Abstract
Ethanol enhancement of 5-hydroxytryptamine (5-HT)3A receptor-mediated responses may have important consequences in the intoxicating and addictive properties of ethanol. Although the exact mechanism is unknown, ethanol-mediated enhancement of 5-HT3 receptor current has been proposed to occur due to stabilization of the open-channel state. It has not been possible to directly measure the open state of the channel due to the extremely low single-channel conductance of 5-HT3A channels. Recently, three arginine residues within the large intracellular loop of the 5-HT3A subunit were substituted by their equivalent residues (glutamine, aspartate, and alanine) of the 5-HT3B subunit to produce a 5-HT3A(QDA) subunit that forms functional homomeric channels exhibiting a measurable single-channel conductance. Using whole-cell rapid-agonist application techniques and the cell-attached single-channel recording configuration, we examined human 5-HT3A(QDA) receptors expressed in human embryonic kidney 293 cells. The agonist sensitivity, macroscopic kinetics, and modulation by ethanol were similar between mutant and wild-type channels, suggesting the substitutions had not altered these channel structure-function properties. The open time histogram for single-channel events mediated by 5-HT3A(QDA) receptors in the presence of maximal 5-HT was best fit by three exponentials, but in the presence of ethanol a fourth open state was evident. In summary, the QDA substitution greatly enhanced single-channel conductance with little effect on 5-HT3A channel's kinetic properties and ethanol enhances agonist action on 5-HT3A receptors by inducing a new, long-lived open-channel state. Furthermore, the 5-HT3A(QDA) receptor appears to be suitable for pharmacological studies of 5-HT3A receptor modulation at a single-channel level.
The 5-hydroxytryptamine (serotonin; 5-HT) type 3 receptor is a member of the Cys-loop ligand-gated ion channel superfamily that includes the nicotinic acetylcholine, GABAA, glycine, and zinc-activated channel ion channels (Davies et al., 2003). Five genes encoding for individual subunits (5-HT3A–E) have been identified within the human genome. However, homomeric 5-HT3A receptors appear to be the dominant receptor in the brain, particularly the rodent brain (Jensen et al., 2008).
5-HT3 receptors are ligand-gated ion channels that play important roles in substance abuse, emesis, inflammatory pain, spinal nociception, and cardiovascular reflexes (Thompson and Lummis, 2007). Activation of 5-HT3 receptors enhances the release of the neurotransmitters dopamine and GABA, which are believed to be the principal neurotransmitters for addiction and intoxication, respectively (McBride et al., 2004). The role of 5-HT3 receptors in the neuronal circuitry of drug dependence has been known for some time (Grant, 1995). Within the mesolimbic dopaminergic system, 5-HT3 receptors located on ventral tegmental area and nucleus accumbens neurons influence dopamine release leading to an enhancement of the reward pathway (De Deurwaerdère et al., 1998). Furthermore, 5-HT3 receptor-mediated dopamine release in these areas is enhanced by ethanol (Campbell et al., 1996). Antagonists to 5-HT3 receptors have been shown to effectively reduce drinking behavior in both rodent and human studies. Recently, ondansetron, a selective 5-HT3 receptor antagonist has been used in clinical studies to reduce the drinking behavior in adolescents and early onset alcoholics (Dawes et al., 2005).
Alcohols and volatile anesthetics of low molecular size enhance 5-HT3A-mediated current amplitudes elicited by low concentrations of 5-HT but are substantially less effective at 5-HT3AB receptors (Hayrapetyan et al., 2005; Stevens et al., 2005a,b). It has been proposed that these compounds stabilize the open state of 5-HT3A channels (Zhou and Lovinger, 1996; Zhou et al., 1998; Lovinger et al., 2000; Solt et al., 2005). However, this hypothesis could never be directly measured due to the small single-channel conductance of the 5-HT3A receptor. This complication can be overcome by using a modified 5-HT3A receptor that has a large single-channel conductance (Kelley et al., 2003; Peters et al., 2004). In this high-conducting 5-HT3A channel, three arginine residues within the membrane-associated helices of the intracellular loop between transmembrane (TM)3 and TM4 have been substituted by the equivalent residues of the 5-HT3B subunit; glutamine (R432Q), aspartate (R436D), and alanine (R440A). The resultant 5-HT3A(QDA) receptor has a single-channel conductance of ∼37 pS, which is 40-fold greater than the wild-type 5-HT3A receptor (Hales et al., 2006; Deeb et al., 2007). However, these studies did not examine the effects of these mutations on channel kinetics. Recent studies on other mutant 5-HT3A receptors have shown that mutant receptors can have different channel kinetic properties compared with wild type and that these differences complicate the interpretation of pharmacological data (Hu et al., 2006). If the QDA mutations do not affect channel kinetics, this 5-HT3A(QDA) receptor has the potential to test the hypothesis that ethanol stabilizes the open conformation of the 5-HT3A channel at a single-channel level.
Here, we assess the properties of the 5-HT3A(QDA) receptor to determine its usefulness in single-channel pharmacological experiments. To demonstrate this potential, we have examined modulation by ethanol at macroscopic and single-channel levels. We determined that mutant 5-HT3A(QDA) and wild-type 5-HT3A receptors have similar 5-HT and alcohol sensitivities and resolve, for the first time, ethanol modulation of 5-HT3 receptors on a single-channel scale.
Materials and Methods
Cell Culture and Transfection
Human embryonic kidney (HEK)293 cells were cultured in media made up of Dulbecco's modified Eagle's medium supplemented with 10% calf serum and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere. Cells were transfected, using the calcium phosphate precipitation method, with cDNA encoding either the 5-HT3A subunit (in pCDM8) or 5-HT3A(QDA) subunit (in GW1) along with green fluorescent protein cDNAs (in pCDM8). Cells were used 24 to 72 h after transfection. Successful transfection of the cells was determined by fluorescence microscopy to identify green fluorescent protein-labeled cells.
Electrophysiology
Macroscopic Currents.
Culture medium was replaced by an extracellular solution that was superfused into the recording chamber at a rate of 5 ml/min. The extracellular solution consisted of 140 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.0 mM glucose, and 10 mM HEPES (pH 7.4 with NaOH). Patch electrodes were filled with intracellular recording solution consisting of 140 mM KCl, 2.0 mM MgCl2, 11 mM EGTA, and 10 mM HEPES (pH 7.4 with KOH), giving a patch electrode resistance of 2 to 4 MΩ. Currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 1 to 2 kHz, and digitized at 10 to 20 kHz with a Digidata 1320 A/D converter (Molecular Devices). For macroscopic current kinetic studies, 5-HT was rapidly applied to cells via a piezo-driven pipette that achieves whole-cell solution exchange times of 1 to 2 ms as measured using the approach described by Li et al., 2000. For alcohol modulation of 5-HT-mediated currents, 5-HT (100 μM) was applied by pressure (70-kPa) ejection (Picospritzer II; General Valve, Fairfield, NJ) from modified patch pipettes for a duration sufficient to evoke ∼10% of the maximal 5-HT (100 μM)-evoked current amplitude. Experiments were performed at room temperature with the cells voltage-clamped at −60 mV.
Single-Channel Recordings.
Cell-attached patches were formed on HEK293 cells expressing 5-HT3A(QDA) receptors. The cell-attached patch configuration allows for a stable, long-lasting patch to be exposed to a constant concentration of agonist and modulator. Cells were bathed in an extracellular recording solution consisting of 140 mM KCl, 4.7 mM NaCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.0 mM glucose 11.0, and 10 mM HEPES (pH 7.4 with NaOH). Sylgard-coated electrodes were filled with extracellular solution containing 5-HT (100 μM) with or without ethanol (25 mM). Single-channel currents were low passed filtered at 2 to 5 kHz and digitized at 40 kHz.
Data Analysis
For analysis of the 5-HT concentration-response relationship, all currents were normalized to the average maximal current elicited by 100 μM 5-HT immediately before and after each measurement. Normalized data were plotted as mean ± S.D. The 5-HT concentration-response curves were fitted to the following Hill equation: I = Imax/[1 + (EC50/[5-HT])n], where I is the peak current at a certain concentration of 5-HT, Imax is the maximum 5-HT-evoked current, EC50 is the concentration of 5-HT that elicits 50% of the maximal response, and n is the Hill coefficient.
The activation phase of whole-cell macroscopic current (from 10 to 90% of peak current amplitude) was fitted by a single exponential function. Measurements of time constants for deactivation and desensitization were determined by fitting the decay of currents (90–10% of peak current amplitude) to exponential functions using the Levenberg-Marquardt algorithm with least-squares minimization (Clampfit version 9.2; Molecular Devices). The number of exponentials that best described the decay of currents was determined from an F-test. Most decay components were best fit to two exponentials, but some were fitted by one or more than two exponentials. To allow for comparisons, a weighted time constant (ΣτnAn/ΣAn) was used.
Single-channel currents were analyzed using the single-channel analysis software in Clampfit 9.2 (Molecular Devices). Records were leak subtracted before analysis. All-points histograms were fitted with multiple Gaussians (least-squares minimization) to determine the unitary current amplitudes. The slope conductance was established by determining the unitary current amplitudes from patches were the pipette potential ranged from 40 to 100 mV.
Single-channel events were detected using the 50% threshold detection method (Colquhoun and Sigworth, 1995). From the single-channel events list, histograms of channel open dwell time distributions were plotted and fitted using a maximum likelihood procedure with correction for missed events (Colquhoun and Sakmann, 1985). The minimal number of exponential components required to fit the distribution was determined by chi-square statistics. A dead time of 90 μs was imposed on the fitting routine. Mean open durations were calculated, from the open dwell time fitted components, as a weighted mean from the open durations and proportions of each component (Krzywkowski et al., 2008).
Data were analyzed post hoc using Prism version 4 software (GraphPad Software Inc., San Diego, CA). Statistical analysis was performed by using an unpaired Student's t test with statistical significance set at p < 0.05.
Drugs and Chemicals
5-Hydroxytryptamine (serotonin) was purchased from Sigma-Aldrich (St. Louis, MO). Ethanol was purchased from American Bioanalytical (Natick, MA).
Results
Agonistic Profiles of 5-HT3A(QDA) Receptors Are Similar to Wild-Type 5-HT3A Receptors.
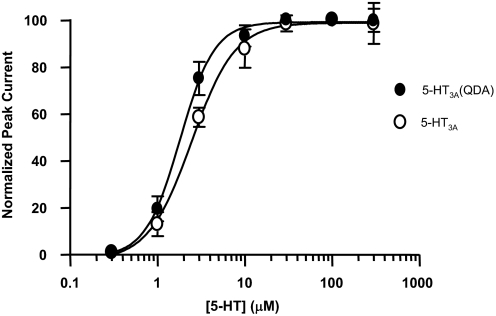
Serotonin (5-HT) elicited a concentration-dependent inward current when applied to HEK293 cells expressing 5-HT3A and 5-HT3A(QDA) receptors (Fig. 1). A fit of the peak current data to a Hill equation yielded EC50 values of 2.5 ± 0.13 and 1.8 ± 0.08 μM for 5-HT3A and 5-HT3A(QDA) receptors, respectively, with Hill coefficients of 1.8 ± 0.16 and 2.2 ± 0.16, respectively (3–8 cells).
Fig. 1.
Serotonin sensitivity of 5-HT3A(QDA) receptors is similar to wild-type 5-HT3A receptors. 5-HT concentration-response relationship for wild-type 5-HT3A receptors (open circles) and 5-HT3A(QDA) receptors (closed circles). Data fit to a Hill equation. For EC50 and Hill coefficient values, see text. Data are from three to eight cells.
Because of the higher single-channel conductance of 5-HT3A(QDA) channels, it is presumed that the 5-HT-evoked currents will be larger than that evoked from 5-HT3A channels. When the whole-cell current densities of the maximally evoked 5-HT response (100 μM) were examined, there was a more than 2-fold increase in whole-cell current density for the QDA containing receptors: 150 ± 22 and 355 ± 67 pA/pF (p = 0.01; n = 9) for 5-HT3A and 5-HT3A(QDA) receptors, respectively.
Macroscopic Kinetics of 5-HT3A(QDA) Receptors Is Similar to Wild-Type 5-HT3A Receptors.
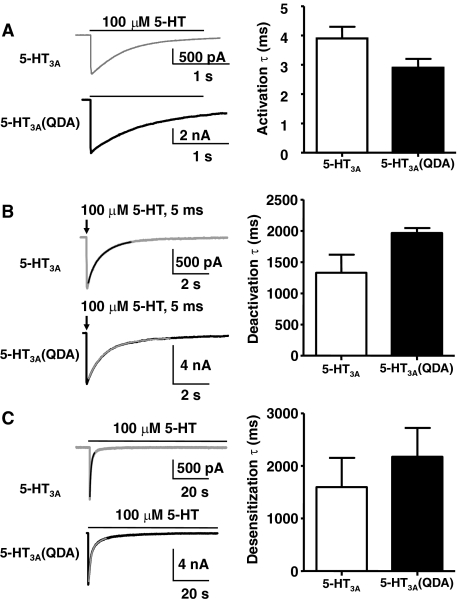
Receptors were activated by rapidly applying 5-HT using a piezo-driven capillary pipette. Figure 2A (left) shows typical currents evoked by the rapid application of 100 μM 5-HT to either 5-HT3A (Fig. 2A, top) or 5-HT3A(QDA) receptors (Fig. 2A, bottom) expressed in HEK293 cells. There was no significant difference in the time for activation between the receptor types at maximal (100 μM) concentrations of 5-HT (Fig. 2A, right). Time constants for receptor activation, at 100 μM 5-HT, were 3.9 ± 0.4 ms (n = 7) for wild-type 5-HT3A receptors and 2.9 ± 0.3 ms (n = 6) for mutant 5-HT3A(QDA) receptors (t test, p = 0.08).
Fig. 2.
The macroscopic kinetics of the 5-HT3A(QDA) receptors are similar to that wild-type 5-HT3A receptors. A, QDA substitutions minimally affect macroscopic current recordings. Left, current traces produced by activation of wild-type 5-HT3A receptors (top; gray trace) and by 5-HT3A(QDA) receptors (bottom; black trace). Currents were evoked by rapidly (solution exchange time, ∼1–2 ms) applying 100 μM 5-HT to HEK293 cells expressing the indicated receptor. Right, mean activation time constants (data are mean ± S.E.M. from three to four cells). B, deactivation. A 5-ms pulse of 100 μM 5-HT was rapidly applied to HEK293 cells expressing 5-HT3A receptors (gray trace) and 5-HT3A(QDA) receptors (black trace). The current decay (between 90 and 10% of maximal current amplitude) immediately after the agonist pulse was fit to an exponential equation; the best fit is shown as a color-contrasted line. The two receptors had similar time constants for deactivation. Data are mean ± S.E.M. from three to four cells. C, desensitization. An exponential fit of the current decay recorded during prolonged exposure to 100 μM 5-HT rapidly applied to HEK293 cells expressing 5-HT3A receptors (gray trace) and 5-HT3A(QDA) receptors (black trace). The color-contrasted line during the decay phase is the exponential fit. No difference to the mean desensitization time constant was detected. Data are mean ± S.E.M. from three cells.
To determine deactivation characteristics of the receptors, a 5-ms pulse of 100 μM 5-HT was rapidly applied to HEK293 cells expressing either 5-HT3A or 5-HT3A(QDA) receptors. The current decay upon agonist termination was fit with an exponential function. The deactivation time constant of 5-HT3A(QDA) receptors was 1900 ± 80 ms (n = 3) compared with 1300 ± 290 ms (n = 4) for wild-type 5-HT3A receptors (t test, p = 0.11; Fig. 2B).
An exponential fit of the current decay recorded during continued exposure to 100 μM 5-HT resulted in a time constant for desensitization of 2100 ± 550 ms (n = 3) for 5-HT3A(QDA) receptors compared with 1600 ± 550 ms (n = 3) for wild-type 5-HT3A receptors (t test, p = 0.5; Fig. 2C).
QDA Mutations Do Not Alter Ethanol Action on 5-HT3A Receptors.
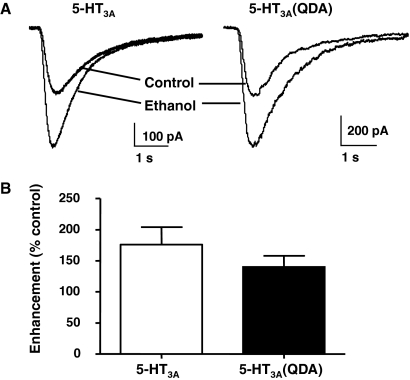
To test whether the QDA mutations alter the action of ethanol on 5-HT3A receptors, we measured the effect of an intoxicating concentration of ethanol on the 5-HT-mediated current that was approximately 10% of the maximal response. The application of 25 mM ethanol by itself did not elicit any current from cells expressing either 5-HT3A receptors or 5-HT3A(QDA) receptors. Figure 3 demonstrates that ethanol similarly enhanced currents mediated by wild-type versus QDA mutant 5-HT3A receptors. At 25 mM, ethanol reversibly increased currents mediated by wild-type 5-HT3A receptors by 165 ± 34% and those by 5-HT3A(QDA) receptors by 139 ± 28% (p = 0.66).
Fig. 3.
Alcohol enhancement of 5-HT3A and 5-HT3A(QDA) receptors. A, representative current recordings showing enhancement of submaximal 5-HT-evoked current amplitudes by 25 mM ethanol. 5-HT3A and 5-HT3A(QDA) receptors were pre-equilibrated with buffer or ethanol and then activated with an EC10 response. B, mean enhancements of wild-type 5-HT3A receptor- (open bars) and mutant 5-HT3A(QDA) receptor (closed bars)-mediated current amplitudes by 25 mM ethanol. Data are mean ± S.E.M. from three to five cells.
Ethanol Does Not Modulate the Single-Channel Conductance of 5-HT3A Receptors Containing the QDA Mutation.
Single-channel currents mediated by 5-HT3A(QDA) receptors expressed in HEK293 cells were recorded using the cell-attached configuration of the patch-clamp technique, where the pipette solution contained 5-HT in the absence or presence of ethanol (25 mM). We used a concentration of 5-HT (100 μM) that in our macroscopic experiments resulted in maximal agonist-evoked current.
Single-channel currents were initially analyzed by creating all-points amplitude histograms and fitting the distribution with multiple Gaussians (least-squares minimization) to determine the unitary current amplitude. Single-channel events were clearly detected in 5-HT3A(QDA) receptor-expressing cells, which is in marked contrast to our previous recordings from cells expressing 5-HT3A receptors where no single-channel events were detected upon 5-HT application (Davies et al., 1999). To investigate whether ethanol modulated single-channel conductance, we determined unitary current amplitudes from patches held at different membrane potentials (−40 to −80 mV) in the absence and presence of ethanol (25 mM). The slope conductance of 5-HT3A(QDA) channels was similar in the absence (28 ± 11 pS; n = 3) and presence (27 ± 2 pS; n = 3) of ethanol (data not shown).
Ethanol Increases the Open State of the 5-HT3A(QDA) Channel.
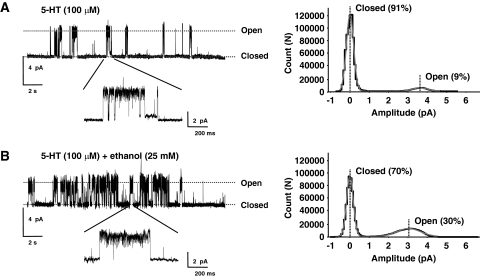
In all patches examined, distinct bursts of channel activity were clearly evident that were terminated by long periods of closure that are thought to represent entry into the desensitized state. A substantial increase in single-channel activity was observed in the presence of ethanol (25 mM) with bursts forming clusters. Figure 4 shows 20-s sweeps of exemplar single-channel activity from two cell-attached patches in the absence and presence of ethanol (25 mM).
Fig. 4.
Ethanol increases the number of open 5-HT3A(QDA) channel events and increases the open probability. Left, cell-attached recordings of 5-HT3A(QDA) receptor-mediated currents evoked by 100 μM 5-HT in the absence (A) or presence (B) of 25 mM ethanol. Twenty-second examples of single-channel activity with an expanded view of a burst. Dotted lines indicating closed and open states. Right, all-points histograms for the 20-s cell-attached recordings of 5-HT3A(QDA) receptor-mediated currents evoked by 100 μM 5-HT shown in the left panel. Histograms were fitted by two Gaussians (gray lines). Percentages of events in the closed and open states are shown.
The ethanol-mediated increase in single-channel activity was not accompanied by a change in unitary current amplitude. At a predicted membrane potential of −80 mV, the unitary current amplitudes evoked by 100 μM 5-HT in the absence and presence of ethanol were 3.1 ± 0.8 and 2.5 ± 0.2 pA, respectively (three patches).
Ethanol Modulates the Channel Open Time Distribution.
Changes to the 5-HT3A(QDA) channel open state were further characterized by examining the open time distribution. Single-channel events were analyzed by a maximal likelihood minimization procedure to fit exponentials to the distribution of open times. The minimal number of exponentials required to fit the distribution was determined by Chi-square statistics.
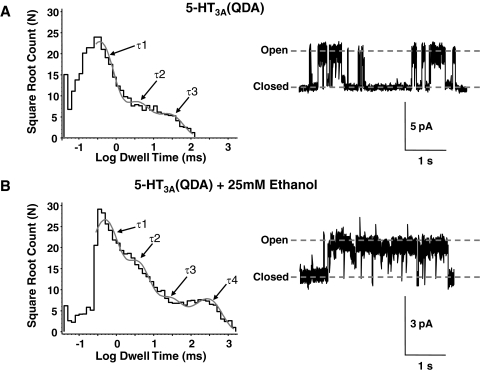
The open time histogram generated from single-channel events mediated by 5-HT3A(QDA) receptors in the presence of 100 μM 5-HT was best fit by three exponentials (Fig. 5B). The three resulting time constants (τ1–3) represent channel open times of brief, medium and long-lasting durations (Table 1).
Fig. 5.
Ethanol induces a fourth open state distinguished by its long open time. Cell-attached recordings of 5-HT3A(QDA) receptor-mediated currents evoked by 100 μM 5-HT (right) in the absence (A) and presence (B) of 25 mM ethanol. Pipette potential was 80 mV. Exponential fits to channel open time histograms (left), data pooled from four patches, reveal three open states in the absence and four open states in the presence of ethanol (gray lines). Resolution of fits was limited to twice the rise time of the filter (0.3 ms).
TABLE 1.
Ethanol evokes a fourth open state
Open times (milliseconds) and their corresponding proportions (in parentheses, percentage) for 5-HT3A(QDA) receptor-mediated currents evoked by 100 μ M 5-HT in the absence and presence of 25 mM ethanol. For each condition, data were collected and pooled from four cell-attached patches at a predicted membrane potential of −80 mV.
| τ1 | τ2 | τ3 | τ4 | |
|---|---|---|---|---|
| 100 μM 5-HT | 0.3 ± 0.05 (63 ± 2) | 2.8 ± 0.13 (20 ± 2) | 24.3 ± 0.11 (17 ± 1) | |
| 100 μM 5-HT + 25 mM ethanol | 0.4 ± 0.1 (42 ± 2) | 2.9 ± 0.1 (29 ± 2) | 20 ± 0.2 (13 ± 1) | 273 ± 0.1 (16 ± 1) |
In the presence of ethanol (25 mM), a fourth open state (τ4) was evident (Fig. 5B). Its open time was 11-fold longer than even the longest open state in ethanol's absence. In addition, the proportion of channels with brief openings was greatly reduced by 25 mM ethanol (Table 1). These changes resulted in the mean open duration of 5-HT3A(QDA) receptors (4.8 ms) to be increased approximately 10-fold in the presence of ethanol (47 ms).
Discussion
We have shown, for the first time, the modulation of single-channel 5-HT3 receptors by ethanol. This was achieved by using a high-conducting mutant subunit that has minimal affect upon the apparent affinity of the receptor for 5-HT, the same kinetic properties compared with wild-type and maintains macroscopic ethanol sensitivity. It is well documented that macroscopic currents mediated by homomeric 5-HT3A receptors are modulated by alcohols both in recombinant expressed receptors and naturally occurring receptors in neuroblastoma cells (Machu and Harris, 1994; Downie et al., 1995; Jenkins et al., 1996; Zhou and Lovinger, 1996; Zhou et al., 1998; Stevens et al., 2005a).
We have shown that the small halogenated volatile anesthetics chloroform and halothane enhanced currents evoked by saturating concentrations of dopamine (a partial agonist at the 5-HT3 receptor) without affecting the dopamine concentration-response relationship (Solt et al., 2005). These data imply that channel gating is enhanced but agonist binding affinity is unaltered. A similar conclusion was drawn by Lovinger et al. (2000) examining ethanol modulation of dopamine activated rodent 5-HT3A receptors.
The increase in channel gating by alcohols strongly suggests that the open state of the channel has been stabilized. However, due to the low single-channel conductance of the 5-HT3A receptor, the direct observation of single open channels is not possible. For this study, we have used a previously described triple mutation in which three arginines in the intracellular loop between TM3 and TM4 of the 5-HT3A subunit have been replaced with equivalent residues from the 5-HT3B subunit (R432Q, R436D, and R440A). The mutant homomeric receptor [5-HT3A(QDA)] has a large measurable single-channel conductance (Kelley et al., 2003; Hales et al., 2006). Although single-channel activity can now be recorded from 5-HT3A-like receptors a potential problem is that such residue substitutions could alter pharmacological and kinetic properties compared with wild-type receptors.
The 5-HT3A(QDA) receptor had a slightly left-shifted 5-HT concentration-response relationship compared with the wild-type 5-HT3A receptors. In addition to similar 5-HT sensitivity, we observed no differences in the rates of activation, deactivation, and desensitization between mutant and wild-type 5-HT3A receptors, indicating that agonist affinity and channel gating have not been drastically altered by the mutations. In support of our observations, a recent study of the nicotinic α7/5-HT3A chimera demonstrated that the QDA substitutions did not alter the affinity of acetylcholine (Rayes et al., 2005).
Data from whole-cell macroscopic recordings have previously shown that enhancement of 5-HT3A receptor-mediated current amplitude by alcohols is dependent upon the concentration of agonist (Zhou et al., 1998). At high concentrations of a highly efficacious agonist (5-HT), alcohols tend to minimally modulate current amplitude (Lovinger and White, 1991; Machu and Harris, 1994; Stevens et al., 2005a,b), but they do slow desensitization (Zhou et al., 1998; Hu et al., 2006). Our data explain these macroscopic observations on a microscopic scale. In our single-channel recordings with maximal agonist concentration, we see three open states in control but an additional, prolonged fourth open event in the presence of ethanol. At saturating 5-HT concentrations, open channels close by entering a period of desensitization, and in the presence of ethanol the extra prolonged open state appears to come at the expense of desensitization because the briefer open states do not change in duration between the absence and presence of ethanol (Table 1). This prolongation of the open state of the channel at the expense of the desensitized state is in agreement with the prolonged desensitization effect of ethanol upon macroscopic currents.
Using the cell-attached patch method, we have examined ethanol effects at steady state that favors the transitions between the open and desensitized states. Ethanol may increase open-channel duration by increasing the activation rate and/or slowing the rate at which the channel deactivates. Having established the usefulness of the 5-HT3A(QDA) receptor in examining the pharmacological properties of 5-HT3A receptors at a single-channel level, future experiments will examine the effects of ethanol upon activation and deactivation kinetics.
The modulation of 5-HT3 receptors by ethanol has a significant role in the plasticity of pathways mediating alcohol addiction and intoxication. Activation of 5-HT3 receptors enhances the release of the neurotransmitters dopamine and GABA, which are thought to be the primary mediators for addiction and intoxication, respectively (McBride et al., 2004). Presynaptic 5-HT3 receptors are known to cause release of GABA from some inhibitory GABAergic interneurons in the amygdala (Koyama et al., 2000), cortex (Zhou and Hablitz, 1999; Puig et al., 2004), and hippocampus (McMahon and Kauer, 1997; Turner et al., 2004), resulting in an increased inhibition of postsynaptic neurons. Such increased GABA release by 5-HT3 receptor activation in the hippocampus is a potential mechanism to explain alcohol-induced memory impairment. The hippocampal tonic current, mediated by extrasynaptic GABAA receptors, has been suggested to be enhanced by ethanol (Choi et al., 2008; Liang et al., 2008; but see Borghese et al., 2006). Consequently, ethanol enhancement of presynaptic 5-HT3-mediated GABA release could result in more GABA release and thus an increase both phasic (synaptic) and tonic (extrasynaptic) inhibition. The tonic inhibition could be further enhanced by the presence of ethanol.
The role of 5-HT3 receptors in the neuronal circuitry of drug dependence has been known for some time (Grant, 1995). Within the mesolimbic dopaminergic system, 5-HT3 receptors located on the ventral tegmental area and nucleus accumbens neurons have been shown to control dopamine release leading to an enhancement of the reward pathway (De Deurwaerdère et al., 1998). Furthermore, 5-HT3 receptor-mediated dopamine release in these areas is enhanced by ethanol (Campbell et al., 1996). By stabilizing the open state of the 5-HT3A channel, ethanol would allow an increase in calcium permeating through the channel, enhancing the calcium cascade that result in increased neurotransmitter release.
Macroscopic currents mediated by heteromeric 5-HT3AB receptors have reduced sensitivity to alcohols compared with homomeric 5-HT3A receptors (Hayrapetyan et al., 2005; Stevens et al., 2005b). A recent study found an association with a variant within HTR3B, the gene encoding for 5-HT3B subunit, and subjects diagnosed for alcohol use disorder with antisocial behavior (Ducci et al., 2009). Alcoholism with antisocial behavior is a characteristic of early onset alcoholics, a subset of alcoholics with which the 5-HT3 receptor antagonist ondansetron was shown in several clinical trials to be effective in reducing the craving for alcohol; reducing the number of drinking episodes (Dawes et al., 2005); and reducing symptoms of anxiety, depression, and hostility in early onset alcoholics (Johnson et al., 2003). The intronic variant found within HTR3B may result in reduced expression of the 5-HT3B subunit and hence an increase in the number of ethanol-sensitive 5-HT3A receptors. This illustrates the need to better understand the interaction between ethanol and 5-HT3A receptors so that novel therapies can be developed to prevent such interactions.
In addition to its treatment for addiction, antagonists of the 5-HT3 receptor are frequently used to treat emesis and irritable bowel syndromes. Modulators of 5-HT3 receptors have also been suggested to have potential therapeutic promise for schizophrenia, anxiety, cognition, and nociception (Thompson and Lummis, 2007). Having demonstrated that 5-HT3A(QDA) receptors closely resemble 5-HT3A receptors except for the ability to have distinguishable single-channel currents makes 5-HT3A(QDA) receptors a valuable recombinant channel to determine the properties of potential new therapeutic agents at a molecular level.
In summary, the QDA mutations within the membrane-associated stretch of the intracellular loop between transmembrane domains III and IV form receptors with high single-channel conductance but with wild-type kinetic properties and alcohol sensitivity. We have, for the first time, directly shown that ethanol stabilizes the open state of 5-HT3A channels.
Acknowledgments
We are grateful to Dr. Timothy Hales for providing the cDNAs for the 5-HT3A(QDA) receptor and to Drs. Timothy Hales, Tarek Deeb, Keith Miller, Stuart Forman, and Douglas Raines for helpful discussions.
This work was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant P01-GM58448]; the National Institutes of Health National Institute of Alcoholism and Alcohol Abuse [Grant R21-AA017938]; and by the Department of Anesthesia and Critical Care, Massachusetts General Hospital (Boston, MA).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.164863.
- 5-HT
- 5-hydroxytryptamine
- TM
- transmembrane
- HEK
- human embryonic kidney.
References
- Borghese et al., 2006.Borghese CM, Stórustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. (2006) The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther 316:1360–1368 [DOI] [PubMed] [Google Scholar]
- Campbell et al., 1996.Campbell AD, Kohl RR, McBride WJ. (1996) Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol 13:569–574 [DOI] [PubMed] [Google Scholar]
- Choi et al., 2008.Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, et al. (2008) Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci 28:11890–11899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun and Sakmann, 1985.Colquhoun D, Sakmann B. (1985) Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol 369:501–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun and Sigworth, 1995.Colquhoun D, Sigworth FJ. (1995) Fitting and statistical analysis of single-channel records, in Single-Channel Recording (Sakmann B, Neher E. eds) pp 483–587 Plenum, New York [Google Scholar]
- Davies et al., 1999.Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. (1999) The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397:359–363 [DOI] [PubMed] [Google Scholar]
- Davies et al., 2003.Davies PA, Wang W, Hales TG, Kirkness EF. (2003) A novel class of ligand-gated ion channel is activated by Zn2+. J Biol Chem 278:712–717 [DOI] [PubMed] [Google Scholar]
- Dawes et al., 2005.Dawes MA, Johnson BA, Ma JZ, Ait-Daoud N, Thomas SE, Cornelius JR. (2005) Reductions in and relations between “craving” and drinking in a prospective, open-label trial of ondansetron in adolescents with alcohol dependence. Addict Behav 30:1630–1637 [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère et al., 1998.De Deurwaerdère P, Stinus L, Spampinato U. (1998) Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: role of 5-HT3 receptors. J Neurosci 18:6528–6538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb et al., 2007.Deeb TZ, Carland JE, Cooper MA, Livesey MR, Lambert JJ, Peters JA, Hales TG. (2007) Dynamic modification of a mutant cytoplasmic cysteine residue modulates the conductance of the human 5-HT3A receptor. J Biol Chem 282:6172–6182 [DOI] [PubMed] [Google Scholar]
- Downie et al., 1995.Downie DL, Hope AG, Belelli D, Lambert JJ, Peters JA, Bentley KR, Steward LJ, Chen CY, Barnes NM. (1995) The interaction of trichloroethanol with murine recombinant 5-HT3 receptors. Br J Pharmacol 114:1641–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci et al., 2009.Ducci F, Enoch MA, Yuan Q, Shen PH, White KV, Hodgkinson C, Albaugh B, Virkkunen M, Goldman D. (2009) HTR3B is associated with alcoholism with antisocial behavior and alpha EEG power–an intermediate phenotype for alcoholism and co-morbid behaviors. Alcohol 43:73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins et al., 1996.Jenkins A, Franks NP, Lieb WR. (1996) Actions of general anaesthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol 117:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al., 2003.Johnson BA, Ait-Daoud N, Ma JZ, Wang Y. (2003) Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res 27:1773–1779 [DOI] [PubMed] [Google Scholar]
- Grant, 1995.Grant KA. (1995) The role of 5-HT3 receptors in drug dependence. Drug Alcohol Depend 38:155–171 [DOI] [PubMed] [Google Scholar]
- Hales et al., 2006.Hales TG, Deeb TZ, Tang H, Bollan KA, King DP, Johnson SJ, Connolly CN. (2006) An asymmetric contribution to gamma-aminobutyric type A receptor function of a conserved lysine within TM2–3 of alpha1, beta2, and gamma2 subunits. J Biol Chem 281:17034–17043 [DOI] [PubMed] [Google Scholar]
- Hayrapetyan et al., 2005.Hayrapetyan V, Jenschke M, Dillon GH, Machu TK. (2005) Co-expression of the 5-HT(3B) subunit with the 5-HT(3A) receptor reduces alcohol sensitivity. Brain Res Mol Brain Res 142:146–150 [DOI] [PubMed] [Google Scholar]
- Hu et al., 2006.Hu XQ, Hayrapetyan V, Gadhiya JJ, Rhubottom HE, Lovinger DM, Machu TK. (2006) Mutations of L293 in transmembrane two of the mouse 5-hydroxytryptamine3A receptor alter gating and alcohol modulatory actions. Br J Pharmacol 148:88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen et al., 2008.Jensen AA, Davies PA, Bräuner-Osborne H, Krzywkowski K. (2008) 3B but which 3B and that's just one of the questions: the heterogeneity of human 5-HT3 receptors. Trends Pharmacol Sci 29:437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley et al., 2003.Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, Peters JA. (2003) A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 424:321–324 [DOI] [PubMed] [Google Scholar]
- Koyama et al., 2000.Koyama S, Matsumoto N, Kubo C, Akaike N. (2000) Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J Physiol 529:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywkowski et al., 2008.Krzywkowski K, Davies PA, Feinberg-Zadek PL, Bräuner-Osborne H, Jensen AA. (2008) High-frequency HTR3B variant associated with major depression dramatically augments the signaling of the human 5-HT3AB receptor. Proc Natl Acad Sci USA 105:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al., 2000.Li X, Czajkowski C, Pearce RA. (2000) Rapid and direct modulation of GABAA receptors by halothane. Anesthesiology 92:1366–1375 [DOI] [PubMed] [Google Scholar]
- Liang et al., 2008.Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. (2008) Functional consequences of GABAA receptor alpha 4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res 32:19–26 [DOI] [PubMed] [Google Scholar]
- Lovinger et al., 2000.Lovinger DM, Sung KW, Zhou Q. (2000) Ethanol and trichloroethanol alter gating of 5-HT3 receptor-channels in NCB-20 neuroblastoma cells. Neuropharmacology 39:561–570 [DOI] [PubMed] [Google Scholar]
- Lovinger and White, 1991.Lovinger DM, White G. (1991) Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol 40:263–270 [PubMed] [Google Scholar]
- Machu and Harris, 1994.Machu TK, Harris RA. (1994) Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther 271:898–905 [PubMed] [Google Scholar]
- McBride et al., 2004.McBride WJ, Lovinger DM, Machu T, Thielen RJ, Rodd ZA, Murphy JM, Roache JD, Johnson BA. (2004) Serotonin-3 receptors in the actions of alcohol, alcohol reinforcement, and alcoholism. Alcohol Clin Exp Res 28:257–267 [DOI] [PubMed] [Google Scholar]
- McMahon and Kauer, 1997.McMahon LL, Kauer JA. (1997) Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol 78:2493–2502 [DOI] [PubMed] [Google Scholar]
- Peters et al., 2004.Peters JA, Kelley SP, Dunlop JI, Kirkness EF, Hales TG, Lambert JJ. (2004) The 5-hydroxytryptamine type 3 (5-HT3) receptor reveals a novel determinant of single-channel conductance. Biochem Soc Trans 32:547–552 [DOI] [PubMed] [Google Scholar]
- Puig et al., 2004.Puig MV, Santana N, Celada P, Mengod G, Artigas F. (2004) In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb Cortex 14:1365–1375 [DOI] [PubMed] [Google Scholar]
- Rayes et al., 2005.Rayes D, Spitzmaul G, Sine SM, Bouzat C. (2005) Single-channel kinetic analysis of chimeric α7–5HT3A receptors. Mol Pharmacol 68:1475–1483 [DOI] [PubMed] [Google Scholar]
- Solt et al., 2005.Solt K, Stevens RJ, Davies PA, Raines DE. (2005) General anesthetic-induced channel gating enhancement of 5-hydroxytryptamine type 3 receptors depends on receptor subunit composition. J Pharmacol Exp Ther 315:771–776 [DOI] [PubMed] [Google Scholar]
- Stevens et al., 2005a.Stevens RJ, Rüsch D, Davies PA, Raines DE. (2005a) Molecular properties important for inhaled anesthetic action on human 5-HT3A receptors. Anesth Analg 100:1696–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens et al., 2005b.Stevens R, Rüsch D, Solt K, Raines DE, Davies PA. (2005b) Modulation of human 5-hydroxytryptamine type 3AB receptors by volatile anesthetics and N-alcohols. J Pharmacol Exp Ther 314:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson and Lummis, 2007.Thompson AJ, Lummis SC. (2007) The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets 11:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner et al., 2004.Turner TJ, Mokler DJ, Luebke JI. (2004) Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience 129:703–718 [DOI] [PubMed] [Google Scholar]
- Zhou and Hablitz, 1999.Zhou FM, Hablitz JJ. (1999) Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol 82:2989–2999 [DOI] [PubMed] [Google Scholar]
- Zhou and Lovinger, 1996.Zhou Q, Lovinger DM. (1996) Pharmacologic characteristics of potentiation of 5-HT3 receptors by alcohols and diethyl ether in NCB-20 neuroblastoma cells. J Pharmacol Exp Ther 278:732–740 [PubMed] [Google Scholar]
- Zhou et al., 1998.Zhou Q, Verdoorn TA, Lovinger DM. (1998) Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol 507:335–352 [DOI] [PMC free article] [PubMed] [Google Scholar]