Abstract
Dendritic cells (DCs)—immunomodulatory cells that initiate adaptive immune responses—have recently been shown to exert proangiogenic effects when infiltrating the tumor microenvironment. As tumors that escape immune surveillance inhibit DC maturation, we explored whether maturation status determines their ability to promote angiogenesis and whether angiogenesis depends on the presence of DCs. Using mouse xenograft models of human tumors, we show that fast-growing “angiogenic” tumors are infiltrated by a more immature DC population than respective dormant avascular tumors. Accordingly, supplementation of immature DCs, but not mature DCs, enhanced tumor growth. When DCs were mixed with Matrigel and injected subcutaneously into mice, only immature DCs promoted the ingrowth of patent blood vessels. Notably, depletion of DCs in a transgenic mouse model that allows for their conditional ablation completely abrogated basic fibroblast growth factor-induced angiogenesis in Matrigel plugs, and significantly inhibited tumor growth in these mice. Because immature DCs actively promote angiogenesis and tumor growth, whereas DC maturation or ablation suppresses this response, we conclude that angiogenesis is dependent on the presence of immature DCs. Thus, cancer immunotherapies that promote DC maturation may act by both augmenting the host immune response to the tumor and by suppressing tumor angiogenesis.—Fainaru, O., Almog, N., Yung, C. W., Nakai, K., Montoya-Zavala, M., Abollahi, A., D’Amato, R., Ingber, D. E. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells.
Keywords: immune cells, vasculogenesis
Dendritic cells (DCs) are a heterogeneous population of antigen-presenting cells that initiate and coordinate innate and adaptive immune responses (1). DCs are not only essential for primary immune responses, but they are also important for the induction of immunological tolerance (2). In most tissues, DCs are present in an “immature” state, which is characterized by the lack of accessory molecules required for T-cell activation, such as CD80 and CD86. These immature cells are potent phagocytes capable of capturing and processing antigens. Detection of damage or pathogen-associated molecular antigens, such as bacterial lipopolysaccharides (LPS) by tissue-resident DCs, initiates DC maturation (1), which is associated with up-regulation of MHCII molecules and costimulatory molecules. Once matured, the DC loses its ability to capture antigens and transforms into an antigen-presenting cell.
Investigations of murine and human cancers indicate that tumor-derived factors and the presence of other myeloid cells, in particular, myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), lead to restricted DC maturation and function (3,4,5,6). Cancers contain far fewer mature DCs compared to corresponding healthy tissues (7) and show an abundance of immature DCs with an impaired capacity to stimulate adaptive antitumor immunity (5). Interestingly, in addition to their classic role as antigen-presenting cells, DCs have been recently found to promote angiogenesis in tumors (8,9,10), endometriosis (10), uterine deciduas (11), and choroidal neovascularization (12). In these studies, DC transplantation led to enhanced angiogenesis and lesion growth. Moreover, immature DCs appear to exhibit higher proangiogenic activity when compared to mature DCs in that they are more effective at inducing capillary endothelial cell migration in vitro (10). However, definitive evidence for differential effects of immature and mature DCs on tumor growth and angiogenesis in vivo and for a dependence of angiogenesis on their presence is lacking.
When neovascularization is suppressed, hyperplastic lesions and solid tumors remain in a dormant state in which growth and death rates are balanced, and they do not transform or expand into clinically detectable cancers (13). We therefore explored whether the maturation state of tumor-infiltrating DCs differs between tumors that are avascular and dormant vs. those that are angiogenic and fast-growing, and whether tumor growth can be modified by altering the maturation state or number of infiltrating DCs. Here, we show that immature DCs are required for neovascularization in general and for growth of various human and murine tumors in mice, whereas DC maturation or ablation inhibits this response.
MATERIALS AND METHODS
Experimental system
Lewis lung carcinoma, B16F10 melanoma, human ovarian carcinoma OVCAR5, and human breast carcinoma MDA-MB-436 [purchased from American Type Culture Collection (ATCC), Manassas, VA, USA] were maintained in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen). Human glioblastoma cells (T98G) (ATCC) were maintained in minimum essential medium (MEM; Invitrogen) supplemented with 10% FBS, 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate. Prior to injection, cells were harvested from subconfluent cultures. Animal studies were carried out using 6- to 8-wk-old male mice. C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and C.B-17 SCID mice from Charles River Laboratories (Wilmington, MA, USA). For DC ablation experiments, we used the CD11c+DTR-Tg mouse (B6.FVB-Tg(Itgax-DTR/EGFP)57LAn/J) (14), in which a transgene was designed to place a simian diphtheria toxin receptor (DTR) under the control of the Itgax = CD11c promoter. On exposure to a single dose (3–4 ng/g mouse weight) of diptheria toxin (Sigma, St. Louis, MO, USA), these mice are depleted of all the DC, whereas DT administration has no effect on CD11c+ cells in wild-type mice. All animal procedures were performed in compliance with Boston Children’s Hospital guidelines and protocols approved by the Institutional Animal Care and Use Committee.
DC culture and transplantation
Bone marrow-derived DCs were prepared as described previously (15). Briefly, mice were euthanized, and bone marrow was extracted from femurs and tibiae by flushing the shafts with 5 ml RPMI 1640. The isolated cells were plated on nonadhesive Petri dishes at a density of 1 × 106 cells/ml in medium (RPMI 1640, 5% FCS, 5×10−5 M 2-mercaptoethanol, and penicillin/streptomycin) containing 10 ng/ml murine recombinant GM-CSF (Peprotech, Rocky Hill, NJ, USA). The medium was replenished every 3 d, and the loosely adherent DCs were collected at the designated times and used for further studies. For bone marrow-derived dendritic cell (BMDC) maturation, the cells were treated overnight with 1 μg/ml LPS (L2654; Sigma). Maturation of BMDCs was verified by analyzing MHCII expression on CD11c+ cells using flow cytometry (10). LPS-treated BMDCs showed increased expression of MHCII when compared to untreated controls.
Flow cytometry
Tumor and Matrigel specimens were treated with collagenase (Liberase Blendzyme 3; Roche Diagnostics, Indianapolis, IN, USA) at 37°C for 30 min. Digested tissue was then filtered through a 40-μm cell strainer and resuspended in FACS buffer (PBS, 5 mM EDTA, and 1% BSA/0.05% sodium azide). Immunostaining was performed in the presence of rat anti-mouse Fc receptor III/II (FcγRIII/II; CD16/32; Pharmingen, San Diego, CA, USA), by incubating the cells with monoclonal antibodies for 30 min on ice. Staining reagents included anti-CD11c allophycocyanin (APC), anti-IA/IE (MHCII) FITC, and anti-CD31 APC, anti-CD45 PE (all purchased from BD Biosciences, San Diego, CA USA). Flow cytometry was performed with a FACS Calibur (Becton Dickinson, Mountain View, CA, USA). Mean fluorescent intensities (MFIs) and geometric means of individual histograms were derived from CellQuest software (Becton Dickinson), and the ratio of MHCII expression on DCs populating pooled dormant tumors and individual angiogenic tumors was calculated. Statistical analysis was performed using paired 2-tailed Student t tests on log2-transformed fluorescence intensity data.
Matrigel plug assay
To directly analyze neovascularization in vivo, 106 immature DCs or LPS-matured DCs were mixed with liquid Matrigel (BD Biosciences) and implanted subcutaneously in C57BL/6 mice. After 7–14 d, animals were sacrificed, Matrigel plugs were removed and subjected to enzymatic digestion, and the percentage of infiltrating endothelial cells were measured by flow cytometry (16). In some experiments, human basic FGF (100 ng/ml; gift from National Institutes of Health, Bethesda, MD, USA) was mixed with Matrigel and implanted subcutaneously into wild-type or CD11cDTR-Tg mice. At d 1, diphteria toxin (3 ng/g mouse weight) in PBS was injected into the mice, and endothelial cell ingrowth into the Matrigel plugs was measured after 7 d using differential interference contrast (DIC) microscopy. Whole-mount preparations of the Matrigel specimens were imaged using an inverted confocal microscope (SP5-AOBS system; Leica Microsystems, Wetzlar, Germany) with an ×20 oil objective, and a series of images was acquired over a thickness of 200 μm.
RESULTS
Immature DCs are enriched within fast-growing angiogenic tumors
The maturation and function of tumor DCs are restricted by the tumor microenvironment (4, 5); however, it is not known whether the maturation status of these immune-modulating cells affects angiogenesis or tumor dormancy in vivo. We first examined whether there is a difference in the maturity of DCs that infiltrate dormant MDA-MB-436 avascular human breast adenocarcinomas or human T98G glioblastomas vs. fast-growing clones of the same tumors that are highly angiogenic when grown in SCID mice (17). In these experimental models, the parental tumor cell line forms dormant tumors that remain occult for long periods of time (>100 d) when inoculated subcutaneously. Over a period of months, some of the tumors switch spontaneously from the dormant state to become angiogenic, which causes them to begin to grow rapidly. Cell lines derived from dormant tumors that had switched to the angiogenic phenotype are termed “angiogenic” because they produce large angiogenic tumors (up to 2000 mm3) within 1 mo of their subsequent inoculation into mice (17).
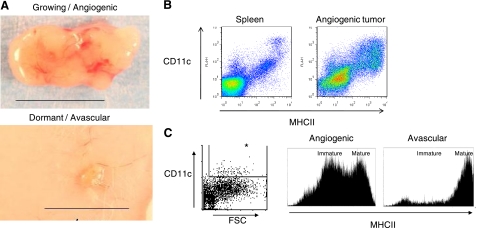
In our experiment, we harvested tumors after 25–28 d. During this time period, subcutaneously injected cells derived from the angiogenic fast-growing tumors generated large palpable tumors, while an equal amount of cells derived from the parental cells generated small nonpalpable dormant tumors (Fig. 1A). Flow cytometric analysis of single-cell suspensions isolated from an enzyme-digested spleen and MDA-MB-436 angiogenic tumors (Fig. 1B) revealed that the tumors are infiltrated by a significant population of CD11c+MHCII+ DCs. Notably, DCs infiltrating dormant tumors were more mature than those infiltrating fast-growing angiogenic tumors, as demonstrated by their higher expression of MHCII (Fig. 1C). The MFI of MHCII expression, an indicator of DC maturity, was ∼2.5-fold higher (2.44±0.77, range 1.54–3.70; P=0.001; n=6) in dormant avascular tumors vs. angiogenic growing cancers, as confirmed in 6 separate experiments (4 involving breast cancer samples, and 2 experiments with the human T98G glioblastoma). Similarly, the geometric means of MHCII expression were 3.2-fold higher in dormant avascular tumors vs. angiogenic growing cancers (3.22±2.09, range 1.23–5.46; P=0.03; n=6).
Figure 1.
Immature DCs are enriched in fast-growing angiogenic tumors. A) Light micrographs showing explants of a fast-growing angiogenic human MDA-MB-436 breast cancer and an avascular dormant tumor after growth subcutaneously in mice for 28 d. Scale bars = 1 cm. B) Scatterplot of data obtained from flow cytometric analysis of cells after they were enzymatically digested from the spleen and from a vascularized breast cancer specimen, and stained with anti-CD11c and anti-IA/IE (MHCII) antibodies. Note the CD11c+MHCII+ DCs in both spleen and tumor. C) Histograms of MHCII expression on tumor infiltrating DCs (box and asterisk indicate DCs that were gated as CD11c+FSChigh cells) isolated from angiogenic cancers compared to avascular tumors; note that avascular tumors exhibit less immature DCs that express lower levels of MHCII. Data are representative of 4 separate experiments.
Immature DCs enhance tumor growth
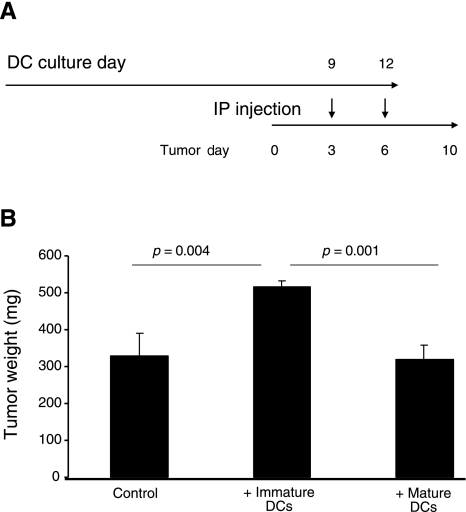
When bone marrow-derived DCs are injected intraperitoneally, they incorporate into intra-abdominal tumors and promote their growth (10). We therefore tested whether the maturation state of DCs influences their ability to promote tumor growth in SCID mice bearing intra-abdominal human ovarian carcinoma OVCAR5. Immature DCs were obtained by culturing bone marrow of C57BL/6 mice in the presence of GMCSF, and a subset of the DCs was induced to mature by incubating them overnight in the presence of LPS. Intraperitoneal injection of mature DCs into mice 3 and 6 d after OVCAR5 tumor implantation (Fig. 2A) did not affect tumor growth when measured after 10 d, whereas injection of immature DCs resulted in significantly larger tumors than those injected with either mature DCs or vehicle alone (Fig. 2B).
Figure 2.
Immature DCs enhance tumor growth when injected in vivo. A) Design of an experiment in which SCID mice (n=4/group) were inoculated first with 1 × 106 intraperitoneal human ovarian carcinoma OVCAR5 cells, and then DCs that were generated by growing bone marrow cells in the presence of GM-CSF for 9 or 12 d were injected into the same animals, with or without overnight exposure to LPS (to stimulate maturation or not, respectively), 3 and 6 d after tumor inoculation. B) At d 10, mice were sacrificed, and the intra-abdominal tumors were removed and weighed; results are presented as mean ± sd weight (mg). Note that injection of immature DCs produced a significant increase in tumor mass compared to control tumors, or tumors injected with the same number of mature DCs. Two-tailed Student’s t test was applied to test for statistical significance.
Immature DCs enhance endothelial cell migration in vivo
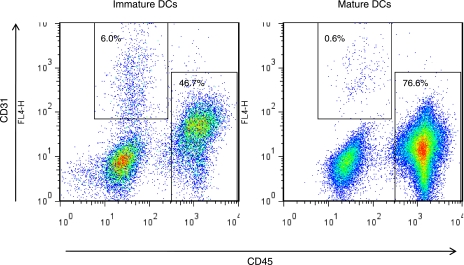
The finding that injection of immature DCs enhanced tumor growth in immune-deficient mice in a short time suggests that this effect on tumor expansion might be due to a nonimmune function of DCs. Given that immature DCs enhance capillary endothelial cell migration in vitro (10), we compared the ability of immature vs. mature DCs to promote neovascularization when implanted within a Matrigel plug (18) and grown subcutaneously in mice for 2 wk. These studies revealed that only immature DCs induced capillary ingrowth, as measured by flow cytometric quantitation of the CD45−CD31+ endothelial cell population isolated from these gels (Fig. 3).
Figure 3.
Immature DCs promote endothelial cell migration in vivo. Matrigel was mixed with 1 × 106 DCs that were cultured in the presence or absence of LPS before being implanted subcutaneously into mice. After 14 d, Matrigel plugs were removed and subjected to enzymatic degradation. Single-cell suspensions were stained with anti-CD31 and anti-CD45 antibodies and analyzed by flow cytometry; endothelial cells are gated as CD45−CD31+ and hematopoietic cells as CD45+CD31−. Note that even though the amount of hematopoietic cells was lower in Matrigel plugs injected with immature DCs, these DCs led to a 10-fold increase in endothelial cell ingrowth as compared to mature DCs. Plot represents pooled single-cell suspensions from 2 Matrigel plugs/mouse, 3 mice/group (n=6/group). *P = 0.007; Fisher’s exact test.
DC depletion abrogates angiogenesis and inhibits tumor growth in vivo
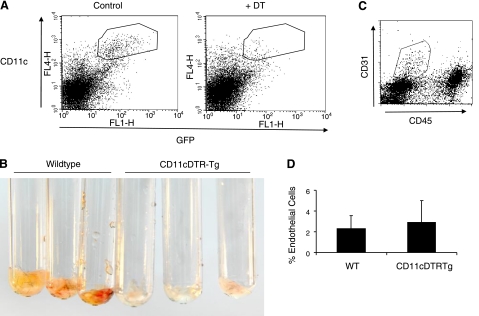
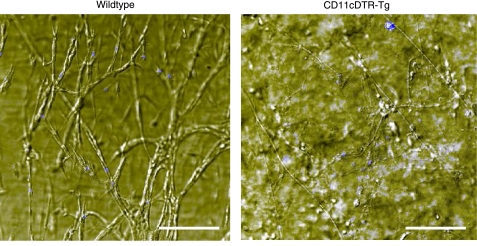
We next investigated whether angiogenesis requires the presence of DCs using CD11c+DTR-Tg (transgenic) mice (14). Flow cytometry was first used to confirm that DCs could be transiently ablated in vivo by administration of diphtheria toxin (DT) (Fig. 4A). Matrigel was then mixed with the angiogenic mitogen, basic fibroblast growth factor (bFGF), before being implanted subcutaneously into wild-type or CD11c+DTR-Tg mice. When we treated mice with a single dose of DT, 1 d after implantation, and analyzed Matrigel plugs 6 d later, we found that angiogenesis was completely inhibited in the DC-depleted CD11c+DTR-Tg mice compared to wild-type controls, as determined by the complete absence of red blood in the explants (Fig. 4B). Flow cytometric analysis of single-cell suspensions isolated from Matrigel plugs and stained for surface expression of CD31 and CD45 showed no effect of DC ablation on the total number of endothelial cells that were recruited to these sites (Fig. 4C, D). However, differential interference contrast microscopy images of Matrigel plugs revealed that these endothelial cells grew as isolated cells in DT-treated mice without DCs, whereas similar numbers of endothelial cells organized within well-formed, functional vascular networks in wild-type mice (Fig. 5).
Figure 4.
DC depletion abrogates angiogenesis in vivo. A) Flow cytometric analysis of single-cell suspensions derived from enzymatically digested spleens stained with anti-CD11c antibodies, which confirm that endogenous DCs (outlined region containing CD11c+GFP+ cells) were depleted within 1 d after of injection of CD11c+DTR-Tg mice with a single dose of DT (3 ng/g). B) Photographs of Matrigel plugs containing bFGF (100 ng/ml) that were implanted subcutaneously for 1 wk in wild-type and CD11c+DTR-Tg mice treated with DT (on d 1; n=3/group). Note the lack of blood, indicating decreased vascularity within the implants removed from animals in which DCs were depleted. C) Flow cytometric analysis of cells removed from Matrigel plugs after staining with anti-CD31 and anti-CD45 antibodies. D) Similar percentages of CD31+CD45− endothelial cells (gated as in C) are recruited to Matrigel plugs regardless of the presence (wild type) or absence (CD11c+DTR-Tg) of DCs caused by DT treatment.
Figure 5.
DC depletion disrupts formation of a vascular network. Differential interference contrast microscopic images of capillary cells grown within whole mounts of Matrigel plugs removed from wild-type animals or CD11c+DTR-Tg mice treated with DT as described in Fig. 4. Cell nuclei are stained with DAPI (blue), and a z stack of images was acquired over a thickness of 200 μm. Scale bars = 150 μm. Note that well-developed microvascular networks can be seen throughout the Matrigel plug from the wild-type mouse, but only single rounded cells appear in the Matrigel plugs from DC-depleted mice (CD11c+DTR-Tg).
Finally, we tested whether tumor growth requires the presence of DCs. When CD11c+DTR-Tg and control wild-type mice were injected intraperitoneally with B16F10 melanoma and treated after 3 d with a single dose of DT, DC ablation led to reduced tumor weight as measured 8 d postinoculation (0.77±0.16 vs. 1.52±0.38 g; P=0.036; n=3 mice/group).
DISCUSSION
Tumor-host interactions manifest dual and seemingly opposing effects in cancer pathogenesis (19). Activation of the immune response results in tumor suppression, whereas the formation of clinically evident disease implies its evasion of immune surveillance. In contrast to the protective function of the immune system, several lines of evidence point to a strong link between unresolved inflammation and tumor progression (20). Multiple cells of the innate immune system, including DCs, have been shown to contribute to tumor cell invasion, expansion, metastasis and angiogenesis via production of cytokines, growth factors, and matrix metalloproteinases (21, 22). Moreover, a recent study showed that the depletion of DCs restores the immune response against the tumor and suppresses tumor growth (23). However, the effects on tumor expansion in that study occurred more rapidly than restoration of tumor immunity, while the growth inhibitory effects correlated with increased endothelial cell apoptosis.
In the present study, we therefore explored whether DCs actively contribute to angiogenesis and tumor growth and whether this effect varies depending on the maturational state of the DC. For a tumor to escape dormancy and develop a malignant phenotype, it must recruit and sustain its own blood supply (24). New blood vessel formation in tumors is dependent on stromal-supporting cells, including bone marrow-derived CD45+ hematopoietic cells (25, 26). Because DC maturation is known to be restricted by the tumor microenvironment (4, 5), we also specifically explored the relation between DC maturity and tumor angiogenesis.
Our work using human breast carcinoma and glioblastoma shows that fast-growing angiogenic tumors in mice are infiltrated by a more immature population of DCs than dormant avascular tumors. Thus, there is a direct correlation between the presence of immature DCs and the expression of the angiogenic phenotype. Because both dormant and angiogenic tumors were harvested after the same time periods in immune-deficient mice, it is tempting to assume that the ability of the tumor to maintain infiltrating DCs in an immature state enables it to induce and sustain angiogenesis that is critical for tumor expansion.
This hypothesis was tested by comparing the effects of injecting mature vs. immature DCs into human SCID mice bearing ovarian carcinoma. When DCs are injected intra-abdominally in animals, they incorporate into intra-abdominal growing tumors (10). Notably, mice that were injected with immature DCs developed significantly larger tumors than controls that were injected with either mature DCs or vehicle. These findings cannot be explained by enhanced antitumor immunity and the “classic” immune functions of DCs, as they were observed in immune-deficient mice. Therefore, we propose that the maturity state of DCs defines their direct proangiogenic and protumorigenic properties.
The peritoneal lining of the abdominal cavity is populated by a very small population of DCs; however, even local injury, such as a silk stitch application, leads to a massive recruitment of immature DCs to that site (unpublished data). It is, therefore, conceivable that these proangiogenic properties are inherent to immature DCs and that tumors take advantage of this property by recruiting them to their microenvironment. To further concentrate on the proangiogenic role of immature DCs, we employed a tumor cell free in vivo angiogenesis model (Matrigel plug assay). Similar to their effect on intra-abdominal tumors, we observed that immature, but not mature DCs, attracted endothelial cells into the Matrigel plugs, providing direct evidence for their ability to stimulate angiogenesis in vivo. This observation is consistent with previously published in vitro data (10). Although we cannot claim that after 2 wk, the cellular composition of the Matrigel plugs nor the maturity of DCs is similar to that at inoculation, we clearly demonstrate that the amount hematopoietic cells at that time is higher with the initial presence of mature DCs. Therefore, differences in hematopoietic cell recruitment or survival cannot explain the differences in endothelial cell recruitment. We thus attribute the differential recruitment of endothelial cells to the maturation status of DCs at the time of inoculation.
Accumulating evidence (8,9,10) suggests that DCs promote angiogenesis in different animal models; however, our data are the first to show that it is specifically the immature form of the DC that is responsible for this effect. Furthermore, by performing DC ablation experiments in CD11c+DTR-Tg mice, we confirmed that angiogenesis is dependent on the presence of these DCs in vivo. One of the limitations of this transgenic mouse system is that the DC population can only be ablated transiently (27) because DCs that are depleted from the spleen within 24 h after a single intraperitoneal injection of DT, begin to repopulate the organ 3 d later. As repeated dosages of DT lead to systemic toxicity, we limited our experiments to a single dose of DT. We have previously observed that DC infiltration peaks 2–4 d after injury in a choroidal neovascularization model (12). To prevent the peak of DC infiltration, we treated the mice with DT 1 d after Matrigel implantation. Strikingly, bFGF-induced vascularization was completely inhibited in the DC-depleted mice, while normal angiogenesis was observed in controls. Interestingly, this effect was not due to inhibition of endothelial cell migration, as similar amounts of these cells were observed in the absence of DCs. Instead, microscopic analysis of these Matrigel plugs revealed that in the absence of DCs, formation of a patent vascular network was completely inhibited. Endothelial cell migration was not inhibited in the absence of DCs, most probably because of the presence of the proangiogenic and chemotactic cytokine bFGF that we premixed with the Matrigel. Although we cannot rule out that a systemic effect of general DC ablation caused the observed decrease in angiogenesis, we postulate that it is the absence of local infiltrating DCs that impairs angiogenesis. First, we observed that Matrigel plugs in the presence of bFGF are heavily infiltrated by hematopoietic cells (data not shown). Furthermore, our data from the choroidal injury model (12) show that DCs are rapidly recruited to sites of active angiogenesis in a transient manner. Finally, it has recently been reported (28) that macrophages promote angiogenesis in Matrigel plugs by secreting IL-1, causing infiltrating cells to secrete endothelial cell-activating factors. It is conceivable that proangiogenic DCs infiltrating the Matrigel are responsive to this form of macrophage signaling. We, therefore, hypothesize that the lack of local infiltration of DCs or their elimination is responsible for the observed decrease in angiogenesis in the mutant mice treated with DT.
Although it has been reported that DCs can integrate into vessel walls, while acquiring an “endothelial cell-like” phenotype (8), we have observed DCs to be strategically located at a perivascular location, suggesting a role for DCs in the support of vessel structure (10). Because immature DCs also lead to endothelial cell migration both in vitro (10)and in vivo, we suggest that these cells play a dual role in promoting angiogenesis. DCs in the tumor microenvironment may serve both to attract circulating endothelial cells to the growing angiogenic tumor and to produce appropriate paracrine signals (e.g., VEGF, IL-8, and bFGF) (23), which support the formation, structure, and survival of newly formed blood vessels. Accordingly, it has been reported by Plaks et al. (11) that DC ablation from the uterus led to perturbed angiogenesis characterized by reduced vascular expansion and attenuated maturation. The researchers suggested that uterine DCs direct decidual angiogenesis by secreting sFlt1 and TGF-β1 that promote coordinated blood vessel maturation. Finally, we demonstrated that ablation of DCs in the same transgenic mouse model led to a rapid reduction in tumor growth when analyzed 5 d post-DC ablation. Similarly, DC ablation led to decreased lesion sizes in mouse models of two more angiogenesis-dependent conditions: endometriosis and choroidal neovascularization (our unpublished observations). All together, these data support a “nonimmune” proangiogenic property of tumor infiltrating DCs.
CONCLUSIONS
Fast-growing angiogenic tumors have an increased capacity to inhibit DC maturation when compared to dormant tumors, and injection of immature DCs, but not mature DCs, enhances the growth of intraperitoneal tumors and promotes angiogenesis. Moreover, neovascularization is completely inhibited, and tumor growth is remarkably impaired when the DC population is ablated. These findings suggest that immature DCs actively promote angiogenesis and tumor growth, whereas DC maturation or ablation suppresses this response. Development of inducers of DC maturation may, therefore, complement current efforts to use DCs for cancer immunotherapy (29), because they would augment the host immune response to the tumor and at the same time suppress tumor angiogenesis. This strategy may also prove to be effective in other angiogenesis-dependent diseases, such as age-related macular degeneration and endometriosis.
Acknowledgments
This work is dedicated to Dr. Judah Folkman, who helped launch and guide this effort. The authors also thank Lauren Bazinet for her expert technical assistance, and Kristin Johnson and Clare Lamont for photography and graphic art. This research was supported by the Fulbright and Rothschild Foundations and the European Molecular Biology Organization (EMBO) Fellowship (O.F.); German Research Council Priority Research Program Tumor-Vessel Interface (DFG-SPP1190) and National Aeronautics and Space Administration Specialized Center of Research (NSCOR) NNJ04HJ12G (A.A.); and U.S. Department of Defense Breast Cancer Innovator award W81XWH-04-1-0316 and U.S. National Institutes of Health award P01 CA045548 (D.I.).
References
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman R M, Hawiger D, Nussenzweig M C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Puig P E, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone D P. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- Conejo-Garcia J R, Benencia F, Courreges M C, Kang E, Mohamed-Hadley A, Buckanovich R J, Holtz D O, Jenkins A, Na H, Zhang L, Wagner D S, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a β-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Coukos G, Benencia F, Buckanovich R J, Conejo-Garcia J R. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainaru O, Adini A, Benny O, Adini I, Short S, Bazinet L, Nakai K, Pravda E, Hornstein M D, D'Amato R J, Folkman J. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. FASEB J. 2008;22:522–529. doi: 10.1096/fj.07-9034com. [DOI] [PubMed] [Google Scholar]
- Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Fainaru O, Bazinet L, Pakneshan P, Benny O, Pravda E, Folkman J, D'Amato R J. Dendritic cells augment choroidal neovascularization. Invest Ophthalmol Vis Sci. 2008;49:3666–3670. doi: 10.1167/iovs.07-1640. [DOI] [PubMed] [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer E G, Littman D R, Lang R A. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M B, Suri R M, Niimi M, Ogilvie A L, Kukutsch N A, Rossner S, Schuler G, Austyn J M. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Adini A, Fainaru O, Udagawa T, Connor K M, Folkman J, D'Amato R J. Matrigel cytometry: a novel method for quantifying angiogenesis in vivo. J Immunol Methods. 2009;342:78–81. doi: 10.1016/j.jim.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Naumov G N, Bender E, Zurakowski D, Kang S Y, Sampson D, Flynn E, Watnick R S, Straume O, Akslen L A, Folkman J, Almog N. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- Passaniti A, Taylor R M, Pili R, Guo Y, Long P V, Haney J A, Pauly R R, Grant D S, Martin G R. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Dranoff G. Triggering tumor immunity through angiogenesis targeting. Clin Cancer Res. 2007;13:3762–3764. doi: 10.1158/1078-0432.CCR-07-0880. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard J W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt S B, Lewis C E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Huarte E, Cubillos-Ruiz J R, Nesbeth Y C, Scarlett U K, Martinez D G, Buckanovich R J, Benencia F, Stan R V, Keler T, Sarobe P, Sentman C L, Conejo-Garcia J R. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–166. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- De Palma M, Venneri M A, Galli R, Sergi Sergi L, Politi L S, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Bennett C L, Clausen B E. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Carmi Y, Voronov E, Dotan S, Lahat N, Rahat M A, Fogel M, Huszar M, White M R, Dinarello C A, Apte R N. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- Melief C J. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]