Abstract
2,2-Bis(bromomethyl)-1,3-propanediol (BMP) is a brominated flame retardant used in unsaturated polyester resins. In a 2-year bioassay BMP was shown to be a multisite carcinogen in rats and mice. Because glucuronidation is the key metabolic transformation of BMP by rats, in this study the in vitro hepatic glucuronidation of BMP was compared across several species. In addition, the glucuronidation activities of human intestinal microsomes and specific human hepatic UDP-glucuronosyltransferase (UGT) enzymes for BMP were determined. To explore other possible routes of metabolism for BMP, studies were conducted with rat and human hepatocytes. Incubation of hepatic microsomes with BMP in the presence of UDP-glucuronic acid resulted in the formation of a BMP monoglucuronide. The order of hepatic microsomal glucuronidation activity of BMP was rats, mice ≫ hamsters > monkeys ⋙ humans. The rate of glucuronidation by rat hepatic microsomes was 90-fold greater than that of human hepatic microsomes. Human intestinal microsomes converted BMP to BMP glucuronide at a rate even lower than that of human hepatic microsomes. Among the human UGT enzymes tested, only UGT2B7 had detectable glucuronidation activity for BMP. BMP monoglucuronide was the only metabolite formed when BMP was incubated with suspensions of freshly isolated hepatocytes from male F-344 rats or with cryopreserved human hepatocytes. Glucuronidation of BMP in human hepatocytes was extremely low. Overall, the results support in vivo studies in rats in which BMP glucuronide was the only metabolite found. The poor glucuronidation capacity of humans for BMP suggests that the pharmacokinetic profile of BMP in humans will be dramatically different from that of rodents.
The brominated flame retardant 2,2-bis(bromomethyl)-1,3-propanediol (BMP) is used in the manufacture of unsaturated polyester resins, molded products, and rigid polyurethane foam. The estimated annual production of BMP in the United States is 3 to 4 million pounds, but current production figures are not available (Elwell et al., 1989). In contrast with other known brominated flame retardants, BMP is a small aliphatic molecule. Its unique structure contains the bromine bonded to the carbon adjacent to the central carbon that has no hydrogen atoms (Fig. 1). This provides a compound that is very resistant to dehydrobromination. It is soluble in organic solvents (acetone, ethanol, and ether) and slightly soluble in water (2 g/l at 25°C) (National Toxicology Program, 1996; U.S. Environmental Protection Agency, 2002).
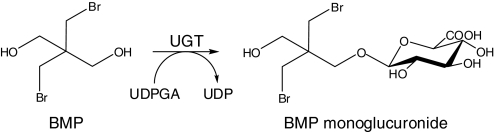
Fig. 1.
UGT-catalyzed glucuronidation of BMP.
No quantitative data were available on human exposure to BMP during the preparation of this article. Occupational exposure as a result of production of flame-retardant resins could occur [National Institute of Occupational Safety and Health, Cincinnati, OH, National Occupational Exposure Survey (1981–1983), unpublished provisional data as of January 24, 1995]. In such situations, the primary routes of human exposure to BMP are expected to be via inhalation and dermal contact (Larsen, 1969; U.S. International Trade Commission, 1994; National Toxicology Program, 1996). BMP may also enter the environment as fugitive dust and through wastewater, where it is expected to be persistent.
Based on animal studies conducted by the National Toxicology Program, BMP is anticipated to be a human carcinogen (Report on Carcinogens, 2004). In the 2-year feeding study of the National Toxicology Program, BMP was found to be a multisite carcinogen in Fischer-344 (F-344) rats and B6C3F1 mice (Dunnick et al., 1997). Increased incidences of neoplasms were observed in a variety of tissues (i.e., esophagus, lung, kidney, and urinary bladder) (National Toxicology Program, 1996). Because of the carcinogenic potential of BMP, it is critical to know whether it is metabolized similarly in rodents and humans to better extrapolate animal toxicity data to humans.
The hydroxyl groups present on BMP (Fig. 1) provide functional groups for glucuronidation and sulfation. Indeed, results of a recent in vivo study with male F-344 rats show that BMP is extensively excreted in the urine (80% in 12 h) solely as a monoglucuronide conjugate (Hoehle et al., 2009). This BMP glucuronide is most likely formed in the liver because it is secreted into the bile at early time points after administration. However, after enterohepatic recycling it is primarily eliminated in the urine.
In the present study, we investigated the in vitro glucuronidation of BMP using hepatic microsomes obtained from a number of species, including humans, as well as six human hepatic recombinant UDP-glucuronosyltransferase (UGT) enzymes. To better assess its metabolism in a more integrated system, BMP was also incubated with rat and human hepatocytes.
Materials and Methods
Chemicals.
[U-14C]BMP (lot 10426-17-34) in absolute ethanol (1 mCi/ml) with a specific activity of 65.1 mCi/mmol (247 μCi/mg) was obtained from Midwest Research Institute (Kansas City, MO). The radiochemical purity of [14C]BMP was determined by reverse-phase HPLC-UV/Vis-radiometric analysis to be 97.3%. Nonradiolabeled BMP (lot 04119MD) was obtained from Sigma-Aldrich (St. Louis, MO). Chemical purity of unlabeled BMP was 98%. [Propyl-2-14C]bisphenol A (BPA) was obtained from Moravek Biochemicals (Brea, CA). Liver digest media (lot 289936) and Williams' E medium (WEM) without phenol red (lot 1282834) were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum was obtained from HyClone (Logan, UT). Flo-Scint III and Pico-Fluor 40 scintillation cocktail solutions were received from PerkinElmer Life and Analytical Sciences (Waltham, MA). 4-(Trifluoromethyl) umbelliferone (TFMU), UDPGA, d-saccharic acid-1,4-lactone, β-glucuronidase (EC 3.2.1.31, type B-1 from bovine liver), sulfatase (EC 3.1.6.1, type VI from Aerobacter aerogenes), acetonitrile, alamethicin, and all other chemicals and reagents were obtained from Sigma-Aldrich. All chemicals and reagents were HPLC or analytical grade.
Biological Materials.
Animals.
Male F-344 rats from Harlan Sprague-Dawley (Indianapolis, IN) weighing 200 to 325 g were used. Animals were fed a standard commercial diet (Harlan Teklad 4% mouse/rat diet; Harlan Teklad, Indianapolis, IN) and were allowed food and water ad libitum. All animals were housed in the University of Arizona Animal Care facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The animals were maintained in a temperature-controlled (25°C) room under a 12-h light/dark cycle and acclimated for at least 5 to 7 days after receipt.
Microsomal fractions.
For the preparation of microsomal fractions, male rats were euthanized by CO2 inhalation, and livers were excised immediately. Pooled F-344 rat liver microsomes were prepared from eight male F-344 rats according to the procedure described by Guengerich (1989). Pooled liver microsomes from female F-344 rats (pool of 105 animals), male B6C3F1 mice (pool of 347 animals), male Golden Syrian hamsters (pool of 101 animals), and male rhesus monkeys (pool of 12 animals) were purchased from XenoTech, LLC (Lenexa, KS).
Pooled human liver microsomes were also purchased from XenoTech, LLC. They were prepared from the livers of 50 donors (29 males and 21 females of white, Hispanic, and African American race, with ages ranging from 7 to 76 years). Pooled human intestinal microsomes were purchased from BD Gentest (Woburn, MA) and contained equal amounts of microsomes prepared from both the duodenum and jejunum of each of the 10 donors (6 males and 4 females of white, Hispanic, and African American race, with ages ranging from 5 to 62 years). Supersomes, i.e., microsomes from insect Sf-9 cells infected with a baculovirus strain containing the cDNA of human UGT1A1, 1A3, 1A4, 1A6, 1A9, or 2B7, were obtained from BD Gentest.
All microsomal preparations were stored at −80°C. Protein concentrations were measured using a BCA Protein Assay Kit from Pierce Chemical (Rockford, IL) with bovine serum albumin as standard (Smith et al., 1985).
Rat and human hepatocyte preparations.
Primary hepatocytes from male F-344 rats were prepared in-house via a two-stage perfusion method as described by Pritchett et al. (2002). Only those cell preparations with >90% viability (as determined by trypan blue exclusion) were used for incubations.
Pooled cryopreserved human hepatocytes were purchased from CellzDirect (Austin, TX) and were prepared from the livers of 10 donors (5 males and 5 females of white and African American race, with ages ranging from 20 to 74 years). The post-thaw viability was determined by trypan blue exclusion to be ≥75%.
Glucuronidation Assays.
To determine UGT activity toward BMP, hepatic microsomes (from male and female F-344 rats, male B6C3F1 mice, male Golden Syrian hamsters, male rhesus monkeys, and humans), intestinal microsomes (humans) or expressed individual UGTs (human) were incubated with [14C]BMP. To ensure activation, microsomes were preincubated on ice with alamethicin (5 μg/ml) for 10 min (Hoehle et al., 2007). Incubations were then performed in a total volume of 0.2 ml, consisting of microsomal protein (0.1–1.0 mg/ml), 0.1 M phosphate buffer, pH 7.4, magnesium chloride (10 mM), the β-glucuronidase inhibitor d-saccharic acid-1,4-lactone (10 mM), UDPGA (4 mM), and [14C]BMP ([50 μM; 1.1 μCi/ml] dissolved in dimethyl sulfoxide; final concentration 1%). The samples were incubated at 37°C for 15 to 360 min, depending on the source of microsomes. A number of control incubations were also performed. These included incubations with the known substrate for a number of UGT enzymes, TFMU, as well as incubations with heat-denatured microsomes or with intact microsomes in the absence of UDPGA. For incubations with recombinant UGTs, the control incubations consisted of Supersomes lacking UGT enzymes, but in the presence of UDPGA. All reactions were terminated with 0.2 ml of ice-cold methanol. Samples were centrifuged (5 min, 14,000 rpm) and the supernatants (50–100 μl) were subjected to HPLC-radiometric or UV/Vis analysis. All incubations were performed in duplicate on at least three different occasions. For concentration-dependent metabolism studies, incubations were conducted with [14C]BMP at final concentrations of 3.5, 7, 15, 25, 50, 100, 250, 500, or 1000 μM (0.2–0.9 μCi/ml) and rat liver microsomes (0.25 mg/ml) as described above. Glucuronidation activities for each substrate concentration were determined in three independent experiments in duplicate.
Hepatocyte Incubations.
Rat hepatocytes (0.25–1 × 106 cells/ml) were incubated in suspension with WEM and [14C]BMP (2, 25, 50, 75, or 100 μM; 0.2–0.6 μCi/ml, 0.25% DMSO-absolute ethanol, 10:1, v/v) at 37°C in a shaking water bath for 0 to 120 min in a total volume of 1 ml. Human hepatocytes (0.25 × 106 cells/ml) were incubated under similar conditions with [14C]BMP (2, 25, or 50 μM) for 0 to 360 min. Aliquots (150–250 μl) were removed at various times, and reactions were terminated by snap-freezing in liquid nitrogen. Samples were thawed and then stored at −20°C until analysis. Before HPLC-radiometric analysis the samples were centrifuged (5 min, 14,000 rpm) to obtain a supernatant. Incubations with rat hepatocytes were conducted three times in duplicate for each BMP concentration. [14C]BPA (50 μM, 0.2 μCi/ml) was included to verify glucuronidation activity of rat and human hepatocytes (Pritchett et al., 2002; Kuester and Sipes, 2007). Negative control incubations were conducted using substrate in WEM without cells.
HPLC Separations and Analyses.
To separate BMP from its metabolite(s), an HP 1100 HPLC system equipped with a quaternary pump, thermostated column compartment, thermostated autosampler, and diode array detector (Agilent Technologies, Palo Alto, CA) was used. The HPLC system was coupled to a flow-through β-RAM detector for 14C radioactivity (IN/US Systems, Tampa, FL) and to an in-line fraction collector (Gilson Inc., Middleton, WI). UV/Vis detection was used to detect TFMU and its metabolites. Data were acquired and analyzed using HP ChemStation software for LC 3D (revision B.01.01; Agilent Technologies) and WinFlow software (version 1.5; LabLogic, Sheffield, UK). Samples (50–100 μl of supernatant) were injected on to a 250 × 4.6-mm i.d., 5 μm, reverse-phase Luna C18 column coupled with a 4.0 × 3.0-mm i.d. SecurityGuard C18 guard cartridge (Phenomenex, Torrance, CA) and eluted with a mobile phase consisting of Nanopure water and acetonitrile, both containing 0.1% formic acid. The gradient was run from 10% acetonitrile and 90% water for the 1st min, then up to 40% acetonitrile over 12 min, and finally up to 90% acetonitrile in 1 min and held for 5 min. The column was re-equilibrated to the initial conditions for 10 min between injections. The flow rate was 0.9 ml/min at 25°C. The autosampler temperature was maintained at 10°C. Because of the low conversion of BMP to BMP glucuronide by human hepatocytes and to confirm the absence or presence of any additional metabolites, the fractions of the radio-HPLC effluent were subjected to further analysis with a Beckman LS 3801 liquid scintillation counter (Beckman Coulter, Fullerton, CA). Fractions were collected at a rate of 1 min/tube. The limit of detection and limit of quantitation for 14C equivalents for the liquid scintillation counter were 0.22 and 0.83 pmol, respectively, as determined by the equation described by Zhu et al. (2005).
Identification of Phase II Metabolites.
For the identification of conjugates of BMP, samples from microsomal and hepatocyte incubations were subjected to enzymatic hydrolysis with β-glucuronidase or sulfatase followed by HPLC analyses. Liquid chromatography-MS and MS/MS analyses of the conjugate peak were conducted using methods described previously by Hoehle et al. (2009). The HP 1100 HPLC system was coupled with an MSD-Trap-SL ion trap mass spectrometer (Agilent Technologies) and LC/MSD Trap software (version 5.3; Bruker Daltonics, Billerica, MA) for data collection and analysis. The mass spectrometer was operated in the positive electrospray ionization mode, and two masses were selected for analysis: m/z = 438.9 (BMP monoglucuronide) and m/z = 262.9 (BMP). The first scan was a full MS scan, and then precursor ions were selected, isolated, and selectively fragmented in the ion trap as described previously (Hoehle et al., 2009).
Data Analysis.
The amount of BMP glucuronide formed and the glucuronidation activity were calculated from the area of the HPLC glucuronide peak using the specific activity of the [14C]BMP stock solution. For the kinetic studies, the data were subjected to analysis based on Michaelis-Menten kinetics with OriginPro 6.0 software (OriginLab Corporation, Northampton, MA). This program determines the Km and Vmax values from enzyme kinetic data using nonlinear regression analysis. The results from the hyperbolic regression were used to determine the in vitro metabolic clearance (CL = Vmax/Km), which is expressed in volume/time. The calculated CL was scaled up to total liver microsomal protein per kilogram (CLint) by assuming a rat liver weight of 30 g/kg b.wt. and a conversion factor of 45 mg of microsomal protein per 1 g of liver (Corley et al., 2005). The total hepatic clearance (CLH) was calculated using the following formula from the well stirred model:
where fu is the unbound fraction of BMP in blood, fuinc is the fraction of BMP unbound to microsomal protein, and CLint is the total in vitro intrinsic clearance (Houston, 1994). The rat hepatic blood flow (Q) was assumed to be 45 ml/min/kg (Corley et al., 2005), and fu was determined to be 0.6 by a method described previously by Cheng et al. (2009). fuinc was estimated to be 0.94 (Hallifax and Houston, 2006). The liver extraction ratio was determined using the CLH/Q ratio.
Results
BMP Glucuronide Formation by Hepatic Microsomes from F-344 Rats.
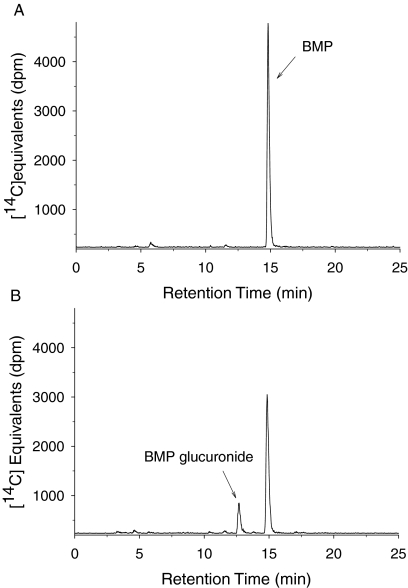
In pilot studies [14C]BMP (or nonradiolabeled BMP for MS analysis) was incubated with various concentrations of hepatic microsomal protein (0.1–0.5 mg/ml) from male F-344 rats in the presence of UDPGA. HPLC analysis of these samples revealed a single product peak (retention time of 12.4 min) eluting earlier than BMP (retention time of 14.4 min) from the reverse-phase column (Fig. 2). This product was tentatively identified as a glucuronide of BMP (Fig. 1) because incubation with β-glucuronidase resulted in a loss of this peak with a corresponding increase in a peak that coeluted with the BMP standard. MS/MS analysis of this metabolite in incubations of rat liver microsomes with BMP revealed a fragmentation pattern identical to that reported previously for the monoglucuronide of BMP (Hoehle et al., 2009). The formation of the BMP glucuronide was stoichiometric with the loss of BMP, and it increased with an increase in concentration of microsomal protein and with time of incubation. Linear product formation for up to 120 min was achieved using a protein concentration of 0.25 mg/ml.
Fig. 2.
Representative HPLC-radiochromatograms from incubations of BMP with hepatic microsomes obtained from male F-344 rats. The 30-min incubations contained 0.25 mg of microsomal protein/ml and 50 μM [14C]BMP. A, incubation without UDPGA. B, incubation in the presence of UDPGA (4 mM). The peak at 5.5 min represents a contaminant (see text).
A single contaminant eluting at 16.4 min was observed in radio-HPLC analyses of [14C]BMP standard (data not shown). This peak was not observed in any incubated samples. However a new peak, which contained the same amount of radiolabel (<3%), eluted at 5.5 min. This peak appears to be a degradation product of the original contaminant as described previously (Hoehle et al., 2009).
Incubation of [14C]BMP with Primary Hepatocytes from Male F-344 Rats.
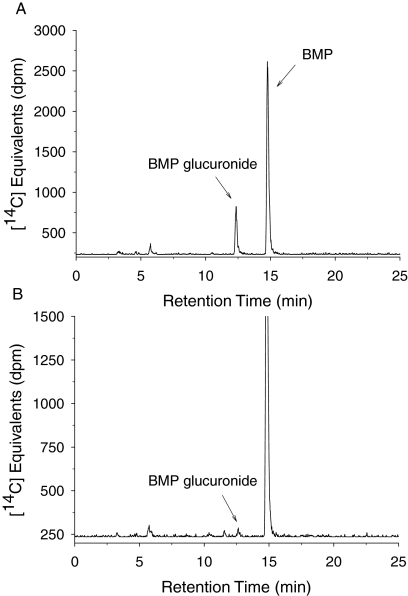
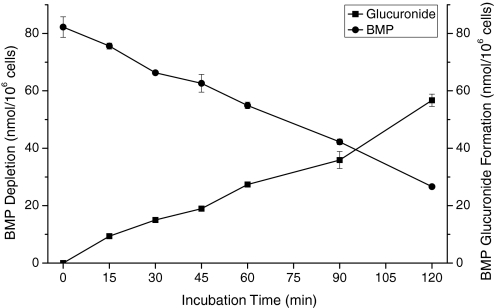
When [14C]BMP was incubated in suspensions of primary hepatocytes obtained from male F-344 rats, the same glucuronide identified in incubations using rat liver microsomes was formed (Fig. 3A). There was no evidence for the formation of other metabolites of BMP by rat hepatocytes (sulfatase treatment, HPLC analyses). A time course showing the conversion of BMP into BMP monoglucuronide is depicted in Fig. 4. The formation of the BMP monoglucuronide was stoichiometric, regardless of the concentration of BMP (2–100 μM) or the concentration of rat hepatocytes (0.25–1.0 × 106 cells/ml). Data are only presented for incubations containing 50 μM BMP.
Fig. 3.
Representative HPLC-radiochromatograms of BMP glucuronide formed after the incubation of 50 μM BMP with freshly isolated male F-344 rat hepatocytes (30 min, 0.5 × 106 cells/ml) (A) and human cryopreserved hepatocytes (240 min, 0.25 × 106 cells/ml) (B). (Note the difference in scale on the y-axis.)
Fig. 4.
Time course of stoichiometric conversion of [14C]BMP (50 μM) into BMP monoglucuronide by freshly isolated hepatocytes (0.5 × 106 cells/ml) from male F-344 rats.
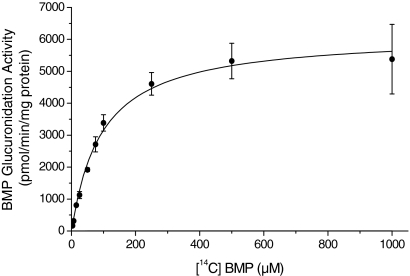
Kinetics of BMP Glucuronidation in Hepatic Microsomes from F-344 Rats.
Kinetic analysis of BMP glucuronide formation was performed using hepatic microsomes from male and female F-344 rats. The formation of the monoglucuronide of BMP followed Michaelis-Menten kinetics (Fig. 5). The Vmax values were 6161 ± 173 and 6339 ± 250 pmol/min/mg protein and Km values were 95 ± 8 and 153 ± 17 μM for hepatic microsomes from male and female F-344 rats, respectively (Table 1). The CL (Vmax/Km) was scaled up to CLint and modeled to estimate CLH (Table 1). The total hepatic clearances (CLH) were found to be 23.5 and 18.5 ml/min/kg for male and female rats, respectively.
Fig. 5.
Representative hyperbolic regression analysis of BMP glucuronidation by hepatic microsomes from male F-344 rats (mean ± S.D. of three independent experiments; incubation time 10 min). Predicted values were calculated based on the Michaelis-Menten equation (OriginPro).
TABLE 1.
Kinetic parameters for the glucuronidation of [14C]BMP by F-344 rat hepatic microsomes
All data were converted to the rate of BMP glucuronide formed per minute per milligram of microsomal protein (mean ± S.D. of three independent experiments). CLint and CLH determinations were performed as described under Materials and Methods.
| Rat Hepatic Microsomes | Vmax | Km | Vmax/Km | Scaled CLint | Scaled CLH | Extraction Ratio |
|---|---|---|---|---|---|---|
| pmol/min/mg protein | μM | μl/min/mg protein | ml/min/kg | |||
| Male | 6161 ± 173 | 95 ± 8 | 65 | 88 | 23.5 | 0.5 |
| Female | 6339 ± 250 | 153 ± 17 | 42 | 56 | 18.5 | 0.4 |
Glucuronidation of BMP by Human Hepatic and Intestinal Microsomes and UGTs.
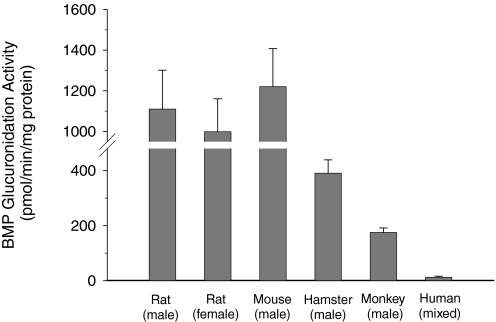
When [14C]BMP (50 μM) was incubated with human liver microsomes and UDPGA under the same conditions used for rat liver microsomes (0.25 mg/ml), no BMP glucuronide was detected. When higher amounts of human hepatic microsomal protein (0.5 and 1.0 mg/ml) and longer incubation times (30–360 min) were used, the HPLC-radiochromatograms revealed a small radioactive peak that coeluted with the BMP glucuronide. In addition, MS/MS analyses confirmed that this peak was the monoglucuronide conjugate. It is remarkable that even after 360 min of incubation at the high microsomal protein concentration of 1.0 mg/ml, only 8% of the initial BMP was converted into BMP glucuronide. The glucuronidation activity of human liver microsomes was 12 pmol/min/mg protein compared with 1110 pmol/min/mg protein for F-344 rat liver microsomes (Fig. 6). Because the rate of BMP glucuronide formation by human hepatic microsomes was so low and did not follow Michaelis-Menten kinetics, CL could not be determined. There was no evidence of saturation even at concentrations up to 1000 μM. Results from positive control incubations conducted with TFMU confirm that these human hepatic microsomes possessed glucuronidation activity. After 10 min of incubation under conditions similar to those with BMP, greater than 90% of TFMU was converted to TFMU glucuronide by human hepatic microsomes.
Fig. 6.
Activities of hepatic microsomes from male and female F-344 rats, male B6C3F1 mice, male Golden Syrian hamsters, male rhesus monkeys, and humans (mixed gender) for the glucuronidation of BMP. Rates of glucuronidation were determined at 50 μM BMP and are expressed as picomoles per minute per milligram of protein (mean ± S.D. of at least three independent experiments).
To further investigate the capacity for human enzymes to glucuronidate BMP, studies were conducted with intestinal microsomes and a series of recombinant cDNA-expressed UGT enzymes (Supersomes). Human intestinal microsomes converted BMP to BMP glucuronide at a slower rate (2.3 pmol/min/mg) than that for hepatic microsomes. Of the six expressed human hepatic UGTs incubated with [14C]BMP, only UGT2B7 was active. It formed BMP glucuronide at a very low rate (7 ± 2 pmol/min/mg protein).
Incubations of [14C]BMP with Human Cryopreserved Hepatocytes.
When [14C]BMP (50 μM) was incubated with suspensions of cryopreserved human hepatocytes, the only metabolite detected was the same monoglucuronide identified in human microsomes (Fig. 3B). The BMP glucuronide was generated at very low rates. Less than 3% of initial BMP concentration was converted to this metabolite over the 6-h incubation at the three BMP concentrations. For example, at 50 μM BMP, the rate of BMP glucuronidation in human hepatocytes (10 pmol/min/106 cells) was approximately 150-fold lower than that of F-344 rat hepatocytes (1498 pmol/min/106 cells). These human hepatocytes possessed efficient UGT activity as demonstrated by the glucuronidation of [14C]BPA. When the human hepatocytes were incubated with 50 μM [14C]BPA, by 360 min, 26% of the starting concentration of BPA was converted to BPA glucuronide. The glucuronidation of BPA by human cryopreserved hepatocytes was reported previously by Kuester and Sipes (2007).
Glucuronidation of BMP by Hepatic Microsomes from Different Species.
In addition to liver microsomes from male and female F-344 rats and from humans, the glucuronidation of BMP was determined in hepatic microsomes obtained from male B6C3F1 mice, male Golden Syrian hamsters, and male rhesus monkeys. Microsomes from all sources catalyzed the formation of the BMP monoglucuronide, although with markedly different activities (Fig. 6). Hepatic microsomes from male mice showed activities similar to those from male and female F-344 rats. Activities of hepatic microsomes from male hamsters were 3-fold lower than those of rats and mice. BMP glucuronidation activities of monkey hepatic microsomes were 6-fold lower than those of rodents but 14-fold higher than the activities in human liver microsomes.
Discussion
The present study was conducted to characterize the in vitro metabolism of the brominated flame retardant BMP. Based on previous in vivo studies indicating that the formation of a BMP glucuronide is the major route of BMP metabolism in rats (Hoehle et al., 2009), the studies reported herein focused on interspecies variations in hepatic glucuronidation of BMP. The in vitro experiments using hepatic microsomes from rats, mice, hamsters, monkeys, and humans as well as those in suspensions of hepatocytes from rats and humans revealed the formation of only one metabolite, BMP monoglucuronide. The extensive glucuronidation of BMP by rat hepatic microsomes and rat hepatocytes is consistent with in vivo results obtained in this species, which revealed the rapid excretion of BMP as a glucuronide conjugate. The rapid glucuronidation of BMP also explains its low oral bioavailability in rats (Hoehle et al., 2009).
Because the glucuronidation of BMP followed Michaelis-Menten kinetics, it was possible to calculate an in vitro intrinsic clearance, CL, and then scale this value to estimate its in vivo hepatic clearance (CLH) via glucuronidation. The mean value for the overall CLH of BMP by hepatic microsomes from male and female F-344 rats was estimated to be 23.5 and 18.5 ml/min/kg. This value indicates a clearance of BMP in rats that corresponds to approximately 50% of the rat liver blood flow that was reported to be 45 ml/min/kg (Corley et al., 2005). These estimated hepatic CLH values suggest a liver extraction ratio of 0.4 to 0.5 for rats. Compounds with extraction ratios greater than 0.7 are considered to be nonrestrictively cleared, whereas those with extraction ratios less than 0.3 are restrictively cleared and are considered to have a low hepatic clearance (Rowland and Tozer, 1995). The hepatic extraction ratios of rat hepatic microsomes for BMP fall into the intermediate range. As stated previously, CL values could not be determined for human hepatic microsomes, and thus extraction ratios were not estimated.
The extrapolated CLH of 18.5 to 23.5 ml/min/kg correlates well with the in vivo plasma clearance of BMP, 22 ml/min/kg, calculated from the data provided by Hoehle et al. (2009). It is somewhat surprising that these values compare so favorably because BMP glucuronide is known to undergo enterohepatic recycling, an event that can prolong plasma half-life and decrease clearance. Because this does not occur in vitro, the extrapolated CLH could exceed in vivo CL. However, it is primarily BMP glucuronide that circulates in plasma after enterohepatic recycling, which would not affect the clearance of the parent compound. In addition, it is possible that tissues other than the liver contribute to the glucuronidation of BMP in vivo and thus increase its overall rate of clearance.
In contrast to the results obtained with rat hepatic preparations, the results of in vitro studies with human liver microsomes and human hepatocytes indicate that the capacity for glucuronidation of BMP by human liver is very low. Incubations of BMP with hepatocytes, which contain a spectrum of metabolic enzymes and cofactors, did not result in the formation of any additional metabolites. Thus, it appears that glucuronidation is the sole route of BMP metabolism by both rat and human hepatocytes. If any additional metabolites are formed, they are formed at levels below the limit of detection. However, recovery of radiolabel from the column as parent and glucuronide was usually greater than 95%.
Because of the minimal metabolism of BMP by human hepatic microsomes and hepatocytes, its glucuronidation by human intestinal microsomes was investigated. Intestinal glucuronidation is known to occur for numerous drugs and chemicals (Cubitt et al., 2009) and may play a more important role in humans than in rats (Dalvie et al., 2008; Wong et al., 2009). Some UGT enzymes (i.e., UGT1A8) are expressed primarily in the intestinal system (Fisher et al., 2001). Although human intestinal microsomes did glucuronidate BMP, the rate was slower than that of hepatic microsomes. Thus, it appears that BMP undergoes minimal glucuronidation by human tissues, and no other significant metabolic pathways are apparent.
The only expressed UGT enzyme to glucuronidate BMP was UGT2B7. This human enzyme glucuronidated BMP at a rate that was similar to that of human hepatic microsomes. Members of the UGT1A family were inactive. Because there is a high homology among members of the UGT2B family (Mackenzie et al., 2005) and UGT2B7 was the only member of the UGT2B family tested, it is not known whether other UGT2B enzymes may contribute somewhat to the low level of BMP glucuronidation observed in human liver and intestine. It is likely that UGT2B enzymes are responsible for the glucuronidation of BMP by hepatic microsomes of rats as well as those of the other species tested. However, the dramatic variation in the extent of BMP glucuronidation between rats and humans suggests that rats may possess a specific UGT2B enzyme that is not or is only poorly expressed in human hepatic microsomes. Other investigators have reported that human liver microsomes possess dramatically less glucuronidation activity toward aliphatic alcohols than rat liver microsomes. For example, the Vmax/Km ratios for the glucuronidation of n-propanol by rat and human hepatic microsomes were 3.0 and 0.5 μl/min/mg, respectively (Iwersen and Schmoldt, 1997; Jurowich et al., 2004).
Because BMP is a dialcohol, it may be metabolized by the same UGT as simple alcohols. Iwersen and Schmoldt (1997) reported that a specific hydroxysteroid UGT (UGT2B3) present in rat liver microsomes catalyzed the glucuronidation of aliphatic alcohols (C1–C7). However, the affinity of this enzyme was low, with Km values ranging from 0.3 to 86 mM (Km values for glucuronosyl transferase(s) decreased with increasing chain length). The alcohol with the highest affinity (heptanol) yielded a Vmax/Km value of 40 μl/min/mg, which is similar to the value of 42 to 65 μl/min/mg obtained for BMP reported here. In studies with human liver microsomes, Jurowich et al. (2004) reported a similar structure-activity relationship for glucuronidation of these alcohols but found their affinities to be 2- to 6-fold lower for human liver microsomes than for rat liver microsomes. Thus, BMP seems to behave similarly to aliphatic alcohols (C5–C7) with respect to glucuronidation by rat and human liver microsomes. An understanding of the molecular aspects of this rodent/human difference in glucuronidation capacity toward chemicals such as BMP has important toxicological ramifications, particularly when metabolic data are used to extrapolate findings in rats to humans.
In conclusion, glucuronidation appears to be the sole route of metabolism for BMP in rodents and humans and most likely for the other species investigated. Among the species tested, rodents displayed the highest glucuronidation capacity. Our data suggest that interspecies differences in UGT enzymes significantly alter the glucuronidation of BMP and result in severely compromised capacity to metabolize BMP in some species. The poor glucuronidation capacity of BMP by humans suggests that its in vivo pharmacokinetic profile will differ dramatically from that obtained in rodents. How such dramatic differences in pharmacokinetics will affect the potential for BMP toxicity in humans remains to be established.
Acknowledgments.
We thank Dr. Michael Cunningham (National Toxicology Program and National Center for Toxicogenomics, National Institute of Environmental Health Sciences) for advice and support and Leigh Jacobs, Gabriel Knudsen, and Matthew Merrell for assistance in various aspects of this project.
This research was supported in part by the National Institutes of Health National Institute of Environmental Health Sciences [Grant P30-ES06694] (Analytical Core of the Southwest Environmental Health Science Center); and the National Institutes of Health National Institute of Environmental Health Sciences [Contract N01-ES45529] (National Toxicology Program).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.032110.
- BMP
- 2;2-bis(bromomethyl)-1;3-propanediol
- UGT
- UDP-glucuronosyltransferase
- HPLC
- high-performance liquid chromatography
- Vis
- visible
- BPA
- bisphenol A
- WEM
- Williams' medium E
- TFMU
- 4-(trifluoromethyl)umbelliferone
- UDPGA
- uridine diphosphate glucuronic acid
- MS
- mass spectrometry
- MS/MS
- tandem mass spectrometry.
References
- Cheng Y, Wright SH, Hooth MJ, Sipes IG. (2009) Characterization of the disposition and toxicokinetics of N-butylpyridinium chloride in male F-344 rats and female B6C3F1 mice and its transport by organic cation transporter 2. Drug Metab Dispos 37:909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley RA, Bartels MJ, Carney EW, Weitz KK, Soelberg JJ, Gies RA, Thrall KD. (2005) Development of a physiologically based pharmacokinetic model for ethylene glycol and its metabolite, glycolic acid, in rats and humans. Toxicol Sci 85:476–490 [DOI] [PubMed] [Google Scholar]
- Cubitt HE, Houston JB, Galetin A. (2009) Relative importance of intestinal and hepatic glucuronidation-impact on the prediction of drug clearance. Pharm Res 26:1073–1083 [DOI] [PubMed] [Google Scholar]
- Dalvie D, Kang P, Zientek M, Xiang C, Zhou S, Obach RS. (2008) Effect of intestinal glucuronidation in limiting hepatic exposure and bioactivation of raloxifene in humans and rats. Chem Res Toxicol 21:2260–2271 [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Heath JE, Farnell DR, Prejean JD, Haseman JK, Elwell MR. (1997) Carcinogenic activity of the flame retardant, 2,2-bis(bromomethyl)-1,3-propanediol in rodents, and comparison with the carcinogenicity of other NTP brominated chemicals. Toxicol Pathol 25:541–548 [DOI] [PubMed] [Google Scholar]
- Elwell MR, Dunnick JK, Brown HR, Montgomery CA. (1989) Kidney and urinary bladder lesions in F344/N rats and B6C3F1 mice after 13 weeks of 2,2-bis(bromomethyl)-1,3-propanediol administration. Fundam Appl Toxicol 12:480–490 [DOI] [PubMed] [Google Scholar]
- Fisher MB, Paine MF, Strelevitz TJ, Wrighton SA. (2001) The role of hepatic and extrahepatic UDP-glucuronosyltransferases in human drug metabolism. Drug Metab Rev 33:273–297 [DOI] [PubMed] [Google Scholar]
- Guengerich FP. (1989) Analysis and characterization of enzymes, in Principles and Methods of Toxicology (Hayes AW. ed) pp 777–814, Raven Press, New York: [Google Scholar]
- Hallifax D, Houston JB. (2006) Binding of drugs to hepatic microsomes: comment and assessment of current prediction methodology with recommendation for improvement. Drug Metab Dispos 34:724–726 [DOI] [PubMed] [Google Scholar]
- Hoehle SI, Knudsen GA, Sanders JM, Sipes IG. (2009) Absorption, distribution, metabolism, and excretion of 2,2-bis(bromomethyl)-1,3-propanediol in male Fischer-344 rats. Drug Metab Dispos 37:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehle SI, Pfeiffer E, Metzler M. (2007) Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol Nutr Food Res 51:932–938 [DOI] [PubMed] [Google Scholar]
- Houston JB. (1994) Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol 47:1469–1479 [DOI] [PubMed] [Google Scholar]
- Iwersen S, Schmoldt A. (1997) A specific hydroxysteroid UGT is responsible for the conjugation of aliphatic alcohols in rats: an estimation of the importance of glucuronidation versus oxidation. Alcohol 5:185–192 [DOI] [PubMed] [Google Scholar]
- Jurowich S, Sticht G, Käferstein H. (2004) Glucuronidation of aliphatic alcohols in human liver microsomes in vitro. Alcohol 32:187–194 [DOI] [PubMed] [Google Scholar]
- Kuester RK, Sipes IG. (2007) Prediction of metabolic clearance of bisphenol A (4,4′-dihydroxy-2,2-diphenylpropane) using cryopreserved human hepatocytes. Drug Metab Dispos 35:1910–1915 [DOI] [PubMed] [Google Scholar]
- Larsen ER. (1969) 2,2-Bis(bromomethyl)propanediol-l,3: a light stable fire retardant monomer for condensation polymers. Org Coatings Plast Chem 29:375 [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15:677–685 [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (1996) Toxicology and carcinogenesis studies of 2,2-bis(bromomethyl)-1,3-propanediol (FR-1138®) (CAS No. 3296-90-0) in F344 rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser 452:1–465 [PubMed] [Google Scholar]
- Pritchett JJ, Kuester RK, Sipes IG. (2002) Metabolism of bisphenol A in primary cultured hepatocytes from mice, rats, and humans. Drug Metab Dispos 30:1180–1185 [DOI] [PubMed] [Google Scholar]
- Report on Carcinogens, 11th ed (2004) U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, Washington, DC: [Google Scholar]
- Rowland M, Tozer TN. (1995) Clinical Pharmacokinetics: Concepts and Applications, 3rd ed, Lippincott, Williams & Wilkins, Baltimore: [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85 [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (2002) Test plan for 2,2-bis(bromomethyl)-1,3-propanediol. CAS No. 3296-90-0, High Production Volume (HPV) Chemical Challenge Program, Washington, DC: [Google Scholar]
- U.S. International Trade Commission (1994) Synthetic Organic Chemicals, U.S. Production and Sales, 1992. USITC Publication No. 2720, USITC, Washington, DC: [Google Scholar]
- Wong YC, Zhang L, Lin G, Zuo Z. (2009) Intestinal first-pass glucuronidation activities of selected dihydroxyflavones. Int J Pharm 366:14–20 [DOI] [PubMed] [Google Scholar]
- Zhu M, Zhao W, Vazquez N, Mitroka JG. (2005) Analysis of low level radioactive metabolites in biological fluids using high-performance liquid chromatography with microplate scintillation counting: method validation and application. J Pharm Biomed Anal 39:233–245 [DOI] [PubMed] [Google Scholar]