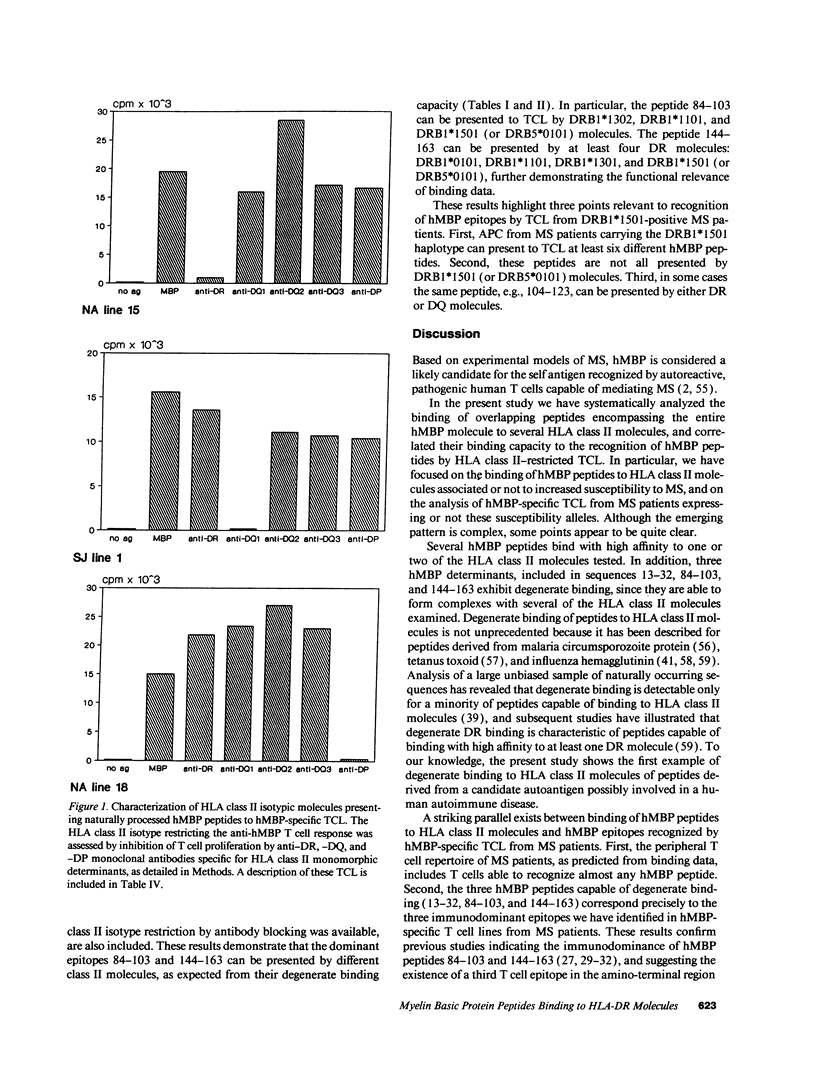
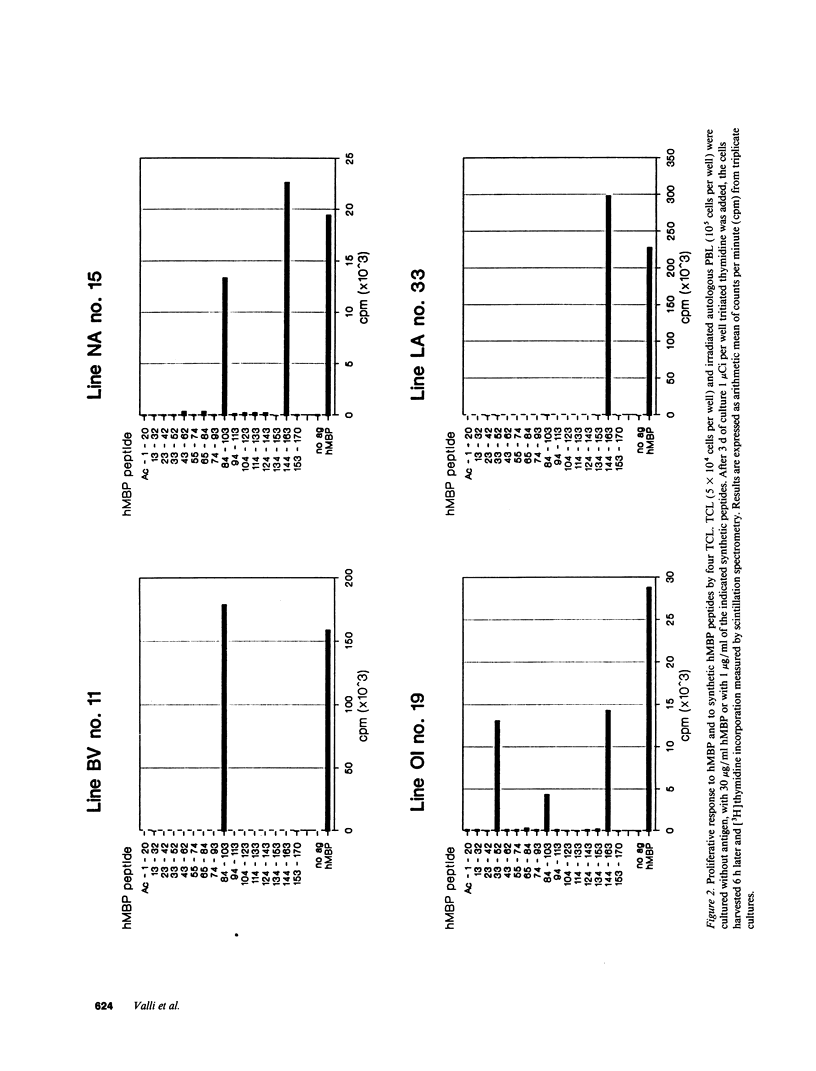
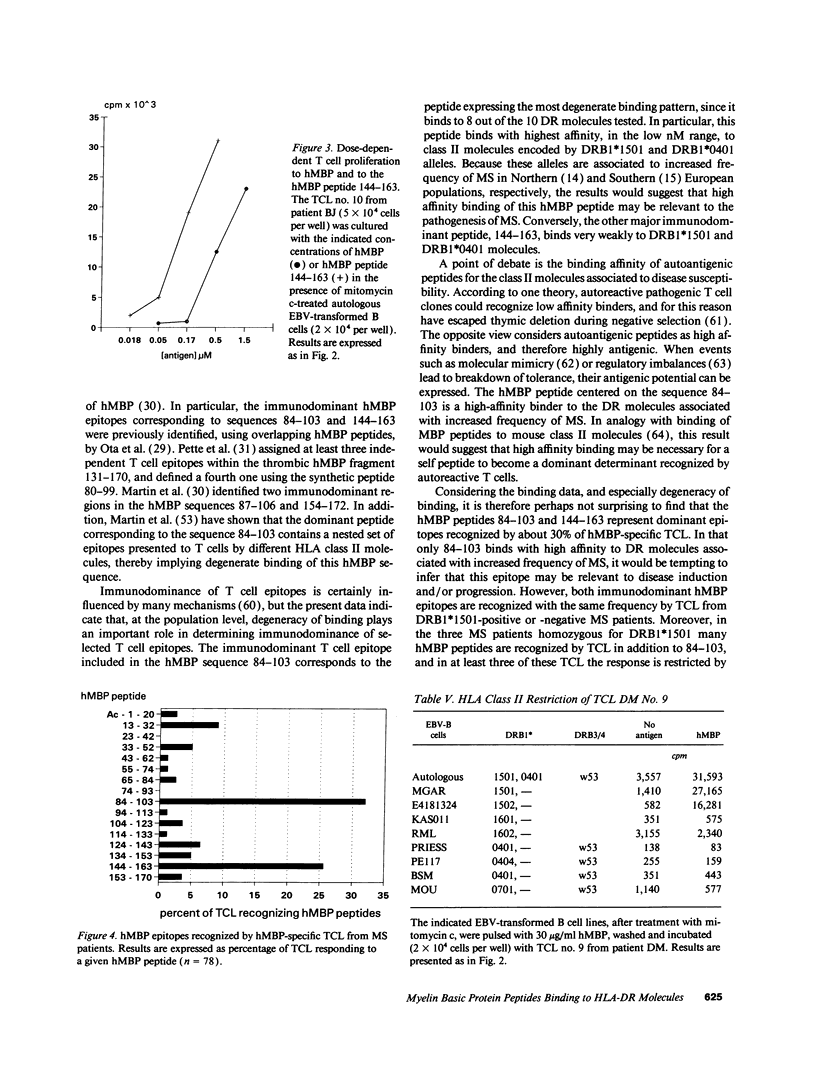
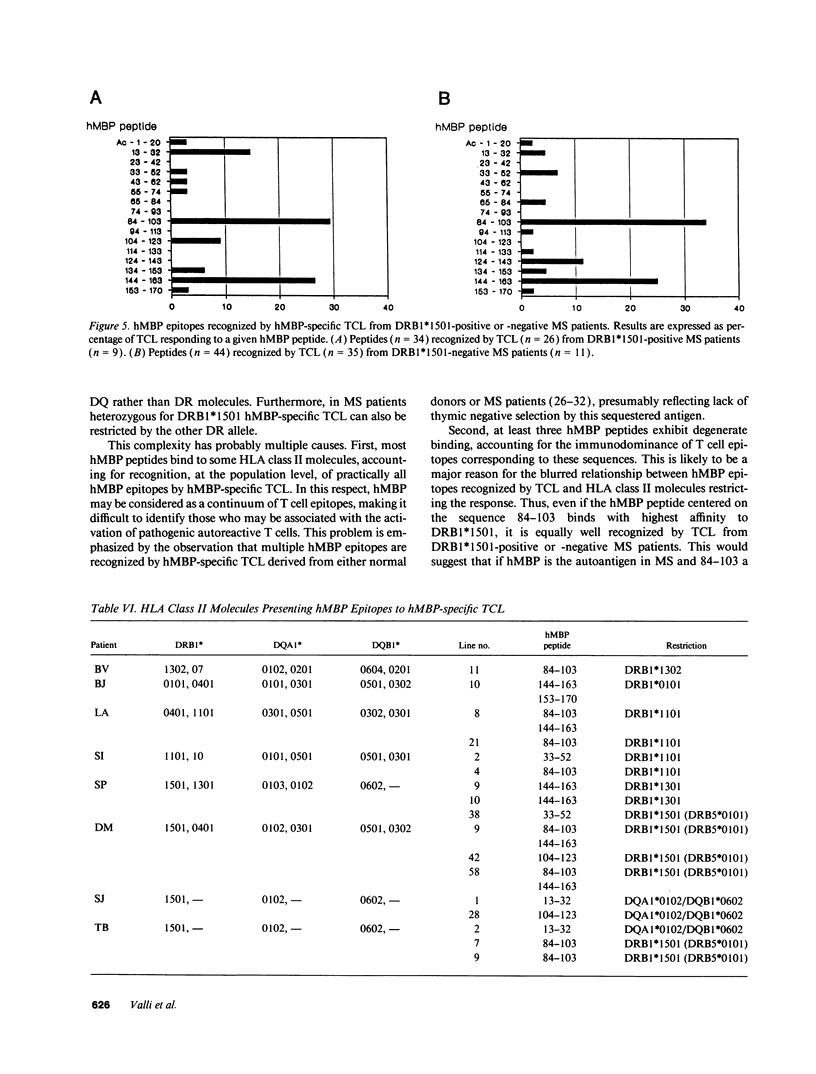
Abstract
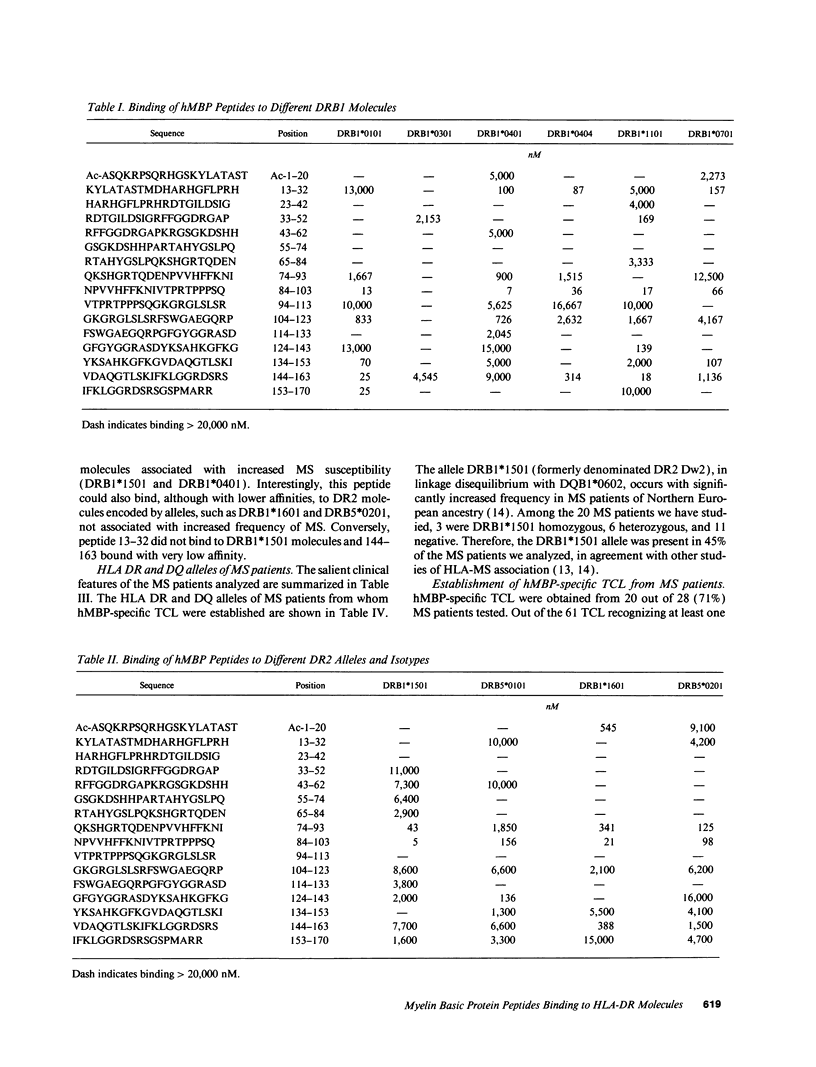
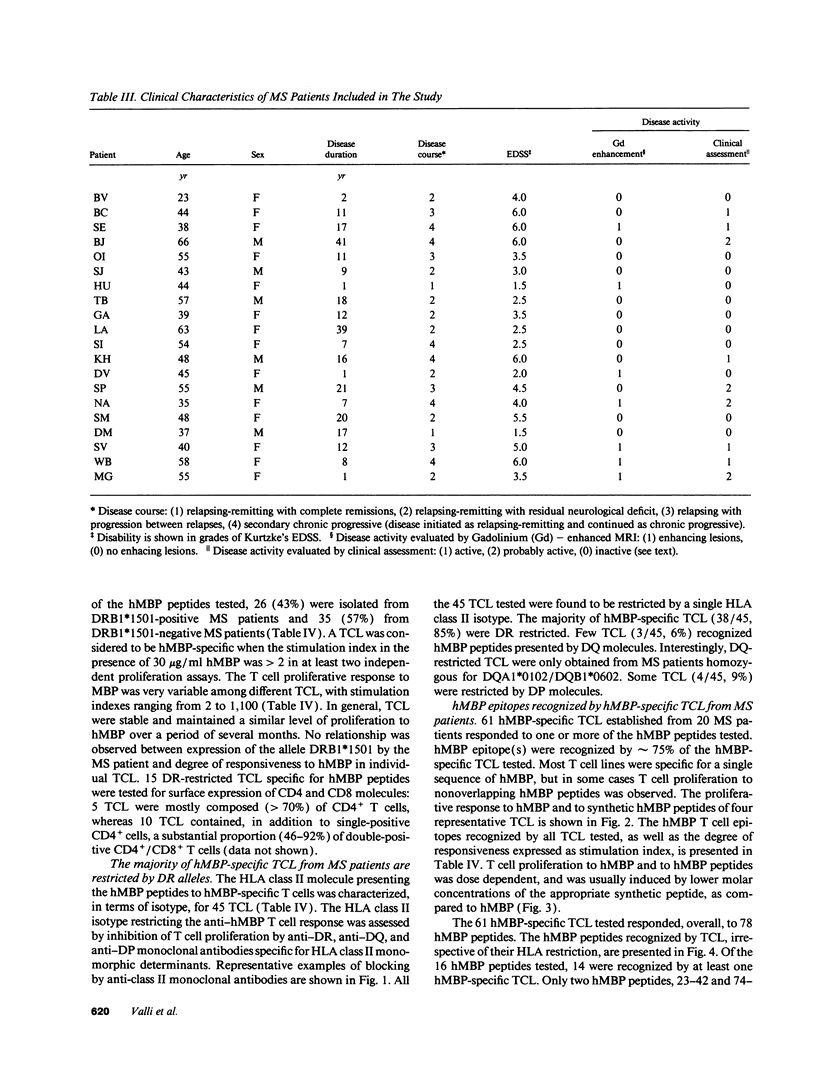
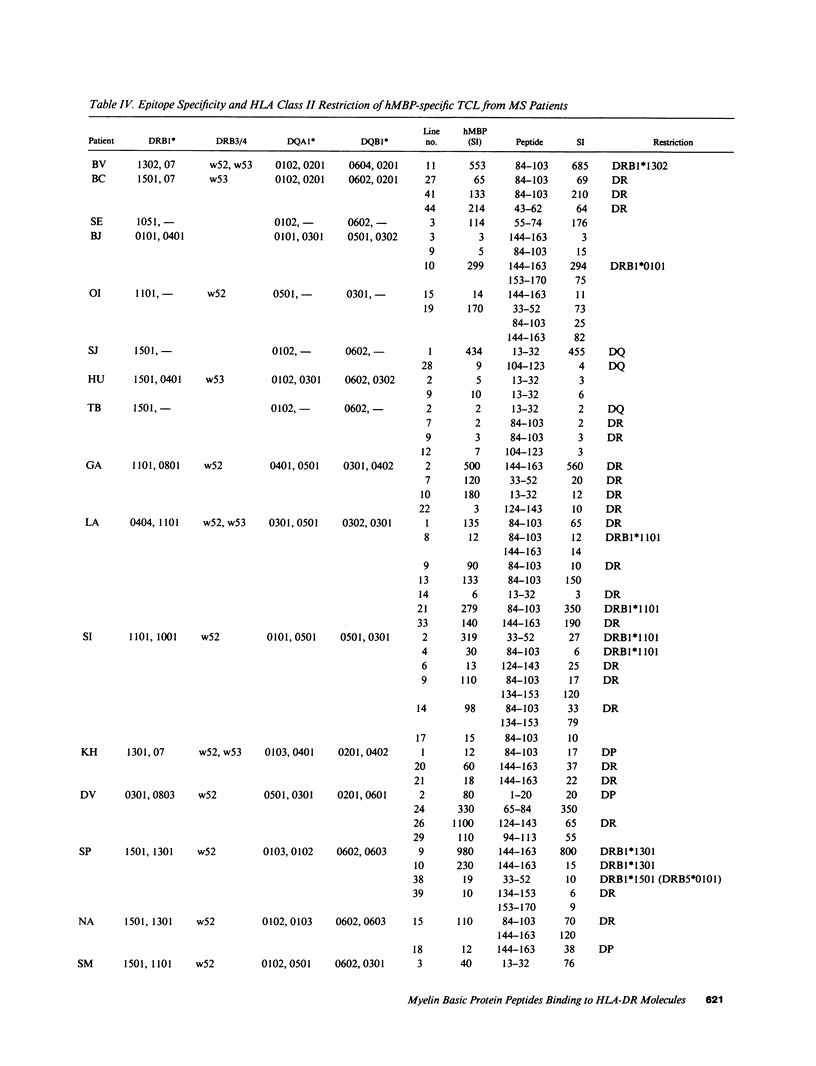
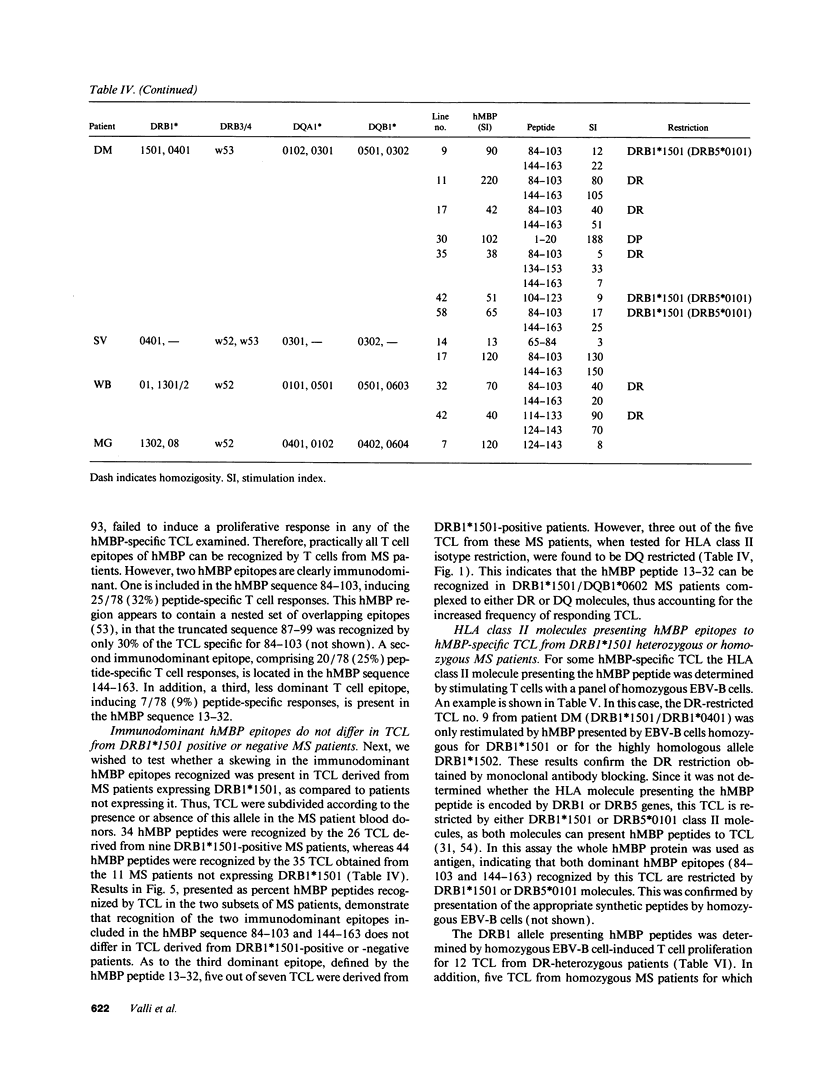
Multiple sclerosis (MS) is an autoimmune disease in which myelin proteins have been implicated as autoantigens recognized by pathogenic autoreactive T cells. To study the relationship between human myelin basic protein (hMBP) and HLA alleles associated to MS susceptibility, such as DRB1*1501, the binding of synthetic peptides spanning the entire hMBP sequence to 10 purified HLA-DR molecules was determined. All the hMBP peptides tested showed binding affinity for at least one of the DR molecules analyzed, but three hMBP peptides, included in sequences 13-32, 84-103, and 144-163 were found capable of binding to three or more DR molecules. The hMBP peptide 84-103 was the most degenerate in binding, in that it bound to 9 out of 10 DR molecules tested. Interestingly, it bound with highest affinity to DRB1*1501 molecules. To correlate the binding pattern of hMBP peptides to HLA class II molecules with their recognition by T cells, 61 hMBP-specific T cell lines (TCL) were established from the peripheral blood of 20 MS patients, who were homozygous, heterozygous, or negative for DRB1*1501. Analysis of hMBP epitopes recognized by these TCL and their HLA restriction demonstrated a very good correlation between binding data and T cell proliferation to hMBP peptides. Although virtually all hMBP peptides tested could be recognized by at least one TCL from MS patients, three immunodominant T cell epitopes were apparent among the TCL examined, corresponding exactly to the hMBP peptides capable of binding to several DR molecules. No major difference could be detected in the recognition of immunodominant hMBP peptides by TCL from DRB1*1501 positive or negative MS patients. These results have implications for the role of hMBP as relevant autoantigen, and of DRB1*1501 as susceptibility allele in MS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Gross N., Carrel S., Corte G. Distinct forms of both alpha and beta subunits are present in the human Ia molecular pool. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4549–4551. doi: 10.1073/pnas.78.7.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acha-Orbea H., Steinman L., McDevitt H. O. T cell receptors in murine autoimmune diseases. Annu Rev Immunol. 1989;7:371–405. doi: 10.1146/annurev.iy.07.040189.002103. [DOI] [PubMed] [Google Scholar]
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegretta M., Nicklas J. A., Sriram S., Albertini R. J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990 Feb 9;247(4943):718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Baxevanis C. N., Reclos G. J., Servis C., Anastasopoulos E., Arsenis P., Katsiyiannis A., Matikas N., Lambris J. D., Papamichail M. Peptides of myelin basic protein stimulate T lymphocytes from patients with multiple sclerosis. J Neuroimmunol. 1989 Mar;22(1):23–30. doi: 10.1016/0165-5728(89)90005-2. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Liblau R. S., Cohen L., Lehmann D., Tournier-Lasserve E., Rosenzweig A., Zhang J. W., Raus J. C., Bach M. A. Restricted T-cell receptor V beta gene usage by myelin basic protein-specific T-cell clones in multiple sclerosis: predominant genes vary in individuals. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2466–2470. doi: 10.1073/pnas.88.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Bodmer J. G., Marsh S. G., Albert E. D., Bodmer W. F., Dupont B., Erlich H. A., Mach B., Mayr W. R., Parham P., Sasazuki T. Nomenclature for factors of the HLA system, 1990. Hum Immunol. 1991 Jul;31(3):186–194. doi: 10.1016/0198-8859(91)90025-5. [DOI] [PubMed] [Google Scholar]
- Chou Y. K., Vainiene M., Whitham R., Bourdette D., Chou C. H., Hashim G., Offner H., Vandenbark A. A. Response of human T lymphocyte lines to myelin basic protein: association of dominant epitopes with HLA class II restriction molecules. J Neurosci Res. 1989 Jun;23(2):207–216. doi: 10.1002/jnr.490230211. [DOI] [PubMed] [Google Scholar]
- Corte G., Calabi F., Damiani G., Bargellesi A., Tosi R., Sorrentino R. Human Ia molecules carrying DC1 determinants differ in both alpha- and beta-subunits from Ia molecules carrying DR determinants. Nature. 1981 Jul 23;292(5821):357–360. doi: 10.1038/292357a0. [DOI] [PubMed] [Google Scholar]
- Ebers G. C., Bulman D. E., Sadovnick A. D., Paty D. W., Warren S., Hader W., Murray T. J., Seland T. P., Duquette P., Grey T. A population-based study of multiple sclerosis in twins. N Engl J Med. 1986 Dec 25;315(26):1638–1642. doi: 10.1056/NEJM198612253152603. [DOI] [PubMed] [Google Scholar]
- Francis D. A., Thompson A. J., Brookes P., Davey N., Lechler R. I., McDonald W. I., Batchelor J. R. Multiple sclerosis and HLA: is the susceptibility gene really HLA-DR or -DQ? Hum Immunol. 1991 Oct;32(2):119–124. doi: 10.1016/0198-8859(91)90108-l. [DOI] [PubMed] [Google Scholar]
- Gammon G., Sercarz E. How some T cells escape tolerance induction. Nature. 1989 Nov 9;342(6246):183–185. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- Giegerich G., Pette M., Meinl E., Epplen J. T., Wekerle H., Hinkkanen A. Diversity of T cell receptor alpha and beta chain genes expressed by human T cells specific for similar myelin basic protein peptide/major histocompatibility complexes. Eur J Immunol. 1992 Mar;22(3):753–758. doi: 10.1002/eji.1830220319. [DOI] [PubMed] [Google Scholar]
- Gorga J. C., Horejsí V., Johnson D. R., Raghupathy R., Strominger J. L. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987 Nov 25;262(33):16087–16094. [PubMed] [Google Scholar]
- Gorga J. C., Knudsen P. J., Foran J. A., Strominger J. L., Burakoff S. J. Immunochemically purified DR antigens in liposomes stimulate xenogeneic cytolytic T cells in secondary in vitro cultures. Cell Immunol. 1986 Nov;103(1):160–173. doi: 10.1016/0008-8749(86)90077-8. [DOI] [PubMed] [Google Scholar]
- Gorodezky C., Najera R., Rangel B. E., Castro L. E., Flores J., Velázquez G., Granados J., Sotelo J. Immunogenetic profile of multiple sclerosis in Mexicans. Hum Immunol. 1986 Aug;16(4):364–374. doi: 10.1016/0198-8859(86)90063-7. [DOI] [PubMed] [Google Scholar]
- Grossman R. I., Gonzalez-Scarano F., Atlas S. W., Galetta S., Silberberg D. H. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986 Dec;161(3):721–725. doi: 10.1148/radiology.161.3.3786722. [DOI] [PubMed] [Google Scholar]
- Hillert J., Leng C., Olerup O. T-cell receptor alpha chain germline gene polymorphisms in multiple sclerosis. Neurology. 1992 Jan;42(1):80–84. doi: 10.1212/wnl.42.1.80. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D., Martin R., Rosen-Bronson S., Flerlage M., McFarland H. F., Long E. O. HLA-DR2a is the dominant restriction molecule for the cytotoxic T cell response to myelin basic protein in DR2Dw2 individuals. J Immunol. 1990 Nov 1;145(9):2880–2885. [PubMed] [Google Scholar]
- Kotzin B. L., Karuturi S., Chou Y. K., Lafferty J., Forrester J. M., Better M., Nedwin G. E., Offner H., Vandenbark A. A. Preferential T-cell receptor beta-chain variable gene use in myelin basic protein-reactive T-cell clones from patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9161–9165. doi: 10.1073/pnas.88.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. I., Karr R. W., Grey H. M., Yu W. Y., O'Sullivan D., Batovsky L., Zheng Z. L., Colón S. M., Gaeta F. C., Sidney J. Single amino acid changes in DR and antigen define residues critical for peptide-MHC binding and T cell recognition. J Immunol. 1991 Apr 1;146(7):2331–2340. [PubMed] [Google Scholar]
- Kurtzke J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Liblau R., Tournier-Lasserve E., Maciazek J., Dumas G., Siffert O., Hashim G., Bach M. A. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991 Jun;21(6):1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- Marrosu M. G., Muntoni F., Murru M. R., Spinicci G., Pischedda M. P., Goddi F., Cossu P., Pirastu M. Sardinian multiple sclerosis is associated with HLA-DR4: a serologic and molecular analysis. Neurology. 1988 Nov;38(11):1749–1753. doi: 10.1212/wnl.38.11.1749. [DOI] [PubMed] [Google Scholar]
- Martin R., Howell M. D., Jaraquemada D., Flerlage M., Richert J., Brostoff S., Long E. O., McFarlin D. E., McFarland H. F. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991 Jan 1;173(1):19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Jaraquemada D., Flerlage M., Richert J., Whitaker J., Long E. O., McFarlin D. E., McFarland H. F. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol. 1990 Jul 15;145(2):540–548. [PubMed] [Google Scholar]
- Martin R., Utz U., Coligan J. E., Richert J. R., Flerlage M., Robinson E., Stone R., Biddison W. E., McFarlin D. E., McFarland H. F. Diversity in fine specificity and T cell receptor usage of the human CD4+ cytotoxic T cell response specific for the immunodominant myelin basic protein peptide 87-106. J Immunol. 1992 Mar 1;148(5):1359–1366. [PubMed] [Google Scholar]
- McDonald W. I., Barnes D. Lessons from magnetic resonance imaging in multiple sclerosis. Trends Neurosci. 1989 Oct;12(10):376–379. doi: 10.1016/0166-2236(89)90075-1. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., Blank S. E., Kibler R. F., McKneally S., Shapira R. Experimental allergic encephalomyelitis in the rat: response to encephalitogenic proteins and peptides. Science. 1973 Feb 2;179(4072):478–480. doi: 10.1126/science.179.4072.478. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Naito S., Kuroiwa Y., Itoyama T., Tsubaki T., Horikawa A., Sasazuki T., Noguchi S., Ohtsuki S., Tokuomi H., Miyatake T. HLA and Japanese MS. Tissue Antigens. 1978 Jul;12(1):19–24. [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Nevinny-Stickel C., Hinzpeter M., Andreas A., Albert E. D. Non-radioactive oligotyping for HLA-DR1-DRw10 using polymerase chain reaction, digoxigenin-labelled oligonucleotides and chemiluminescence detection. Eur J Immunogenet. 1991 Oct-Dec;18(5-6):323–332. doi: 10.1111/j.1744-313x.1991.tb00032.x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D., Arrhenius T., Sidney J., Del Guercio M. F., Albertson M., Wall M., Oseroff C., Southwood S., Colón S. M., Gaeta F. C. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991 Oct 15;147(8):2663–2669. [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Appella E., Walker L., Phillips L., Colón S. M., Miles C., Chesnut R. W., Sette A. Characterization of the specificity of peptide binding to four DR haplotypes. J Immunol. 1990 Sep 15;145(6):1799–1808. [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Del Guercio M. F., Colón S. M., Sette A. Truncation analysis of several DR binding epitopes. J Immunol. 1991 Feb 15;146(4):1240–1246. [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry as a mechanism for the cause and a probe uncovering etiologic agent(s) of autoimmune disease. Curr Top Microbiol Immunol. 1989;145:127–135. doi: 10.1007/978-3-642-74594-2_11. [DOI] [PubMed] [Google Scholar]
- Ota K., Matsui M., Milford E. L., Mackin G. A., Weiner H. L., Hafler D. A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990 Jul 12;346(6280):183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Pette M., Fujita K., Wilkinson D., Altmann D. M., Trowsdale J., Giegerich G., Hinkkanen A., Epplen J. T., Kappos L., Wekerle H. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7968–7972. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Richert J. R., Reuben-Burnside C. A., Deibler G. E., Kies M. W. Peptide specificities of myelin basic protein-reactive human T-cell clones. Neurology. 1988 May;38(5):739–742. doi: 10.1212/wnl.38.5.739. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER G. A., BEEBE G., KIBLER R. F., KURLAND L. T., KURTZKE J. F., MCDOWELL F., NAGLER B., SIBLEY W. A., TOURTELLOTTE W. W., WILLMON T. L. PROBLEMS OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS: REPORT BY THE PANEL ON THE EVALUATION OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS. Ann N Y Acad Sci. 1965 Mar 31;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- Sette A., Sidney J., Albertson M., Miles C., Colón S. M., Pedrazzini T., Lamont A. G., Grey H. M. A novel approach to the generation of high affinity class II-binding peptides. J Immunol. 1990 Sep 15;145(6):1809–1813. [PubMed] [Google Scholar]
- Sette A., Southwood S., O'Sullivan D., Gaeta F. C., Sidney J., Grey H. M. Effect of pH on MHC class II-peptide interactions. J Immunol. 1992 Feb 1;148(3):844–851. [PubMed] [Google Scholar]
- Sherritt M. A., Oksenberg J., de Rosbo N. K., Bernard C. C. Influence of HLA-DR2, HLA-DPw4, and T cell receptor alpha chain genes on the susceptibility to multiple sclerosis. Int Immunol. 1992 Feb;4(2):177–181. doi: 10.1093/intimm/4.2.177. [DOI] [PubMed] [Google Scholar]
- Sinha A. A., Lopez M. T., McDevitt H. O. Autoimmune diseases: the failure of self tolerance. Science. 1990 Jun 15;248(4961):1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Tuohy V. K., Lu Z., Sobel R. A., Laursen R. A., Lees M. B. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol. 1989 Mar 1;142(5):1523–1527. [PubMed] [Google Scholar]
- Wall M., Southwood S., Sidney J., Oseroff C., del Guericio M. F., Lamont A. G., Colón S. M., Arrhenius T., Gaeta F. C., Sette A. High affinity for class II molecules as a necessary but not sufficient characteristic of encephalitogenic determinants. Int Immunol. 1992 Jul;4(7):773–777. doi: 10.1093/intimm/4.7.773. [DOI] [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Weiner H. L., Hafler D. A. T-cell recognition of myelin basic protein. Immunol Today. 1991 Aug;12(8):277–282. doi: 10.1016/0167-5699(91)90126-E. [DOI] [PubMed] [Google Scholar]
- Yaqub B. A., Daif A. K. Multiple sclerosis in Saudi Arabia. Neurology. 1988 Apr;38(4):621–623. doi: 10.1212/wnl.38.4.621. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Moore A. C., Kitamura K., Steinman L., Rothbard J. B. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986 Nov 20;324(6094):258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. 1985 Sep 26-Oct 2Nature. 317(6035):355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]



