Abstract
The present studies were conducted to investigate the relationship between discriminative stimulus effects of indirectly acting monoaminergic psychostimulants and their ability to increase extracellular levels of dopamine (DA) in the nucleus accumbens (NAcb) shell. First, the behavioral effects of methamphetamine (MA), cocaine (COC), 1-[2-[bis(4-fluorophenyl-)methoxy]ethyl]-4-(3-phenylpropyl)piperazine (GBR 12909), d-amphetamine, and methylphenidate were established in rats trained to discriminate intraperitoneal injections of 0.3 mg/kg MA from saline. In other studies, in vivo microdialysis was used to determine the effects of MA, COC, and GBR 12909 on extracellular DA levels in the NAcb shell. Results show that all drugs produced dose-related and full substitution for the discriminative stimulus effects of 0.3 mg/kg MA. In microdialysis studies, cumulatively administered MA (0.3–3 mg/kg), COC (3–56 mg/kg), and GBR 12909 (3–30 mg/kg) produced dose-dependent increases in DA efflux in the NAcb shell to maxima of approximately 1200 to 1300% of control values. The increase in DA levels produced by MA and COC was rapid and short-lived, whereas the effect of GBR 12909 was slower and longer lasting. Dose-related increases in MA lever selection produced by MA, COC, and GBR 12909 corresponded with graded increases in DA levels in the NAcb shell. Doses of MA, COC, and GBR 12909 that produced full substitution increased DA levels to approximately 200 to 400% of control values. Finally, cumulatively administered MA produced comparable changes in DA levels in both naive and 0.3 mg/kg MA-trained rats. These latter results suggest that sensitization of DA release does not play a prominent role in the discriminative stimulus effects of psychomotor stimulants.
Drug discrimination procedures have been widely used to characterize behavioral effects of MA, COC, and other monoaminergic psychostimulants that may be related to their subjective effects in humans and their abuse liability. Pharmacological studies with different types of selective agonists and antagonists have provided considerable evidence that the dopaminergic actions of monoaminergic psychostimulants play a prominent role in their discriminative stimulus effects (e.g., Callahan et al., 1997; Tidey and Bergman, 1998). Human brain imaging studies also have identified a positive relationship between increases in neurotransmission in DA-rich brain regions and the intensity of subjective effects of COC (e.g., Volkow et al., 1997), further supporting the prominence of dopaminergic mechanisms among the several types of actions that mediate the internal stimulus effects of monoaminergic psychostimulant drugs (Walsh and Cunningham, 1997; Czoty et al., 2004).
Direct evidence from microdialysis studies has shown that MA and other monoaminergic psychostimulants increase extracellular concentrations of DA in brain regions that may mediate their abuse-related behavioral effects (Kuczenski et al., 1995; Schad et al., 1995; Czoty et al., 2004). For example, using self-administration procedures to study drug-maintained behavior and in vivo microdialysis procedures to measure the DA efflux, a close association between the reinforcing effects of COC or amphetamine and increases in extracellular DA levels from several different brain regions have been observed both in rodents (Pettit and Justice, 1989; Ranaldi et al., 1999; Di Chiara et al., 2004; Munzar et al., 2004) and nonhuman primates (Kimmel et al., 2005, 2007; Bradberry and Rubino, 2006). A similar association also has been reported between increased DA efflux and increased locomotor activity or frequency of observable stereotypic behaviors in rats (Cadoni et al., 2000; Baumann et al., 2002; Di Chiara, 2002; Tanda et al., 2007). Finally, sensitization to abuse-related behavioral effects (i.e., motor, stereotypic, and self-administration behavior) of monoaminergic psychomotor stimulants seems to be accompanied by sensitization to their effects on DA efflux in rats (Cadoni et al., 2000; Di Chiara, 2002), although similar findings have not been reported in nonhuman primates (Bradberry, 2000; Bradberry and Rubino, 2006).
In view of the above-mentioned studies, it is noteworthy that there is little comparable evidence for a relationship between the discriminative stimulus effects of psychomotor stimulant drugs and their effects on extracellular concentrations of DA. In one study conducted in nonhuman primates, Czoty et al. (2004) reported that MA and other psychomotor stimulant drugs produced comparable increases in the levels of caudate DA at doses that substituted for MA in drug discrimination procedures, consistent with the idea that changes in brain DA plays a prominent role in such behavioral effects. However, these effects were not clearly dose-related, because doses of MA that only partially substituted for the training dose increased DA to levels similar to those seen with drugs that fully substituted for the training stimulus. In addition, responding on the MA-associated lever decreased over a period of time during which DA levels remained significantly above baseline values, suggesting that other mechanisms may come to regulate the initial dopaminergic actions of MA (Czoty et al., 2004).
The present study was conducted to further examine the discriminative stimulus effects of monoaminergic psychostimulants and, for MA, COC, and 1-[2-[bis(4-fluorophenyl) methoxy]ethyl]-4-(3-phenylpropyl)piperazine (GBR 12909), the relationship between such effects and changes in extracellular concentrations of DA in the shell of the NAcb as measured by in vivo microdialysis in rats. These latter three drugs were chosen to represent a monoamine-releasing compound (MA), a monoamine transport blocker (COC), and, with GBR 12909, a monoamine transport blocker that is generally considered to be DA-selective (Baumann et al., 2002; Howell and Kimmel, 2008). To evaluate the generality of such relationships across dissimilar drug discrimination procedures, two different groups of rats were trained to discriminate MA from saline using either a discrete-trial avoidance/escape procedure (Shannon and Holtzman, 1976; Holtzman, 2001) or a 20-response fixed-ratio (FR 20) schedule of food reinforcement to maintain behavior (Katz et al., 2004). Subsequently, the effects of monoamine releasers (MA and d-amphetamine) and transport blockers (COC, GBR 12909, and methylphenidate) were studied in both groups of trained rats. In other groups, the effects of cumulative doses of MA, COC, and GBR 12909 on extracellular concentrations of DA in the NAcb shell were measured over time and evaluated with regard to the results of drug discrimination experiments. Finally, the view that sensitization of the dopaminergic neurotransmitter system after repeated psychostimulant treatment may play a significant role in addiction-related effects of stimulant drugs (Ito et al., 2000; Robinson and Berridge, 2000) was examined by comparing data from microdialysis experiments in naive rats and drug-experienced rats after months of repeated exposure to MA in drug discrimination studies.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) weighing 275 g at the start of the study were used as subjects. Per institutional guidelines for cage density and depending on weight, subjects were housed singly or in groups of two or four per cage) in a climate-controlled environment under a 12-h light/dark cycle: lights on at 7:00 AM. All experiments were conducted 5 days/week from Monday to Friday between 8:00 AM and 6:00 PM. Subjects in the discrete-trial avoidance/escape and microdialysis procedures had unlimited access to food except during testing, whereas subjects in the food-reinforcement procedure were fed a daily ration of approximately 15 g of standard rodent chow at least 30 min after testing to maintain their body weights at a constant level. All subjects had unlimited access to water and were maintained in accordance with the guidelines of the Institute of Laboratory Animal Resources (1996). In addition, all research procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee.
Drugs
MA, COC, d-amphetamine, and methylphenidate (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline. GBR 12909 (Sigma-Aldrich) was dissolved in vehicle which was, by volume, a 20:60:20 mixture of 95% ethanol, 0.9% saline, and Emulphor (Alkamuls EL-620; Rhône-Poulenc, Cranbury, NJ). Doses of each drug were calculated on the basis of free base weight and expressed either as milligrams per kilogram or as micromoles per kilogram (1 mg/kg = 6.7 μmol/kg MA, 3.3 μmol/kg COC, 7.4 μmol/kg d-amphetamine, 4.3 μmol/kg methylphenidate, and 2.2 μmol/kg GBR 12909). All drugs were administered by intraperitoneal injection.
Apparatus
Drug discrimination experimental chambers were housed in light- and sound-attenuating enclosures, each provided with a fan for circulation (Med Associates, Inc., St. Albans, VT). The chambers used for the discrete-trial avoidance/escape procedure contained a single lever (observation lever) on the back wall and two levers (response levers) on the front wall; the two response levers were separated by a Plexiglas divider that extended approximately 5 cm into the chamber. The divider ran from the chamber ceiling to a grid floor through which a brief low-intensity electric stimuli (111 Hz in 250-ms pulses) could be delivered (Med Associates, Inc.). Under the food reinforcement procedure, the operant chamber was equipped with a feeder and a tray for food delivery that was centered on the front wall beneath the two response levers; continuous white noise masked extraneous sounds (Med Associates, Inc.). In both types of chamber, the two response levers were set 17 cm apart and required a downward force of 0.4 N to produce an audible click in the chamber and to be recorded as a response. A lamp located on the back panel provided ambient illumination (house light), and light-emitting diodes (LEDs) located directly above the response levers served as stimulus lights. Experimental variables were controlled, and data were collected by personal computers with interfacing equipment and operating software from Med Associates, Inc.
MA Discrimination
Discrete-Trial Avoidance/Escape.
A discrete-trial avoidance/escape procedure previously described by Holtzman and colleagues (Shannon and Holtzman, 1976; Holtzman, 2001) was used to train rats to discriminate intraperitoneal injections of 0.3 mg/kg MA (D) from saline (S) by differentially reinforcing responding on the two response levers after intraperitoneal injections. One response lever was associated with injections of MA, whereas the other response lever was associated with injections of saline; the assignment of MA- and saline-associated levers was counterbalanced across rats. MA and saline were administered under a double alternation schedule (DDSSD).
During training, rats were injected with drug or saline and then returned to the home cage for 15 min. At the end of the 15-min pretreatment period, rats were placed in the experimental chamber, and the first trial of the session was initiated by illumination of the ambient light and the onset of white noise. After 5 s, current (0.6–1.2 mA, adjusted for the individual subject) was delivered to the grid floor for 0.5 s out of every 3 s. Rats could end the trial and terminate the schedule of shock delivery by completing a two-response sequence: first, pressing the single observing lever, which turned off the white noise and house light and turned on the LEDs above the two response levers and, second, pressing the injection-associated (correct) response lever. A correct response turned off the LEDs above the levers and initiated a 60-s timeout (TO) period during which the chamber was dark, shock was not delivered, and responding had no scheduled consequences. A trial was recorded as “correct” if the two-response sequence was completed correctly on the first attempt; if the subject pressed the observation lever first followed by the incorrect response lever before pressing the correct response lever, the trial was recorded as “incorrect.” An incorrect response turned off the LEDs above the two response levers and restarted the house light and white noise, requiring another observing response by the subject before a choice could be registered. If this condition was not met, the trial terminated automatically after 60 s, initiating the 60-s TO that preceded the next trial. Each training session consisted of 20 trials.
Drug testing began after responding met the criterion of ≥85% correct (i.e., correct in at least 17 of 20 trials) in five consecutive training sessions. Initially, dose-response data were obtained for MA in each rat. Thereafter, substitution tests with other drugs were conducted when the above criterion was met in four of five successive training sessions, including the day immediately preceding the test session.
Test sessions were similar to training sessions with the following provisos: 1) the first press on either response lever after a response on the observing lever ended the trial; and 2) sessions consisted of four cycles with 15 trials per cycle. Each cycle was preceded by a 15-min period (30 min for GBR 12909) during which subjects were placed in their home cages; cumulative doses of drugs or consecutive injections of saline were administered intraperitoneally at the onset of the 15-min periods. Testing within a session was discontinued if 13 of the 15 trials (87%) were completed on the MA-associated lever or if the subject failed to respond on either lever for three consecutive trials within a cycle.
Fixed-Ratio Schedule of Food Reinforcement.
Subjects were first trained to press each of the two response levers under an FR 20 schedule of food reinforcement. Completion of the FR 20 response requirement occasioned the delivery of one 45-mg pellet (BioServe, Frenchtown, NJ) into the food tray. Subsequently, subjects were trained to discriminate intraperitoneal injections of 0.3 mg/kg MA from saline. After an intraperitoneal injection of MA, responses only on one lever were reinforced; after intraperitoneal injection of saline, responses only on the other lever were reinforced. As under the discrete-trial avoidance/escape schedule, the assignment of MA-associated and saline-associated levers was counterbalanced across rats and training sessions occurred in a double alternation sequence (e.g., SDDSS).
Each session began by placing the subject inside the experimental chamber 5 min after intraperitoneal injection. After a TO period during which all lights were extinguished and responding had no scheduled consequences (5 min), the house light and LEDs were illuminated and only completion of the FR 20 on the injection-appropriate (correct) lever was reinforced. Responses on the other lever (incorrect) reset the FR response requirement for food delivery. Presentation of each food pellet was followed by a 20-s timeout period during which all lights were extinguished and lever responses had no scheduled consequences. Sessions ended after each rat obtained 20 food pellets or after 15 min, whichever occurred first.
Testing began when the performance criteria of at least 85% injection-associated responding during the first FR 20 and throughout the session were met over four consecutive training sessions. Thereafter, test sessions were conducted whenever subjects met the above criteria for 2 consecutive training days in the sequence SD or DS. Test sessions consisted of four cycles of 25 min (TO plus session time during which the FR 20 schedule was in effect). Schedule parameters and contingencies during test sessions were identical to those in training sessions, with the exception that 20 successive responses on either lever were reinforced. During the test session, incremental doses of the test drug were administered at the start of each 25-min cycle (cumulative dosing). This procedure allowed determination of the effects of up to four cumulative doses during a single test session. When necessary, overlapping ranges of cumulative doses were studied in separate sessions to determine the effects of five or more doses of a drug. The effects of four consecutive intraperitoneal injections of saline or of a full range of doses of MA were determined for all rats before studies with other drugs were begun.
Microdialysis
Surgery.
Rats were injected with an anesthetic mixture of ketamine and xylazine (60 and 12 mg/kg i.p., respectively) and positioned in a stereotaxic apparatus where an incision was made in the scalp and a section of the skull was exposed. A small hole, approximately 2 mm in diameter, was drilled in the skull to expose the dura. Concentric dialysis probes (see below), aimed at the NAcb shell as described previously by Tanda et al. (2005), were then implanted in one hemisphere of the brain, chosen randomly. The probe was aimed at the NAcb shell according to the rat brain atlas of Paxinos and Watson (1987) (uncorrected coordinates: anterior = +2.0 mm, lateral = ±1.0 mm, and vertical = 7.9 mm; anterior and lateral coordinates were measured from bregma, and the vertical coordinate was measured from the dura).
In Vivo Microdialysis.
Using AN69 fibers (Hospal Dasco, Lyon, France), concentric dialysis probes were generated as described by Tanda et al. (2005). The probe materials included two 4-cm pieces of silica-fused capillary tubes; one tube designated as the inlet and the other as the outlet. Both the inlet and the outlet tubes were inserted into a 22-gauge stainless steel needle and were fixed to the needle at, respectively, 10 and 8 mm from the end of the needle by a small drop of glue. The inlet and outlet tubes were carefully inserted into a 5.5- to 6-mm capillary dialyzing fiber with the other opening closed by a drop of glue. The inlet tubing was set at approximately 0.1 mm from the closed end of the fiber, and the outlet was set at approximately 2.0 mm from the inlet tip. Once tubes were inserted, the end of the dialyzing fiber was glued to the 22-gauge needle (approximately 2.4 mm in length). The exposed dialyzing surface of the fibers not covered by the glue was limited to the lowest 2.0-mm portion of the probes. During surgery, the needle was held by a CMA/10 clip (CMA/Microdialysis AB, Solna, Sweden) mounted on a stereotaxic holder and inserted to the coordinates listed above. The probes were held in place with cement (GlasIonomer Cement CX-Plus, Henry Schein, Melville, NY). After surgery, rats were placed into hemispherical CMA-120 cages (CMA/Microdialysis AB) and allowed to recover overnight. The CMA-120 cages were equipped with overhead fluid swivels (Instech Laboratories Inc., Plymouth, PA) for connection to the dialysis probes.
Approximately 24 h after implantation of probes, microdialysis studies were conducted on freely moving rats in the same CMA-120 hemispherical cages in which the rats recovered overnight from surgery. Ringer's solution (147.0 mM NaCl, 2.2 mM CaCl2, and 4.0 mM KCl) was delivered at a constant flow rate of 1 μl/min through the dialysis probes using a 2.5-ml syringe (Hamilton Co., Reno, NV) attached to a CMA/102 Microdialysis Pump (CMA Microdialysis AB). The first dialysate sample (10 μl) was taken approximately 20 to 30 min after the pump was started, and thereafter samples were taken every 10 min and DA content was analyzed immediately as described below. Drug or vehicle testing began after approximately 1 h and after three successive DA values with less than 10% variability were obtained. Samples were then taken every 10 min for approximately 300 min after the first injection. Each subject was used only once and received drug treatments as detailed below.
Analytical Procedure.
Dialysate samples of 10 μl without purification were injected into a high-performance liquid chromatography system consisting of an MD 150 × 3.2 mm column (particle size 3.0 μm; ESA Inc., Chelmsford, MA) and a coulometric detector (5200a Coulochem II; ESA) to quantify DA. Oxidation and reduction electrodes of the analytical cell (5014B; ESA Inc.), respectively, were set at +125 mV and −175 mV. The mobile phase consisting of 100 mM NaH2PO4, 0.1 mM Na2EDTA, 0.5 mM n-octyl sulfate, and 18% (v/v) methanol (pH adjusted to 5.5 with Na2HPO4) was pumped at 0.6 ml/min by a solvent delivery module (582; ESA Inc.). DA assay sensitivity was 2 fmol/sample.
Histology
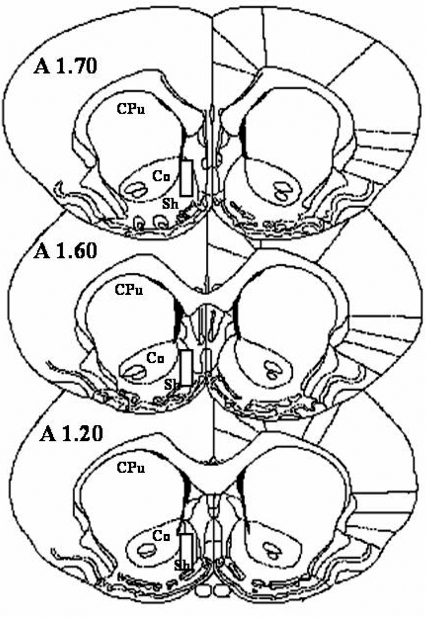
At the end of the experiment, rats were euthanized with an overdose of pentobarbital and decapitated, and the brains were removed and fixed in 4% formaldehyde in saline solution. After approximately 3 to 5 days, brains were placed in a 30% sucrose-0.1 M phosphate-buffered saline solution, pH 7.4, for at least 2 days. Using a sliding microtome, brains were then cut into 50-μm serial coronal sections oriented according to Paxinos and Watson (1987) and observed under a microscope to identify the location of the probes. The position of the probe was verified in all rats, and the dialyzing portion of all probes implanted in the NAcb shell is shown schematically in Fig. 1. For simplicity, all probe placements are shown on only one side, whereas the hemisphere into which the probe was implanted varied between left and right sides randomly across subjects. The anterior coordinates measured from bregma are shown in Fig. 1. Data for rats with probe placements outside the NAcb shell were excluded from the study.
Fig. 1.
The NAcb, the major terminal areas of the mesolimbic dopaminergic system. The superimposed rectangles show the limits of the positions of the dialyzing portions of the microdialysis probes. Sh, NAcb shell; Co, nucleus accumbens core; CPu, caudate putamen. [Based on Paxinos and Watson (1987).]
Drug Testing
Using cumulative dosing procedures, incremental doses of test drugs or vehicle were administered every 30 min after collection of baseline DA samples, and samples were collected at 10, 20, and 30 min after each injection. This procedure allowed determination of the effects of up to three or four cumulative doses during a single test session. Although differences in onset to action among drugs preclude comparisons of absolute potency with cumulative dosing, these procedures allowed us to evaluate the association between changes in DA levels and MA-like discriminative stimulus effects at comparable time points within experimental sessions.
Data Analysis
MA Discrimination.
The effects of drugs in MA discrimination studies are expressed in terms of their ability to elicit responding on the MA-associated lever and alter either latencies to complete the response requirement (discrete-trial avoidance/escape) or baseline rates of responding (food presentation). Under discrete-trial avoidance/escape conditions, the percentage of responding on the MA-associated lever in each component of a test session was calculated by dividing the number of trials completed on the MA-associated lever by the total number of trials (i.e., correct responses/15); the time from the onset of each trial to the completion of the two-response chain was recorded as the latency of the trial. Under food reinforcement conditions, the percentage of responses on the MA-associated lever and the overall response rate in a test component were calculated for each subject by dividing, respectively, the number of responses on the MA-associated lever by the total number of responses and the total number of responses by the time during which responses were recorded. Data for each dose of a drug were averaged across individual rats to provide mean values (±S.E.M.) for the group (n = 6–11) of subjects. Under the food reinforcement procedure, data for any rat that failed to emit at least 20 responses on either lever were excluded from the calculation of the mean percent MA-associated responding. If fewer than three subjects responded at a particular dose, no mean value was calculated for percent MA-associated responding at that dose. Data were further analyzed by comparison to the effects of vehicle injection and 0.3 mg/kg MA. Doses of a drug that produced a mean of >80% responding on the MA-associated lever were considered to substitute fully for 0.3 mg/kg MA.
When appropriate, averaged dose-effect data for the two groups were analyzed using standard linear regression and analysis of variance (ANOVA) techniques. ED50 values and their 95% confidence limits were determined from points on the linear part of the ascending portions of the dose-effect curves (Snedecor and Cochran, 1967) and converted to micromoles per kilogram values. In all cases, significance was defined at the 95% level of confidence (p < 0.05). Thus, when the effects of drugs under the different procedures were compared, pairs of ED50 values were considered to differ significantly only if their 95% confidence limits did not overlap. The effects of all drugs on mean latency and rates of responding under the discrete-trial avoidance/escape procedure and the food presentation procedure, respectively, were analyzed using paired t test comparisons with control values.
In Vivo Microdialysis.
Data from in vivo microdialysis experiments are expressed as a percentage of basal levels of DA. Basal levels were calculated as the mean of values from three to five consecutive samples that were taken immediately before the first drug or vehicle injection and displayed <10% variability. All results are presented as group means (± S.E.M.), and data were analyzed using a two-way ANOVA (drug and time as factors) for repeated measures over time; overall changes from basal levels were subjected to Tukey post hoc analyses. The effects of each cumulative dose of a drug were determined by averaging the three samples after its injection. ED50 values and the percentage increases in stimulation of DA levels at effective doses for each drug were determined as detailed above.
Results
Drug Discrimination
Acquisition of MA Discrimination under Different Training Conditions.
Based on averaged data (mean ± S.E.M.), subjects trained to discriminate injections of 0.3 mg/kg MA from vehicle under the discrete-trial avoidance/escape procedure and under the schedule of food presentation reached criterion levels of lever selection in approximately the same number of daily sessions, 45 ± 3 and 38 ± 5 days, respectively.
Discriminative Stimulus Effects of Monoaminergic Stimulants in MA-Trained Subjects.
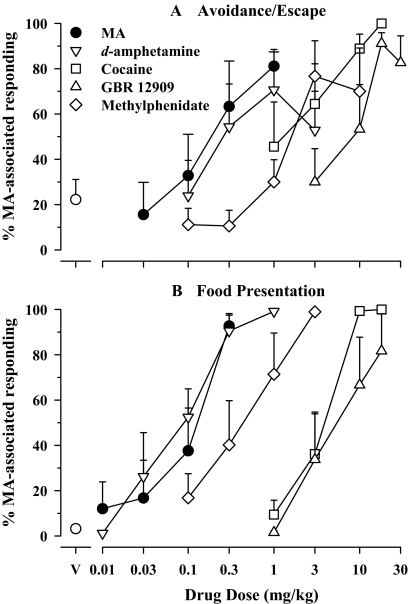
Cumulatively administered MA (0.03–1.0 mg/kg) produced significant and dose-dependent increases in MA-associated responding in rats trained to discriminate 0.3 mg/kg MA under the discrete-trial avoidance/escape and food presentation schedules (Fig. 2, A and B, respectively, ●). Grouped data suggest some differences in the potency of MA in the two groups; mean ED50 values for substitution by MA under the discrete-trial avoidance/escape and food presentation schedules were 1.27 and 0.57 μmol/kg, respectively (Table 1), suggesting an approximately 2-fold greater potency under the latter schedule. In agreement with this difference in potency during testing, a cumulative intraperitoneal dose of 0.3 mg/kg MA, which served as the training dose for both groups, met the criterion for full substitution under the food presentation schedule but produced only approximately 65% responding on the MA-associated lever under the discrete-trial avoidance/escape schedule. Notwithstanding these observations, confidence intervals for ED50 values under the two schedules overlapped, precluding statistical confirmation of difference in potency.
Fig. 2.
Effects of MA and related compounds in rats trained to discriminate injections of MA (0.3 mg/kg) from saline under a discrete-trial avoidance/escape procedure (top) and under an FR 20 schedule of food reinforcement (bottom). A, ordinates: percentage of trials completed on the MA-associated key. B, percentage of responses on the MA-associated key. Abscissae, cumulative drug dose in milligrams per kilogram (log scale). Each point represents the mean (±S.E.M.) effect in at least six rats. V, vehicle.
TABLE 1.
Doses calculated to produce 50% responding on the MA-associated lever (ED50) and their 95% confidence limits for MA, d-amphetamine, COC, methylphenidate, and GBR 12909 in rats trained to discriminate intraperitoneal injections of 0.3 mg/kg (2.01 μmol/kg) MA from saline under the two training procedures
| Drug | Avoidance/Escape |
Food Presentation |
||
|---|---|---|---|---|
| Dose | ED50 (95% CL) | Dose | ED50 (95% CL) | |
| mg/kg (μmol/kg) | μmol/kg | mg/kg (μmol/kg) | μmol/kg | |
| MA | 0.03–1.0 (0.20–6.70) | 1.27 (0.45–4.07) | 0.01–0.3 (0.07–2.01) | 0.57 (0.30–1.45) |
| d-Amphetamine | 0.1–1.0 (0.74–7.40) | 1.65 (0.03–4.88) | 0.01–1.0 (0.07–7.40) | 0.39 (0.16–0.70) |
| COC | 1.0–18 (3.3–59.34) | 4.33 (0.23–9.79) | 1.0–10 (3.3–32.96) | 11.50 (8.28–15.27) |
| Methylphenidate | 0.3–3.0 (1.29–12.86) | 6.08 (3.71–13.28) | 0.1–3.0 (0.43–12.86) | 1.78 (0.79–3.37) |
| GBR 12909 | 3.0–30 (6.66–66.58) | 14.53 (4.32–24.73) | 1.0–18 (2.22–39.95) | 12.39 (6.05–29.28) |
d-Amphetamine, COC, and methylphenidate also produced dose-related increases in responding on the MA-associated lever and fully substituted for the MA training dose under both schedules (Fig. 2, A and B, ▿, □, and ♢, respectively). As with MA, potencies tended to differ somewhat between the two schedules. Based on mean ED50 values, d-amphetamine and methylphenidate were approximately 4-fold more potent under the schedule of food presentation (0.39 and 1.78 μmol/kg, respectively) than under the discrete-trial avoidance/escape procedure (1.65 and 6.08 μmol/kg, respectively), whereas COC was approximately 3-fold more potent under conditions involving discrete-trial avoidance/escape (4.33 μmol/kg) than food reinforcement (11.5 μmol/kg) (Table 1). However, as with MA, the confidence intervals for d-amphetamine and COC, but not for methylphenidate, overlapped, suggesting that the observed differences in intraperitoneal potency of drugs under the two experimental conditions were not significant (Table 1). Like the other monoaminergic psychomotor stimulants, GBR 12909 also produced dose-related increases in responding on the MA-associated lever; the cumulative dose of 18 mg/kg resulted in ≥90% responses on the MA-associated lever under both schedules (Fig. 2, A and B, ▵). Mean ED50 values for GBR 12909 were highly comparable under the discrete-trial avoidance/escape and food presentation schedules (14.5 and 12.4 μmol/kg, respectively).
Under the discrete-trial avoidance/escape procedure, the average latency to complete the two-response chain after the highest cumulative dose of drugs did not vary significantly from latency values during the corresponding component of test sessions when vehicle was studied (t ≤ 2.08; p > 0.05) (Table 2). Analogously, although drugs may have produced some fluctuations in response rates under the schedule of food presentation, response rates after the highest doses of drugs (except COC) did not differ significantly from values obtained for injections of vehicle before the corresponding components of test sessions (t ≤ 2.01; p > 0.05) (Table 2). The highest cumulative dose of intraperitoneal COC, 18 mg/kg, decreased response rates by approximately 40% from values obtained with vehicle injection.
TABLE 2.
Effects of MA, d-amphetamine, COC, methylphenidate, and GBR 12909 on mean latency to complete a trial and rates of responding as a percentage of control values under the discrete-trial avoidance/escape procedure and the food presentation procedure
Data are means ± S.E.M.
| Drug | Dose | Mean Latency | Dose | Response Rate |
|---|---|---|---|---|
| mg/kg | s | mg/kg | responses/min | |
| Vehicle | 11.48 (±1.81) | 94.94 (±3.13) | ||
| MA | 1.0 | 7.30 (±1.57) | 0.3 | 108.20 (±12.14) |
| d-Amphetamine | 3.0 | 15.11 (±6.26) | 1.0 | 84.94 (±19.57) |
| COC | 18 | 8.16 (±1.66) | 18 | 59.23 (±13.47) |
| Methylphenidate | 10 | 14.31 (±7.44) | 3.0 | 104.02 (±17.37) |
| GBR 12909 | 30 | 13.37 (±7.37) | 18 | 101.17 (±17.48) |
In Vivo Microdialysis
Control Values for Extracellular DA in NAcb Shell.
Control values obtained after acclimatization to the experimental environment showed little variability across subjects. Five successive injections of saline administered every 30 min did not significantly increase extracellular levels of DA in dialysate samples from the NAcb shell above basal values (Fig. 3, ○). Absolute values for levels of extracellular DA levels were 11.3 ±2 (S.E.M.) fmol/sample.
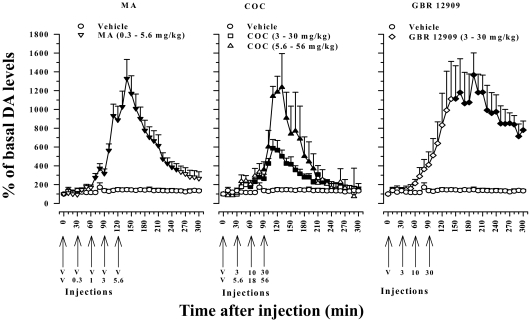
Fig. 3.
Time course of the effects of systemic cumulative administration of vehicle (V), MA (left, 0.3–5.6 mg/kg), COC (center, 3–30 mg/kg; 5.6–56 mg/kg), and GBR 12909 (right, 3–30 mg/kg) on extracellular levels of DA in dialysates samples taken from the NAcb. Arrows indicate the time at which incremental cumulative injections were administered. Ordinates, percentage of basal DA levels; abscissae, time in minutes after injection. Each point represents the mean (±S.E.M.) effect expressed as a percentage of basal values, uncorrected for probe recovery. All data points for all drugs are generated from n of at least four to six subjects. Filled symbols represent values significantly different from basal DA values (p < 0.05).
Effects of MA, COC, and GBR 12909 on Extracellular DA Levels in NAcb Shell.
As shown in Fig. 3 and confirmed by two-way ANOVA, cumulative doses of MA and COC produced dose-related increases in DA levels above vehicle values [drug (F1,12 = 11.82, p < 0.05), time (F30,360 = 9.01, p < 0.05), and drug × time interaction (F30,360 = 9.17, p < 0.05)]. Thus, the lowest dose of MA (0.3 mg/kg; 2.01 μmol/kg) and COC (3.0 mg/kg; 8.83 μmol/kg) did not consistently increase levels of DA above vehicle values (p > 0.05), whereas higher doses of cumulatively administered MA (1.0–5.6 mg/kg; 6.70–37.52 μmol/kg) or COC (10–56 mg/kg; 32.96–184.60 μmol/kg) produced rapid increases in levels of DA after each injection (p < 0.05). The maximal effects of the highest cumulative doses of MA (5.6 mg/kg) and COC (56 mg/kg) were comparable and evident within 20 min after administration as an approximately 10-fold or greater increase in extracellular levels of NAcb DA (Fig. 3). Levels of DA returned to near baseline vehicle values approximately 120 min after administration of 5.6 mg/kg MA or 56 mg/kg COC and remained at that level for the remainder of the observation periods (Fig. 3, left and center).
As for MA and COC, cumulative administration of GBR 12909 (3.0–30 mg/kg; 6.66–66.58 μmol/kg) also produced significant and dose-dependent increases in extracellular levels of DA in the NAcb shell [two-way ANOVA, main effect of drug (F1,6 = 12.24, p < 0.05), time (F30,180 = 7.29, p < 0.05), and drug × time interaction (F30,180 = 7.61, p < 0.05)] (Fig. 3, right panel, ♢). Variability among samples precluded statistical significance in the effects of each cumulative dose of GBR 12909, although increases in DA levels approached significance in one or more of the three samples collected after each injection (p = 0.061–0.094). As shown in Fig. 3, a >10-fold increase in extracellular levels of DA above mean (± S.E.M.) baseline values (1367 ± 235%) occurred approximately 70 to 100 min after the last cumulative dose of GBR 12909 (30 mg/kg). The effects of this cumulative dose of GBR 12909 were significant and persisted over the 5-h observation period (p < 0.05).
Relationship between Behavior and Extracellular Levels of DA.
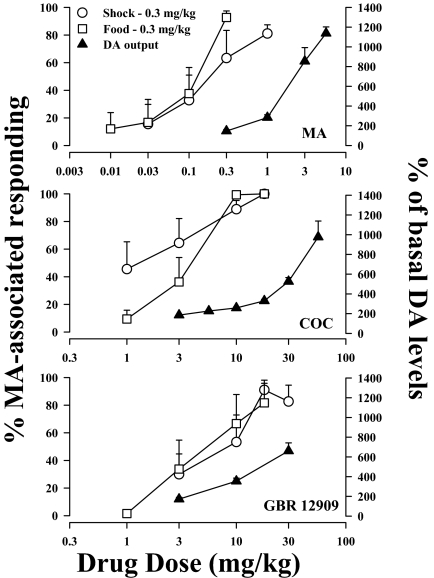
Figure 4 shows the relationships between drug dose, the MA-like discriminative stimulus effects of MA, COC, and GBR 12909, and their effects on DA efflux as a percentage of basal values. As stated above, cumulative administration of MA, COC, and GBR 12909 produced dose-dependent increases in extracellular levels of DA in the NAcb shell (Fig. 4, ▴). In the samples collected within 30 min after each cumulative dose, the highest cumulative doses of MA, COC, and GBR 12909 increased mean (± S.E.M.) levels of DA to 1138 ± 65, 976 ± 162, and 661 ± 81% of basal values, respectively. The ED50 values, reflecting the potency with which MA, COC, and GBR 12909 increased levels of DA in the NAcb shell, are 9.3 μmol/kg (1.4 mg/kg) (95% confidence limits 6.6–13.0), 59.3 μmol/kg (18 mg/kg) (45.9–80.7), and 16.2 μmol/kg (7.3 mg/kg) (219.8–2821), respectively. Doses of drugs that had comparable effects in MA discrimination experiments generally produced similar increases in extracellular levels of DA, although some differences in the effects of COC and GBR 12909 compared with MA are apparent (Fig. 4; Table 3). For example, doses of MA associated with maximum MA-like discriminative stimulus effects under the schedule of food presentation were associated with an approximate doubling of mean basal levels of DA in the NAcb shell whereas, for COC and GBR 12909, comparable behavioral effects were associated with an approximately 4-fold increase in extracellular levels of DA in the NAcb shell (Fig. 4; Table 3).
Fig. 4.
Behavioral effects of MA, COC, and GBR 12909 in rats trained to discriminate MA under the two drug discrimination procedures and changes in extracellular levels of DA in the NAcb of drug-naive rats. Left ordinates, percentage of responding on the MA-associated key; right ordinates, percentage of basal DA levels. Each point in the dialysis data represents the average of the three samples taken at each time point (10, 20, and 30 min) after each cumulative injection; abscissae, cumulative drug dose in milligrams per kilogram.
TABLE 3.
Percentage increases in the levels of extracellular concentrations of DA in the NAcb shell at ED50 doses (from Table 1) and at doses that produce maximal MA-associated responding in subjects trained to discriminate 0.3 mg/kg MA (see Fig. 4)
| Avoidance/Escape |
Food Presentation |
|||
|---|---|---|---|---|
| ED50 | Maximum Effect | ED50 | Maximal Effect | |
| % basal DA (95% CI) | ||||
| MA | 174 (5–342) | 329 (191–468) | 153 (−19 to 326) | 195 (31–359) |
| COC | 134 (47–220) | 380 (320–441) | 166 (85–247) | 380 (320–441) |
| GBR 12909 | 255 (14–497) | 449 (240–659) | 239 (−12 to 490) | 449 (240–659) |
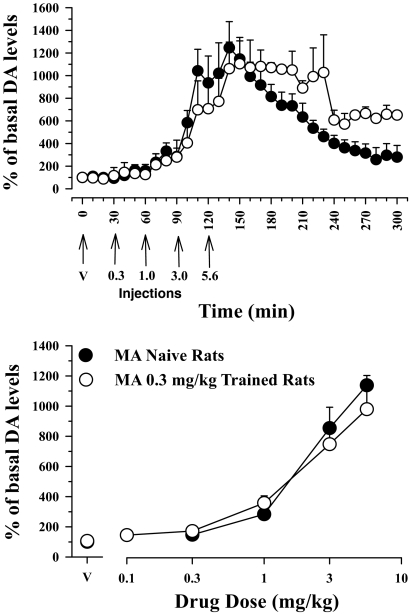
DA Levels in Naive and MA-Trained Subjects.
Figure 5 shows the effects of cumulative doses of MA on extracellular levels of DA in the NAcb shell in naive rats (data from Fig. 4) and rats that served in the MA discrimination studies using food reinforcement. The effects of vehicle injections at the outset of the session were similar in the two groups of subjects (Fig. 5, compare ● and ○); absolute values for levels of extracellular DA levels were 11.3 ± 2 and 13.6 ± 4 fmol/sample. Cumulative administration of MA in MA-trained rats produced a significant dose- and time-dependent increase in extracellular levels of DA in dialysate from the NAcb shell. Data in Fig. 5 show that the effects of lower cumulative doses of MA (0.1 and 0.3 mg/kg) were generally comparable in naive and MA-trained rats but that some slight differences may have emerged as the cumulative dose of MA increased. For example, although variability among individual subjects did not permit statistical confirmation in grouped data, the effects of the high cumulative dose of 5.6 mg/kg MA appear somewhat diminished in MA-trained rats. Further examination of the data indicates that 5.6 mg/kg MA in MA-trained rats produced increases in mean (± S.E.M.) extracellular levels of DA in the NAcb shell that persisted for nearly 3 h [from approximately 50 min (1081 ± 146%) after injection of this dose until 230 min (1028 ± 333%) of the observation period] (Fig. 5, top). In contrast, the effects of the same cumulative dose of MA in MA-naive subjects declined in an orderly fashion within the same time period (50 min = 916 ± 132%; 230 min = 461 ± 67%) (Fig. 5, top). The ED50 values for the MA dose-effect functions in naive rats and in MA-trained rats were, respectively, 9.32 μmol/kg (1.39 mg/kg; 95% confidence limits: 6.56–13.04) and 7.87 μmol/kg (1.17 mg/kg; confidence limits 4.85–12.48), confirming that the position and slope of the two functions were not significantly different.
Fig. 5.
Effects of cumulatively administered MA on extracellular levels of DA in the NAcb in drug-naive rats (data from Fig. 4; n = 6) and rats trained to discriminate 0.3 mg/kg MA under the food reinforcement schedule (n = 6). Ordinates, percentage of basal DA levels; abscissae top, time in minutes after injection; abscissae bottom, cumulative drug dose in milligrams per kilogram (log scale). V, vehicle.
Discussion
The main objective of the present research was to further characterize the relationship between behavioral effects of MA and related psychomotor stimulant drugs that may be associated with their influence on extracellular concentrations of DA. In these studies, reliable discriminative stimulus control over behavior was established by 0.3 mg/kg MA in rats trained under both a discrete-trial avoidance/escape procedure and an FR schedule of food reinforcement. The number of training sessions required to achieve criterion levels of discrimination were comparable under the two sets of conditions. These observations are consistent with previous findings indicating that differences in drug discrimination procedures do not appreciably alter the course of acquisition (Holtzman, 1986; Munzar and Goldberg, 2000).
Dose-related increases in responding on the injection-associated lever and full substitution for the training dose of MA by each indirect DA agonist were evident under both schedule conditions. However, some apparent differences in drug potency were observed under the two schedules. Based on ED50 values, for example, COC was approximately 3 times more potent under discrete-trial avoidance/escape conditions than under the schedule of food reinforcement. In contrast, other drugs were either equipotent under the two schedule conditions (GBR 12909) or approximately 4 times more potent under the schedule of food presentation than under the discrete-trial avoidance/escape schedule (MA, d-amphetamine, and methylphenidate). In the absence of studies to control or systematically vary potentially contributing factors, the basis for apparent differences in potency in the two procedures are unclear. Drugs were tested in irregular order in the two groups; thus, it is unlikely that prior testing experience played a role in differences in drug potency. Pretreatment times were time-locked across procedures, and it is unlikely that differences in potency can be explained by schedule-related differences (i.e., avoidance/escape versus food presentation). Differences in onset of action and dopaminergic mechanism also do not seem to explain the apparent differences in potency. For example, COC and the amphetamines have very quick onset to action by the intraperitoneal route, yet their relative potencies were in opposite directions in the two drug discrimination procedures. Methylphenidate and COC are transport blockers; again, they displayed relative potencies that were directionally opposite in the two procedures. On the other hand, GBR 12909, which can have somewhat variable onset to action, was similar in potency in the two procedures.
The effects of drugs in the present study can be compared with previous findings in rats trained to discriminate a higher dose of MA (1.0 mg/kg) from vehicle under a schedule of food reinforcement similar to the one used in the present studies (Munzar and Goldberg, 2000). Based on ED50 values, MA appears to be approximately 6-fold more potent under the lower of the two training dose conditions (0.3 versus 1.0 mg/kg), whereas COC and GBR 12909 appear to be <2-fold more potent under the lower training dose condition. Such differences in the potency of MA are not surprising and probably reflect dose-related differences in the stimulus intensity of the training dose of MA. However, it is somewhat surprising that potency differences of a comparable magnitude were not observed with either COC or GBR 12909. Although speculative in the absence of additional data, it is possible that the relationship between drug dose and stimulus intensity function differs between drugs that primarily release monoamines (e.g., MA) and drugs that block their uptake into the presynaptic neuron (e.g., COC or GBR 12909).
A close association between extracellular levels of DA in striatal areas and abuse-related behavioral effects of MA and other psychomotor stimulant drugs has been observed previously in intravenous self-administration studies but has not been extensively studied in drug discrimination procedures. To our knowledge, only one study has documented the relationship between levels of DA in targeted brain regions and the discriminative stimulus effects of MA and other psychomotor stimulant drugs (Czoty et al., 2004). As in that and related studies (Kuczenski et al., 1991; Schad et al., 1995; Baumann et al., 2002; Tanda et al., 2007), cumulative doses of MA, COC, and GBR 12909 in the present experiments produced significant dose- and time-dependent increases in DA levels in the NAcb shell. The graded increases in DA levels in the NAcb shell that were observed in the present study corresponded with graded increases in MA lever selection produced by cumulative doses of MA, COC, and GBR 12909. For example, doses of all three drugs that produced less than 40% MA-associated responding failed to substantially increase extracellular levels of DA in the NAcb shell, whereas doses of drugs that fully substituted for the training dose most often increased levels of DA in the NAcb shell to ≥300% of control values (Table 3). In conjunction with previous findings in monkeys, the present results in rats indicate, first, the general nature of the relationship between the stimulant-like discriminative stimulus effects of indirect monoaminergic agonists and their ability to increase extracellular DA and, second, the fact that a relatively moderate increase in extracellular DA suffices to produce such behavioral effects. This latter point is highlighted by the substantial difference in magnitude of effect on extracellular levels of DA after doses of stimulant drugs that fully substituted for the training dose of MA and doses of each drug that were 3- to 10-fold higher (Fig. 4).
The time courses for the accumulation of extracellular DA after treatment with the different psychomotor stimulants in the present experiments also are in general agreement with previous reports. Thus, MA and COC elicited relatively rapid increases in DA levels, whereas GBR 12909 produced a slow, but longer lasting effect on peak increases in extracellular levels of DA. Peak levels of DA in the NAcb shell were observed within approximately 30 min after the highest cumulative doses of MA and COC and approximately 90 min after the highest cumulative dose of GBR 12909. Unlike COC and MA, GBR 12909 led to levels of DA that remained at >800% 5 h after the highest dose, providing further support for the view that GBR 12909 enters the brain more slowly than MA or COC and has effects that may be more long-lived (Baumann et al., 2002; Desai et al., 2005).
The discriminative stimulus effects of MA and related psychomotor stimulants were assessed in rats that were behaviorally trained and repeatedly exposed to MA, whereas DA levels were primarily measured in experimentally naive rats. In this regard, previous studies have shown that repeated treatment with psychomotor stimulant drugs may lead to sensitization to their DA-mediated behavioral and neurochemical effects. However, such actions usually occur under relatively restrictive circumstances and in particular brain regions. For example, Cadoni et al. (2000) and Di Chiara (2002) reported that, in behaviorally sensitized rats, repeated treatment with either amphetamine (single injection) or COC (twice a day) for 10 or 14 days, respectively, led to sensitization to their effects on DA transmission in the core portion of the NAcb, whereas the efflux of DA was not significantly increased, and, after a high dose of cocaine, was even reduced in the shell portion (Cadoni et al., 2000). Notwithstanding some evidence for prolonged effects of the highest dose of MA, the results of the present experiments generally agree with those findings and do not reveal clear evidence of neurochemical sensitization after extensive exposure to MA in drug discrimination studies. The reason for the absence of clear signs of neurochemical sensitization in the present results is not immediately apparent. Previous studies have highlighted the special importance of context and temporal factors in sensitization to psychomotor stimulant drugs. From this perspective, it may be that sensitization was not observed in the current experiments because DA levels were measured in an environment different from that in which subjects received daily drug injections (Ito et al., 2000; Weiss et al., 2000). However, subjects in the present experiments had received injections of MA in different environments (holding cages and experimental chamber) over the course of drug discrimination studies. The length of time between the last injection and measurement of DA levels (3–7 days) also is not likely to explain the lack of a sensitized DA response, because several studies have shown sensitization to psychomotor stimulant drugs when daily treatment of up to 10 days was followed by a challenge dose of a psychomotor stimulant drug up to 3 days after the final pretreatment (Reith et al., 1987; Kalivas et al., 1988; White and Kalivas, 1998). Although the possibility cannot be discounted, it would be surprising for environmental considerations per se to so overwhelmingly mask neurochemical sensitization, especially after the long history of repeated exposures to MA in the present studies.
Sensitization of the dopaminergic response in the NAcb shell has been implicated in addictive properties of psychomotor stimulant drugs, including an increase in intravenous self-administration behavior (Robinson and Berridge, 2000; Weiss et al., 2000; Everitt et al., 2001; Koob and Le Moal, 2001). It is perhaps noteworthy that most of this work has been conducted in rodent species and that addiction-related studies in other species have not provided parallel evidence. For example, Bradberry (2000, 2007) and Bradberry and Rubino (2006) reported no increase in DA response over time in striatal regions of rhesus monkeys after 32 weeks of cocaine self-administration. Similarly, imaging studies in drug-experienced humans have reported a lack of a sensitized DA response after chronic exposure to psychomotor stimulant drugs (Volkow et al., 1997; Castner et al., 2000). As in the present experiments, each of these studies involved testing after prolonged exposure to psychomotor stimulant drugs, which may have reduced the expression of sensitization. Taken together, these findings indicate that the expression of sensitization is not a necessary feature of addiction-related stimulus effects of psychomotor stimulant drugs.
Acknowledgments
We thank Dr. Gianluigi Tanda for his invaluable assistance during the conduct of these studies.
This work was supported by in part by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA07658, R01-DA10566] (to J.B.) and the Ruth L. Kirschstein National Service Award (to Nancy K. Mello).
Parts of this work were previously presented: Desai RI, Paronis CA, Makriyannis A, Thakur GA, and Bergman J (2007) Discriminative stimulus effects of methamphetamine and in vivo microdialysis in rats. Experimental Biology 2007; 2007 Apr 28–May 2; Washington, DC. American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.165746.
- MA
- methamphetamine
- COC
- cocaine
- DA
- dopamine
- GBR 12909
- 1-[2-[bis(4-fluorophenyl-)methoxy]ethyl]-4-(3-phenylpropyl)piperazine
- NAcb
- nucleus accumbens
- FR
- fixed-ratio
- LED
- light-emitting diode
- TO
- timeout
- ANOVA
- analysis of variance.
References
- Baumann et al., 2002.Baumann MH, Ayestas MA, Sharpe LG, Lewis DB, Rice KC, Rothman RB. (2002) Persistent antagonism of methamphetamine-induced dopamine release in rats pretreated with GBR12909 decanoate. J Pharmacol Exp Ther 301:1190–1197 [DOI] [PubMed] [Google Scholar]
- Bradberry, 2000.Bradberry CW. (2000) Applications of microdialysis methodology in nonhuman primates: practice and rationale. Crit Rev Neurobiol 14:143–163 [PubMed] [Google Scholar]
- Bradberry, 2007.Bradberry CW. (2007) Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology 191:705–717 [DOI] [PubMed] [Google Scholar]
- Bradberry and Rubino, 2006.Bradberry CW, Rubino SR. (2006) Dopaminergic responses to self-administered cocaine in Rhesus monkeys do not sensitize following high cumulative intake. Eur J Neurosci 23:2773–2778 [DOI] [PubMed] [Google Scholar]
- Cadoni et al., 2000.Cadoni C, Solinas M, Di Chiara G. (2000) Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol 388:69–76 [DOI] [PubMed] [Google Scholar]
- Callahan et al., 1997.Callahan PM, De La Garza R, 2nd, Cunningham KA. (1997) Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav 57:601–607 [DOI] [PubMed] [Google Scholar]
- Castner et al., 2000.Castner SA, al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB, Goldman-Rakic PS. (2000) Behavioral changes and [123I]IBZM equilibrium SPECT measurement of amphetamine-induced dopamine release in rhesus monkeys exposed to subchronic amphetamine. Neuropsychopharmacology 22:4–13 [DOI] [PubMed] [Google Scholar]
- Czoty et al., 2004.Czoty PW, Makriyannis A, Bergman J. (2004) Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology 175:170–178 [DOI] [PubMed] [Google Scholar]
- Desai et al., 2005.Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. (2005) Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther 315:397–404 [DOI] [PubMed] [Google Scholar]
- Di Chiara, 2002.Di Chiara G. (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137:75–114 [DOI] [PubMed] [Google Scholar]
- Di Chiara et al., 2004.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47:227–241 [DOI] [PubMed] [Google Scholar]
- Everitt et al., 2001.Everitt BJ, Dickinson A, Robbins TW. (2001) The neuropsychological basis of addictive behaviour. Brain Res Rev 36:129–138 [DOI] [PubMed] [Google Scholar]
- Holtzman, 1986.Holtzman SG. (1986) Discriminative stimulus properties of caffeine in the rat: noradrenergic mediation. J Pharmacol Exp Ther 239:706–714 [PubMed] [Google Scholar]
- Holtzman, 2001.Holtzman SG. (2001) Differential interaction of GBR 12909, a dopamine uptake inhibitor, with cocaine and methamphetamine in rats discriminating cocaine. Psychopharmacology 155:180–186 [DOI] [PubMed] [Google Scholar]
- Howell and Kimmel, 2008.Howell LL, Kimmel HL. (2008) Monoamine transporters and psychostimulant addiction. Biochem Pharmacol 75:196–217 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, 1996.Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Ito et al., 2000.Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. (2000) Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 20:7489–7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas et al., 1988.Kalivas PW, Duffy P, DuMars LA, Skinner C. (1988) Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther 245:485–492 [PubMed] [Google Scholar]
- Katz et al., 2004.Katz JL, Kopajtic TA, Agoston GE, Newman AH. (2004) Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309:650–660 [DOI] [PubMed] [Google Scholar]
- Kimmel et al., 2005.Kimmel HL, Ginsburg BC, Howell LL. (2005) Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse 56:129–134 [DOI] [PubMed] [Google Scholar]
- Kimmel et al., 2007.Kimmel HL, O'Connor JA, Carroll FI, Howell LL. (2007) Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav 86:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob and Le Moal, 2001.Koob GF, Le Moal M. (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129 [DOI] [PubMed] [Google Scholar]
- Kuczenski et al., 1991.Kuczenski R, Segal DS, Aizenstein ML. (1991) Amphetamine, cocaine, and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci 11:2703–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski et al., 1995.Kuczenski R, Segal DS, Cho AK, Melega W. (1995) Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci 15:1308–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzar and Goldberg, 2000.Munzar P, Goldberg SR. (2000) Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology 148:209–216 [DOI] [PubMed] [Google Scholar]
- Munzar et al., 2004.Munzar P, Tanda G, Justinova Z, Goldberg SR. (2004) Histamine H3 receptor antagonists potentiate methamphetamine self-administration and methamphetamine-induced accumbal dopamine release. Neuropsychopharmacology 29:705–717 [DOI] [PubMed] [Google Scholar]
- Paxinos and Watson, 1987.Paxinos G, Watson C. (1987) The Rat Brain in Stereotaxic Coordinates, Academic Press, Sydney, Australia [Google Scholar]
- Pettit and Justice, 1989.Pettit HO, Justice JB., Jr (1989) Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav 34:899–904 [DOI] [PubMed] [Google Scholar]
- Ranaldi et al., 1999.Ranaldi R, Pocock D, Zereik R, Wise RA. (1999) Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous d-amphetamine self-administration. J Neurosci 19:4102–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith et al., 1987.Reith ME, Benuck M, Lajtha A. (1987) Cocaine disposition in the brain after continuous or intermittent treatment and locomotor stimulation in mice. J Pharmacol Exp Ther 243:281–287 [PubMed] [Google Scholar]
- Robinson and Berridge, 2000.Robinson TE, Berridge KC. (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95 (Suppl 2):S91–S117 [DOI] [PubMed] [Google Scholar]
- Schad et al., 1995.Schad CA, Justice JB, Jr, Holtzman SG. (1995) Naloxone reduces the neurochemical and behavioral effects of amphetamine but not those of cocaine. Eur J Pharmacol 275:9–16 [DOI] [PubMed] [Google Scholar]
- Shannon and Holtzman, 1976.Shannon HE, Holtzman SG. (1976) Evaluation of the discriminative effects of morphine in the rat. J Pharmacol Exp Ther 198:54–65 [PubMed] [Google Scholar]
- Snedecor and Cochran, 1967.Snedecor GW, Cochran WG. (1967) Statistical Methods, 6th ed, Iowa State University Press, Ames [Google Scholar]
- Tanda et al., 2005.Tanda G, Ebbs A, Newman AH, Katz JL. (2005) Effects of 4′-chloro-3α-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther 313:613–620 [DOI] [PubMed] [Google Scholar]
- Tanda et al., 2007.Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. (2007) Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther 321:334–344 [DOI] [PubMed] [Google Scholar]
- Tidey and Bergman, 1998.Tidey JW, Bergman J. (1998) Drug discrimination in methamphetamine-trained monkeys: agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther 285:1163–1174 [PubMed] [Google Scholar]
- Volkow et al., 1997.Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830 [DOI] [PubMed] [Google Scholar]
- Walsh and Cunningham, 1997.Walsh SL, Cunningham KA. (1997) Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology 130:41–58 [DOI] [PubMed] [Google Scholar]
- Weiss et al., 2000.Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA 97:4321–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White and Kalivas, 1998.White FJ, Kalivas PW. (1998) Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend 51:141–153 [DOI] [PubMed] [Google Scholar]