Abstract
Hepatic ischemia reperfusion (IR) injury causes acute kidney injury (AKI). However, the contribution of AKI to the pathogenesis of liver IR injury is unclear. Furthermore, controversy still exists regarding the role of A1 adenosine receptors (A1ARs) in AKI. In this study, we determined whether exogenous and endogenous A1AR activation protects against AKI with subsequent liver protection after hepatic IR in mice. We found that after hepatic IR A1 knockout (KO) mice and A1AR antagonist-treated A1 wild-type (WT) mice developed worse AKI and liver injury compared with vehicle-treated A1WT mice. Moreover, a selective A1AR agonist protected against hepatic IR-induced AKI and liver injury in A1WT mice. Renal A1AR-mediated kidney protection plays a crucial role in protecting the liver after IR because: 1) selective unilateral renal lentiviral overexpression of human A1ARs [enhanced green fluorescent protein (EGFP)-huA1AR] in A1KO mice protected against both kidney and liver injury sustained after liver IR, 2) removal of the EGFP-huA1AR lentivirus-injected kidney from A1KO mice abolished both renal and hepatic protection after liver IR, and 3) bilateral nephrectomy before hepatic ischemia abolished the protective effects of A1AR activation in A1WT mice. Finally, inhibition of Akt, but not extracellular signal-regulated kinase mitogen-activated protein kinase, prevented the kidney and liver protection afforded by A1AR agonist treatment. Taken together, we show that endogenous and exogenous activation of renal A1ARs protect against liver and kidney injury after liver IR in vivo via pathways involving Akt activation.
Hepatic ischemia reperfusion (IR) is a frequent cause of acute liver failure during the perioperative period and occurs frequently after major liver resection or liver transplantation (Davis et al., 2002; Lee et al., 2009). Acute kidney injury (AKI) is common in patients who sustain hepatic IR injury, and the development of AKI in addition to liver injury greatly increases mortality and morbidity during the perioperative period (Davis et al., 2002). We recently developed a murine model of liver IR-induced AKI characterized by early renal endothelial cell death and severe renal vascular impairment with subsequent renal inflammation caused by cytokine and neutrophil infiltration, filamentous (F)-actin degradation, and proximal tubular necrosis (Lee et al., 2009).
Our laboratory also previously demonstrated that exogenous and endogenous A1 adenosine receptor (AR) activation protected against direct renal IR injury in vivo (Lee and Emala, 2000, 2001; Lee et al., 2004; Joo et al., 2007). We also demonstrated that selective renal expression of human A1ARs (huA1ARs) via lentiviral gene delivery attenuated renal IR injury in mice lacking A1ARs (Kim et al., 2009). Furthermore, we showed that A1AR activation can modulate liver IR injury in mice (Kim et al., 2008). However, other investigators have reported that a nonselective AR antagonist (theophylline) or selective A1AR antagonists [8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 8-(noradamantan-3-yl)-1,3-dipropylxanthine (KW-3902)] improved renal function, urine output, and renal hemodynamics against direct renal injury induced by insults such as cisplatin, gentamicin, or glycerol (Bowmer et al., 1986; Heidemann et al., 1989; Kellett et al., 1989; Yao et al., 1994). Therefore, the role of A1ARs in renal injury remains controversial, and furthermore it is in unknown whether A1ARs protect against renal injury induced by remote liver injury after liver IR. In addition, it is unclear whether the renal-protective effect of renal A1AR activation directly contributes to the reduction of liver injury after hepatic IR.
Activation of A1ARs in renal proximal tubule cells and vascular endothelial cells initiates several cytoprotective kinase signaling cascades including extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) and Akt (Joo et al., 2007). Because ERK MAPK and Akt signaling pathways are known to protect against endothelial cell apoptosis (Buckley et al., 1999, Kennedy et al., 1999) and hepatic IR-induced AKI directly causes renal endothelial cell apoptosis with subsequent vascular dysfunction and neutrophil infiltration (Lee et al., 2009), we hypothesized that the A1AR-mediated activation of ERK MAPK and Akt signaling pathways may protect against renal endothelial cell apoptosis and reduce AKI after liver IR.
In this study, we sought to further elucidate the role of renal A1AR activation in attenuating renal and hepatic injury caused by hepatic IR. We used A1AR knockout (A1KO) mice in addition to the pharmacological manipulation of A1ARs with a selective agonist and a selective antagonist in A1AR wild-type (A1WT) mice. We also achieved selective renal expression of huA1ARs in the kidneys of A1KO mice. We tested the following hypotheses: 1) genetic deletion or pharmacologic blockade of A1ARs in mice would exacerbate AKI after hepatic IR, 2) preischemic activation of A1ARs would protect against AKI after hepatic IR in A1WT mice, and 3) A1AR-mediated protection against hepatic IR-induced AKI is via activation of pre-existing cytoprotective kinases including ERK MAPK and Akt. We also tested the hypothesis that renal protection with A1AR activation is directly responsible for the hepatic protection after liver IR via two approaches: 1) we bilaterally nephrectomized mice to determine whether the hepatic protection with A1AR agonist treatment is attenuated or eliminated in these cohorts of mice and 2) we determined whether selective renal expression of huA1ARs in A1KO mice would reduce both kidney and liver injury after liver IR.
Materials and Methods
Detailed methods describing mice, surgery and anesthesia protocols, immunohistochemistry, and RNA isolation are available in Supplemental Data.
Murine Model of Hepatic IR.
After Columbia University Institutional Animal Care and Use Committee approval, male A1WT or A1KO mice (20–25 g) were subjected to partial 60-min liver IR as described previously (Kim et al., 2008; Lee et al., 2009). To determine the role of exogenous manipulations of A1ARs in hepatic IR injury, some mice were treated with a single dose of a selective A1AR agonist, 2-chloro-N6-cyclopentyladenosine (CCPA; 0.1 mg/kg i.p.), or a selective A1AR antagonist, DPCPX (0.4 mg/kg i.p.), 15 min before hepatic ischemia. CCPA and DPCPX were dissolved first in dimethyl sulfoxide and then further diluted in saline for a final dimethyl sulfoxide concentration of approximately 0.5%. Sham-operated mice were treated with vehicle, CCPA, or DPCPX and subjected to laparotomy and identical liver manipulations without vascular occlusion. Five and 24 h after reperfusion, plasma was collected for the measurement of creatinine and alanine aminotransferase (ALT). In separate cohorts of mice, kidneys were collected at 5 h after reperfusion to measure the expression of proinflammatory mRNA induction and vascular permeability, and they were collected at 24 h after reperfusion to measure vascular permeability, neutrophil infiltration, apoptosis, and histological evaluation of renal tubular injury as described below and in Supplemental Data.
In a separate cohort of A1WT mice, we removed both kidneys before liver ischemia to determine whether renal A1AR activation is directly responsible for reducing liver and kidney injury after liver IR. Preliminary studies demonstrated that mice subjected to bilateral nephrectomy and 60 min of liver ischemia had significantly worse liver injury with high mortality. These findings support the hypothesis that impaired or lack of renal function increases hepatic injury in mice. Therefore, in binphrectomized mice, we reduced the hepatic ischemia time to 45 min.
Intrarenal Lentivirus Delivery in Vivo in A1KO Mice.
Generation of lentivirus encoding EGFP or EGFP-huA1AR and in vivo transduction have been described previously (Kim et al., 2009) (see Supplemental Data). We used three techniques to detect the expression of EGFP or EGFP-huA1AR in the kidney and liver after intrarenal injection of lentivirus: 1) direct visualization of EGFP in frozen sections, 2) immunohistochemistry for huA1ARs, and 3) reverse transcription-polymerase chain reaction (RT-PCR) (Table 1) for the EGFP-A1AR transgene in the liver and kidney tissues as described previously (Kim et al., 2009). Two days after intrarenal injection of lentivirus encoding EGFP (100 μl) or EGFP-huA1AR (20 or 100 μl) into the left kidney of A1KO mice, we induced liver IR injury. In some mice, we removed the EGFP or EGFP-huA1AR lentivirus-injected left kidney before liver ischemia to determine whether the EGFP-huA1AR-overexpressing kidneys are directly responsible for reducing liver and kidney injury after liver IRI.
TABLE 1.
RT-PCR primers used in this study
| Gene | Species | Amplicon Size | Primer Sequences (Sense/Antisense) | Annealing | Cycle Number |
|---|---|---|---|---|---|
| bp | °C | ||||
| GAPDH | Mouse | 450 | 5′-ACCACAGTCCATGCCATCAC-3′ | 65 | 15 |
| 5′-CACCACCCTGTTGCTGTAGCC-3′ | |||||
| A1AR | Mouse/human | 340 | 5′-CATTGGGCCACAGACCTACT-3′ | 60 | 22 |
| 5′-GAAGTAGACCATGTACTCCA-3′ | |||||
| TNF-α | Mouse | 290 | 5′-TACTGAACTTCGGGGTGATTGGTCC-3′ | 65 | 24 |
| 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ | |||||
| ICAM-1 | Mouse | 409 | 5′-TGTTTCCTGCCTCTGAAGC-3′ | 60 | 21 |
| 5′-CTTCGTTTGTGATCCTCCG-3′ | |||||
| KC | Mouse | 202 | 5′-CAATGAGCTGCGCTGTCAGTG-3′ | 60 | 26 |
| 5′-CTTGGGGACACCTTTTAGCATC-3′ | |||||
| MCP-1 | Mouse | 312 | 5′-ACCTGCTGCTACTCATTCAC-3′ | 60 | 22 |
| 5′-TTGAGGTGGTTGTGGAAAAG-3′ | |||||
| MIP-2 | Mouse | 282 | 5′-CCAAGGGTTGACTTCAAGAAC-3′ | 60 | 28 |
| 5′-AGCGAGGCACATCAGGTACG-3′ |
Plasma ALT Activity and Creatinine Level.
The plasma ALT activities were measured by using the Infinity ALT assay kit according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA). Plasma creatinine was measured by an enzymatic creatinine reagent kit according to the manufacturer's instructions (Thermo Fisher Scientific). This method of creatinine measurement largely eliminates the interference from mouse plasma chromagens well known to the Jaffe method (Slot, 1965).
Enzyme-Linked Immunosorbent Assay for Plasma TNF-α and IL-6 after Liver IR.
Twenty-four hours after liver reperfusion, the plasma tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) levels were measured with mouse-specific enzyme-linked immunosorbent assay kits according to the manufacturer's instructions (eBioscience, San Diego, CA).
Histological Analysis of Renal Injury.
For histological preparations, kidney tissues were fixed in 10% formalin solution overnight. After automated dehydration through a graded alcohol series, transverse kidney slices were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin. Renal hematoxylin-eosin sections were evaluated for the severity of renal proximal tubule injury in the cortico-medullary junction by counting the number of hypereosinophilic (necrotic) cells in 100× fields by an experienced pathologist (V.D.D.), who was blinded to the treatment each animal had received, as described previously (Lee et al., 2009).
Assessment of Kidney Inflammation.
Kidney inflammation was determined by the detection of neutrophil infiltration by immunohistochemistry 24 h after hepatic IR and the measurement of mRNA-encoding markers of inflammation, including keratinocyte-derived cytokine (KC), intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractive protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), and TNF-α 5 h after liver IR as described previously (Lee et al., 2009) (see Supplemental Data).
Assessment of Kidney and Liver Vascular Permeability.
Changes in kidney and liver vascular permeability were assessed by quantitating extravasation of Evans blue dye (EBD) into the tissue as described by Awad et al. (2006) with minor modifications (Lee et al., 2009) (see Supplemental Data).
Detection of Kidney Apoptosis.
We used in situ terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) assay and DNA laddering assay to detect renal apoptosis after liver IR as described previously (Chen et al., 2008, 2009) (see Supplemental Data).
F-Actin Staining of Kidney Sections.
Because breakdown of F-actin occurs early after IR, we visualized the F-actin cytoskeleton by staining with phalloidin as an early index of renal injury (Molitoris, 1997) (see Supplemental Data).
Potential Roles of ERK MAPK and Akt in A1AR-Mediated Renal Protection after Liver IR.
Inhibitors of ERK MAPK (PD98059) and Akt (wortmannin) signaling intermediates were used in this protocol. The doses of inhibitors of PD98059 and wortmannin were selected based on previous in vivo studies (Joo et al., 2006, 2007). In addition, we performed preliminary experiments to demonstrate that the dosage and method of administration of PD98059 and wortmannin we used effectively blocked the phosphorylation of ERK and Akt in vivo, respectively (Joo et al., 2007). To test the hypothesis that ERK MAPK and/or Akt participate in A1AR-mediated protection against liver IR-induced AKI, we pretreated the mice with PD98059 (an inhibitor of MEK1 to inhibit ERK phosphorylation, 1 mg/kg i.p.) or wortmannin [an inhibitor of phosphoinositide 3 kinase (PI3K) to inhibit Akt phosphorylation, 1 mg/kg i.p.] 15 min before CCPA injection.
Protein Determination and Reagents.
Protein contents were determined with a bicinchoninic acid protein assay kit (Thermo Fisher Scientific), using bovine serum albumin as a standard. Unless otherwise specified, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis.
The data were analyzed with t tests when means between two groups were compared or with one-way (e.g., plasma creatinine or ALT) ANOVA plus Tukey post hoc multiple comparison test to compare mean values across multiple treatment groups. The ordinal values of the kidney injury scores were analyzed by the Kruskal-Wallis nonparametric test with Dunn posttest comparison between groups. In all cases, P < 0.05 was taken to indicate significance. All data are expressed as mean ± S.E.M.
Results
A1AR Modulation Affects Renal and Hepatic Function after Liver IR Injury.
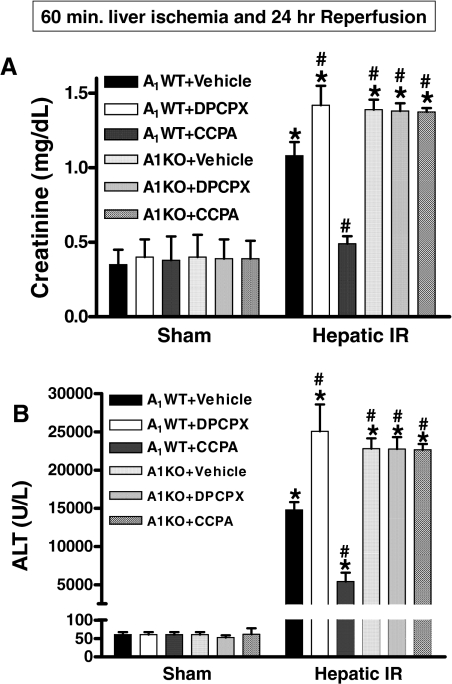
A1WT and A1KO mice that underwent sham operations had similar baseline renal and hepatic function (Fig. 1). Our model of hepatic IR resulted in severe kidney dysfunction 24 h after reperfusion indicated by significant increases in plasma creatinine levels as described previously (Kim et al., 2008, Lee et al., 2009). However, 24 h after hepatic IR injury the A1KO mice had significantly higher plasma Cr and ALT compared with the A1WT mice (Fig. 1). A1WT mice pretreated with DPCPX before hepatic ischemia also had significantly higher Cr and ALT at 24 h compared with vehicle-treated A1WT mice subjected to liver IR (Fig. 1). Exogenous A1AR activation with CCPA treatment protected the A1WT mice against AKI and liver injury after hepatic IR injury (Fig. 1). We determined that DPCPX- or CCPA-treated A1KO mice subjected to IR had similar Cr and ALT compared with the A1KO mice subjected to liver IR alone, confirming the in vivo selectivity of these drugs for the A1AR (Fig. 1). Injection of CCPA or DPCPX alone without hepatic IR (CCPA-sham or DPCPX-sham) had no effect on renal or hepatic function (Fig. 1).
Fig. 1.
Comparison of mean plasma creatinine (A) and ALT activity (B) measured from sham-operated and vehicle-treated A1WT mice (n = 4) and A1KO mice (n = 4), sham-operated A1WT mice given injections of 0.1 mg/kg CCPA (n = 4) or 0.4 mg/kg DPCPX (n = 4), A1WT mice (n = 6–10) or A1KO mice (n = 6–10) pretreated with vehicle, and A1WT mice (n = 6–9) or A1KO mice (n = 6) pretreated with DPCPX or CCPA and subjected to liver IR. Plasma creatinine and ALT activity were measured at 24 h after reperfusion for each mouse. *, P < 0.05 versus A1WT or A1KO sham group. #, P < 0.05 versus A1WT+vehicle hepatic IR group. Error bars represent 1 S.E.M.
Bilateral Nephrectomy before Hepatic Ischemia Abolishes CCPA-Mediated Hepatic Protection after Liver IR.
Pilot studies demonstrated that mice subjected to bilateral nephrectomy and 60 min of liver ischemia had significantly worse liver injury (ALT = 29,587 ± 4252 U/liter, n = 6) with high mortality (50%). These findings support the hypothesis that the kidneys modulate liver injury after hepatic IR in mice. Therefore, in mice subjected to bilateral nephrectomy, we reduced the hepatic ischemia time to 45 min. Bilateral nephrectomy caused significant rises in plasma Cr in both vehicle-treated (Cr = 3.1 ± 0.2 mg/dl, n = 5) and CCPA-treated A1WT mice (Cr = 3.1 ± 0.1 mg/dl, n = 5) in 24 h. In addition, bilateral nephrectomy before 45 min of hepatic ischemia caused significant liver injury (ALT = 21,086 ± 1594 U/liter, n = 5) 24 h after reperfusion. Furthermore, bilateral nephrectomy before 45 min of hepatic ischemia abolished the hepatic protective effects of CCPA (ALT = 26,212 ± 1802 U/liter, n = 5, P = 0.073 versus bilateral nephrectomy and 45-min hepatic IR), demonstrating that the renal activation of A1AR is critical in producing both renal and hepatic protection after liver IR.
Unilateral Renal Injection of EGFP-huA1AR Lentivirus in A1KO Mice Protects against Hepatic and Renal Injury after Liver IR.
We demonstrated previously that selective in vivo renal expression of EGFP or EGFP-huA1AR after intrarenal lentiviral gene delivery in mice is possible without major expression in the contralateral kidney or liver (Kim et al., 2009). We confirmed selective left renal expression of EGFP-huA1AR via 1) direct visualization of EGFP in frozen sections of the injected kidney, contralateral kidney, or liver, 2) immunohistochemistry for huA1ARs, and 3) RT-PCR for the EGFP-A1AR transgene in the liver and kidney tissues (data not shown) as described previously (Kim et al., 2009).
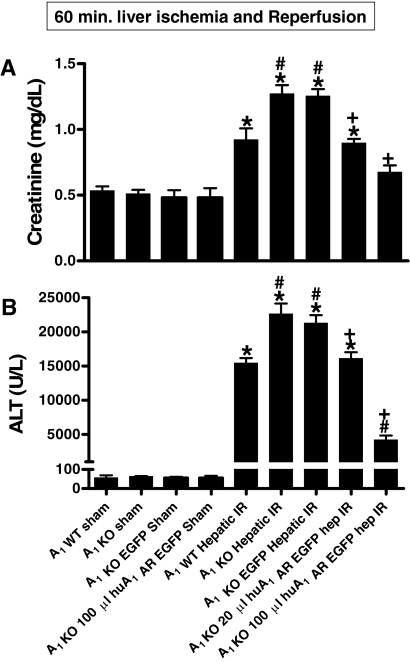
The average plasma level of ALT and Cr in the sham-operated A1KO mice renally injected with lentivirus encoding EGFP or EGFP-huA1AR was similar to the levels obtained from A1KO sham-operated mice not renally injected with lentivirus (Fig. 2). In A1KO mice renally injected with EGFP lentivirus, the plasma level of Cr and ALT significantly increased at 24 h after liver IR. The increases in ALT and Cr were significantly suppressed in A1KO mice renally injected (48 h before liver IR) with EGFP-huA1AR lentivirus in a dose-dependent manner at 24 h after liver IR.
Fig. 2.
Comparison of mean plasma creatinine (A; mg/dl) and ALT activity (B; U/liter) measured from sham-operated A1WT mice (n = 4) and A1KO mice (n = 4), sham-operated A1KO mice renally injected with EGFP-encoding lentivirus (100 μl, n = 5), A1WT mice (n = 6) or A1KO mice (n = 6) subjected to hepatic IR, and A1KO mice renally injected with EGFP (n = 5) or EGFP-huA1AR mice encoding lentivirus (20 or 100 μl, n = 5 each) and subjected to hepatic IR. Mice were renally injected with lentivirus 48 h before sham surgery or hepatic IR. Plasma ALT and creatinine was measured at 24 h after reperfusion. *, P < 0.05 versus sham-operated mice. #, P < 0.05 versus A1WT mice subjected to liver IR. +, P < 0.05 versus A1KO mice injected with EGFP lentivirus. Error bars represent 1 S.E.M.
Removing the EGFP-huA1AR Lentivirus-Injected Kidney before Hepatic Ischemia Abolishes both Renal and Hepatic Protection after Liver IR in A1KO Mice.
Unilateral nephrectomy of the EGFP-huA1AR lentivirus (100 μl)-injected kidney before hepatic ischemia completely abolished the hepatic (ALT = 22,447 ± 2544 U/liter, n = 4) and renal (Cr = 1.26 ± 0.07 mg/dl, n = 4) protective effects of renal huA1AR overexpression in A1KO mice (shown in Fig. 2), demonstrating that the overexpression of huA1AR in the injected kidney plays a crucial role in protecting the kidney and liver after liver IR. Unilateral nephrectomy of the EGFP lentivirus-injected kidney in A1KO mice before hepatic ischemia did not significantly change the hepatic injury (ALT = 19,356 ± 1032 U/liter, n = 4) and renal injury (Cr = 1.37 ± 0.07 mg/dl, n = 4) compared with the A1KO mice with two intact kidneys 24 h after reperfusion (Fig. 3).
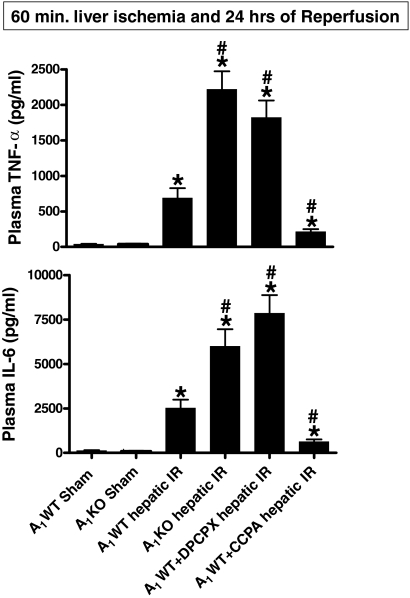
Fig. 3.
Plasma TNF-α (top) and IL-6 levels (bottom) (in pg/ml) in sham-operated A1WT mice (A1WT sham, n = 4) and A1KO mice (A1KO sham, n = 4), A1WT mice (A1WT hepatic IR, n = 6) or A1KO mice (A1KO hepatic IR, n = 5–8) subjected to 60 min of hepatic ischemia and 24 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX, n = 5–8) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA, n = 5) and subjected to 60 of min hepatic ischemia and 24 h of reperfusion. Data are presented as means ± S.E.M. *, P < 0.05 versus A1WT or A1KO sham group. #, P < 0.01 versus A1WT hepatic IR group.
A1AR Modulation Affects Plasma TNF-α and IL-6 after Liver IR.
In sham-operated animals, the plasma TNF-α (n = 4) and plasma IL-6 (n = 4) levels were very low (Fig. 3). The plasma TNF-α (n = 6, P < 0.01) and IL-6 (n = 6, P < 0.01) levels increased markedly in A1WT mice 24 h after liver IR (Fig. 3). The A1KO mice and DPCPX-treated A1WT mice showed significantly higher plasma TNF-α and plasma IL-6 levels compared with the A1WT mice 24 h after liver IR. In contrast, the A1WT mice treated with CCPA showed significantly reduced plasma levels of TNF-α (n = 5) and IL-6 levels compared with A1WT mice 24 h after liver IR.
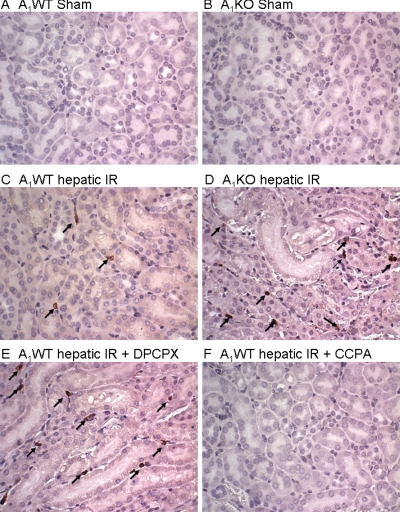
A1AR Modulation Affects Renal Tubular Necrosis after Liver IR.
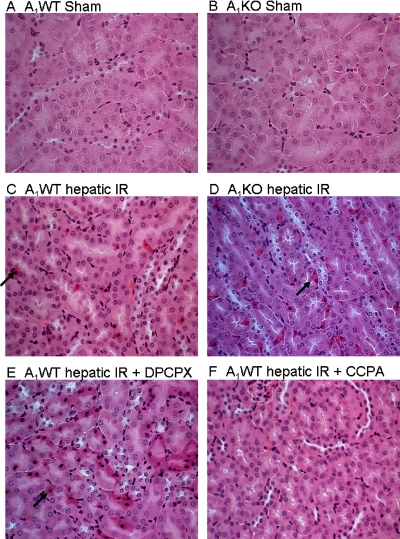
Representative kidney histological slides (cortico-medullary junction) from A1WT mice, A1KO mice, DPCPX-treated A1WT mice, and CCPA-treated A1WT mice subjected to 60 min of ischemia and 24 h of reperfusion and A1WT mice or A1KO mice subjected to the sham operation are shown in Fig. 4. In kidneys from the A1WT mice subjected to liver IR, we observed multifocal acute tubular injury, including S3 segment proximal tubule necrosis indicated by hypereosinophilia (arrow showing a single, hypereosinophilic cell in Fig. 4C). Correlating with significantly worsened renal function, significantly increased numbers of hypereosinophilic necrotic proximal tubules were observed in A1KO mice and DPCPX-pretreated A1WT mice subjected to hepatic IR injury compared with the A1WT mice (Fig. 4, D and E). Furthermore, A1WT mice pretreated with the A1AR agonist CCPA showed significantly fewer hypereosinophilic necrotic proximal tubule cells (Fig. 4F). Quantifications of hypereosinophilic necrotic proximal tubule cells (400× field) in the S3 segment of the kidney were performed. We failed to detect hypereosinophilic necrotic cells from sham-operated mice (A1WT sham, n = 4; A1KO sham, n = 4). The A1WT mice subjected to hepatic IR resulted in a significant number of hypereosinophilic necrotic renal proximal tubule cells 24 h after hepatic IR (8.4 ± 1.1 hypereosinophilic necrotic cells/400× field, n = 5, p < 0.01 versus sham). A significantly higher percentage of proximal tubule necrosis developed in the A1KO mice (15.7 ± 2.9 hypereosinophilic necrotic cells/400× field, n = 5, p < 0.05 versus A1WT mice subjected to liver IR) or DPCPX-pretreated A1WT mice (14.3 ± 2.0 hypereosinophilic cells/400× field, n = 5, p < 0.05 versus A1WT mice subjected to liver IR) compared with A1WT mice subjected to IR. A1WT mice pretreated with the A1AR agonist (CCPA) before IR injury showed reduced proximal tubule hypereosinophilic necrotic cells (5.3 ± 0.8 hypereosinophilic cells/400× field, P < 0.05 versus A1WT mice subjected to liver IR). Injection of CCPA or DPCPX alone (without hepatic IR) had no effect on kidney histology.
Fig. 4.
Representative (four to five slides) hematoxylin and eosin-stained photomicrographs (magnification: 400×) in kidney sections from sham-operated A1WT (A1WT sham; A) and A1KO mice (A1KO sham; B), A1WT (A1WT hepatic IR; C), or A1KO mice (A1KO hepatic IR; D) subjected to 60 min of liver ischemia and 24 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX; E) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA; F) and subjected to 60 min of liver ischemia and 24 h of reperfusion (magnification 400×, cortico-medullary junction). Hypereosinophilic proximal tubules (arrows) visible in A1WT mice subjected to liver IR are increased in A1KO mice or DPCPX-treated A1WT mice subjected to liver IR.
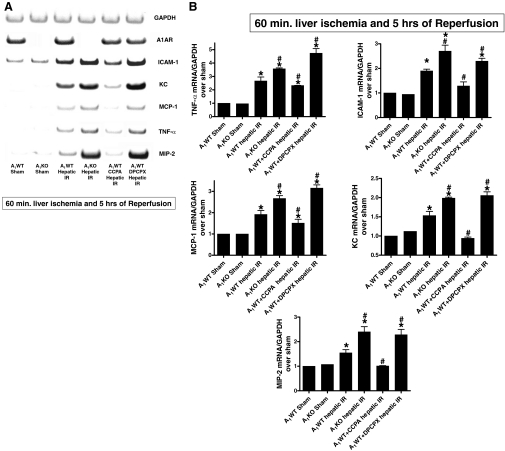
A1AR Modulation Affects Renal Proinflammatory mRNA Expression after Liver IR Injury.
Hepatic IR injury was associated with significantly increased renal proinflammatory mRNA expression (ICAM-1, TNF-α, KC, MCP-1, and MIP-2) 5 h after hepatic IR. Kidneys from A1KO or A1WT mice treated with DPCPX showed increased expression of all proinflammatory mRNAs studied after liver IR (Fig. 5). In contrast, CCPA pretreatment significantly suppressed the increases in proinflammatory mRNA expression 5 h after hepatic IR. Injection of CCPA or DPCPX alone (without hepatic IR) had no effect on proinflammatory gene expression in the kidney.
Fig. 5.
A, representative gel images of semiquantitative RT-PCR results for GAPDH, murine A1AR, TNF-α, ICAM-1, KC, MCP-1, and MIP-2 mRNAs of kidney tissues from sham-operated A1WT and A1KO mice (A1WT sham, n = 3; A1KO sham, n = 3), A1WT or A1KO mice subjected to 60 min of hepatic ischemia and 5 h of reperfusion (A1WT hepatic IR, n = 6; A1KO hepatic IR, n = 5), and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX, n = 5) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA, n = 5) and subjected to 60 min of hepatic ischemia and 5 h of reperfusion. B, densitometric quantification of relative proinflammatory mRNA band intensities normalized to GAPDH from RT-PCRs. Data are presented as means ± S.E.M. *, P < 0.05 versus A1WT or A1KO sham group. #, P < 0.05 versus A1WT hepatic IR group.
A1AR Modulation Affects Renal Neutrophil Infiltration 24 h after IR Injury.
Figure 6 shows representative images of neutrophil immunohistochemitry of kidney sections from sham-operated A1WT (A) or A1KO mice (B), A1WT or A1KO mice subjected to liver IR (C and D, respectively), or A1WT mice subjected to IR after DPCPX or CCPA pretreatment (E and F, respectively). In sham-operated mice, we were unable to detect neutrophils in the kidney (n = 4 each). Sixty minutes of hepatic ischemia and 24 h of reperfusion resulted in significant neutrophil recruitment into the kidneys of A1WT mice. In the A1WT mice subjected to liver IR, we detected 9.25 ± 2 neutrophils/field (100× magnification, n = 6). The A1KO mice subjected to liver IR injury had significantly higher neutrophil counts (30.3 ± 3.3 neutrophils/field, n = 5) compared with the A1WT mice 24 h after IR (P < 0.05). The A1WT mice pretreated with DPCPX before hepatic IR injury also had increased neutrophil infiltration (31.3 ± 6.7 neutrophils/field, n = 5) compared with the A1WT mice 24 h after IR (P < 0.05). In contrast, the A1WT mice pretreated with an A1AR agonist (CCPA) and subjected to hepatic IR injury had significantly reduced neutrophil infiltration (1.1 ± 1 neutrophils/field, n = 5). Injection of CCPA or DPCPX alone (without hepatic IR) had no effect on neutrophil infiltration into the kidney.
Fig. 6.
Representative photomicrographs (three to five experiments) of immunohistochemistry for neutrophils (arrows indicating brown granules) in kidney (400×) sections from sham-operated A1WT mice (A1WT sham; A) and A1KO mice (A1KO sham; B), A1WT (A1WT hepatic IR; C) or A1KO (A1KO hepatic IR; D) subjected to 60 min of hepatic ischemia and 24 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX; E) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA; F) and subjected to 60 min of hepatic ischemia and 24 h of reperfusion.
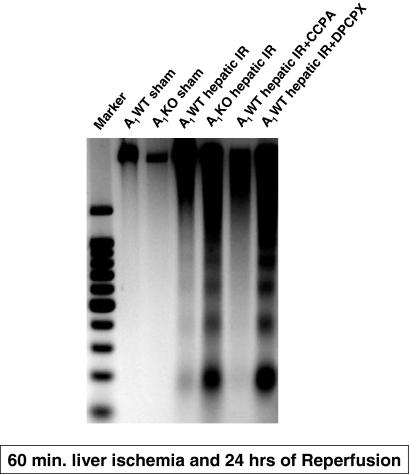
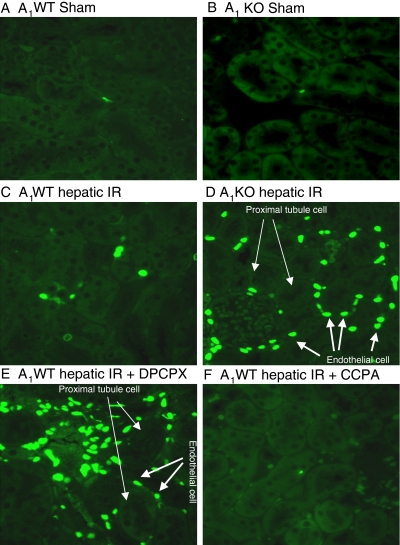
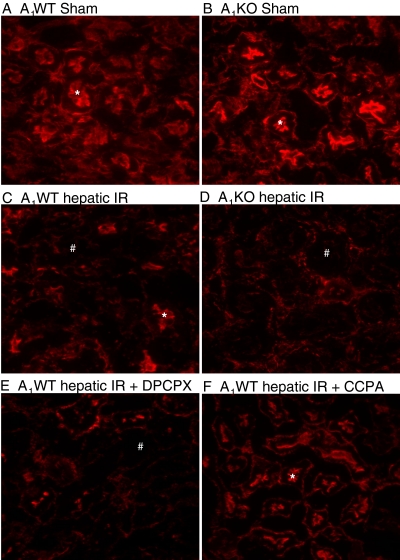
A1AR Modulation Affects the Severity of Renal Apoptosis after Liver IR Injury.
Sham-operated A1WT or A1KO mice did not exhibit DNA laddering in kidneys (representative of four experiments; Fig. 7). However, 60 min of hepatic ischemia and 24 h of reperfusion resulted in DNA fragmentation in the kidneys of A1WT mice (Fig. 7). The A1KO mice subjected to liver IR showed increased renal DNA laddering in kidneys compared with the A1WT mice subjected to liver IR. Moreover, DNA from mice pretreated with the A1AR antagonist (DPCPX) or agonist (CCPA) displayed increased or decreased fragmentation in the kidneys, respectively compared with the A1WT mice subjected to liver IR. Sham-operated mice demonstrated few TUNEL-positive cells in the kidneys 24 h after hepatic ischemia (Fig. 8, A and B). The TUNEL staining (representative of four experiments) showed that endothelial cell apoptosis was predominant in the kidney after 60 min of hepatic ischemia and 24 h of reperfusion (Fig. 8, C–F). However, the degree of endothelial apoptosis in A1KO mice was significantly higher at 24 h after reperfusion (Fig. 8D). Moreover, DPCPX-pretreated A1WT mice showed an increased number of TUNEL-positive cells compared with A1WT mice (Fig. 8E), whereas the A1WT mice pretreated with the A1AR agonist (CCPA) and subjected to IR injury resulted in reduced TUNEL-positive endothelial cells (Fig. 8F). Injection of CCPA or DPCPX alone (without hepatic IR) had no effect on renal endothelial apoptosis.
Fig. 7.
Representative gel images (of four experiments) demonstrating DNA laddering as an index of DNA fragmentation in the kidney tissues from sham-operated A1WT (A1WT sham) and A1KO mice (A1KO sham), A1WT (A1WT hepatic IR) or A1KO mice (A1KO hepatic IR) subjected to 60 min of hepatic ischemia and 24 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA) and subjected to 60 min of hepatic ischemia and 24 h of reperfusion. Apoptotic DNA fragments were extracted according to the methods of Herrmann et al. (1994). This method of DNA extraction selectively isolates apoptotic, fragmented DNA and leaves behind the intact chromatin.
Fig. 8.
Representative fluorescent photomicrographs (of four experiments) illustrate apoptotic nuclei (TUNEL fluorescent stain; magnification 400×) in kidney sections from sham-operated A1WT mice (A1WT sham; A) and A1KO mice (A1KO sham; B), A1WT mice (A1WT hepatic IR; C) or A1KO mice (A1KO hepatic IR, D) subjected to 60 min of hepatic ischemia and 24 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX; E) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA; F) and subjected to 60 min of hepatic ischemia and 24 h of reperfusion. In the kidney, endothelial cells predominantly underwent apoptotic death (short, thick arrows) with sparing of renal proximal tubule cells (long, thin arrows) as illustrated in Fig. 6D.
A1AR Modulation Affects Renal and Hepatic Vascular Permeability 5 h after Liver IR.
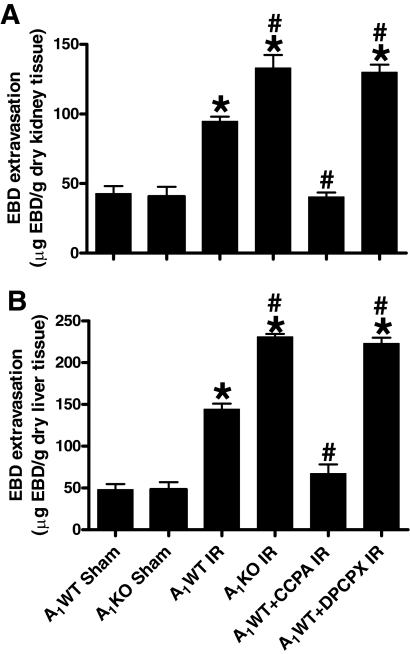
Analyses of EBD extravasations in mice subjected to sham operation or liver IR are shown in Fig. 9. The increase in EBD contents was significantly higher for A1KO mice and A1WT mice treated with DPCPX and subjected to liver IR. In contrast, the A1WT mice treated with CCPA before liver IR had significantly reduced EBD extravasation 5 h after liver IR. Injection of CCPA or DPCPX alone (without hepatic IR) had no effect on renal and hepatic vascular permeability.
Fig. 9.
Quantification of EBD extravasations as indices of vascular permeability of kidney (A) and liver (B) tissues from sham-operated A1WT mice (A1WT sham, n = 4) and A1KO mice (A1KO sham, n = 4), A1WT mice (A1WT hepatic IR, n = 7) or A1KO mice (A1KO hepatic IR, n = 7) subjected to 60 min of hepatic ischemia and 5 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX, n = 6) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA, n = 6) and subjected to 60 min of hepatic ischemia and 5 h of reperfusion. Data are presented as means ± S.E.M. *, P < 0.05 versus A1WT or A1KO sham group. #, P < 0.05 versus A1WT hepatic IR group.
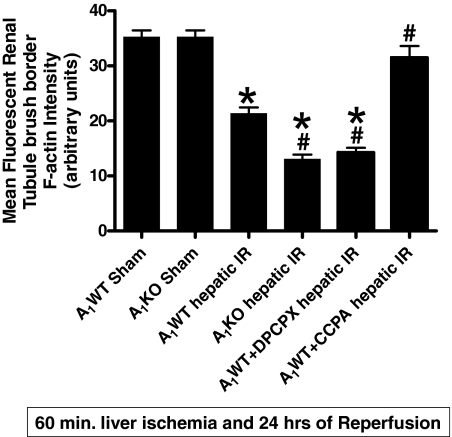
A1AR Modulation Affects the Degradation of Renal F-Actin after Liver IR.
In Fig. 10, 24-h posthepatic IR-induced disruptions of the F-actin cytoskeleton in renal tubular epithelial cells are shown. A1WT or A1KO mice subjected to sham surgery showed intense staining in tubular epithelial and the basal plasma membrane (Fig. 10, A and B). In contrast, kidneys from A1WT mice subjected to liver IR showed loss of F-actin staining in the tubular epithelial cells (Fig. 10C). In addition, the A1KO mice and the A1WT mice treated with DPCPX and subjected to liver IR showed even more loss of F-actin integrity (Fig. 10, D and F, respectively). In contrast, the A1WT mice treated with CCPA before liver IR showed significantly better preserved F-actin structure after liver IR because the staining is quite similar to that of sham-operated mice (Fig. 10E). Mean fluorescent intensity analysis for proximal tubule F-actin is shown in Fig. 11. Injection of CCPA or DPCPX alone (without hepatic IR) had no effect on renal F-actin integrity.
Fig. 10.
Representative fluorescent photomicrographs (of four experiments) of phalloidin staining of the kidney tissues (magnification: 400×) from sham-operated A1WT mice (A1WT sham; A) and A1KO mice (A1KO sham; B), A1WT mice (A1WT hepatic IR; C) or A1KO mice (A1KO hepatic IR; D) subjected to 60 min of hepatic ischemia and 24 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX; E) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA; F) and subjected to 60 min of hepatic ischemia and 24 h of reperfusion. In the kidney, F-actin stains of proximal tubular epithelial cells are prominent in the brush border from sham-operated mice (*), which is severely degraded in the kidneys of mice subjected to liver IR (#).
Fig. 11.
Quantification of mean renal proximal tubule F-actin intensity in kidney tissues from sham-operated A1WT mice (A1WT sham) and A1KO mice (A1KO sham), A1WT mice (A1WT hepatic IR) or A1KO mice (A1KO hepatic IR) subjected to 60 min of hepatic ischemia and 24 h of reperfusion, and A1WT mice pretreated with 0.4 mg/kg DPCPX (A1WT hepatic IR+DPCPX) or 0.1 mg/kg CCPA (A1WT hepatic IR+CCPA) and subjected to 60 min of hepatic ischemia and 24 h of reperfusion. *, P < 0.05 versus A1WT or A1KO sham group. #, P < 0.05 versus A1WT hepatic IR group.
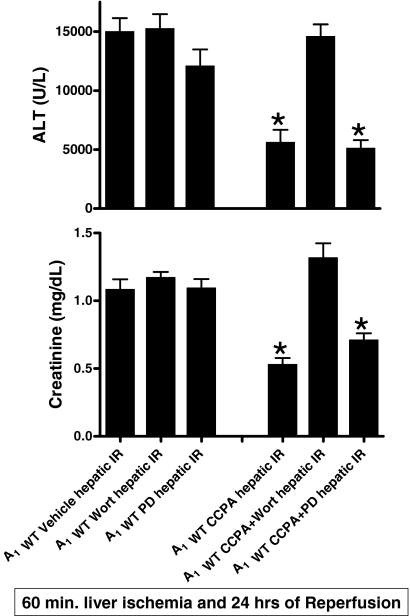
Signaling Pathways of A1AR-Mediated Renal Protection: Critical Role for the Akt Pathway.
We probed the renal protective signaling pathways activated by acute A1AR activation in mice subjected to liver IR. We have demonstrated previously (Joo et al., 2007) that acute A1AR activation resulted in rapid phosphorylation of ERK MAPK and Akt in A1WT but not A1KO mice. To determine whether ERK and/or Akt phosphorylation mediate the cytoprotective signaling of acute A1AR activation-mediated renal protection after hepatic IR, A1WT mice were pretreated with PD98059 (a MEK1 inhibitor) or wortmannin (a PI3K inhibitor) before CCPA treatment. We have demonstrated previously that the doses of PD98059 and wortmannin used effectively blocked phosphorylation of ERK and Akt, respectively, in mice in vivo (Joo et al., 2006, 2007). We found that the inhibition of PI3K, but not MEK1, prevented the renal and hepatic protection with acute A1AR activation after hepatic IR (Fig. 12). Although the dose of PD98059 was effective in inhibiting MEK1 (Joo et al., 2006), inhibition of ERK phosphorylation did not prevent the renal protection with acute A1AR activation. Inhibitors alone had no effect on renal or hepatic function (Fig. 12) after IR injury.
Fig. 12.
Plasma creatinine (top; mg/dL) and ALT activity (bottom; U/liter) in A1WT mice after injection with vehicle (A1WT Vehicle hepatic IR) or 0.1 mg/kg CCPA 15 min (A1WT CCPA hepatic IR) before 60 min of hepatic ischemia and 24 h of reperfusion. Some A1WT mice were pretreated with PD98059 (PD, an inhibitor of MEK1 to inhibit ERK phosphorylation, 1 mg/kg i.p.) or wortmannin (an inhibitor of PI3K to inhibit Akt phosphorylation, 1 mg/kg i.p.) 15 min before vehicle [A1WT Wort hepatic IR (n = 5) or A1WT PD hepatic IR (n = 5)] or CCPA treatment [A1WT CCPA+Wort hepatic IR (n = 5) or A1WT CCPA+PD hepatic IR (n = 5)]. Data are presented as means ± S.E.M. Inhibition of PI3K → Akt pathway but not MEK → ERK MAPK prevents acute A1AR activation-induced renal protection after hepatic IR. *, P < 0.05 versus A1WT Vehicle hepatic IR group.
Discussion
We demonstrate in this study that renal A1AR activation is directly responsible for both hepatic and renal protection after liver IR in mice because 1) removal of both kidneys before liver IR completely abolished the hepatic and renal protective effects observed in A1WT mice, 2) selective renal expression of huA1ARs in A1KO mice reduced both kidney and liver injury after liver IR, and 3) removal of the EGFP-huA1AR lentivirus-injected kidney before liver IR in A1KO mice abolished the hepatic and renal protective effects of huA1AR expression. In addition, 1) mice deletionally lacking the A1ARs (A1KO mice) or A1WT mice pretreated with an A1AR antagonist (DPCPX) demonstrate greater renal injury, 2) kidneys from A1KO or DPCPX-pretreated A1WT mice showed increased tubular necrosis (hypereosinophilia), apoptosis (DNA laddering and TUNEL staining), and inflammation (neutrophil infiltration and proinflammatory mRNA and cytokine expression), 3) exogenous activation of A1ARs with CCPA before hepatic ischemia attenuated renal injury in A1WT mice, and 4) blocking Akt prevented A1AR-mediated renal protection after hepatic IR.
AKI caused by hepatic IR injury results in rapid, immediate apoptotic destruction of renal endothelial cells with subsequent individual cell necrosis of proximal tubules and interstitial neutrophil infiltration and inflammation (Lee et al., 2009). This is clinically significant as hepatic IR is a frequent cause of AKI during the perioperative period, and the incidence of renal dysfunction after major liver surgery or liver transplantation approaches 40 to 80% (Davis et al., 2002; Betrosian et al., 2007). Furthermore, our model of hepatic IR-induced AKI appears to resemble clinically observed AKI in humans manifesting as a combination of apoptosis, necrosis, and inflammation. The hepatic IR-induced murine AKI model may lead to development of pharmacological therapies capable of attenuating endothelial cell apoptosis, renal tubular necrosis/inflammation, and cytokine-mediated attack observed in human AKI.
Release of adenosine after stress (e.g., hypoxia, IR) with subsequent activation of ARs has been proposed to auto-protect against cell death in several cell types (Dinour and Brezis, 1991; Walsh et al., 1995). Indeed, recent elegant studies combining genetic deletion and pharmacological inhibition have demonstrated that ecto-5′-nucleotidase (CD73), the enzyme involved in extracellular adenosine production by converting AMP to adenosine, is critical for hepatic (Hart et al., 2008) and renal protection (Grenz et al., 2007) by ischemic preconditioning in mice. Furthermore, it has been demonstrated that diminished adenosine uptake via hypoxia-inducible factor-1-dependent repression of equilibrative nucleoside transporter types 1 and 2 greatly enhances extracellular adenosine levels (Eltzschig et al., 2005, Morote-Garcia et al., 2009). These studies imply that endogenous adenosine production is critical in protecting against hypoxia- or ischemia-induced organ injury. Previous studies also have demonstrated that activation of cell surface A1ARs, in particular, produces cytoprotective effects against IR injury in many organ systems, including the heart, kidney, and brain (Heurteaux et al., 1995; Lee et al., 2004; Lankford et al., 2006; Kim et al., 2009). Mechanistically, A1AR activation produces several cellular effects that are suited to attenuate the multifaceted pathophysiology of AKI (endothelial and renal tubular cell apoptosis, inflammation, and necrosis).
Complicating the issues, however, is that several investigators have reported that a nonselective AR antagonist (theophylline) or selective A1AR antagonists (DPCPX, KW-3902) improved renal function, urine output, and renal hemodynamics in several models of nephrotoxin AKI induced by cisplatin, gentamicin, or glycerol (Bidani and Churchill, 1983; Yao et al., 1994). In addition, Lin et al. (1988) have demonstrated that theophylline increased renal plasma flow and glomerular filtration rate after ischemic renal injury. These studies were performed based on the observation that A1AR antagonism increases urine output, solute transport, and renal blood flow. Indeed, A1AR antagonists reversed these indices of renal injury in toxin and ischemic models of ARF (Yao et al., 1994, 2000).
We have previously demonstrated that the degree of renal injury is directly proportional to the degree of hepatic injury after liver IR (Lee et al., 2009). We have also previously demonstrated that A1KO mice and A1WT mice treated with a selective A1AR antagonist (DPCPX) developed significantly worse liver injury (alanine aminotransferase, liver necrosis, neutrophil infiltration, and apoptosis) compared with the A1WT mice 24 h after liver IR injury (Kim et al., 2008). Taken together, our previous and current findings imply that endogenous and exogenous A1AR plays an important role in hepatic and renal protection. Furthermore, we now show in this study that renal A1AR activation is directly responsible for both hepatic and renal protection after liver IR in mice by three direct experimental data: 1) removal of the kidneys before liver IR completely abolished the hepatic and renal protective effects CCPA in A1WT mice, 2) selective expression of huA1ARs in A1KO mice decreases AKI and liver injury, and 3) removal of the EGFP-huA1AR lentivirus-injected kidney before liver IR in A1KO mice abolished the hepatic and renal protective effects of huA1AR expression. We confirmed that systemic spillover of EGFP-huA1AR lentivirus (to the contralateral kinder and/or the liver) cannot explain the hepatic and renal protective effects with EGFP-huA1AR lentivirus injection. Instead, we propose that the direct EGFP-huA1AR-mediated reduction in AKI provided hepatic protection after IR. Taken together, our findings imply that liver IR-mediated AKI potentiates the liver injury further and protecting the kidney reduces liver damage after IR.
Acute renal protection after hepatic IR with A1AR activation is mediated by Akt activation because the inhibition of phosphoinositide 3-kinases with wortmannin prevented CCPA-mediated renal protection after hepatic IR. The serine/threonine kinase Akt is an important component of cell survival pathways in many cell types (Hausenloy et al., 2004, 2005). In particular, Akt has diverse functions to counteract apoptosis including inhibition of mitochondrial cytochrome c and phosphorylation of several proapoptotic factors (e.g., bad, caspase 9, glycogen synthase kinase 3) (Cross et al., 2000). Akt can also increase the activity of heat shock protein 27 in certain cell types (Konishi et al., 1997; Rane et al., 2001, 2003) promoting F-actin stability. Better preserved F-actin cytoskeleton in the kidneys of mice subjected to liver IR after CCPA treatment may have contributed to reduced renal tubular necrosis and apoptosis observed in these mice.
Our results show that bilateral nephrectomy exacerbates hepatic IR injury in mice. Bilateral nephrectomy-induced extrarenal organ dysfunction has been described (Paladino et al., 2009). Reduced renal cytokine elimination after nephrectomy may contribute to increased plasma and hepatic proinflammatory cytokine levels and exacerbate hepatic IR injury. Indeed, increased plasma IL-6 after bilateral nephrectomy contributes to lung injury in mice (Klein et al., 2008). Taken together, it is possible that loss of renal cytokine clearance may induce extrarenal organ (e.g., intestine, lung) injury that can potentiate cytokine release, further exacerbating systemic inflammatory response.
One of the limitations of this study is that the cell types targeted in the kidney via A1AR activation were not directly elucidated. A1AR signaling has been extensively characterized in various nonimmune cells and includes modulation of adenylyl cyclase, protein kinase C, PI3K, and ERK MAPKs (Haskó et al., 2008). Hepatic IR-induced AKI is characterized by early renal endothelial cell apoptosis with subsequent proximal tubule necrosis (Lee et al., 2009). Furthermore, we have previously showed direct in vitro renal tubular protective effects of A1ARs against both anoxic and oxidant-induced necrosis (Lee and Emala, 2002a,b). Therefore, we propose that both renal endothelial and renal tubule cells are targeted by the A1ARs. It remains to be determined in future in vitro studies whether hepatocytes are also directly targeted via A1AR activation.
In summary, we demonstrate in this study that endogenous A1AR activation provides protection against hepatic IR-induced AKI injury by reducing endothelial apoptosis, renal proximal tubular necrosis, and inflammatory changes. We propose that renal endothelial and/or tubular A1ARs serve to protect against renal insults that occur after hepatic IR via Akt-dependent mechanisms. Given the protective benefit of endogenous and exogenous A1AR activation against hepatic IR-induced AKI and that hepatic IR is common in patients after liver surgery, liver transplantation, or sepsis, our findings may have important future therapeutic implications. The finding that loss of renal function (after bilateral nephrectomy) potentiated liver injury after IR is an interesting finding that requires further investigation.
Supplementary Material
This work was supported by the National Institutes of Health [Grant DK58547].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.166884.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- IR
- ischemia reperfusion
- A1AR
- A1 adenosine receptor
- huA1AR
- human A1AR
- AKI
- acute kidney injury
- WT
- wild type
- KO
- knockout
- F-actin
- filamentous actin
- CCPA
- 2-chloro-N6-cyclopentyladenosine
- DPCPX
- 8-cyclopentyl-1,3-dipropylxanthine
- EGFP
- enhanced green fluorescent protein
- KW-3902
- 8-(noradamantan-3-yl)-1,3-dipropylxanthine
- ERK
- extracellular signal-regulated kinase
- MAPK
- mitogen-activated protein kinase
- PD98059
- 2′-amino-3′-methoxyflavone
- RT-PCR
- reverse transcription-polymerase chain reaction
- ICAM-1
- intercellular adhesion molecule-1
- EBD
- Evans blue dye
- PI3K
- phosphoinositide 3 kinase l
- TUNEL
- terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling
- ALT
- alanine aminotransferase
- TNF-α
- tumor necrosis factor α
- IL-6
- interleukin-6
- KC
- keratinocyte-derived cytokine
- MCP-1
- monocyte chemoattractive protein-1
- MIP-2
- macrophage inflammatory protein-2
- MEK1
- meiosis-specific serine/threonine-protein kinase 1
- Cr
- creatinine
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase.
References
- Awad et al., 2006.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. (2006) Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290:F1516–F1524 [DOI] [PubMed] [Google Scholar]
- Betrosian et al., 2007.Betrosian AP, Agarwal B, Douzinas EE. (2007) Acute renal dysfunction in liver diseases. World J Gastroenterol 13:5552–5559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidani and Churchill, 1983.Bidani AK, Churchill PC. (1983) Aminophylline ameliorates glycerol-induced acute renal failure in rats. Can J Physiol Pharmacol 61:567–571 [DOI] [PubMed] [Google Scholar]
- Bowmer et al., 1986.Bowmer CJ, Collis MG, Yates MS. (1986) Effect of the adenosine antagonist 8-phenyltheophylline on glycerol-induced acute renal failure in the rat. Br J Pharmacol 88:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley et al., 1999.Buckley S, Driscoll B, Barsky L, Weinberg K, Anderson K, Warburton D. (1999) ERK activation protects against DNA damage and apoptosis in hyperoxic rat AEC2. Am J Physiol Lung Cell Mol Physiol 277:L159–L166 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2009.Chen SW, Kim M, Kim M, Song JH, Park SW, Wells D, Brown K, Belleroche J, D'Agati VD, Lee HT. (2009) Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int 75:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2009.Chen SW, Park SW, Kim M, Brown KM, D'Agati VD, Lee HT. (2009) Human heat shock protein 27 overexpressing mice are protected against hepatic ischemia and reperfusion injury. Transplantation 87:1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross et al., 2000.Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. (2000) Serine/threonine protein kinases and apoptosis. Exp Cell Res 256:34–41 [DOI] [PubMed] [Google Scholar]
- Davis et al., 2002.Davis CL, Gonwa TA, Wilkinson AH. (2002) Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Part I. Liver Transpl 8:91–109 [DOI] [PubMed] [Google Scholar]
- Dinour and Brezis, 1991.Dinour D, Brezis M. (1991) Effects of adenosine on intrarenal oxygenation. Am J Physiol Renal Physiol 261:F787–F791 [DOI] [PubMed] [Google Scholar]
- Eltzschig et al., 2005.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J, et al. (2005) HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med 202:1493–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz et al., 2007.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Köhle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. (2007) Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18:833–845 [DOI] [PubMed] [Google Scholar]
- Hart et al., 2008.Hart ML, Much C, Gorzolla IC, Schittenhelm J, Kloor D, Stahl GL, Eltzschig HK. (2008) Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology 135:1739–1750 [DOI] [PubMed] [Google Scholar]
- Haskó et al., 2008.Haskó G, Linden J, Cronstein B, Pacher P. (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy et al., 2004.Hausenloy D, Mocanu M, Yellon D. (2004) Cross-talk between the survival kinases during reperfusion in ischaemic preconditioning. Cardiovasc J S Afr 15:S11. [DOI] [PubMed] [Google Scholar]
- Hausenloy et al., 2005.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. (2005) Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288:H971–H976 [DOI] [PubMed] [Google Scholar]
- Heidemann et al., 1989.Heidemann HT, Müller S, Mertins L, Stepan G, Hoffmann K, Ohnhaus EE. (1989) Effect of aminophylline on cisplatin nephrotoxicity in the rat. Br J Pharmacol 97:313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann et al., 1994.Herrmann M, Lorenz HM, Voll R, Grünke M, Woith W, Kalden JR. (1994) A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 22:5506–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux et al., 1995.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. (1995) Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA 92:4666–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo et al., 2006.Joo JD, Kim M, D'Agati VD, Lee HT. (2006) Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol 17:3115–3123 [DOI] [PubMed] [Google Scholar]
- Joo et al., 2007.Joo JD, Kim M, Horst P, Kim J, D'Agati VD, Emala CW, Sr, Lee HT. (2007) Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol 293:F1847–F1857 [DOI] [PubMed] [Google Scholar]
- Kellett et al., 1989.Kellett R, Bowmer CJ, Collis MG, Yates MS. (1989) Amelioration of glycerol-induced acute renal failure in the rat with 8-cyclopentyl-1,3-dipropylxanthine. Br J Pharmacol 98:1066–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy et al., 1999.Kennedy SG, Kandel ES, Cross TK, Hay N. (1999) Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 19:5800–5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2009.Kim M, Chen SW, Park SW, Kim M, D'Agati VD, Yang J, Lee HT. (2009) Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75:809–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2008.Kim J, Kim M, Song JH, Lee HT. (2008) Endogenous A1 adenosine receptors protect against hepatic ischemia reperfusion injury in mice. Liver Transpl 14:845–854 [DOI] [PubMed] [Google Scholar]
- Klein et al., 2008.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. (2008) Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 74:901–909 [DOI] [PubMed] [Google Scholar]
- Konishi et al., 1997.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. (1997) Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett 410:493–498 [DOI] [PubMed] [Google Scholar]
- Lankford et al., 2006.Lankford AR, Yang JN, Rose'meyer R, French BA, Matherne GP, Fredholm BB, Yang Z. (2006) Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. Am J Physiol Heart Circ Physiol 290:H1469–H1473 [DOI] [PubMed] [Google Scholar]
- Lee and Emala, 2000.Lee HT, Emala CW. (2000) Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am J Physiol Renal Physiol 278:F380–F387 [DOI] [PubMed] [Google Scholar]
- Lee and Emala, 2001.Lee HT, Emala CW. (2001) Protein kinase C and G(i/o) proteins are involved in adenosine- and ischemic preconditioning-mediated renal protection. J Am Soc Nephrol 12:233–240 [DOI] [PubMed] [Google Scholar]
- Lee and Emala, 2002a.Lee HT, Emala CW. (2002a) Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A(1) and A(2a) adenosine receptor activation. Am J Physiol Renal Physiol 282:F844–F852 [DOI] [PubMed] [Google Scholar]
- Lee and Emala, 2002b.Lee HT, Emala CW. (2002b) Preconditioning and adenosine protect human proximal tubule cells in an in vitro model of ischemic injury. J Am Soc Nephrol 13:2753–2761 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2004.Lee HT, Gallos G, Nasr SH, Emala CW. (2004) A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15:102–111 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2009.Lee HT, Park SW, Kim M, D'Agati VD. (2009) Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest 89:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al., 1988.Lin JJ, Churchill PC, Bidani AK. (1988) Theophylline in rats during maintenance phase of postischemic acute renal failure. Kidney Int 33:24–28 [DOI] [PubMed] [Google Scholar]
- Molitoris, 1997.Molitoris BA. (1997) Putting the actin cytoskeleton into perspective: pathophysiology of ischemic alterations. Am J Physiol Renal Physiol 272:F430–F433 [DOI] [PubMed] [Google Scholar]
- Morote-Garcia et al., 2009.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. (2009) Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136:607–618 [DOI] [PubMed] [Google Scholar]
- Paladino et al., 2009.Paladino JD, Hotchkiss JR, Rabb H. (2009) Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc Res 77:8–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane et al., 2001.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. (2001) p38 kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem 276:3517–3523 [DOI] [PubMed] [Google Scholar]
- Rane et al., 2003.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278:27828–27835 [DOI] [PubMed] [Google Scholar]
- Slot, 1965.Slot C. (1965) Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 17:381–387 [DOI] [PubMed] [Google Scholar]
- Walsh et al., 1995.Walsh RS, Borges M, Thornton JD, Cohen MV, Downey JM. (1995) Hypoxia preconditions rabbit myocardium by an adenosine receptor-mediated mechanism. Can J Cardiol 11:141–146 [PubMed] [Google Scholar]
- Yao et al., 2000.Yao K, Heyne N, Osswald H. (2000) Effect of the selective adenosine A1-receptor antagonist KW-3902 on tubuloglomerular feedback in radiocontrast-media-induced nephropathy in rats with chronic nitric-oxide deficiency. Jpn J Pharmacol 84:347–350 [DOI] [PubMed] [Google Scholar]
- Yao et al., 1994.Yao K, Kusaka H, Sato K, Karasawa A. (1994) Protective effects of KW-3902, a novel adenosine A1-receptor antagonist, against gentamicin-induced acute renal failure in rats. Jpn J Pharmacol 65:167–170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.