Abstract
Patients with type 2 diabetes lose β cells, but the underlying mechanisms are incompletely understood. Glucose-6-phosphate dehydrogenase (G6PD) is the principal source of the major intracellular reductant, NADPH, which is required by many enzymes, including enzymes of the antioxidant pathway. Previous work from our laboratory has shown that high glucose impairs G6PD activity in endothelial and kidney cells, which leads to decreased cell survival. Pancreatic β cells are highly sensitive to increased ROS. This study aimed to determine whether G6PD and NADPH play central roles in β-cell survival. Human and mouse islets, MIN6 cell line, and G6PD deficient mice were studied. High glucose inhibited G6PD expression and activity. Inhibition of G6PD with siRNA led to increased ROS and apoptosis, decreased proliferation, and impaired insulin secretion. High glucose decreased insulin secretion, which was improved by overexpressing G6PD. G6PD-deficient mice had smaller islets and impaired glucose tolerance compared with control mice, which suggests that G6PD deficiency per se leads to β-cell dysfunction and death. G6PD plays an important role in β-cell function and survival. High-glucose-mediated decrease in G6PD activity may provide a mechanistic explanation for the gradual loss of β cells in patients with diabetes.—Zhang, Z., Liew, C. W., Handy, D. E., Zhang, Y., Leopold, J. A., Hu, J., Guo, L., Kulkarni, R. N., Loscalzo, J., Stanton, R. C. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and β-cell apoptosis.
Keywords: diabetes mellitus, islets, antioxidants, pentose phosphate pathway
Oxidative stress mediates glucose toxicity to cells and tissues and occurs as a result of an imbalance between processes that produce reactive oxygen species (ROS) and processes that reduce ROS (antioxidants). The major components of the antioxidant system are catalase, superoxide dismutases (SODs), glutathione system, and glucose-6-phosphate dehydrogenase (G6PD). Although any cell is potentially vulnerable to increased ROS, β cells are particularly vulnerable to ROS cytotoxicity, which has been attributed to the relatively low levels of antioxidant enzymes in islets (1,2,3). For example, Tiedge et al. (3) have shown that (cytoplasmic) Cu/Zn SOD and (mitochondrial) Mn SOD expression levels in islets were in the range of 30–40% of those in the liver. In other studies, these investigators have found that glutathione peroxidase-1 (GPx-1) gene expression was 15% of those in liver and that catalase gene expression was not detectable in pancreatic islets (2).
Both type 1 and type 2 diabetes lead to loss of β cells. In type 1 diabetes, β cells are damaged initially by an immune-mediated process (4). In type 2 diabetes, β-cell function decreases gradually over years. Moreover, β-cell mass diminishes over time (5). No definitive causes for loss of β cells have been determined, but it is likely that chronic exposure to elevated blood glucose contributes to decreased β-cell survival. As β cells are highly sensitive to increased ROS, it is likely that increased ROS play a role in the loss of β cells. Indeed, many in vivo and in vitro studies have shown that treatments targeting oxidative stress improve both β-cell function and survival (5,6,7).
Although all components of the antioxidant system are important for cell survival, G6PD has a unique role, as it is the principal source of NADPH, which is the main intracellular reductant that promotes the antioxidant action of peroxidases (8,9,10,11). G6PD is the rate-limiting enzyme in the pentose-phosphate pathway, which produces ribose-5-phosphate and NADPH. Although other sources for NADPH exist, studies by our laboratory and others have shown that G6PD is the major source of NADPH for the antioxidant system and other critical enzymes (9, 12,13,14,15,16,17,18). NADPH is used by the glutathione and thioredoxin systems to regenerate reduced forms that will then be used in antioxidant roles. Catalase, which converts hydrogen peroxide to water and oxygen, does not use NADPH directly, but an essential allosteric binding site for NADPH maintains catalase in its most active tetrameric conformation and protects it against the toxicity of hydrogen peroxide (H2O2) (19). The other major component of the antioxidant system, SOD, which converts superoxide to hydrogen peroxide, does not use NADPH. However, the SOD-produced H2O2 is then reduced by either catalase or GPxs. Hence, SODs become ultimately dependent on NADPH as lack of it will lead to a decrease in catalase and the level of reduced glutathione and a resultant increase in hydrogen peroxide levels. Increased hydrogen peroxide then inhibits SOD activity by a product inhibition mechanism. Therefore, decreases in G6PD activity and, as a result, NADPH level will impair the entire antioxidant system.
Work from our laboratory and others has shown that high glucose and diabetes decrease G6PD activity in endothelial cells, kidney, liver, and red blood cells, which leads to oxidative damage, cellular dysfunction, and organ damage (20,21,22). Previous work has suggested that the inhibition of the pentose phosphate pathway (G6PD is the rate-limiting enzyme of this metabolic pathway) leads to β-cell dysfunction (23). Taken together, all of these data led to our hypothesis that high-glucose-mediated decrease in G6PD would lead to impaired β-cell function and cell death.
MATERIALS AND METHODS
Cell culture and human islet culture
MIN6 β cells were incubated at 37°C and 5% CO2 in DMEM supplemented with 15% fetal bovine serum, penicillin, and streptomycin. Human islets were provided by the Islet Cell Resource Centers Program and cultured in Miami medium 1A containing 5.6 mM glucose (Mediatech Inc., Herndon, VA,USA).
G6PD activity measurement
G6PD activity of MIN6 cells and islets were measured as described previously (22).
ROS measurement
ROS production was measured with the dye CM-H2DCFDA (Invitrogen, Carlsbad, CA, USA). Fluorescence was read with a microplate fluorometer (Victor2 fluorometer, PerkinElmer, Wellesley, MA, USA). After the fluorescence reading, cells were collected to measure protein concentration, which was used to normalize the readings.
Construction of adenoviral-hG6PD vector
Human G6PD cDNA was excised from pCMV-XL4-G6PD (OriGene, Rockville, MD, USA) and was confirmed by sequencing. The construction of adenoviral-hG6PD expression vector was described previously (24). The titer of adenovirus was determined with a commercial kit (Adeno-X™ Rapid Titer Kit; Clontech, Palo Alto, CA, USA), following the manufacturer’s instructions. Empty vector was used for control experiments. MOI 15 was used for all experiments.
siRNA Transfection
Transfection of siRNA for G6PD was performed according to the Lipofectamine™ RNAiMAX transfection protocol (Invitrogen), and then the cells were cultured for 48 h. The sequences designed for inhibiting G6PD gene expression (cat. no. s66341; Invitrogen) in mouse β cells are AAUCAACUGUCGAACCACAtt (sense) and UGUGGUUCGACAGUUGAUUgg (antisense). A scrambled siRNA (4390846; Invitrogen) without biological effects was used as control.
Cell-death detection
Histone/DNA complexes released from the nucleus to the cytosol (DNA fragmentation), a marker for apoptosis, were measured according to the manufacturer’s instructions (Cell Death Detection ELISAPlus; Roche, Indianapolis, IN, USA). In brief, cell lysates were incubated with anti-DNA-peroxidase and anti-histone-biotin and transferred to streptavidin-coated 96-well plates. Absorbance was measured after addition of the peroxidase substrate ABTS (2,2′-azino-bis-3-ethylbenzthiazoline-6-sufonate).
Measurement of cellular proliferation
MIN6 cells were seeded in a 96-well plate, and siRNA transfection was done the next day. Then cells were incubated for 48 h in DMEM medium with 10% FBS. The cellular proliferation was measured with cell proliferation ELISA BrdU assay (Roche Diagnostics) according to the manufacturer’s instructions.
Ki67 staining
Min6 cells were seeded in coverslips, and the next day siRNA transfection was done. After 48 h, cells were fixed with methanol and washed. Cells were blocked with 10% BSA for 20 min. Subsequently, the cells were incubated overnight with Ki67 antibody (BD Pharmingen, San Jose, CA, USA) in antibody buffer solution (10% BSA/PBS). Excess primary antibodies were washed off with 10% BSA/PBS solution. The cells were then incubated with Alexa-conjugated secondary antibody (Molecular Probes, Eugene, OR, USA) for 1 h. Highly cross-adsorbed Alexa594 goat anti-mouse IgG (H+L) (Invitrogen) was used. The coverslips were mounted with antifade mounting medium. To determine nonspecific binding, staining with normal IgG was also performed. Slides were viewed under a fluorescence microscope.
Glucose-stimulated insulin secretion (GSIS) in MIN6 cells and isolated islets
MIN6 cells were grown in 12-well plates until 80% confluent and were then starved with DMEM supplemented with 0.1% bovine serum albumin and 2.8 mM glucose for 14 h. Cells were washed with PBS and starved again with KRB buffer (125 mM NaCl, 4.74 mM KCl, 1 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5 mM NaHCO3, and 25 mM HEPES, pH 7.4) supplemented with 2.8 mM glucose for 1 h. Medium was then changed to KRB buffer with either 2.8 mM or 16.7 mM glucose. Medium was collected after 1 h and centrifuged. The supernatant was used for insulin assay. Cells were lysed to measure protein concentration. Insulin was measured with an ELISA kit (Crystal Chem Inc., Downers Grove, IL, USA), and the results were normalized with protein concentrations. Islets isolated from mice were cultured in RPMI 1640 medium containing 10% FBS. The same GSIS procedure was done as described for the MIN6 cells.
Western blotting
Protein (40 μg/ lane) was size-fractionated electrophoretically using 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes blocked with a 5% nonfat milk solution. The membranes were incubated with antibodies to G6PD (Bethyl Laboratories, Montgomery, TX, USA) and actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and visualized with the ECL detection system (Amersham, Piscataway, NJ, USA).
Animal model
All animal experiments were approved by the Institutional Animal Care and Use Committee at Harvard Medical School and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The G6PD-deficient mouse model was recovered in the offspring of 1-ethyl-nitrosourea-treated male mice on a C3H murine background by Pretsch et al. (25). Later, Sanders et al. (26) showed that there is a single-point mutation (A to T transversion) in the 5′ splice site consensus sequence at the 3′ end of the exon1. The mice were bred at Harvard Medical School from frozen embryos obtained from the Medical Research Council (Harwell, U.K.). G6PD is an X-linked gene, and thus heterozygotes and homozygotes are female and hemizygotes are male. Wild-type C3H control mice and hemizygous G6PD-deficient male mice were studied. Animals were genotyped and characterized as described previously (14).
Islet isolation
Pancreatic islets were isolated by the collagenase digestion method (27). After injection of collagenase (2 mg/ml) into pancreatic ducts, pancreases were harvested and incubated at 37°C in a water bath for 12 min to digest. Ficoll gradient solution (Sigma, St. Louis, MO, USA) was used to separate islets from exocrine tissues. Islets were hand-picked under a dissection microscope. Exocrine tissue was collected for Western blot experiments.
Relative islet mass calculation and immunostaining of pancreas
Mice were anesthetized, and the pancreas was immediately dissected and fixed in Z-fix solution. Three sections (separated by 25 μm) were obtained from each pancreas. Immunoperoxidase staining of sections was done with a guinea pig polyclonal antibody against mouse insulin (Zymed, Burlingame, CA, USA). Relative islet mass was evaluated by point-counting morphometry. Images were acquired using a BX60 microscope (Olympus, Melville, NY, USA) and were analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA). Immunofluorescence staining of pancreas sections of the mice was done with a rabbit polyclonal antibody against mouse G6PD (Bethyl Laboratories).
Glucose tolerance test (GTT)
GTT was performed on animals that had been deprived of food overnight for 16 h. Glucose levels were measured from blood collected from the tail immediately before and at 15, 30, 60, and 120 min after i.p. glucose injection (2 g/kg body weight).
Statistical analysis
Data were expressed as means ± sd. Comparison between groups was performed by Student’s paired 2-tailed t test. ANOVA was used for comparisons across groups with post hoc analysis using the Newman-Keuls test. Values of P < 0.05 were considered significant.
RESULTS
High glucose inhibits G6PD activity and protein expression
To determine whether high glucose affects G6PD expression and activity in β cells, human islets and mouse islets were cultured in 5.6 and 25 mM glucose for 72 h. As compared to the islets in normal glucose, G6PD protein expression was decreased by high glucose (Fig. 1A) in both models, and G6PD activity was also reduced (Fig. 1B). These data show that high glucose leads to a decrease in G6PD activity and protein level in islets, which likely contributes to increased ROS generation.
Figure 1.
High glucose decreases G6PD expression and activity in human and mouse islets. Human and mouse islets were exposed to either 5.6 or 25 mM glucose for 72 h, after which islets were collected for protein extraction. G6PD activity was measured, and Western blot was performed to detect G6PD protein expression. A) G6PD protein expression was lower in islets exposed to 25 mM glucose as compared to 5.6 mM glucose. A total of 200 islets was used for each sample; n = 3. *P < 0.05 vs. 5.6 mM. B) G6PD activity of both human islets and mouse islets exposed to high glucose was lower than with 5.6 mM glucose. n = 4. *P < 0.05 vs. 5.6 mM.
Inhibition of G6PD leads to increased ROS and apoptosis
To determine further whether decreased G6PD activity could contribute to increased ROS in β cells, MIN6 cells were transfected with either specific siRNA targeted to G6PD or scrambled siRNA as control (Fig. 2A). Specific knockdown of G6PD protein in MIN6 cells led to a significant increase of ROS in MIN6 cells (Fig. 2B), illustrating that inhibition of G6PD per se can lead to increased ROS.
Figure 2.
Inhibition of G6PD causes increased ROS generation and increased apoptosis in MIN6 cells. A) MIN6 cells were seeded in 6-well plates, and the next day siRNA transfection was done. After 48 h, cells were harvested for protein extraction, after which Western blot was performed to detect G6PD and actin. G6PD protein expression decreased significantly by specific siRNA knockdown. n = 6. *P < 0.01. B) MIN6 cells were prepared as in A, except that cells were seeded in 24-well plates. ROS accumulation was measured with CM-H2DCFDA. Inhibition of G6PD led to significant increases in ROS; n = 12. *P < 0.05. C) MIN6 cells were prepared as in A. Apoptosis was measured as described in Materials and Methods. Inhibition of G6PD led to increased DNA fragmentation, a marker of apoptosis; n = 6. *P < 0.001. D) Cells were prepared as in A. Western blot was performed to detect cleaved caspase-3, total caspase 3, and actin. Inhibition of G6PD led to increased cleaved caspase-3, a marker of apoptosis. n = 4. *P < 0.01.
As increased ROS may lead to cell death, and since research from our laboratory has shown an important role for G6PD and NADPH in preventing ROS-mediated cell death (28), studies on cell survival were also performed. As a marker for apoptosis, histone/DNA complexes released from the nucleus to the cytosol were measured (DNA fragmentation). Cell death was increased significantly by inhibiting G6PD (Fig. 2C). Caspase 3 is an important mediator of apoptosis, and cleaved caspase 3 protein is observed in apoptosis (29). Inhibition of G6PD increased cleaved-caspase 3 protein in MIN6 cells, suggesting that inhibition of G6PD promotes apoptosis of β cells (Fig. 2D).
Inhibition of G6PD decreases cellular proliferation and insulin secretion in MIN6 cells
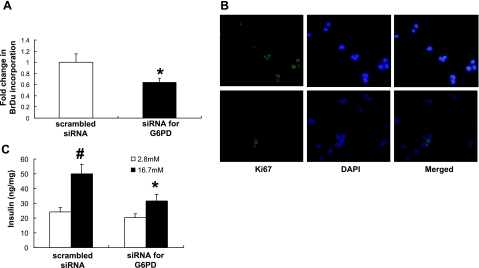
In addition to playing an important role in cell death, previous research from our laboratory has shown that G6PD plays a central role in cell growth (17). To determine whether inhibition of G6PD would affect cellular proliferation, two markers of cell proliferation were studied: BrDu incorporation assay and immunostaining of Ki67. Inhibition of G6PD reduced BrDu incorporation (Fig. 3A), which suggests decreased proliferation. In addition, Ki67, a cell cycle marker of proliferation (30), was detected using immunofluorescence staining. In cells transfected with scrambled siRNA, a pattern of nuclear localization suggests proliferation (Fig. 3B). However, on knockdown of G6PD, the pattern changed in the cells to a diffuse staining, which could indicate no nuclear localization and reduced proliferation (Fig. 3B).
Figure 3.
Inhibition of G6PD causes decreased cellular proliferation and impaired insulin secretion in MIN6 cells. A) Cells were prepared as in Fig. 1A. BrDu incorporation was used to measure cellular proliferation. Inhibition of G6PD significantly decreased cell proliferation; n = 24. *P < 0.001. B) Ki67 staining was performed as another marker of cellular proliferation. In control cells, Ki67 protein is clearly present in the nucleus, consistent with proliferating cells. Inhibition of G6PD led to a diffuse pattern of staining with no clear nuclear localization, consistent with impaired proliferation. C) GSIS assay was performed in MIN6 cells as described in Materials and Methods. Inhibition of G6PD led to a significant impairment in glucose-stimulated insulin release; n = 8. #P < 0.05 vs. 2.8 mM. *P < 0.05 vs. 16.7 mM and scrambled siRNA.
Inhibition of G6PD decreases GSIS
To investigate the effects of inhibiting G6PD on pancreatic function, GSIS was measured. Specific inhibition of G6PD using the siRNA construct led to a significant decrease in insulin secretion (Fig. 3C), which suggests that G6PD activity plays a role in insulin secretion.
Overexpression of G6PD lowers ROS level in β cells
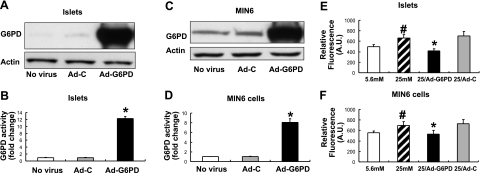
High glucose has been shown to cause ROS accumulation in β cells, leading to cell death and dysfunction (1,2,3). As our previous studies (Fig. 2B) determined that inhibition of G6PD leads to increased ROS level, we tested whether overexpression of G6PD would decrease ROS accumulation in β cells exposed to high glucose. Adenoviral-human G6PD expression vector was constructed to overexpress G6PD in MIN6 cells and islets. As shown, infection of Ad-G6PD increased both G6PD protein expression (Fig. 4A, C) and activity (Fig. 4B, D). In both islets (Fig. 4E) and MIN6 cells (Fig. 4F), 25 mM glucose significantly increased ROS level by 20%, compared to 5.6 mM glucose. Overexpression of G6PD in both models completely prevented the high-glucose-induced increase in ROS level, which suggested that G6PD is critical for the regulation of redox homeostasis in β cells.
Figure 4.
Overexpressing G6PD decreases ROS levels in Islets and MIN6 cells. Adenoviral-G6PD vector (Ad-G6PD) was constructed to overexpress human G6PD (see Materials and Methods). Empty adenoviral vector (Ad-C) was used as control. A–D) Adenoviral overexpression led to increased G6PD protein expression and activity. A, B) Islets were infected with Ad-G6PD vector or Ad-C for 48 h. G6PD protein expression and activity was measured; n = 6. *P < 0.05 vs. Ad-C and no virus controls. C, D) MIN6 cells were infected with Ad-G6PD vector or Ad-C for 48 h. G6PD protein expression and activity was measured; n = 8. *P < 0.05 vs. Ad-C and no virus controls. E, F) Islets and MIN6 cells were exposed to either 5.6 or 25 mM glucose for 72 h, and ROS generation was measured. Data show that 25 mM glucose increased ROS level in both islets (E) and MIN6 cell (F) by 20%, compared with 5.6 mM glucose. In both models, ROS level was reduced by overexpression of G6PD. #P < 0.05 vs. 5.6 mM. *P < 0.05 vs. 25 mM and 25 mM with Ad-C infection.
Overexpression of G6PD enhances insulin secretion in MIN6 cells
Figure 3C showed that inhibition of G6PD impaired GSIS. Previous studies have shown a role for the pentose phosphate pathway (although G6PD was not specifically studied) in insulin secretion (23, 31, 32); thus, cells overexpressing G6PD were used to determine whether overexpression of G6PD would improve insulin secretion. In MIN6 cells, high glucose decreased both basal and stimulated insulin release (Fig. 5). Cells were infected with either empty vector (Ad-C) as a control or an adenovirus containing G6PD (Ad-G6PD). Cells were then incubated with either 5.6 or 25 mM glucose for 48 h. After the 48 h incubation period, GSIS measurement was done. Cells incubated in 5.6 mM glucose showed similar GSIS response in Ad-C- or Ad-G6PD-infected cells. Cells incubated in 25 mM glucose had decreased insulin release as compared to cells incubated in 5.6 mM glucose. Cells incubated in 25 mM glucose had a blunted GSIS that was rescued by overexpression of G6PD, suggesting that the high-glucose-mediated decrease in GSIS is due, at least in part, to increased oxidative stress, which can be ameliorated by increasing G6PD activity.
Figure 5.
Overexpression of G6PD ameliorates the high-glucose-mediated impairment of insulin secretion in MIN6 cells. MIN6 cells were infected with either Ad-C or Ad-G6PD and cultured in 5.6 or 25 mM glucose for 48 h. Cells were then starved, and GSIS was ascertained, as described in Materials and Methods. High glucose decreased both basal and stimulated insulin release. Overexpression of G6PD led to a restoration of GSIS in cells cultured in 25 mM glucose. n = 6. *P < 0.05 vs. 2.8 mM. #P < 0.05 vs. 16.7 mM and 25 mM with Ad-C.
Islets express low levels of G6PD as compared to the exocrine pancreas, and G6PD-deficient mice have decreased islet mass and impaired glucose tolerance as compared to mice with wild-type G6PD activity
Lastly, studies were performed in mice (wild-type and G6PD deficient) to determine whether G6PD deficiency per se leads to changes in pancreatic islets. First, the protein expression level of G6PD in β cells was determined in wild-type mice. Coimmunostaining for G6PD (red) and insulin (green) showed that islet cells have very low expression of G6PD, as compared to exocrine pancreatic cells (Fig. 6A). To further confirm this, G6PD protein expression was detected by Western blotting in both isolated islets and exocrine pancreas (Fig. 6B). G6PD protein in islets is significantly lower than in exocrine pancreas. Compared to wild-type mice, hemizygous G6PD-deficient mice have 15% G6PD activity (14). The relative islet mass of G6PD-deficient mice was significantly smaller than that of wild-type mice (Fig. 7A, B). Furthermore, a GTT showed that the glucose levels in G6PD-deficient mice were significantly higher than in wild-type mice (Fig. 7C). Finally, GSIS was assayed in islets isolated from both wild-type and hemizygous mice. Insulin secretion of islets from hemizygous mice was reduced compared with wild-type mice (Fig. 7D) These data from G6PD-deficient mice suggest that G6PD activity plays a role in β-cell survival and function in vivo.
Figure 6.
Islets have very low levels of G6PD protein, as determined by coimmunostaining of G6PD (red) and insulin (green) in pancreas of C3H mice. A) Left panel: there is little expression of G6PD in the islet, whereas nonislet cells have much higher levels of G6PD protein. Right panel: insulin staining localizes islet tissue. B) Western blot shows that G6PD protein expression in isolated islets (I) is significantly lower than in exocrine pancreas (Exo); n = 3.
Figure 7.
G6PD deficiency is associated directly with decreased islet mass and impaired glucose tolerance. Islet mass is decreased significantly in mice expressing lower levels of G6PD activity. A) Immunostaining of paraffin-embedded pancreatic sections (n=3×3 slides) with antibody against mouse insulin was performed on pancreas from wild-type control mice and G6PD-deficient mice. Hemizygous mice have much smaller islets as compared to wild-type mice. Original view ×20. B) Quantitation of relative islet mass. Islet mass was measured as described in Materials and Methods. Hemizygous mice have significantly smaller islets as compared to wild-type mice. *P < 0.05 vs. WT. C) Mice expressing lower levels of G6PD activity have impaired glucose tolerance. Intraperitoneal GTTs show that G6PD-deficient mice had impaired glucose tolerance as compared to wild-type control mice. *P < 0.01 vs. WT. D) GSIS was measured with islets isolated from wild-type and G6PD-deficient mice. G6PD-deficient mice had significantly impaired GSIS as compared to wild-type mice. Mice were studied at age of 6 mo. #P < 0.05 vs. 2.8 mM. *P < 0.05 vs. 16.7mM WT. WT, wild type; Hemi, hemizygous (15% of WT G6PD activity).
DISCUSSION
The loss of pancreatic β-cell function is a central feature of both type 1 and type 2 diabetes. In type 1 diabetes, an autoimmune process leads to insulitis and loss of β cells. In type 2 diabetes, loss of β cells occurs over a period of years due to a process that has not been elucidated fully. Glucotoxicity and lipotoxicity are thought to play a major role, at least in significant part by increasing oxidative stress, leading to apoptosis of β cells (33, 34). High glucose has been shown to increase ROS in many cell types in patients with diabetes due to a combination of increased production of ROS along with decreased antioxidant function (1, 21, 33,34,35,36,37). Many laboratories have shown that pancreatic β cells are very sensitive to oxidant damage, which has been attributed to the low expression levels of antioxidant enzymes (1,2,3). Thus, β cells are likely at higher risk of oxidant-mediated cellular damage and death as compared to other mammalian cell types.
The evidence for accumulation of ROS leading to β-cell dysfunction and apoptosis has been reviewed recently (38, 39). Increased ROS occurs due to an imbalance of processes that produce ROS and processes that reduce ROS (antioxidant systems). Possible sources of increased ROS production in diabetes include glyceraldehyde autooxidation, increased aldose reductase reactivity and resultant decrease in NADPH, increased production of superoxide from mitochondria, and others (1, 33, 34, 38). Examples of decreased antioxidant function are as follows. Pancreatic β cells have low levels of GPx, catalase, and SOD activities under normal glucose conditions as compared to other cell types. Lenzen et al. (2) have reported levels of 30–40% of liver enzyme levels for SOD, 15% of liver levels for GPx, and nondetectable catalase activity. Robertson and colleagues (40) have shown that β cells express very low levels of GPx. Decreased levels of antioxidant enzymes have been reported in diabetes as well. In the pancreas of type 2 diabetic patients, SOD expression was decreased (41). In the pancreas of diet-induced diabetic mice, GPx was down-regulated (42). Thus, the combination of high-glucose-mediated increase in ROS and low levels of antioxidants renders the β cell to be unusually sensitive to the deleterious effects of increased ROS as compared to other cell types. Further support of increased ROS playing an important role in β-cell dysfunction, and cell death has been demonstrated by studies involving overexpression of antioxidants. For example, adenoviral-mediated GPx overexpression protects β cells against oxidative stress (43). Catalase overexpression protects islets against oxidative stress (44). Overexpression of GPx provides protection against the deleterious effects of hyperglycemia (45).
Two major roles for G6PD in β cells have been suggested in the present study: maintenance of β-cell function and preservation of β cells. Figures 3 and 5 show that G6PD activity and ROS likely play important roles in GSIS. A role for G6PD and NADPH in regulating insulin secretion has been suggested previously through indirect studies. Ammon, Steinke, and Patel (23, 31, 32) reported that 6-aminonicotinamide, a general inhibitor of the pentose phosphate pathway, decreased insulin release in isolated rat islets. More recently, a role for ROS in regulating β-cell function has been shown by Robertson and colleagues (46) in HIT-T15 cells cultured in increased glucose concentrations. These studies determined that the activities of two important regulators of the insulin promoter, PDX-1 and MafA, were undetectable (46) and that antioxidant treatment improved this defect. Recently, a role for NADPH has also been shown in insulin secretion and another NADPH producing enzyme, mitochondrial associated-isocitrate dehydrogenase (ICDH). This form of ICDH is primarily associated with mitochondria and is not a source of NADPH for cytoplasmic enzymes. In this study, mitochondrial-based shuttling mechanisms (ICDH production of NADPH) were determined to regulate insulin secretion (47). Taken together, these results suggest that NADPH is important for proper β-cell function by maintaining proper ROS levels in the cytoplasm and by providing reducing power to mitochondrial shuttle reactions.
To treat and prevent diabetes, it is essential to preserve β cells. The results presented in the present work show that G6PD is important for β-cell proliferation and prevention of β-cell death. Past research has shown that hyperglycemia stimulates apoptosis in islets along with expression of proapoptotic genes (38). Pancreatic β cells from humans with type 2 diabetes have been shown to have increased expression of the apoptotic mediators, caspase 3 and caspase 6 (48). Butler et al. (49) determined that pancreatic tissue from autopsy specimens from people with type 2 diabetes showed a 3–10 fold increase in β-cell apoptosis as compared to people without diabetes. The data reported here show that inhibition of G6PD increases the expression of cleaved caspase 3 and increased histone/DNA complex release from the nucleus, which are markers of apoptotic cell death. Our laboratory has previously shown that G6PD is of central importance in cell survival in that G6PD is critical for cell growth (17) and essential for protecting against various forms of cell death (28). The results in Fig. 3 demonstrated that decreased G6PD impaired BrdU incorporation and altered Ki67 staining pattern, both of which are markers of decreased cell proliferation (50). Furthermore, the data reported from the G6PD-deficient animals are particularly interesting, in that islet mass was directly proportional to G6PD activity. These results suggest that G6PD activity per se can control islet cell mass. Thus, considering the central role that G6PD plays in maintaining the entire antioxidant system by providing NADPH along with the low level of G6PD expression, it is intriguing to speculate that enhancing G6PD would have a highly beneficial, protective effect by enhancing catalase, glutathione reductase, and SOD, as the handling of hydrogen peroxide by either catalase or the glutathione system will enhance the flux of partially reduced forms of oxygen through to water and lipid alcohols.
G6PD is the main source of NADPH used by the cellular antioxidant systems, as discussed in the introduction. Information reported in the present study illustrate, we believe for the first time, that G6PD protein expression is very low in β cells (Fig. 6). Considering that the entire antioxidant system is dependent on NADPH (as discussed in the introduction), even modest decreases in G6PD activity could significantly increase the ROS level and lead to cell damage and death. In addition, the data reported here support the following conclusions. 1) High glucose impairs G6PD activity and increases ROS in β cells (Fig. 1). 2) Specific inhibition of G6PD alone leads to decreased β-cell survival and decreased β-cell proliferation (Figs. 2 and 3). 3) Overexpression of G6PD prevents the high-glucose-mediated increase in ROS (Fig. 4), and overexpression of G6PD restores GSIS (Fig. 5). 4) The islet mass is directly associated with G6PD activity in G6PD-deficient mice. Taken together, these data support a significant role for decreased G6PD playing a central role in the high-glucose-mediated increase in ROS, which leads to β-cell dysfunction and death. Increasing G6PD activity would likely offer a new approach for protecting pancreatic β cells from dysfunction and death.
Acknowledgments
The authors thank Dan Kawamori, Xiaodan Wang, Wan-Chun Li, Jiang Hu, and Hui Li for technical assistance. This work was supported by U.S. National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK54380 to R.C.S.; NIH/National Heart, Lung, and Blood Institute (NHLBI) grants HL 61795, N01 HV 28178, P01 HL 81857, and U54 HL 070819 to J.A.L.; and NIH/NHLBI grant HL 089734 to D.E.H. with Jacob Joseph (Brigham and Women’s Hospital, Boston, MA, USA). R.N.K. was supported by NIH grants RO1 DK 68721 and RO1 DK 67536; and C.L.W. is supported by NIH training grant T32DK007260. The Joslin Core Facilities were used for islet isolation and other measurements, which are funded by Diabetes Endocrinology Research Center (DERC) grant P30DK036836. The authors declare no conflicts of interests.
References
- Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G S. Update in type 1 diabetes. J Clin Endocrinol Metab. 2007;92:2403–2407. doi: 10.1210/jc.2007-0339. [DOI] [PubMed] [Google Scholar]
- Wajchenberg B L. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- Robertson R P, Tanaka Y, Takahashi H, Tran P O, Harmon J S. Prevention of oxidative stress by adenoviral overexpression of glutathione-related enzymes in pancreatic islets. Ann N Y Acad Sci. 2005;1043:513–520. doi: 10.1196/annals.1333.058. [DOI] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Munday R, Lenzen S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes. 1998;47:1578–1585. doi: 10.2337/diabetes.47.10.1578. [DOI] [PubMed] [Google Scholar]
- Martini G, Ursini M V. A new lease of life for an old enzyme. BioEssays. 1996;18:631–637. doi: 10.1002/bies.950180806. [DOI] [PubMed] [Google Scholar]
- Pandolfi P P, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini F, Franze A, Iervolino A, Filosa S, Salzano S, Ursini M V. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J Biol Chem. 1999;274:2750–2757. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- Ursini M V, Parella A, Rosa G, Salzano S, Martini G. Enhanced expression of glucose 6-phosphate dehydrogenase in human cells sustaining oxidative stress. Biochem J. 1997;323:801–806. doi: 10.1042/bj3230801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R S, Vijay V, Marks B, Levine R J, Sabbah H N, Wolin M S, Recchia F A, Gupte S A. Up-regulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J Card Fail. 2007;13:497–506. doi: 10.1016/j.cardfail.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Jain M, Brenner D A, Cui L, Lim C C, Wang B, Pimentel D R, Koh S, Sawyer D B, Leopold J A, Handy D E, Loscalzo J, Apstein C S, Liao R. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res. 2003;93:e9–16. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- Leopold J A, Walker J, Scribner A W, Voetsch B, Zhang Y Y, Loscalzo A J, Stanton R C, Loscalzo J. Glucose-6-phosphate dehydrogenase modulates vascular endothelial growth factor-mediated angiogenesis. J Biol Chem. 2003;278:32100–32106. doi: 10.1074/jbc.M301293200. [DOI] [PubMed] [Google Scholar]
- Matsui R, Xu S, Maitland K A, Mastroianni R, Leopold J A, Handy D E, Loscalzo J, Cohen R A. Glucose-6-phosphate dehydrogenase deficiency decreases vascular superoxide and atherosclerotic lesions in apolipoprotein E(-/-) mice. Arterioscler Thromb Vasc Biol. 2006;26:910–916. doi: 10.1161/01.ATV.0000205850.49390.3b. [DOI] [PubMed] [Google Scholar]
- Park J, Choe S S, Choi A H, Kim K H, Yoon M J, Suganami T, Ogawa Y, Kim J B. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes. 2006;55:2939–2949. doi: 10.2337/db05-1570. [DOI] [PubMed] [Google Scholar]
- Tian W-N, Braunstein L D, Pang J, Stuhlmeier K, Xi Q-C, Tian X, Stanton R C. Importance of glucose 6-phosphate dehydrogenase activity for cell growth. J Biol Chem. 1998;273:10609–10617. doi: 10.1074/jbc.273.17.10609. [DOI] [PubMed] [Google Scholar]
- Tian W-N, Pignatare J N, Stanton R C. Signal transduction proteins that associate with the platelet-derived growth factor (PDGF) receptor mediate the PDGF-induced release of glucose 6-phosphate dehydrogenase. J Biol Chem. 1994;269:14798–14805. [PubMed] [Google Scholar]
- Kirkman H N, Rolfo M, Ferraris A M, Gaetani G F. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J Biol Chem. 1999;274:13908–13914. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores M, Ibanez-Hernandez M A, Galvan R E, Gutierrez M, Duran-Reyes G, Medina-Navarro R, Pascoe-Lira D, Ortega-Camarillo C, Vilar-Rojas C, Cruz M, Baiza-Gutman L A. Glucose-6-phosphate dehydrogenase activity and NADPH/NADP+ ratio in liver and pancreas are dependent on the severity of hyperglycemia in rat. Life Sci. 2006;78:2601–2607. doi: 10.1016/j.lfs.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Xu Y, Osborne B W, Stanton R C. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of protein kinase A which contributes to oxidative stress in rat kidney cortex. Am J Physiol (Renal Physiol) 2005;289:F1040–F1047. doi: 10.1152/ajprenal.00076.2005. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Apse K, Pang J, Stanton R C. High glucose inhibits glucose 6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem. 2000;275:40042–40047. doi: 10.1074/jbc.M007505200. [DOI] [PubMed] [Google Scholar]
- Ammon H P, Steinke J. 6-Amnionicotinamide (6-AN) as a diabetogenic agent. In vitro and in vivo studies in the rat. Diabetes. 1972;21:143–148. doi: 10.2337/diab.21.3.143. [DOI] [PubMed] [Google Scholar]
- Leopold J A, Zhang Y Y, Scribner A A, Stanton R C, Loscalzo J. Glucose 6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler Thromb Vasc Biol. 2003;23:411–417. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- Pretsch W, Charles D J, Merkle S. X-linked glucose-6-phosphate dehydrogenase deficiency in Mus musculus. Biochem Genet. 1988;26:89–103. doi: 10.1007/BF00555491. [DOI] [PubMed] [Google Scholar]
- Sanders S, Smith D P, Thomas G A, Williams E D. A glucose-6-phosphate dehydrogenase (G6PD) splice site consensus sequence mutation associated with G6PD enzyme deficiency. Mutat Res. 1997;374:79–87. doi: 10.1016/s0027-5107(96)00222-9. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco A P. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- Tian W-N, Braunstein L D, Apse K, Pang J, Rose M, Tian X, Stanton R C. Importance of glucose 6-phosphate dehydrogenase activity in cell death. Am J Physiol (Cell) 1999;276:C1121–C1131. doi: 10.1152/ajpcell.1999.276.5.C1121. [DOI] [PubMed] [Google Scholar]
- Mazumder S, Plesca D, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2008;414:13–21. doi: 10.1007/978-1-59745-339-4_2. [DOI] [PubMed] [Google Scholar]
- Starborg M, Gell K, Brundell E, Hoog C. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci. 1996;109:143–153. doi: 10.1242/jcs.109.1.143. [DOI] [PubMed] [Google Scholar]
- Ammon H P, Patel T N, Steinke J. The role of the pentose phosphate shunt in glucose induced insulin release: in vitro studies with 6-aminonicotinamide, methylene blue, NAD +, NADH, NADP +, NADPH, and nicotinamide on isolated pancreatic rat islets. Biochim Biophys Acta. 1973;297:352–367. doi: 10.1016/0304-4165(73)90083-4. [DOI] [PubMed] [Google Scholar]
- Ammon H P, Steinke J. Effect of 6-aminonicotinamide on insulin release and C-14 glucose oxidation by isolated pancreatic rat islets: difference between glucose, tolbutamide, and aminophylline. Endocrinology. 1972;91:33–38. doi: 10.1210/endo-91-1-33. [DOI] [PubMed] [Google Scholar]
- Robertson R P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- Robertson R P, Harmon J, Tran P O, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53:S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- Baynes J W, Thorpe S R. Role of oxidative stress in diabetic complications. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Forbes J M, Coughlan M T, Cooper M E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- Maritim A C, Sanders R A, Watkins J B., III Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Prato S. Beta-cell apoptosis in type 2 diabetes: quantitative and functional consequences. Diabetes Metab. 2008;34:S56–64. doi: 10.1016/S1262-3636(08)73396-2. [DOI] [PubMed] [Google Scholar]
- Robertson R, Zhou H, Zhang T, Harmon J S. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. 2007;48:139–146. doi: 10.1007/s12013-007-0026-5. [DOI] [PubMed] [Google Scholar]
- Robertson R P, Harmon J S. Pancreatic islet beta-cell and oxidative stress: the importance of glutathione peroxidase. FEBS Lett. 2007;581:3743–3748. doi: 10.1016/j.febslet.2007.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- Qiu L, List E O, Kopchick J J. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005;4:1311–1318. doi: 10.1074/mcp.M500016-MCP200. [DOI] [PubMed] [Google Scholar]
- Moriscot C, Richard M J, Favrot M C, Benhamou P Y. Protection of insulin-secreting INS-1 cells against oxidative stress through adenoviral-mediated glutathione peroxidase overexpression. Diabetes Metab. 2003;29:145–151. doi: 10.1016/s1262-3636(07)70021-6. [DOI] [PubMed] [Google Scholar]
- Benhamou P Y, Moriscot C, Richard M J, Beatrix O, Badet L, Pattou F, Kerr-Conte J, Chroboczek J, Lemarchand P, Halimi S. Adenovirus-mediated catalase gene transfer reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia. 1998;41:1093–1100. doi: 10.1007/s001250051035. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tran P O, Harmon J, Robertson R P. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon J S, Stein R, Robertson R P. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280:11107–11113. doi: 10.1074/jbc.M410345200. [DOI] [PubMed] [Google Scholar]
- Ronnebaum S M, Ilkayeva O, Burgess S C, Joseph J W, Lu D, Stevens R D, Becker T C, Sherry A D, Newgard C B, Jensen M V. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281:30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89:5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- Butler A E, Janson J, Bonner-Weir S, Ritzel R, Rizza R A, Butler P C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Iatropoulos M J, Williams G M. Proliferation markers. Exp Toxicol Pathol. 1996;48:175–181. doi: 10.1016/S0940-2993(96)80039-X. [DOI] [PubMed] [Google Scholar]