Abstract
Conventionally, G protein-coupled receptors are thought to increase calcium via inositol 1,4,5-trisphosphate (InsP3). More recent evidence shows that an alternative second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP), also has a role to play, causing researchers to question established calcium releasing pathways. With the recent development, by our group, of cell-permeant NAADP (NAADP-aceteoxymethyl ester) and a selective NAADP receptor antagonist (Ned-19; 1-(3-((4-(2-fluorophenyl)piperazin-1-yl)methyl)-4-methoxyphenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid),the ability to investigate this signaling pathway has improved. Therefore, we investigated a role for NAADP in oxytocin-mediated responses in the rat uterus. Oxytocin- and NAADP-mediated effects were investigated by using contractile measurements of whole uterine strips from rat in organ baths. Responses were correlated to calcium release in cultured rat uterine smooth muscle cells measured by fluorescence microscopy. Inhibition of both oxytocin-induced contraction and calcium release by the traditional NAADP-signaling disrupter bafilomycin and the NAADP receptor antagonist Ned-19 clearly demonstrated a role for NAADP in oxytocin-induced signaling. A cell-permeant form of NAADP was able to produce both uterine contractions and calcium release. This response was unaffected by depletion of sarcoplasmic reticulum stores with thapsigargin, but was abolished by both bafilomycin and Ned-19. Crucially, oxytocin stimulated an increase in NAADP in rat uterine tissue. The present study demonstrates directly that NAADP signaling plays a role in rat uterine contractions. Moreover, investigation of this signaling pathway highlights yet another component of oxytocin-mediated signaling, stressing the need to consider the action of new components as they are discovered, even in signaling pathways that are thought to be well established.
The requirement for calcium in uterine contractions attracted attention as early as 1909 (Blair-Bell and Hick, 1909). During subsequent years, great progress has been made in understanding the mechanisms of action of calcium leading to this contraction. It is now well established that a rise in intracellular calcium in uterine smooth muscle causes activation of myosin light chain kinase and phosphorylation of myosin light chain, leading to contraction. This rise in calcium, as in many different cell types, is traditionally believed to be from two sources: extracellular calcium entry though plasma membrane calcium channels and release of calcium from the sarcoplasmic reticulum (Noble et al., 2009). Release from the sarcoplasmic reticulum is mediated through the action of agonists on plasma membrane G protein-coupled receptors predominantly leading to the production of the second messenger inositol 1,4,5-trisphosphate (InsP3). InsP3 acts at InsP3 receptors on the sarcoplasmic reticulum, causing them to open, allowing efflux of calcium into the cytosol. In addition, there are data supporting a role for the second messenger cyclic ADP-ribose (cADPR) acting at the alternative ryanodine receptors on the sarcoplasmic reticulum (Barata et al., 2004; Thompson et al., 2004).
There is, however, a growing body of evidence for an additional intracellular store. This store is found within acidic organelles and is accessed via production of the alternative second messenger nicotinic acid adenine dinucleotide phosphate (NAADP) (Guse and Lee, 2008). Since its discovery (Lee and Aarhus, 1995), NAADP signaling has been characterized in a number of invertebrate systems (Churchill et al., 2003; Santella, 2005) and mammalian systems (Cancela et al., 1999). This research has established NAADP as a unique calcium-signaling molecule (Patel, 2004; Lee, 2005; Galione et al., 2009) that releases calcium from a store independent of InsP3 and cADPR (Genazzani and Galione, 1996; Lee, 2000). Indeed, a number of recent studies have demonstrated that G protein-coupled signaling can occur not only via InsP3 but also through a NAADP component (Masgrau et al., 2003; Yamasaki et al., 2005; Macgregor et al., 2007; Naylor et al., 2009), which surprisingly includes the classical neurotransmitter glutamate (Pandey et al., 2009).
Oxytocin-mediated signaling highlighted one such possibility. The oxytocin receptor belongs to the G protein-coupled receptor family. It stimulates uterine contraction in a concentration-dependent manner by increasing intracellular calcium primarily through production of InsP3 and release from the sarcoplasmic reticulum (Wray, 2007). It has, however, been noted that prolonged treatment with thapsigargin to deplete the sarcoplasmic reticulum does not eliminate oxytocin-induced contractions (Shmygol et al., 2006), suggesting other processes may be involved. The non-InsP3 component has been most commonly explained by the action of RhoA, which activates Rho kinase to sensitize the contractile machinery to calcium (Kimura et al., 1996). Nevertheless, a study using isolated human myometrial cells demonstrated a role for lysosome-related, acidic calcium stores and NAADP in oxytocin-induced calcium increases (Soares et al., 2007).
Since the first report by Soares et al. (2007) of a role for NAADP in oxytocin-induced calcium increases in isolated human myometrial cells, we now know that the target of NAADP is the two-pore channel (Brailoiu et al., 2009; Calcraft et al., 2009; Galione et al., 2009; Zong et al., 2009), and we have developed selective chemical probes for agonizing (cell-permeant NAADP; Parkesh et al., 2008) or antagonizing (Ned-19; Naylor et al., 2009) the NAADP receptor. Using these selective pharmacological agents, we were able to investigate the role of NAADP in oxytocin action at not just the level of isolated cells, but also at the level of intact strips of rat uterine tissue. We conclude that NAADP plays a role in oxytocin-mediated contraction in the rat uterus.
Materials and Methods
Tissue Preparation.
Contractile studies were performed on samples of rat uterine tissue obtained from ex-breeder Wistar rats (Harlan UK Limited, Bicester, Oxon, UK). Rats were killed by using a rising concentration of CO2 followed by cervical dislocation. Uteri were excised and cleaned of adhering fat and mesentery. Whole tissue strips were cut into 10-mm lengths and mounted in organ baths (ADInstruments Ltd., Chalgrove, Oxfordshire, UK) that contained 2 ml of modified Krebs' solution. Strips were attached to an isometric force transducer (ADInstruments Ltd.) by 0.3-mm silk thread (Pearsalls Ltd, Taunton, UK). Transducer voltages were amplified and converted to digital signals, and measurements were recorded by using Chart version 5.5.6 (ADinstruments Ltd.). Strips were maintained at 37°C, aerated with 95% O2/5% CO2, and tensioned to 0.5 g. Preparations were allowed to equilibrate until the spontaneous phasic contractions became stable (60–90 min). The tissue was then challenged with 60 mM KCl to ensure contractile viability and determine maximum contraction.
Measurement of Uterine Contraction.
To determine the role of intracellular calcium in oxytocin-induced contractions strips were incubated with calcium-free Krebs solution containing 1 mM EGTA for 5 min followed by 10 min with oxytocin (remaining in calcium-free Krebs solution). After each addition of oxytocin strips were incubated in calcium containing Krebs solution for 10 min to replenish the stores before repeating the protocol with a higher concentration of oxytocin.
To determine the role of NAADP in oxytocin-induced contraction, strips were incubated for 1 h with either vehicle, 1 μM thapsigargin (sarco-endoplasmic reticulum calcium ATPase inhibitor), 3 μM bafilomycin (V-H+ ATPase inhibitor), or varying concentrations of Ned-19 (selective NAADP receptor antagonist; Naylor et al., 2009). After this incubation, strips were exposed to cumulative concentrations of oxytocin for 10 min at each concentration to produce a concentration-response curve.
To determine whether NAADP can induce contractions, strips of rat uterus were exposed to cumulative concentrations of NAADP-acetoxymethyl ester (NAADP-AM) for 1 h at each concentration. The pharmacology of the NAADP-induced contractions was determined by preincubating strips for 1 h with either vehicle, 1 μM thapsigargin, 3 μM bafilomycin, or 1 μM Ned-19, followed by the addition of cumulative concentrations of NAADP-AM every hour.
Smooth Muscle Cell Preparation.
Primary smooth muscle cells were prepared from rat uterine myometrium according to procedures described previously (Krizsan-Agbas et al., 2008) with some modifications. After adult female Sprague-Dawley rats were anesthetized with urethane (1.2 g/kg i.p.), the uteri were immediately excised and removed into cold Hanks' balanced salt solution (Mediatech, Herndon, VA). The myometrium was rapidly separated from the endometrium and stroma, and then minced into fragments (approximately 1 mm3). The tissues were digested for 1.5 h at 37°C by collagenase type II (600 units/ml; Sigma-Aldrich, St. Louis, MO) in Dulbecco's modified Eagle's medium/F12 culture medium, followed by trituration with a sterile Pasteur pipette and filtration through a cell strainer with 70-μm pores. After collection and two washes by centrifugation (100g, 10 min), the cells were resuspended in fresh Dulbecco's modified Eagle's medium/F12 medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin, and then plated on collagen-coated coverslips. The cultures were maintained at 37°C in an atmosphere of 5% CO2 and 95% humidified air, and the medium was changed every 2 to 3 days.
Measurement of Changes in Intracellular Calcium.
Cultured rat uterine smooth muscle cells were loaded with the fluorescent calcium indicator fura-2 (3 μM) by incubation of the cells in Hanks' balanced salt solution plus fura-2-acetoxymethyl ester for 45 min and Hanks' balanced salt solution alone for an additional 15 to 60 min to allow de-esterification of the dye. Coverslips were mounted in a 500-μl bath on the stage of a TE2000U Eclipse Nikon (Tokyo, Japan) inverted microscope equipped with a Photometrics (Tucson, AZ) CoolSnap HQ charge-coupled device camera. Fura-2 fluorescence (excitation at 340 and 380 nm, emission at 520 nm) of single cells was acquired at a frequency of 0.3 Hz. Experiments were analyzed off-line by using MetaFluor software (Molecular Devices, Sunnyvale, CA). Cells from four animals were used in this study.
To distinguish between oxytocin-mediated calcium release from intracellular stores and calcium influx from the extracellular space, 10 nM oxytocin was added to the bath in the presence of Hanks' balanced salt solution either with or without calcium (Mediatech).
To determine the role of NAADP in oxytocin-mediated calcium release, cells were incubated for 15 min with 1 μM thapsigargin, 60 min with 1 μM bafilomycin, or 15 min with 5 μM Ned-19, followed by the addition of 10 nM oxytocin.
To determine whether NAADP can induce calcium release, rat uterine smooth muscle cells were exposed to 4, 40, and 400 nM NAADP-AM. To ensure NAADP-AM was acting via the NAADP receptor, cells were preincubated with 5 μM Ned-19 for 15 min before the addition of 400 nM NAADP-AM.
To determine the selectivity of Ned-19, isolated uterine smooth muscle cells from rat were injected with either 5 μM NAADP or 5 μM InsP3 (final concentration of approximately 50 nM as 1% of the cellular volume is injected) after 10-min preincubation with 100 nM Ned-19. Injections were performed by using Femtotips II and the InjectMan NI2 and FemtoJet systems (Eppendorf AG, Hamburg, Germany). Pipettes were back-filled with an intracellular solution composed of 110 mM KCl, 10 mM NaCl, and 20 mM HEPES (pH 7.2) and supplemented with or without NAADP or InsP3 at the concentrations indicated. The injection time was 0.5 s at 60 hectopascals with a compensation pressure of 20 hectopascals.
Imaging of Two Distinct Stores.
Cultured rat uterine smooth muscle cells were incubated with 100 nM ER Tracker Green and 50 nM LysoTracker red or a combination of the two for 15 to 20 min. Cells were fixed in 4% (w/v) paraformaldehyde, mounted with Citifluor, and examined under a confocal laser-scanning microscope [Leica (Wetzlar, Germany) TCS SL] with excitation/emission wavelengths set to 488/520 nm for ER Tracker green and 543/620 nm for LysoTracker red in the sequential mode. Pinhole size was 1 Airy disc (95.5 μm), and focal depth was 616 nm.
Measurement of NAADP Levels.
Rat uterine tissue slices (650-μm thickness), prepared using a tissue chopper (Mickle Engineering, Surrey, United Kingdom), were triturated, and the resulting suspension was washed by centrifugation (800g for 3 min at 4°C). Cells were incubated at 37°C for 15 min before measurement of NAADP. The suspensions were then maintained in Hanks' balanced salt solution or stimulated with 10 nM oxytocin. Aliquots (200 μl) from each suspension were removed just before stimulation to determine the resting levels of NAADP and then at the times indicated. The samples were quenched by the addition of an equal volume of 1.5 M HClO4 followed by sonication [Jencons (Leicestershire, United Kingdon) Vibracell; amplitude 60 for 5 s]. The denatured protein was pelleted by centrifugation (9000g for 10 min). Supernatant fractions, containing the NAADP, were neutralized with an equal volume of 2 M KHCO3, and the samples were centrifuged (9000g for 10 min) to remove the resulting KClO4 precipitate. NAADP content was determined by using a radioreceptor assay based on binding sites for [32P]NAADP in sea urchin egg homogenates (Aarhus et al., 1996; Billington and Genazzani, 2000; Patel et al., 2000) as reported in detail (Lewis et al., 2007). In brief, the homogenates were incubated with the radioligand, either in the absence or presence of the extracts, and bound radioactivity was measured after rapid filtration. The NAADP content was then quantified by comparison of the reduction in radioligand binding by known concentrations of authentic NAADP. The results were normalized to the total protein content of the samples determined by using bichinconinic acid (Sigma-Aldrich).
Data Analysis.
Tension after the addition of either vehicle or antagonist was averaged over 10 min before the addition of stimulant (oxytocin or NAADP). This tension was subtracted from all subsequent measurements to correct for any effects of pretreatment. Contractile responses in rat uterine strips were then normalized. Zero percent was taken as 0 g tension. Maximum contraction (100%) was taken as the maximum contractile tension after the addition of 60 mM KCl. Contraction induced by oxytocin in calcium-containing buffer was measured as the average tension during the 10-min incubation. Contraction induced by oxytocin in calcium-free buffer was measured as the maximum tension during the 10-min incubation. Contraction induced by NAADP-AM was measured as the average tension during the final 10 min of the 1-h incubation. Data and representative traces were plotted, and EC50 values (geometric mean with 95% confidence intervals) were calculated with Prism software (GraphPad Software Inc., San Diego, CA). EC50 values, along with maximal responses to oxytocin, were compared with control (vehicle-treated strips) by using unpaired Student's t test (Prism; GraphPad Software Inc.).
Calcium responses in isolated cells from rat uterine smooth muscle were normalized to resting calcium levels. To determine the fold increase in intracellular calcium, the maximum ratio unit after addition was divided by the basal ratio units before addition. Bar charts and representative traces were plotted by using Prism software (GraphPad Software Inc.) Statistical significance was determined by using an unpaired Student's t test (Prism; GraphPad Software Inc.).
Materials.
Krebs' solution was composed of 125 mM NaCl, 4.7 mM KCl, 1.26 mM CaCl2, 2.3 mM MgSO4, 1.17 mM KH2PO4, 25 mM NaHCO3, and 2.8 mM glucose. All constituents were purchased from Sigma-Aldrich. Hanks' balanced salt solution was purchased from Mediatech. Bafilomycin was purchased from LC Laboratories (Woburn, MA). Ned-19 was purchased from InterBioScreen (Moscow, Russia) or synthesized as reported (Naylor et al., 2009). NAADP-AM was synthesized in the laboratory as described previously (Parkesh et al., 2008).
Stock solution of thapsigargin, bafilomycin, and Ned-19 were made up in dimethyl sulfoxide (DMSO) and stored at −20°C. NAADP-AM was stored as powder, under argon, at −20°C, and made up in DMSO on the day of experimentation. All concentrations of oxytocin were made up in ultra-pure water and stored at −20°C.
Results
Calcium from Intracellular Stores Is Sufficient for Oxytocin-Induced Contractions.
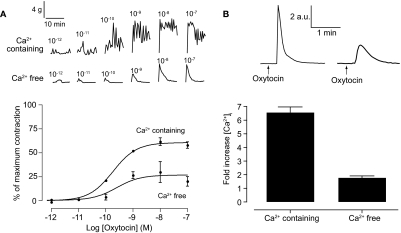
Two distinct components can be discerned in the effect of oxytocin on whole uterine strips. First, there is an increase in the amplitude of contraction. Second, at higher concentrations there is an increase in basal tone (Fig. 1A). Overall, oxytocin-induced contractions shows a concentration-dependent increase in the mean amplitude of contraction with an EC50 of 182 pM (120–276 pM; Fig. 1A). Removing extracellular calcium abolished spontaneous contractions. Subsequent addition of oxytocin caused transient increases in contraction in a concentration-dependent manner [EC50 of 251 pM (156–4040 pM); Fig. 1A]. These increases were significantly smaller than those seen in the presence of extracellular calcium (26 ± 3.9% compared with 60 ± 1.3% maximum; p < 0.001). In both the presence and absence of calcium, oxytocin produced a maximal contraction at 10 nM; therefore, this concentration was used to look at changes in calcium in isolated uterine smooth muscle.
Fig. 1.
Intracellular calcium is sufficient for oxytocin-induced contractions. Top, representative traces of oxytocin-induced contractions in whole uterine strips (A) and oxytocin-induced changes in intracellular calcium in isolated uterine smooth muscle cells (B) in the presence and absence of extracellular calcium. A, bottom, concentration-response curves for oxytocin-induced contractions in the presence (n = 4 rats) and absence (n = 3 rats) of extracellular calcium. Symbols represent mean ± S.E.M. normalized to maximum contraction induced by 60 mM KCl. B, bottom, bar chart representing oxytocin-induced changes in calcium in the presence (n = 14 cells) and absence (n = 17 cells) of extracellular calcium. Bars represent the mean ± S.E.M. normalized to basal levels before the addition of oxytocin.
Oxytocin (10 nM) induced a 6.6 ± 0.4-fold transient increase (n = 14 cells) in intracellular calcium in the presence of calcium (Fig. 1B). Removal of extracellular calcium caused a significant decrease in the oxytocin-induced rise in calcium (1.79 ± 0.12-fold increase; n = 17; p < 0.001). Taken together, these data provide evidence that extracellular calcium is not required for oxytocin-induced responses in uterus. Moreover, as expected, there is a clear correlation with the decrease in contraction and a decrease in calcium release. It was noted that responses in the absence of extracellular calcium were relatively weak, reducing the range of responses, as also noted in isolated human myometrial cells (Soares et al., 2007). Therefore, to maximize contractile responses, we carried out all future experiments in the presence of extracellular calcium.
Oxytocin-Induced Calcium Has a NAADP Component Acting on Acidic Organelles.
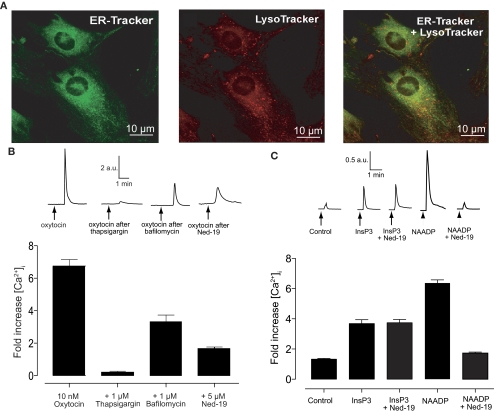
Uterine smooth muscle cells were labeled with ER Tracker and LysoTracker (Fig. 2A) to observe their localization. Both stores were distributed evenly throughout the smooth muscle with no actual colocalization, consistent with the findings from isolated human myometrial cells (Soares et al., 2007). Although the images show some apparent colocalization, it is likely caused by the limit of resolution in these confocal images (approximately 0.5 μm). Consistent with these images, the endoplasmic reticulum and lysosomes have previously been reported to form synapses at this scale in pulmonary arterial smooth muscle cells (Kinnear et al., 2008).
Fig. 2.
Oxytocin-induced calcium release has a NAADP component. A, isolated uterine smooth muscle cells show two, evenly distributed, distinct calcium stores in smooth muscle. Cells were loaded with 100 nM ER Tracker green (left) for 20 min and 50 nM LysoTracker red (center) for 15 min before images were taken with a confocal microscope. There is no colocalization of the two stores (right). B, oxytocin-induced calcium release in isolated uterine smooth muscle cells has an NAADP component. Top, representative traces of oxytocin-induced calcium release either before or after pretreatment with 1 μM thapsigaragin (15 min), 1 μM bafilomycin (60 min), or 5 μM Ned-19 (15 min). Bottom, bar chart representing oxytocin-induced changes in calcium either before (n = 19 cells) or after pretreatment with 1 μM thapsigaragin (n = 12 cells), 1 μM bafilomycin (n = 16 cells), or 5 μM Ned-19 (n = 17 cells). Bars represent the mean ± S.E.M. normalized to basal levels before the addition of oxytocin. C, Ned-19 is a selective inhibitor of NAADP in uterine smooth muscle. Top, representative traces of calcium release after injection with vehicle, InsP3, or NAADP with or without pretreatment with 100 nM Ned-19 (10 min). Bottom, bar chart representing changes in calcium induced by injection with either vehicle, 5 μM InsP3, 5 μM InsP3 after pretreatment with 100 nM Ned-19 (10 min), 5 μM NAADP, or 5 μM NAADP after pretreatment with 100 nM Ned-19 (10 min). Bars represent the mean ± S.E.M. normalized to basal levels before addition (n = 6 cells each).
Application of either 1 μM thapsigargin or 1 μM bafilomycin caused a transient increase in cytosolic calcium in cultured uterine smooth muscle cells. The length of time taken for this transient to return to baseline was used to predict the time needed for these agents to completely empty their respective calcium stores, and this was used as the time of preincubation before the addition of 10 nM oxytocin. In cultured uterine smooth muscle cells thapsigargin (1 μM, 15-min preincubation) caused an almost complete abolition of any subsequent oxytocin-induced calcium release. There was, however, evidence for a sarcoplasmic reticulum-independent component. Indeed both 1 μM bafilomycin (60-min preincubation) or 5 μM Ned-19 (15-min preincubation) caused an inhibition to the oxytocin-induced rise in intracellular calcium of 49 and 75%, respectively (p < 0.001; Fig. 2B).
Although Ned-19 has been shown to be highly selective over InsP3 and cADPR in sea urchin egg homogenate (Naylor et al., 2009), to demonstrate the selectivity of Ned-19 in the uterus, isolated smooth muscle cells were injected with either InsP3 or NAADP after pretreatment with 100 nM Ned-19. Ned-19 had no effect on the calcium increase induced by the injection of InsP3, but completely abolished NAADP-induced calcium release (Fig. 2C). Having shown selectivity, Ned-19 was used to further demonstrate that the action of oxytocin at acidic organelles is via NAADP.
Oxytocin-Induced Contractions Use Both the Sarcoplasmic Reticulum and Acidic Organelles.
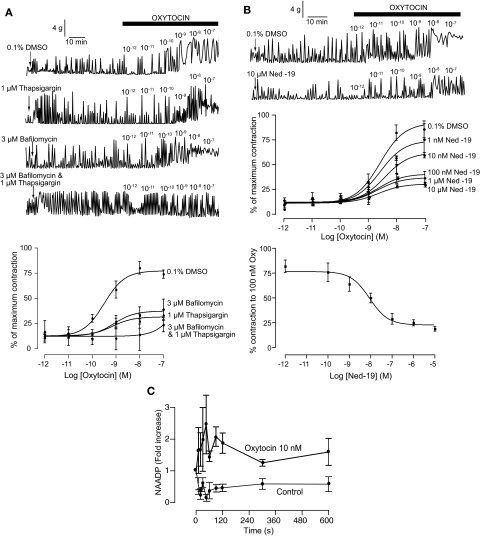
To determine a role for sarcoplasmic reticulum in oxytocin-induced contractions, uterine strips were incubated with 1 μM thapsigargin for 1 h to completely deplete sarcoplasmic reticulum calcium. The addition of thapsigargin in itself caused a transient increase in contraction within the first 10 min of application (Fig. 3A). Thapsigargin had little effect on the EC50 to oxytocin [706 pM (84–5900 pM); n = 4; p = 0.68], but resulted in a 46% decrease in the maximum contraction obtained (p < 0.001; Fig. 3A).
Fig. 3.
Oxytocin-induced contractions have a NAADP component. A, top, representative traces of oxytocin-induced contractions after 1-h pretreatment with either 0.1% DMSO, 1 μM thapsigaragin, 3 μM bafilomycin, or both 1 μM thapsigaragin and 3 μM bafilomycin. Bottom, concentration-response curves for oxytocin-induced contractions after 1-h pretreatment with either 0.1% DMSO (n = 5 rats) 1 μM thapsigaragin (n = 5 rats), 3 μM bafilomycin (n = 3 rats), or both 1 μM thapsigaragin and 3 μM bafilomycin (n = 3 rats). Symbols represent mean ± S.E.M. normalized to maximum contraction induced by 60 mM KCl. B, oxytocin-induced contractions are inhibited by the selective NAADP receptor antagonist Ned-19. Top, representative traces of oxytocin-induced contractions after 1-h pretreatment with either 0.1% DMSO or 10 μM Ned-19. Middle, concentration-response curves for oxytocin-induced contractions after 1-h pretreatment with 0.1% DMSO (n = 3 rats) or varying conentrations of Ned-19 (1 nM–10 μM; n = 3 rats for each concentration). Symbols represent mean ± S.E.M. normalized to maximum contraction induced by 60 mM KCl. Bottom, concentration-response curve for Ned-19 inhibition of oxytocin-induced contractions (100 nM oxytocin). Symbols represent mean ± S.E.M. normalized to maximum contraction induced by 60 mM KCl. C, oxytocin induces an increase in NAADP levels in rat uteri. Symbols represent mean ± S.E.M. (n = 3 for control and n = 5 for oxytocin) normalized to the absolute level of NAADP at time 0 (20.0 ± 4.2 pmol/mg protein.).
To determine a role for acidic organelles in oxytocin-induced contractions, uterine strips were incubated with 3 μM bafilomycin for 1 h to completely deplete these stores. Calcium loading of these stores depends on the proton gradient (Docampo and Moreno, 1999). The addition of bafilomycin did not itself cause a change in uterine spontaneous contractions (Fig. 3A). Nor did its application affect the EC50 response to oxytocin [822 pM (71–9500 pM); n = 3; p = 0.59]. However, bafilomycin did cause a significant decrease in the maximum contraction obtained by oxytocin with responses plateauing at 25 ± 4.9% (p < 0.001; Fig. 3A).
To demonstrate that thapsigargin and bafilomycin were acting independently, uterine strips were incubated with both agents together. As with thapsigargin alone, application of these agents caused a large transient increase in contraction within 10 min of application (Fig. 3A). There was a clear shift in the subsequent oxytocin-induced concentration-response curve with the initial increase in contraction being observed at oxytocin concentrations three orders of magnitude higher (Fig. 3A).
Oxytocin-Induced Contractions Are Inhibited by the Selective NAADP Receptor Antagonist Ned-19.
Ned-19, at all concentrations tested, had little effect on spontaneous contractions (Fig. 3B) and did not affect the EC50 for oxytocin (P > 0.05 for all concentrations). However, increasing concentrations of Ned-19 caused a significant decrease in the maximal contractile response to oxytocin. Indeed, as little as 1 nM Ned-19 (p < 0.05) caused a decrease in the oxytocin-induced contractions, with 10 μM Ned-19 causing a 77% inhibition at 100 nM oxytocin (p < 0.001; Fig. 3B). Ned-19 proved to be highly potent in inhibiting oxytocin-induced contractions with an IC50 of 9 ± 0.2 nM (Fig. 3B).
Oxytocin Stimulates an Increase in NAADP Levels.
One of the criteria for a chemical to be considered a second messenger is that its levels increase in response to agonist stimulation (Robison et al., 1971). A sensitive and selective assay for NAADP has been described based on the sea urchin binding protein (Aarhus et al., 1996; Billington and Genazzani, 2000; Patel et al., 2000), which we have optimized (Lewis et al., 2007). Incubation of chopped rat uteri with buffer alone showed a stable basal NAADP over time (Fig. 3C), whereas tissue incubated with 10 nM oxytocin resulted in a significant (p ≤ 0.0001, all oxytocin versus all control) increase in NAADP levels over time (Fig. 3C). These data demonstrate that NAADP is a bona fide second messenger for oxytocin.
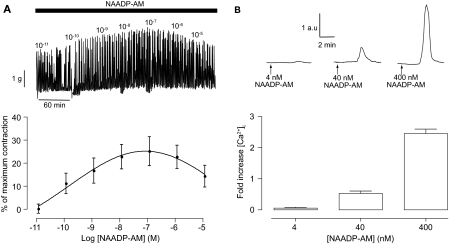
A Cell-Permeant Form of NAADP Can Induce Uterine Contractions through Calcium Release.
Having established a role for NAADP in oxytocin-induced uterine contractility we decided to consider the action of NAADP more directly. Therefore, a cell-permeant form of NAADP (NAADP-AM) (Parkesh et al., 2008) was applied to uterine strips. Addition of NAADP-AM caused a small, but significant, increase in the amplitude and frequency of contractions, but unlike oxytocin it did not increase the basal tone (Fig. 4A). This change in contractions was noted to show the characteristic “bell-shaped” concentration-response (Cancela et al., 1999) in that higher concentration of NAADP-AM produced less contraction than the optimum concentration of 100 nM (Fig. 4A). Consistent with its ability to increase contraction, NAADP-AM produced an increase in intracellular calcium in isolated uterine smooth muscle cells in a concentration-dependent manner with 400 nM NAADP-AM producing a 2.5- ± 0.14-fold (n = 23) increase in intracellular calcium (Fig. 4B).
Fig. 4.
A cell-permeable form of NAADP can induce uterine contractions through calcium release. Top, representative traces of NAADP-AM-induced contractions in whole uterine strips (A) and NAADP-AM-induced changes in intracellular calcium in isolated uterine smooth muscle cells (B). A, bottom, concentration-response curves for NAADP-AM-induced contractions (n = 4 rats). Symbols represent mean ± S.E.M. normalized to maximum contraction induced by 60 mM KCl. B, bottom, bar chart representing NAADP-AM-induced changes in calcium. Bars represent the mean ± S.E.M. normalized to basal levels before the addition of NAADP-AM.
NAADP-AM-Induced Contraction Involves Calcium from Acidic Organelles but Not Sarcoplasmic Reticulum.
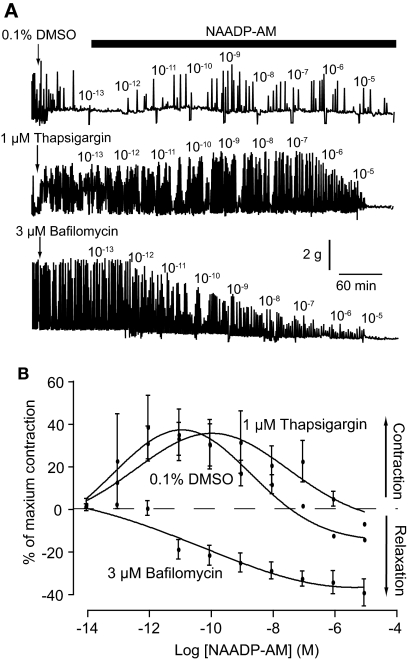
Pretreatment with 0.1% DMSO (vehicle) for 1 h caused a leftward shift in the NAADP-AM concentration-response curve, producing a maximum contraction at 10 pM NAADP-AM (p < 0.05; Fig. 5). Application of 1 μM thapsigargin for 1 h before NAADP-AM caused a slight rightward shift in the concentration-response curve (maximum contraction at 1 nM NAADP-AM), but did not affect the amplitude of the contraction. By comparison, application of 3 μM bafilomycin for 1 h not only completely inhibited NAADP-AM-induced contractions but also reversed the response from contraction to relaxation (Fig. 5).
Fig. 5.
NAADP-AM-induced contraction involves calcium from acidic organelles but not sarcoplasmic reticulum. A, representative traces of NAADP-AM-induced contractions after 1-h pretreatment with either 0.1% DMSO, 1 μM thapsigaragin, or 3 μM bafilomycin. B, concentration-response curves for NAADP-AM-induced contractions after 1-h pretreatment with either 0.1% DMSO (n = 3 rats), 1 μM thapsigaragin (n = 3 rats), or 3 μM bafilomycin (n = 5 rats). Symbols represent mean ± S.E.M. normalized to maximum contraction induced by 60 mM KCl.
NAADP-AM-Induced Contractions Are Inhibited by a Selective NAADP Receptor Antagonist.
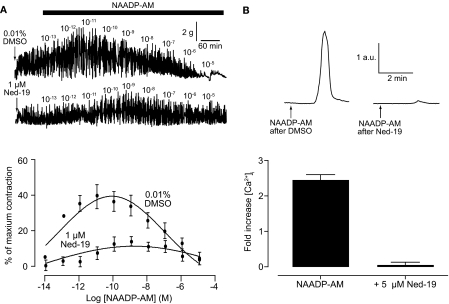
Ned-19 was used to confirm that NAADP-AM is acting via the established NAADP signaling pathway. Incubation for 1 h with 1 μM Ned-19 before producing a concentration-response curve to NAADP-AM clearly inhibited contraction (Fig. 6A). Uterine strips exposed to Ned-19 only produced a maximal contraction of 11 ± 4.4% compared with 39 ± 11.6% in strips pretreated with 0.01% DMSO (p < 0.05; Fig. 6A). Consistent with the abolition of NAADP-AM-induced contractions, 15-min pretreatment with 5 μM Ned-19 completely abolished the rise in calcium seen in uterine smooth muscle cells (Fig. 6B).
Fig. 6.
The selective NAADP receptor antagonist Ned-19 inhibits the effects of NAADP-AM. Top, representative traces of NAADP-AM-induced contractions in whole uterine strips after 1-h pretreatment with 0.01% DMSO or 1 μM Ned-19 (A) and NAADP-induced changes in intracellular calcium in isolated uterine smooth muscle cells before and after 15-min pretreatment with 5 μM Ned-19 (B). A, bottom, concentration-response curves for NAADP-AM-induced after 1-h pretreatment with 0.01% DMSO (n = 3 rats) or 1 μM Ned-19 (n = 3 rats). Symbols represent mean ± S.E.M. normalized to maximum contraction induced by 60 mM KCl. B, bottom, bar chart representing NAADP-induced changes in intracellular calcium in isolated uterine smooth muscle cells before (n = 23 cells) and after pretreatment with 5 μM Ned-19 (n = 18 cells). Bars represent the mean ± S.E.M. normalized to basal levels before the addition of NAADP-AM.
Discussion
The effect of oxytocin on uterine contractions is of major pharmacological importance. It is the strongest uterotonic agent known and is commonly used in obstetrical practice to augment labor (Shmygol et al., 2006). In addition, the synthetic long-acting oxytocin analog carbetocin is widely used to prevent or treat postpartum hemorrhage (Peters and Duvekot, 2009). Moreover, the efficacy of the oxytocin antagonist atosiban to inhibit premature uterine contractions in humans is indicative of a role for oxytocin in human labor (de Heus et al., 2008).
Given the clinical importance of oxytocin, multiple studies have been carried out to obtain a clear understanding of its signaling cascade (Wray, 2007). With regard to intracellular calcium release, the emphasis of this research has been on the sarcoplasmic reticulum, InsP3 receptors, and, more recently, ryanodine receptors. Although the role for InsP3 is undisputed, ryanodine provides a more controversial story. Taggart and Wray (1998) found that ryanodine had little, if any, effect in isolated rat uterus, and a recent review (Noble et al., 2009) concluded that in intact myometrium, from a variety of species, ryanodine receptors appear to have little function. However, Barata et al. (2004) demonstrated a role for cADPR and hence ryanodine receptors in intracellular calcium and contraction of isolated human myometrial cells. Similarly, Soares et al. (2007) found that the oxytocin-induced calcium response was reduced by ryanodine and 8-bromo-cADPR, implicating the involvement of ryanodine receptors.
This conundrum highlights a number of unresolved issues linked to myometrial contractions. One such issue is why depleting calcium from the sarcoplasmic reticulum does not abolish oxytocin-mediated contractions (Shmygol et al., 2006). As discussed earlier, this nonsarcoplasmic reticulum component of oxytocin signaling has been linked to the action of RhoA (Kimura et al., 1996). However, it was noted that G protein-coupled receptors, to which subclass the oxytocin receptor belongs, can increase the production of the calcium-releasing second messenger NAADP (Masgrau et al., 2003; Yamasaki et al., 2005; Macgregor et al., 2007; Naylor et al., 2009). Importantly, histamine has been shown to increase NAADP levels in isolated human myometrial cells (Soares et al., 2007). We have now demonstrated that oxytocin also stimulates an increase in NAADP in rat uteri, demonstrating that NAADP is a bona fide messenger for oxytocin. As NAADP releases calcium from acidic organelles (Churchill et al., 2002), depletion of the sarcoplasmic reticulum would not affect its signaling capabilities and may provide an additional explanation for the non-InsP3 component of oxytocin-mediated contractions.
In this article we directly demonstrate a role for NAADP in oxytocin-induced contractions in rat uteri. These data are consistent with and build on the earlier findings of Soares et al. (2007), who demonstrated the involvement of NAADP in oxytocin-induced calcium increases in isolated human myometrial cells. There was no absolute requirement for extracellular calcium for oxytocin-induced contraction of uterine strips or oxytocin-induced calcium rises in uterine smooth muscle cells (Fig. 1). In addition, it was clear that there was a choice of at least two distinct calcium stores: the sarcoplasmic reticulum or acidic lysosomes (Fig. 2). Thapsigargin, an inhibitor of the sarcoplasmic reticulum calcium pump, almost completely abolished oxytocin-induced calcium rises (Fig. 2). There was, however, evidence of a small residual thapsigargin-insensitive increase consistent with that seen by Calcraft et al. (2009) when measuring pure NAADP responses in cells overexpressing the NAADP receptor. Consistent with previous reports (Shmygol et al., 2006), thapsigargin-treated uterine strips still contracted in response to oxytocin although to a lesser extent (Fig. 3). Taken together, these data suggest a role for NAADP as the “trigger” for a larger global calcium release as hypothesized previously (Cancela et al., 1999; Kinnear et al., 2008), with a small release of calcium from acidic organelles triggering a secondary larger increase from the sarcoplasmic reticulum. The discrepancy in the larger inhibitory response of thapsigargin on calcium release compared with contraction may simply demonstrate that small calcium changes are sufficient to evoke the contractile machinery. More likely is the fact that the contractile response has already been shown to possess a calcium-independent component in the form of activation of Rho A, and this mechanism would not be affected by thapsigargin.
Depletion of calcium in acidic organelles, using bafilomycin (V-H+ ATPase inhibitor; Docampo and Moreno, 1999), caused a decrease in both oxytocin-induced calcium release and oxytocin-induced contractions, further demonstrating a role of calcium from acidic organelles (Figs. 2 and 3). To determine whether the signaling pathways used by NAADP and InsP3 were synergistic or dependent, we depleted both stores. This resulted in almost a complete abolition of the oxytocin-induced contraction with a small increase at 100 nM oxytocin (Fig. 3). These data provided clear evidence that complete oxytocin-induced contractions require both NAADP and InsP3. We hypothesize that the action of oxytocin at its receptor results in production of both InsP3 and NAADP, as reported for several other G protein-coupled receptors (Cancela et al., 1999; Yamasaki et al., 2005; Soares et al., 2007; Pandey et al., 2009). NAADP produces a local release of calcium from acidic organelles, which is then amplified by InsP3 and its receptors to provide a global release (Cancela et al., 1999). Our results are completely consistent with the earlier results of Soares et al. (2007), who showed that oxytocin-induced calcium increases in isolated human myometrial cells were greatly reduced by either bafilomycin or thapsigargin (they did not report their combined effect).
Indeed, by targeting the NAADP receptor using Ned-19, a selective receptor antagonist (Naylor et al., 2009), we were able to reduce both oxytocin-induced calcium release (Fig. 2) and oxytocin-induced contractions (Fig. 3), proving that NAADP signaling is involved in oxytocin-induced contractions. Unsurprisingly, Ned-19 inhibited both calcium release and contractile response to a greater extent compared with depletion of the acidic store by using bafilomycin. The latter method, although useful for an initial query, causes an increase in basal calcium levels and can amplify contractile responses to other components.
To look at the effect of NAADP more directly we used NAADP-AM, a cell-permeant form of NAADP (Parkesh et al., 2008). NAADP-AM was able to produce contraction of whole uterine strips (Fig. 4), providing the first direct evidence that NAADP may have a role to play in uterine smooth muscle contraction. The induced contraction showed a classic bell-shaped concentration-response curve, diagnostic of NAADP (Cancela et al., 1999; Masgrau et al., 2003; Calcraft et al., 2009). In contrast, NAADP-induced calcium release shows a concentration-dependent increase up to 400 nM, a concentration that had reached the downward slope of the bell-shaped curve in intact tissue. Unfortunately, when working with NAADP-AM it is impossible to determine precise concentrations of cytosolic NAADP because of the competing activity of esterases freeing NAADP versus metabolizing enzymes clearing NAADP (Berridge et al., 2002). However, it is clear that NAADP-AM could produce an increase in calcium in uterine smooth muscle that is sufficient to activate the contractile machinery. This response was not inhibited by depleting the sarcoplasmic reticulum but was completely abolished by depleting the acidic organelles (Fig. 5) or inhibiting the NAADP receptor (Fig. 6). Surprisingly, depletion of acidic organelles not only completely inhibited NAADP-AM-induced contractions but also reversed the response from contraction to relaxation. At this time it is not clear why this was the case; however, it was specific to the emptying of the acidic organelles because direct inhibition at the receptor using Ned-19 merely abolished the contractile response.
Contraction of uterine tissue has been subdivided into a number of components, including increases in amplitude, frequency, and basal tone. It has been suggested that changes in frequency and basal tone of contraction are mediated by calcium release, whereas potentiation of contraction amplitude is achieved by sensitization of contractile machinery to calcium (Shmygol et al., 2006). Although detailed coupling of each contractile component to specific calcium signals has not been elucidated, various types of calcium signals could be involved to modulate such multicomponent contraction. Indeed, it was noted that, of the two calcium-dependent components, NAADP-AM did not cause an increase in basal tone as seen with oxytocin-induced contractions. This implies that NAADP is not involved in the mechanism for increasing basal tone (slowing relaxation).
In conclusion, our study directly demonstrated the involvement of NAADP signaling in both calcium responses in isolated cells and contractions of intact uterine tissue from rat. Our findings are consistent with a previous report showing a role for NAADP in oxytocin-induced calcium increases in isolated human myometrial cells (Soares et al., 2007). Combining our results reported here with previously published studies (Churchill et al., 2003; Yamasaki et al., 2005; Soares et al., 2007; Pandey et al., 2009) highlights the need to consider potential action of NAADP, even in G protein-signaling pathways that are traditionally linked to InsP3 signaling.
Acknowledgments
We thank Clive Garnham for technical assistance and Alison Brading and Keith Brain for helpful discussions.
This work was supported by the Biotechnology and Biological Sciences Research Council [Grant BB/D012694/1] and the National Institutes of Health [Grant R01 HL90804].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.165837.
- InsP3
- inositol 1,4,5-trisphosphate
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- NAADP-AM
- NAADP-aceteoxymethyl ester
- cADPR
- cyclic ADP-ribose
- DMSO
- dimethyl sulfoxide
- Ned-19
- 1-(3-((4-(2-fluorophenyl)piperazin-1-yl)methyl)-4-methoxyphenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid.
References
- Aarhus et al., 1996.Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. (1996) Activation and inactivation of Ca2+ release by NAADP. J Biol Chem 271:8513–8516 [DOI] [PubMed] [Google Scholar]
- Barata et al., 2004.Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, Feitoza S, Sieck G, Chini EN. (2004) The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology 145:881–889 [DOI] [PubMed] [Google Scholar]
- Berridge et al., 2002.Berridge G, Cramer R, Galione A, Patel S. (2002) Metabolism of the novel Ca2+-mobilizing messenger nicotinic acid-adenine dinucleotide phosphate via a 2′-specific Ca2+-dependent phosphatase. Biochem J 365:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington and Genazzani, 2000.Billington RA, Genazzani AA. (2000) Characterization of NAADP(+) binding in sea urchin eggs. Biochem Biophys Res Commun 276:112–116 [DOI] [PubMed] [Google Scholar]
- Blair-Bell and Hick, 1909.Blair-Bell W, Hick P. (1909) Report CXII. Observations on the physiology of the female genital organs. Br Med J 1:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu et al., 2009.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, et al. (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol 186:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft et al., 2009.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela et al., 1999.Cancela JM, Churchill GC, Galione A. (1999) Coordination of agonist-induced Ca2+-signaling patterns by NAADP in pancreatic acinar cells. Nature 398:74–76 [DOI] [PubMed] [Google Scholar]
- Churchill et al., 2003.Churchill GC, O'Neill JS, Masgrau R, Patel S, Thomas JM, Genazzani AA, Galione A. (2003) Sperm deliver a new second messenger: NAADP. Curr Biol 13:125–128 [DOI] [PubMed] [Google Scholar]
- Churchill et al., 2002.Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. (2002) NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111:703–708 [DOI] [PubMed] [Google Scholar]
- de Heus et al., 2008.de Heus R, Mulder EJ, Derks JB, Visser GH. (2008) Acute tocolysis for uterine activity reduction in term labor: a review. Obstet Gynecol Surv 63:383–388; quiz 405 [DOI] [PubMed] [Google Scholar]
- Docampo and Moreno, 1999.Docampo R, Moreno SN. (1999) Acidocalcisome: A novel Ca2+ storage compartment in trypanosomatids and apicomplexan parasites. Parasitol Today 15:443–448 [DOI] [PubMed] [Google Scholar]
- Galione et al., 2009.Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, Zhu MX. (2009) The acid test: the discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca2+ release channels. Pflügers Arch 458:869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani and Galione, 1996.Genazzani AA, Galione A. (1996) Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem J 315:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse and Lee, 2008.Guse AH, Lee HC. (2008) NAADP: a universal Ca2+ trigger. Sci Signal 1:re10. [DOI] [PubMed] [Google Scholar]
- Kimura et al., 1996.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245–248 [DOI] [PubMed] [Google Scholar]
- Kinnear et al., 2008.Kinnear NP, Wyatt CN, Clark JH, Calcraft PJ, Fleischer S, Jeyakumar LH, Nixon GF, Evans AM. (2008) Lysosomes colocalize with ryanodine receptor subtype 3 to form a trigger zone for calcium signaling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 44:190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsan-Agbas et al., 2008.Krizsan-Agbas D, Pedchenko T, Smith PG. (2008) Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J Neurosci Res 86:3086–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, 2000.Lee HC. (2000) Multiple calcium stores: separate but interacting. Sci STKE 2000:pe1. [DOI] [PubMed] [Google Scholar]
- Lee, 2005.Lee HC. (2005) Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J Biol Chem 280:33693–33696 [DOI] [PubMed] [Google Scholar]
- Lee and Aarhus, 1995.Lee HC, Aarhus R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem 270:2152–2157 [DOI] [PubMed] [Google Scholar]
- Lewis et al., 2007.Lewis AM, Masgrau R, Vasudevan SR, Yamasaki M, O'Neill JS, Garnham C, James K, Macdonald A, Ziegler M, Galione A, et al. (2007) Refinement of a radioreceptor binding assay for nicotinic acid adenine dinucleotide phosphate. Anal Biochem 371:26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor et al., 2007.Macgregor A, Yamasaki M, Rakovic S, Sanders L, Parkesh R, Churchill GC, Galione A, Terrar DA. (2007) NAADP controls cross-talk between distinct Ca2+ stores in the heart. J Biol Chem 282:15302–15311 [DOI] [PubMed] [Google Scholar]
- Masgrau et al., 2003.Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJ, Galione A. (2003) NAADP: a new second messenger for glucose-induced Ca2+ responses in clonal pancreatic β cells. Curr Biol 13:247–251 [DOI] [PubMed] [Google Scholar]
- Naylor et al., 2009.Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, et al. (2009) Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol 5:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble et al., 2009.Noble K, Matthew A, Burdyga T, Wray S. (2009) A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. Eur J Obstet Gynecol Reprod Biol 144 (Suppl 1):S11–S9 [DOI] [PubMed] [Google Scholar]
- Pandey et al., 2009.Pandey V, Chuang CC, Lewis AM, Aley PK, Brailoiu E, Dun NJ, Churchill GC, Patel S. (2009) Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem J 422:503–512 [DOI] [PubMed] [Google Scholar]
- Parkesh et al., 2008.Parkesh R, Lewis AM, Aley PK, Arredouani A, Rossi S, Tavares R, Vasudevan SR, Rosen D, Galione A, Dowden J, et al. (2008) Cell-permeant NAADP: a novel chemical tool enabling the study of Ca2+ signaling in intact cells. Cell Calcium 43:531–538 [DOI] [PubMed] [Google Scholar]
- Patel, 2004.Patel S. (2004) NAADP-induced Ca2+ release: a new signaling pathway. Biol Cell 96:19–28 [DOI] [PubMed] [Google Scholar]
- Patel et al., 2000.Patel S, Churchill GC, Galione A. (2000) Unique kinetics of nicotinic acid-adenine dinucleotide phosphate (NAADP) binding enhance the sensitivity of NAADP receptors for their ligand. Biochem J 352:725–729 [PMC free article] [PubMed] [Google Scholar]
- Peters and Duvekot, 2009.Peters NC, Duvekot JJ. (2009) Carbetocin for the prevention of postpartum hemorrhage: a systematic review. Obstet Gynecol Surv 64:129–135 [DOI] [PubMed] [Google Scholar]
- Robison et al., 1971.Robison GA, Butcher RW, Sutherland EW, Posternak T, Hardman JG. (1971) Cyclic AMP, Academic Press, New York [Google Scholar]
- Santella, 2005.Santella L. (2005) NAADP: a new second messenger comes of age. Mol Interv 5:70–72 [DOI] [PubMed] [Google Scholar]
- Shmygol et al., 2006.Shmygol A, Gullam J, Blanks A, Thornton S. (2006) Multiple mechanisms involved in oxytocin-induced modulation of myometrial contractility. Acta Pharmacol Sin 27:827–832 [DOI] [PubMed] [Google Scholar]
- Soares et al., 2007.Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, Lund FE, Galione A, Chini EN. (2007) NAADP as a second messenger: neither CD38 nor base-exchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am J Physiol Cell Physiol 292:C227–C239 [DOI] [PubMed] [Google Scholar]
- Taggart and Wray, 1998.Taggart MJ, Wray S. (1998) Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational dependence in isolated rat uterus. J Physiol 511:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson et al., 2004.Thompson M, Barata da Silva H, Zielinska W, White TA, Bailey JP, Lund FE, Sieck GC, Chini EN. (2004) Role of CD38 in myometrial Ca2+ transients: modulation by progesterone. Am J Physiol Endocrinol Metab 287:E1142–E1148 [DOI] [PubMed] [Google Scholar]
- Wray, 2007.Wray S. (2007) Insights into the uterus. Exp Physiol 92:621–631 [DOI] [PubMed] [Google Scholar]
- Yamasaki et al., 2005.Yamasaki M, Thomas JM, Churchill GC, Garnham C, Lewis AM, Cancela JM, Patel S, Galione A. (2005) Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ spiking in mouse pancreatic acinar cells. Curr Biol 15:874–878 [DOI] [PubMed] [Google Scholar]
- Zong et al., 2009.Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. (2009) The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflügers Arch 458:891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]