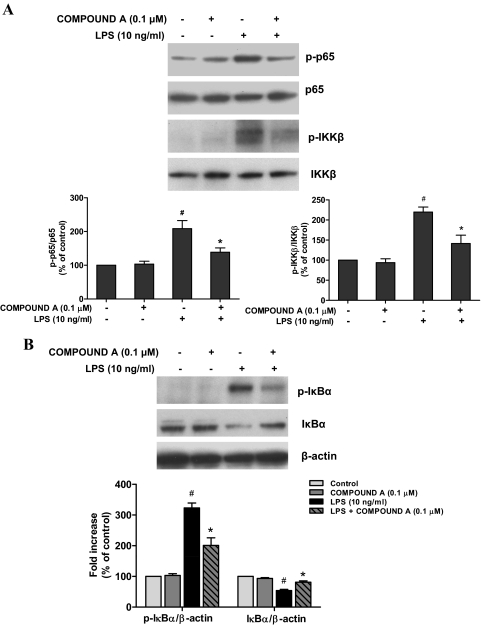
NF-κB is a key transcriptional factor for the gene expression of proinflammatory factors, such as iNOS and TNFα. Given the dramatic suppression of the production of these proinflammatory mediators in microglia by compound A (Fig. 3), we therefore investigated how compound A influences LPS-induced activation of NF-κB. Western blotting analysis revealed that pretreatment with 0.1 μM compound A significantly inhibited LPS-elicited p65 and IKK-β phosphorylation in the primary microglia-enriched cultures (Fig. 4A). Furthermore, LPS induced a rapid phosphorylation of cytosolic IκBα followed by the degradation of IκBα, and these effects were attenuated by the treatment of compound A (Fig. 4B).
Fig. 4.
Compound A inhibited NF-κB signaling pathway by Western blot analysis. Primary microglia-enriched cultures were pretreated with compound A (0.1 μM) for 30 min and then stimulated with LPS for 15 min. The levels of phosphorylated p65 and IKK-β compared with total p65 and IKK-β (A) and phosphorylated IκBα and total IκBα relative to β-actin (B) were investigated by Western blot analysis. Quantified results are expressed as a percentage of the vehicle control cultures and are the mean ± S.E. from three independent experiments. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
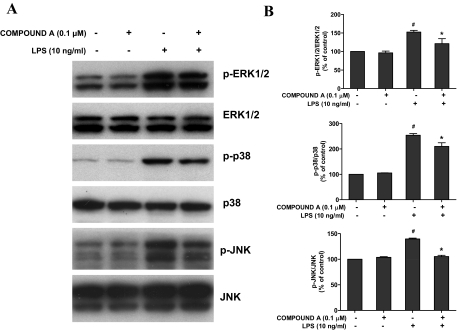
Compound A Suppressed LPS-Induced Phosphorylation of MAPKs in Microglia.
It is well known that the MAPK pathway, including ERK1/2, p38, and JNK, is involved in the regulation of immune responses (Bardwell, 2006). Therefore, we determined the effects of compound A on the phosphorylation of MAPKs induced by LPS. Primary microglia-enriched cultures were pretreated with 0.1 μM compound A for 30 min followed by LPS treatment for another 15 min. Results showed that compound A treatment inhibited LPS-induced phosphorylation of ERK1/2, p38 MAPK, and JNK in microglia (Fig. 5).
Fig. 5.
Compound A suppressed MAPK phosphorylation in activated microglia by Western blot analysis. Primary microglia-enriched cultures were pretreated with compound A (0.1 μM) for 30 min and then stimulated with LPS for 15 min. After the treatment, the whole cell protein was harvested. A, the levels of phosphorylated MAPKs (ERK1/2, p38, and JNK) compared with total MAPKs were investigated by Western blot analysis. B, autoradiographs were analyzed by densitometry. Quantified results are expressed as a percentage of the vehicle control cultures and are the mean ± S.E. from three independent experiments. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
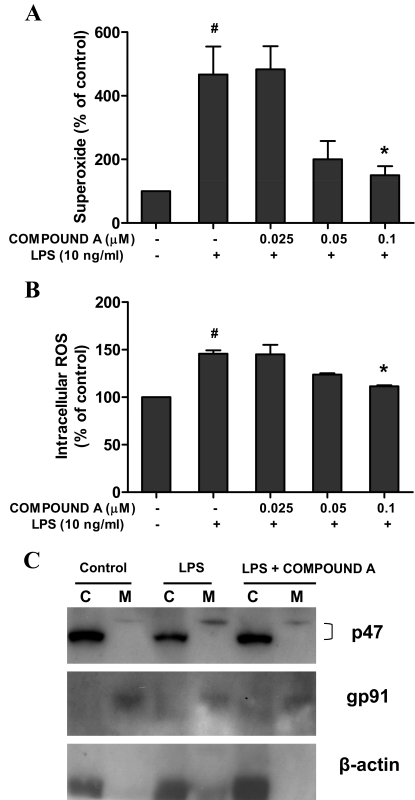
Compound A Attenuated LPS-Induced Production of ROS Through the Inhibition of NADPH Oxidase Activity.
Previous studies have found that ROS including extracellular superoxide and intracellular ROS produced by LPS-activated microglia appeared to be a pivotal effector of DA neurodegeneration (Gao and Hong, 2008). To examine whether compound A was capable of decreasing LPS-induced production of ROS, primary microglia-enriched cultures were used to measure the production of extracellular superoxide and intracellular ROS. The results showed a significant increase in the production of superoxide (Fig. 6A) and intracellular ROS (Fig. 6B) in microglia stimulated with LPS, which could be attenuated by the application of compound A.
Fig. 6.
Compound A attenuated LPS-induced production of ROS through the inhibition of NADPH oxidase. Primary microglia-enriched cultures were pretreated with compound A (0.1 μM) for 30 min followed by LPS treatment. A and B, the release of superoxide was determined immediately by measuring the SOD-inhibitable reduction of WST-1 (A), and the levels of intracellular ROS were detected with DCFH-DA (B). After LPS treatment for 15 min, subcellular fractions were isolated for Western blot analysis for p47PHOX levels in membrane and cytosolic fractions of microglia. Lanes C, cytosolic extract; M, membrane extract. C, β-actin and gp91PHOX were used as internal cytosolic and membrane controls, respectively. Results are expressed as a percentage of the vehicle control cultures and are the mean ± S.E. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
Because NADPH oxidase is the key enzyme required for the production of superoxide and intracellular ROS in activated immune cells, we then examined whether compound A could inhibit NADPH oxidase activity. The activation of NADPH oxidase requires translocation of phosphorylated cytosolic subunits (p47PHOX, p67PHOX, and p40PHOX) to the cell membrane and binding to cytochrome b558 composed of p22PHOX and gp91PHOX. Western blot assay indicated an increase in the immunoreactivity of p47PHOX in the membrane of microglia after LPS stimulation in primary microglia-enriched cultures, and this increase could be reduced by treatment with compound A (Fig. 6C). These results indicate that compound A decreased LPS-induced ROS production at least partly through preventing the translocation of p47PHOX from the cytosol to the membrane, thereby inhibiting NADPH oxidase activation after LPS treatment.
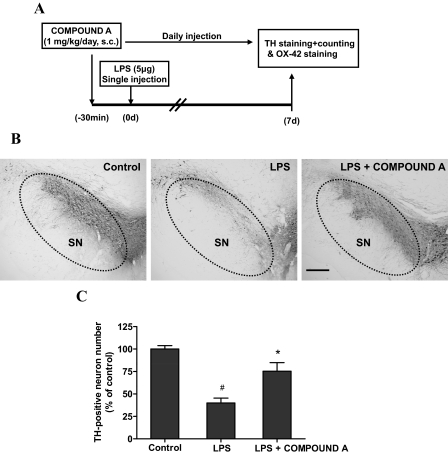
Compound A Attenuated LPS Injection-Induced DA Neuronal Loss and Microglia Activation in the SN.
After demonstrating neuroprotective effects of compound A in neuron-glia cultures, we extended our findings in the in vivo studies. Compound A (1 mg/kg/day s.c.) was administrated 30 min before a single stereotaxical injection of LPS (5 μg in 2 μl of saline) into the rat SN pars compacta. After seven daily injections of compound A, the brains were removed, sectioned, and processed for quantification of DA neurons by immunostaining using an anti-TH antibody. Injection of LPS led to a 60% loss of nigral TH-positive neurons compared with the vehicle control, whereas the loss of TH-neurons was only 20% in the compound A treatment group. Morphologically, injection of compound A significantly prevented the degeneration of TH-positive neuronal fibers in the SN induced by LPS (Fig. 7).
Fig. 7.
Compound A attenuated DA neuronal loss in the SN induced by LPS in vivo. A, the treatment schedule of LPS-intoxicated rats with compound A is shown. Rats were pretreated with compound A (1 mg/kg/day s.c.) before a single intranigral injection of LPS (5 μg) into the SN pars compacta on the right side of the rat brain. Seven days after LPS injection, brains were harvested and sectioned. B and C, the sections were immunostained with an anti-TH antibody (B) and the number of TH-positive neurons in the SN was counted (C). Scale bar, 200 μm. Results are expressed as a percentage of the TH-positive number of the vehicle control group and are the mean ± S.E. from six rats. #, p < 0.05 compared with control groups; *, p < 0.05 compared with LPS-treated groups.
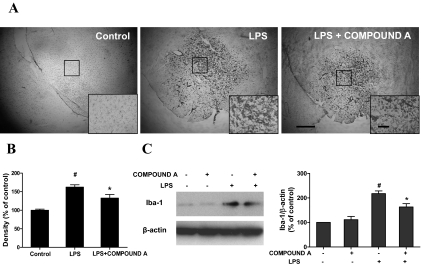
To further investigate the basis of these neuroprotective effects of compound A on LPS-induced neurodegeneration, we detected the ability of compound A to inhibit LPS-induced microglia activation. Seven days after LPS injection, the brain sections were immunostained with an antibody against OX-42. Representative photomicrographs of OX-42-immunoreactive microglia are shown in Fig. 8A. In the vehicle control sections, microglia exhibited a resting status with a ramified morphology. Upon activation with LPS injection, microglia were characterized by larger size, thicker processes, and more rounded cell body. Microglia activation and morphologic changes were significantly inhibited by the treatment of compound A. The quantification of the OX-42 staining revealed an approximate 30% reduction in the SN of rats injected with both LPS and compound A compared with rats injected with LPS alone (Fig. 8B). To further confirm the inhibitory effects of compound A on LPS-induced microglia activation, Western blot analysis was performed by using an antibody against Iba-1, another marker of microglia. As shown in Fig. 8C, compound A pretreatment obviously suppressed LPS-induced elevation of Iba-1 expression in primary neuron-glia cultures. These in vitro findings are in agreement with results obtained from the in vivo studies. Taking these data together, clear evidence indicates that compound A significantly inhibited the LPS-induced neuroinflammation.
Fig. 8.
Compound A inhibited LPS-induced microglial activation. Rats were treated with compound A (1 mg/kg/day s.c.) before the injection of LPS (5 μg). A and B, 7 days later, the brains were harvested, sectioned, and immunostained with OX-42 antibody. The images are representative of six rats in each group. Scale bar, 200 μm; inset, 100 μm. B, the quantification of the OX-42 staining. Densitometry analysis of nigral OX-42-positive microglia from six evenly spaced brain sections from each rat was performed with Image J software. Quantified results are expressed as a percentage of the control and are the mean ± S.E. from six rats. To further confirm the inhibitory effects of compound A on LPS-induced microglia activation, primary midbrain neuron-glia cultures were pretreated with compound A (0.1 μM) for 30 min followed by LPS (10 ng/ml) treatment. C, 7 days later the levels of Iba-1 were determined by Western blot analysis. Quantified results are expressed as a percentage of the vehicle control cultures and are the mean ± S.E. from three independent experiments. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
Discussion
Several lines of evidence presented in this study demonstrate that compound A provided significant neuroprotection on DA neurons against LPS-induced neurotoxicity through the inhibition of IKK-β and the suppression of proinflammatory factor production. In in vitro studies, compound A protected DA neurons against LPS-induced neurodegeneration in the mesencephalic neuron-glia cultures through inhibiting the production of several proinflammatory factors. In animal studies, compound A suppressed the LPS-induced activation of microglia and significantly attenuated the LPS-induced loss of nigral DA neurons. These findings suggest that anti-inflammatory properties underlie the neuroprotective effects of compound A.
Substantial reports have documented that neuroinflammatory mechanisms contribute to the cascades leading to neuronal degeneration (Gao and Hong, 2008). The innate immune surveillance in the central nervous system is mediated primarily by microglia. Once activated by immunological challenges, environmental stresses, and neuronal injuries, microglia are considered to be closely associated with neuronal damage, especially in neurodegenerative diseases (Ransohoff and Perry, 2009). In this study, we found that compound A protected DA neurons against LPS- and MPP+-induced neurodegeneration. LPS directly induces the activation of microglia and the release of proinflammatory factors and consequently produces DA neurotoxicity. In contrast, MPP+ directly damages DA neurons and causes the release of toxic substances, which in turn activate microglia, termed reactive microgliosis (Gao et al., 2003b). Either kind of microglial activation induced by LPS and MPP+ will further cause the DA neuronal loss in a “self-propelling” way and finally result in progressive DA neurodegeneration. However, without the presence of microglia, compound A failed to exhibit any neuroprotection on DA neurons, suggesting microglia participated in compound A-mediated neuroprotection. Based on these results, we concluded that compound A exerted neuroprotection against LPS-induced neurotoxicity through the inhibition of microglia activation.
Moreover, activated microglia induce neurodegeneration via the release of many proinflammatory factors, including cytokines such as TNFα and IL-1β, ROS, and reactive nitrogen species such as superoxide and NO. Analysis of postmortem brains indicated that microglia activation, elevated production of ROS, and proinflammatory factors such as TNFα, IL-1β, and NO have been discerned in the SN (McGeer et al., 1988). Therefore, inhibition of these proinflammatory factors can be beneficial for combating PD. In accordance with previous findings that compound A reduced the production of TNFα and IL-6 in a mouse model of acute ischemia–reperfusion injury (Moss et al., 2007), we found compound A decreased the production of TNFα, IL-1β, and NO (Fig. 3). These findings suggest that the inhibition of microglia activation and the decrease of proinflammatory factors contribute to compound A-mediated neuroprotection on DA neurons.
NF-κB is known to play an important role in regulating many genes involved in cell survival, immunity, and inflammation (Moynagh, 2005; Hoffmann and Baltimore, 2006). For instance, NF-κB activation suppresses TNFα-induced apoptosis in various cell types (Van Antwerp et al., 1996). NF-κB is broadly expressed in the central nervous system, including neurons and glia. Recently, increasing emphasis has been placed on the role of NF-κB in the innate immune system. NF-κB is the most important transcription factor in inflammatory responses by regulating the expression of various proinflammatory factors such as NO, TNFα, and IL-1β (Jana et al., 2001; Lee et al., 2004; Moynagh, 2005; Moriyama et al., 2006). Nurr1, an orphan nuclear receptor, exerts anti-inflammatory effects by docking to NF-κB-p65 on target inflammatory gene promoters, recruiting the CoREST corepressor complex, and subsequently resulting in clearance of NF-κB-p65 and transcriptional repression in astrocytes and microglia. By reducing NF-κB-mediated production of proinflammatory and neurotoxic factors, Nurr1 protects against loss of DA neurons in PD (Saijo et al., 2009). Moreover, several lines of evidence show that selective inhibition of NF-κB activation prevented DA neuronal loss against MPTP-intoxicated PD mice (Ghosh et al., 2007). Thus, attempts to develop drugs targeting NF-κB for the treatment of neurodegenerative diseases have become an area of great interest.
Given the marked inhibition of compound A on the production of several proinflammatory mediators in microglia (Fig. 3), we therefore investigated the effects of compound A on LPS-induced activation of NF-κB and pinpointed the targeting site responsible for neuroprotective effects of compound A on LPS- and MPTP-induced neurotoxicity. In resting conditions, NF-κB is sequestered in the cytoplasm by binding to its inhibitor IκB. In response to inflammatory stimuli, IκBs are rapidly phosphorylated and then degraded via the IKK complex, followed by the release and translocation of NF-κB dimers (p50 and p65) to the nucleus and thus regulating the expression of target genes. IKK complex is comprised of two catalytic subunits, IKK-α and IKK-β, and a regulatory subunit, IKK-γ/NEMO (Yamamoto and Gaynor, 2004). Despite high sequence homology and similar structural domains between the two catalytic subunits, IKK-β has a 20- to 50-fold higher level of kinase activity for IκB than does IKK-α (Li et al., 1999). IKK-β knockout mice have completely compromised the NF-κB pathway and die in utero of liver failure from massive hepatocyte apoptosis (Li et al., 1999). In addition, the conditional knockout of the IKK-β gene in myeloid-lineage cells, including microglia, reduced IKK activity in cultured primary microglia and blunted LPS-induced proinflammatory gene expression. Importantly, mice with deficient IKK-β genes are more resistant to kainic acid-induced hippocampal neuronal death and revealed decreased glial activation and expression of proinflammatory genes such as TNFα and IL-1β (Cho et al., 2008).
Compound A has been reported to specifically inhibit IKK-β to suppress NF-κB signaling pathway and provide cardioprotection (Ziegelbauer et al., 2005; Moss et al., 2007, 2008). In the present study, we found that compound A significantly suppressed LPS-elicited phosphorylation of p65 and IKK-β in the primary microglia-enriched cultures (Fig. 4A) and stabilized the inhibitor IκB (Fig. 4B), which in turn will prevent the nuclear translocation of the NF-κB p50–p65 complex.
In addition to IKK-β inhibition, compound A suppressed LPS-induced phosphorylation of MAPKs including ERK1/2, p38 MAPK, and JNK in microglia (Fig. 5). Based on previous work from Ziegelbauer et al. (2005), compound A inhibited human recombinant IKK-β with a Ki of 2 nM for ATP and 4 nM for the substrate GST-IkBα, whereas it inhibited IKK-α at higher concentrations (Ki for ATP: 135 nM), but did not inhibit (IC50 > 10 μM) MAPK kinase 4, MAPK kinase 7, ERK-1, phosphoinositide 3-kinase γ, protein kinase A, and protein kinase C (Ziegelbauer et al., 2005). Therefore, the observed inhibition of MAPK by compound A in this study may be secondary to its suppression on NF-κB. Certainly, there is close cross-talk among key inflammatory signaling pathways during inflammation. For example, TNFα, an important downstream product of NF-κB activation, can activate ERK1/2 in keratinocytes during epidermal inflammation or induce a more than 10-fold increase in p38 MAPK phosphorylation in neutrophil (Lokuta and Huttenlocher, 2005).
We also observed the unexpected inhibition of compound A on NADPH oxidase (Fig. 6), a key superoxide-generating enzyme during inflammation in various immune cells such as microglia, macrophages, and neutrophils (Gao et al., 2002). The treatment of compound A suppressed LPS-induced p47PHOX translocation from cytosol to the cell membrane and led to the reduction in ROS production from microglia (Fig. 6). There was overwhelming evidence indicating that ROS can induce the activation of MAPK cascades and NF-κB pathway in activated microglia (Ramanan et al., 2008). On the other hand, patients with deficiency in NEMO, the regulatory subunit of the IKK complex, have impaired activation of neutrophil NADPH oxidase (Singh et al., 2009). Currently, it is not clear whether compound A at the concentration used in this study directly interacts with one or more subunits of NADPH oxidase to affect its activation. Further studies are warranted to define precise mechanisms underlying the inhibition of compound A on NADPH oxidase.
Taken together, the inhibitory effects of compound A on LPS-induced activation of NF-κB pathways are responsible mainly for the neuroprotection of compound A. The inhibition, either direct or indirect, of compound A on NADPH oxidase partly contributes to the neuroprotective effect of compound A, which reinforces the critical role of oxidative damage in inflammation-mediated neurodegeneration. In summary, our studies not only indicate an important contribution of NF-κB-mediated inflammatory process in PD neurodegeneration, but also imply that modulation of the NF-κB pathway by using drugs such as compound A may potentially better treat PD patients.
This work was supported by The Michael J. Fox Foundation for Parkinson's Research and the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.165829.
- PD
- Parkinson's disease
- DA
- dopamine
- SN
- substantia nigra
- ROS
- reactive oxygen species
- ERK
- extracellular signal-regulated kinase
- LPS
- lipopolysaccharide
- NF-κB
- nuclear factor-κB
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NO
- nitric oxide
- iNOS
- inducible NO synthase
- MAPK
- mitogen-activated protein kinase
- MPP+
- 1-methyl-4-phenylpyridinium
- DCFH-DA
- dichlorodihydrofluorescein diacetate
- SOD
- superoxide dismutase
- TH
- tyrosine hydroxylase
- OX-42
- anti-CR3 complement receptor antibody
- PCR
- polymerase chain reaction
- TNF
- tumor necrosis factor
- IL
- interleukin
- HBSS
- Hanks' balanced salt solution
- JNK
- c-Jun N-terminal kinase
- IκB
- inhibitor of κB
- IKK
- IκB kinase
- Iba-1
- ionized calcium-binding adapter molecule-1
- PHOX
- phagocytic oxidase
- compound A
- 7-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-5-[(3S)-3-piperidinyl]-1,4-dihydro-2H-pyrido[2,3-d][1,3]oxazin-2-one hydrochloride.
References
- Bardwell, 2006.Bardwell L. (2006) Mechanisms of MAPK signaling specificity. Biochem Soc Trans 34:837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho et al., 2008.Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, et al. (2008) Role of microglial IKKβ in kainic acid-induced hippocampal neuronal cell death. Brain 131:3019–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao and Hong, 2008.Gao HM, Hong JS. (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al., 2002.Gao HM, Hong JS, Zhang W, Liu B. (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al., 2003a.Gao HM, Liu B, Zhang W, Hong JS. (2003a) Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci 24:395–401 [DOI] [PubMed] [Google Scholar]
- Gao et al., 2003b.Gao HM, Liu B, Zhang W, Hong JS. (2003b) Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. FASEB J 17:1954–1956 [DOI] [PubMed] [Google Scholar]
- Ghosh et al., 2007.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. (2007) Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci USA 104:18754–18759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch and Hunot, 2009.Hirsch EC, Hunot S. (2009) Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 8:382–397 [DOI] [PubMed] [Google Scholar]
- Hoffmann and Baltimore, 2006.Hoffmann A, Baltimore D. (2006) Circuitry of nuclear factor κB signaling. Immunol Rev 210:171–186 [DOI] [PubMed] [Google Scholar]
- Hunot et al., 1997.Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. (1997) Nuclear translocation of NF-κB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci USA 94:7531–7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis and Smeyne, 2005.Jackson-Lewis V, Smeyne RJ. (2005) MPTP and SNpc DA neuronal vulnerability: role of dopamine, superoxide, and nitric oxide in neurotoxicity. Mini-review. Neurotox Res 7:193–202 [DOI] [PubMed] [Google Scholar]
- Jana et al., 2001.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. (2001) Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem 276:44527–44533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin, 1999.Karin M. (1999) How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18:6867–6874 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2004.Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ. (2004) Spinal NF-κB activation induces COX-2 up-regulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci 19:3375–3381 [DOI] [PubMed] [Google Scholar]
- Li et al., 1999.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. (1999) The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med 189:1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2000a.Liu B, Du L, Hong JS. (2000a) Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther 293:607–617 [PubMed] [Google Scholar]
- Liu et al., 2000b.Liu B, Jiang JW, Wilson BC, Du L, Yang SN, Wang JY, Wu GC, Cao XD, Hong JS. (2000b) Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J Pharmacol Exp Ther 295:125–132 [PubMed] [Google Scholar]
- Lokuta and Huttenlocher, 2005.Lokuta MA, Huttenlocher A. (2005) TNF-α promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J Leukoc Biol 78:210–219 [DOI] [PubMed] [Google Scholar]
- McGeer et al., 1988.McGeer PL, Itagaki S, Boyes BE, McGeer EG. (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38:1285–1291 [DOI] [PubMed] [Google Scholar]
- Moriyama et al., 2006.Moriyama N, Taniguchi M, Miyano K, Miyoshi M, Watanabe T. (2006) ANP inhibits LPS-induced stimulation of rat microglial cells by suppressing NF-κB and AP-1 activations. Biochem Biophys Res Commun 350:322–328 [DOI] [PubMed] [Google Scholar]
- Moss et al., 2007.Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. (2007) IKKβ inhibition attenuates myocardial injury and dysfunction following acute ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 293:H2248–H2253 [DOI] [PubMed] [Google Scholar]
- Moss et al., 2008.Moss NC, Tang RH, Willis M, Stansfield WE, Baldwin AS, Selzman CH. (2008) Inhibitory κB kinase-β is a target for specific nuclear factor κB-mediated delayed cardioprotection. J Thorac Cardiovasc Surg 136:1274–1279 [DOI] [PubMed] [Google Scholar]
- Moynagh, 2005.Moynagh PN. (2005) The NF-κB pathway. J Cell Sci 118:4589–4592 [DOI] [PubMed] [Google Scholar]
- Nagatsu and Sawada, 2006.Nagatsu T, Sawada M. (2006) Cellular and molecular mechanisms of Parkinson's disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol 26:781–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan et al., 2008.Ramanan S, Kooshki M, Zhao W, Hsu FC, Robbins ME. (2008) PPARα ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-κB and AP-1 pathways. Free Radic Biol Med 45:1695–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff and Perry, 2009.Ransohoff RM, Perry VH. (2009) Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27:119–145 [DOI] [PubMed] [Google Scholar]
- Saijo et al., 2009.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh et al., 2009.Singh A, Zarember KA, Kuhns DB, Gallin JI. (2009) Impaired priming and activation of the neutrophil NADPH oxidase in patients with IRAK4 or NEMO deficiency. J Immunol 182:6410–6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp et al., 1996.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. (1996) Suppression of TNF-α-induced apoptosis by NF-κB. Science 274:787–789 [DOI] [PubMed] [Google Scholar]
- Werner et al., 2008.Werner SL, Kearns JD, Zadorozhnaya V, Lynch C, O'Dea E, Boldin MP, Ma A, Baltimore D, Hoffmann A. (2008) Encoding NF-κB temporal control in response to TNF: distinct roles for the negative regulators IκBα and A20. Genes Dev 22:2093–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing et al., 2007.Xing B, Liu M, Bing G. (2007) Neuroprotection with pioglitazone against LPS insult on dopaminergic neurons may be associated with its inhibition of NF-κB and JNK activation and suppression of COX-2 activity. J Neuroimmunol 192:89–98 [DOI] [PubMed] [Google Scholar]
- Yamamoto and Gaynor, 2004.Yamamoto Y, Gaynor RB. (2004) IκB kinases: key regulators of the NF-κB pathway. Trends Biochem Sci 29:72–79 [DOI] [PubMed] [Google Scholar]
- Zhang and Ghosh, 2001.Zhang G, Ghosh S. (2001) Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest 107:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2006.Zhang W, Shin EJ, Wang T, Lee PH, Pang H, Wie MB, Kim WK, Kim SJ, Huang WH, Wang Y, et al. (2006) 3-Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro. FASEB J 20:2496–2511 [DOI] [PubMed] [Google Scholar]
- Ziegelbauer et al., 2005.Ziegelbauer K, Gantner F, Lukacs NW, Berlin A, Fuchikami K, Niki T, Sakai K, Inbe H, Takeshita K, Ishimori M, et al. (2005) A selective novel low-molecular-weight inhibitor of IκB kinase-β (IKK-β) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol 145:178–192 [DOI] [PMC free article] [PubMed] [Google Scholar]