Abstract
Research investigating CYP2C8 as a drug-metabolizing enzyme has gained momentum over the past few years. CYP2C8 is estimated to oxidatively metabolize approximately 5% of therapeutically prescribed drugs. It is polymorphically expressed, and several single nucleotide polymorphisms have been identified with varying effects on the clearance of CYP2C8 substrates. However, the human liver expression of CYP2C8 and effects of genetic variation, age, and gender on mRNA and protein levels have not been fully explored. In this report, interindividual variation in CYP2C8 mRNA and protein expression in 60 livers from white individuals was examined. The livers were genotyped for CYP2C8*3 and CYP2C8*4 polymorphisms. The effects of genotype, age, and gender on hepatic CYP2C8 expression and the correlation of CYP2C8 mRNA expression with CYP3A4 and other CYP2C members were evaluated. The mean ± S.D. protein levels in CYP2C8*1/*1 livers was 30.8 ± 17.5 pmol/mg protein, and a trend for decreased protein levels was observed for CYP2C8*1/*4 livers (15.8 ± 9.7 pmol/mg, p = 0.07). The mean expression levels of CYP2C8 was comparable in males and females (p = 0.18). The mRNA expression of CYP2C8, CYP2C9, CYP2C19, and CYP3A4, but not CYP2C18, was highly correlated (p < 0.0001). Moreover, the hepatic CYP2C8 and CYP3A4 protein levels were strongly correlated (r = 0.76, p < 0.0001). This correlation is most likely due to common regulation factors for both genes. CYP2C8 mRNA or protein expression levels were not significantly affected by CYP2C8*3 or *4 genotype, gender, or age, and variation observed clinically in CYP2C8 activity warrants further investigation.
The involvement of CYP2C8 in the metabolic clearance of drugs, drug interactions, and its pharmacogenetics has been increasingly recognized in the last several years (Niemi et al., 2003, 2005; Totah and Rettie, 2005; Kirchheiner et al., 2006; Tornio et al., 2008). CYP2C8 accounts for 7% of total microsomal cytochrome P450 (P450) content of the liver (Shimada et al., 1994; Rendic and Di Carlo, 1997) and is estimated to be involved in the oxidative metabolism of at least 5% of therapeutically prescribed drugs, including amodiaquine, amiodarone, cerivastatin, paclitaxel, repaglinide, pioglitazone, rosiglitazone, and verapamil. CYP2C8 is also involved in the endogenous metabolism of arachidonic acid and all-trans-retinoic acid to epoxyeicosatrienoic acids and 4-hydroxy-all-trans-retinoic acid, respectively (Zeldin et al., 1996; McSorley and Daly, 2000). CYP2C8 shares more common substrates with CYP3A4 than it does with CYP2C9, despite having 70% sequence homology and 80% sequence similarity to CYP2C9 (Totah and Rettie, 2005). In addition, similar transcription factors, including pregnane X receptor, constitutive androstane receptor, and glucocorticoid receptor, are involved in the induction of CYP2C8 and CYP3A4 isozymes (Bahadur et al., 2002; Raucy et al., 2002; Ferguson et al., 2005).
There is conflicting data in the literature with regards to in vitro and in vivo metabolic effects of CYP2C8 single nucleotide polymorphisms (SNPs). For example, individuals with the CYP2C8*1/*3 or CYP2C8*3/*3 genotypes have increased clearance of CYP2C8 substrates, such as repaglinide, rosiglitazone, and pioglitazone, compared with individuals carrying the CYP2C8*1/*1 genotype (Niemi et al., 2005; Kirchheiner et al., 2006; Tornio et al., 2008). In contrast, in vitro experiments and some in vivo pharmacokinetic studies that involved the CYP2C8*3 allele showed contradictory results (Dai et al., 2001; Bahadur et al., 2002; Parikh et al., 2007; Daily and Aquilante, 2009). Niemi et al. (2003) reported that the CYP2C8*4 allele did not influence the pharmacokinetics of repaglinide. Accordingly, the metabolic activity of CYP2C8 alleles is somewhat controversial, although ostensibly these CYP2C8 variants may have altered protein expression and/or function.
There is limited characterization of hepatic CYP2C8 protein expression in individuals with different CYP2C8 genotypes. This information would be valuable for studies that scaled metabolism from human liver microsomes (HLM) to whole liver, to determine the relationship between genotype-phenotype and explain the interindividual variability in pharmacokinetics of various CYP2C8 substrates. In addition, data pertaining to CYP2C8 ontogeny or gender differences are lacking. To address some of these issues, we genotyped for CYP2C8*3 and CYP2C8*4 (the most common SNPs in whites), evaluated CYP2C8 mRNA and protein expression, and examined the effect of age and gender on CYP2C8 expression in 60 liver samples from white individuals.
Materials and Methods
The CYP2C8 primary antibody (rabbit antibody to human CYP2C8) was purchased from GeneTex, Inc. (San Antonio, TX). Secondary antibodies (IRDye 680 goat anti-rabbit and IRDye 800CW goat anti-mouse) and Odyssey blocking reagent were obtained from LI-COR Biosciences (Lincoln, NE). Western blotting reagents and NuPAGE Novex Bis-Tris mini gels were purchased from Invitrogen (Carlsbad, CA). Immobilon-FL-PVDF transfer membrane was obtained from Millipore Corporation (Billerica, MA). Recombinant P450 enzymes, cDNA-expressed human P450 reductase, and human cytochrome b5 were obtained from BD Gentest (Woburn, MA).
Human Liver Samples.
Samples of human liver (n = 60) from white donors were obtained from the University of Washington School of Pharmacy Human Liver Tissue Bank (Seattle, WA). HLM were prepared according to previously published protocols (Paine et al., 1997). Protein concentrations were determined by the method of Lowry et al. (1951). The expression levels of CYP3A4 were determined previously (Lin et al., 2002). For the 60 livers, genotyping results were available for 57 livers, and CYP2C8 mRNA and protein expression were determined for 55 and 53 livers, respectively.
Genotyping for CYP2C8*3 and CYP2C8*4.
All genotyping assays were performed at the DNA Sequencing and Gene Expression Center, Department of Pharmaceutics, University of Washington. The CYP2C8*3 (416G>A and 1196A>G; R139K; K399R) and CYP2C8*4 (792C>G; I264M) SNPs were genotyped by using validated TaqMan assays from Applied Biosystems (Foster City, CA). The cycling conditions for polymerase chain reaction (PCR) amplification were one cycle at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min in a reaction volume of 10 μl that contained 1 μl of genomic DNA (∼25–100 ng) and 1× final concentrations of TaqMan universal PCR master mix and SNP assay primers and probes. The allelic discrimination was determined in a post-PCR analysis on an Applied Biosystems 7900 HT instrument. Internal controls of sequence-verified genotypes were included for each assay.
Quantitation of CYP2C8 mRNA.
Total mRNA from human liver (n = 55) was extracted and normalized to ∼200 ng/μl. Using 750 ng of total RNA, whole genome expression measurement was carried out using Illumina (San Diego, CA) HumanRef-8 version 2 Expression BeadChips according to the manufacturer's protocol. All liver samples were measured in duplicate, with each sample and replicate randomized between processed batches of 24 arrays done on different days. Raw signal intensity measurements from each sample were processed using the Illumina BeadStudio software version 2.3.41 using the “average” normalization function, and replicate data from each liver was averaged to obtain a final value.
Protein Quantitation.
Immunoquantitation of CYP2C8 in human liver samples (n = 53) was performed by Western blot analysis as described by Lin et al. (2002), with minor modifications. In brief, 20 μg of liver microsomal protein was resolved on NuPAGE Novex Bis-Tris mini gels by electrophoresis and transferred simultaneously onto PVDF membranes. The membrane was blocked overnight with Odyssey blocking reagent, incubated with the primary anti-CYP2C8 antibody (1:10,000 dilution) followed by the secondary antibody (1:15,000 dilution). The imaging of the membrane was performed on the Odyssey Infrared Imaging Systems (LI-COR Biosciences).
Recombinant human CYP2C8 was used as standard for the protein-calibration curve (0.312–1.25 pmol of CYP2C8). Standards and liver samples were run in duplicate. A selected liver sample (HL-105) served as a quality sample and was added to each gel to control for the interday variation. CYP2C8 protein levels in liver samples were quantified by comparing the unknown band image intensity to the appropriate standard curve using Odyssey version 2.1.12. The cross-reactivity of the CYP2C8 antibody was examined with other P450s (CYP2C9, CYP2C19, CYP3A4, CYP3A5, CYP2B6, and CYP2J2).
Statistical and Data Analysis.
Values for protein content are expressed as picomoles of CYP2C8 per milligram of microsomal protein, whereas arbitrary absolute values for mRNA are reported. Statistical significance was tested by using a Student's t test, where p < 0.05 was considered statistically significant. For association tests, we determined the Spearman's rank correlation using Stata/SE (version 10; College Station, TX). An r value of <0.5, 0.51 to 0.7, and 0.71 to 1.0 were designated as weak, modest, and strong correlation, respectively. Mean ± S.D.s are reported where appropriate.
Results and Discussion
Liver samples from 60 white individuals were included in this study. The median age was 46 years (range, 7–70 years) with nearly equal distribution of male and female donors (29 and 31, respectively). The observed allelic frequencies for CYP2C8*1, CYP2C8*3, and CYP2C8*4 were 0.82, 0.14, and 0.04, respectively. The CYP2C8*3 allele frequency determined in this study was comparable to the frequency of 0.15 reported in white British and white Spanish individuals (Bahadur et al., 2002). The CYP2C8 genotype frequencies are summarized in Table 1.
TABLE 1.
Genotype frequency and mRNA and protein expression by CYP2C8 genotypes for human livers from 60 white individuals
Mean ± S.D. reported where appropriate.
| Genotype | CYP2C8*1/*1 | CYP2C8*1/*1 | CYP2C8*1/*1 | CYP2C8*3/*1 |
|---|---|---|---|---|
| Genotype frequency | ||||
| Number of livers (of 57 total genotyped) | 38 | 12 | 5 | 2 |
| Frequency | 0.67 | 0.21 | 0.09 | 0.04 |
| mRNA expression | ||||
| Number of livers | 33 | 12 | 5 | 2 |
| Relative value | 18181 ± 9912 | 22482 ± 13294 | 18587 ± 10884 | 10279 |
| Protein expression | ||||
| Number of livers | 33 | 11 | 5 | 2 |
| Amount (pmol/mg protein) | 30.8 ± 17.5 | 37.3 ± 15.2 | 15.8 ± 9.7 | 21.3 |
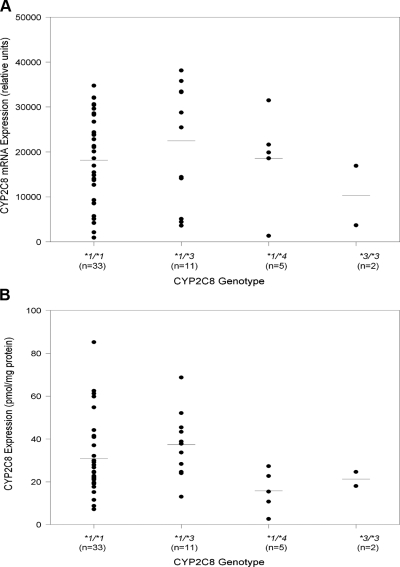
To identify the influence of different CYP2C8 SNPs on CYP2C8 transcript, mRNA levels were quantified in the liver samples. CYP2C8 mRNA was abundant in all the livers. The mean absolute value for CYP2C8 mRNA for each genotype is presented in Fig. 1A. Compared with livers with a CYP2C8*1/*1 genotype, mean mRNA levels of livers with CYP2C8*1/*3 or CYP2C8*1/*4 genotypes were comparable (Fig. 1A; Table 1). The interindividual variation for CYP2C8 mRNA levels was 44-fold in the liver samples. For CYP2C8*3/*3 livers, the mean mRNA levels were 48% lower compared with CYP2C8*1/*1 livers. However, the statistical significance of this reduction cannot be estimated due to small sample size (n = 2).
Fig. 1.
A, CYP2C8 mRNA levels (absolute values) for liver samples with different genotypes (n = 55). The mRNA levels were determined as described under Materials and Methods. B, CYP2C8 protein contents of liver samples with different genotypes (n = 53). Genotype and microsomal CYP2C8 content were determined as described under Materials and Methods. Data points for individual liver samples are the average result of at least two Western blots.
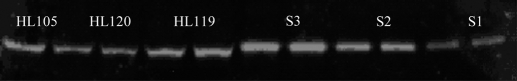
CYP2C8 in the microsomal fractions of liver samples was detected as an immunoreactive band at 55 kDa by using a commercially available CYP2C8-specific polyclonal antibody (Fig. 2). The antibody used was specific to CYP2C8 and did not cross-react with any of the other recombinant P450 proteins tested (data not shown). The interday variation for the control liver sample (HL-105) was 17.2%. CYP2C8 mRNA levels were poorly correlated to CYP2C8 protein levels (r = 0.48, p = 0.0005), although Rodríguez-Antona et al. (2008) reported a stronger correlation in their liver samples (r = 0.62).
Fig. 2.
Representative Western blot of HLM for HL119 and HL120 (20 μg of microsomal protein loaded per lane). S1 (0.312 pmol), S2 (0.625 pmol), and S3 (1.25 pmol) are the recombinant human CYP2C8 standards. CYP2C8 was observed at 55 kDa. HL-105 was included on each gel as a quality control sample for interday variation. The quantified levels of HL119 and HL120 using the standard curve was 43.2 and 22.4 pmol/mg, respectively.
CYP2C8 protein was detected in all liver samples and CYP2C8 protein expression as a function of CYP2C8 genotypes is presented in Table 1 and Fig. 1B. A 33-fold interindividual variation in protein expression is observed. CYP2C8 protein is comparable in livers with the CYP2C8*1/*3 or CYP2C8*1/*1 genotypes. Because there were only two livers with the CYP2C8*3/*3 genotype, no conclusions as to the effect of the CYP2C8*3 allele on protein expression can be made. Garcia-Martin et al. (2004) reported decreased clearance of the R-ibuprofen in CYP2C8*3/*3 individuals, whereas Kirchheiner et al. (2006) reported significantly increased metabolic activity for CYP2C8*3/*3 individuals when rosiglitazone was administered. A larger number of livers with the CYP2C8*3/*3 genotype are needed to clarify the functional consequences of the CYP2C8*3 allele on CYP2C8 protein expression and catalytic activity.
In livers with the CYP2C8*1/*4 genotype, there was a trend for decreased CYP2C8 protein (15.8 ± 9.7 pmol/mg microsomal protein, n = 5, p = 0.07) compared with CYP2C8*1/*1 livers. No livers with the CYP2C8*4/*4 genotype were found among the liver samples. Despite the findings of decreased protein expression in livers with the CYP2C8*1/*4 genotype, Niemi et al. (2003) failed to see a difference in ibuprofen clearance in CYP2C8*1/*4 subjects (n = 3) compared with CYP2C8*1/*1 subjects (n = 19). However, this null result may be due to the fact that CYP2C8 may only play a minor role in the metabolism of ibuprofen, as determined in more recent in vitro studies (Chang et al., 2008).
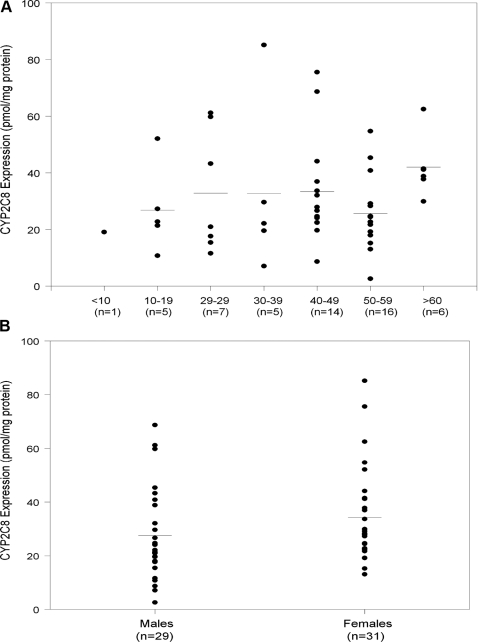
The effects of age and gender on the CYP2C8 protein expression are shown in Fig. 3, A and B, respectively. The ages of the donors ranged from 7 to 70 years and had no observable effect on the expression of CYP2C8. Rodríguez-Antona (2008) also found that age did not alter CYP2C8 expression. With regard to gender, the mean expression levels of CYP2C8 protein was comparable in females (34.3 ± 17.7, n = 28) and males (27.7 ± 17.4, n = 25, p = 0.18) (Fig. 3B). However, it seems that females have slightly higher (22%) expression than males. At the mRNA transcript levels, there was no difference between females (19,965 ± 10,463, n = 28) and males (19,680 ± 12,186, n = 27). In these same sets of livers, CYP3A4 expression in females is higher by 57% compared with males, although there is high variability in overall expression between the two groups. CYP3A4 expression levels in females was 101.3 ± 82.2 (n = 31) compared with 57.5 ± 63.9 (n = 29) in males (Lin et al., 2002). Similar findings of 2-fold increased levels of CYP3A4 expression were reported in 44 female livers compared with 48 male livers (Wolbold et al., 2003).
Fig. 3.
A, relation of age on CYP2C8 protein expression in livers samples from white individuals. Microsomal CYP2C8 content was determined as described under Materials and Methods. Data points for individual liver samples are the average result of at least two Western blots. B, relation of gender on CYP2C8 protein expression (female n = 28, male n = 25). Microsomal CYP2C8 content were determined as described under Materials and Methods. Data points for individual liver samples are the average result of at least two Western blots.
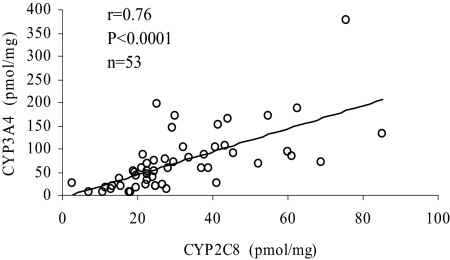
As shown in Table 2, CYP2C8 mRNA expression was highly correlated with the mRNA levels of CYP2C9 (rs = 0.88), CYP2C19 (rs = 0.68), and CYP3A4 (rs = 0.71), but not CYP2C18 (rs = 0.11). Similar transcription factors (pregnane X receptor, constitutive androstane receptor, and glucocorticoid receptor) are involved in the induction and possibly basal regulation of various CYP2C family members and CYP3A4 (Bahadur et al., 2002; Raucy et al., 2002; Ferguson et al., 2005). Liver samples from individuals exposed to inducers (such as dexamethasone, phenytoin, or phenobarbital) before organ procurement had 45% higher levels of CYP2C8 mRNA (p = 0.034), although CYP2C8 protein levels were not significantly different (p = 0.48) compared to those not exposed. Similar to the mRNA expression data, CYP2C8 protein correlated strongly with CYP3A4 protein expression (Fig. 4) (r = 0.74, p < 0.0001). Note that from the entire set of human livers, 17% (n = 9) of the livers show higher CYP2C8 protein levels than CYP3A4. This result could complicate studies on drug-drug interactions in which compounds of interest are both CYP2C8 and CYP3A4 substrates and under conditions where CYP3A4 is inhibited.
TABLE 2.
Correlations among CYP2C family and CYP3A4 mRNA expression
| CYP2C8 | CYP2C9 | CYP2C18 | CYP2C19 | CYP3A4 | |
|---|---|---|---|---|---|
| CYP2C8 | 1.0 | 0.88* | 0.11 | 0.68* | 0.71* |
| CYP2C9 | 1.0 | 0.14 | 0.67* | 0.70* | |
| CYP2C18 | 1.0 | −0.03 | 0.15 | ||
| CYP2C19 | 1.0 | 0.70* | |||
| CYP3A4 | 1.0 |
Significant at p < 0.0001 by Spearman's rank test.
Fig. 4.
CYP2C8 and CYP3A4 protein content is highly correlated in human liver samples. The samples were previously characterized for CYP3A4 protein content (Lin et al., 2002). From the entire set of human livers, 9 of 53 (17%) show higher CYP2C8 expression levels than CYP3A4 expression.
From the recent P450 identification studies, there is a large degree of overlapping substrate specificity between CYP2C8 and CYP3A4. These substrates are structurally diverse from various therapeutic classes. Substrates that are predominantly metabolized by CYP2C8 with minor CYP3A4 contribution are as follows: paclitaxel (Sonnichsen et al., 1995), cerivastatin (Boberg et al., 1997; Mück, 2000), repaglinide (Bidstrup et al., 2003), and rosiglitazone. CYP3A4 substrates with minor CYP2C8 contribution include carbamazapine (Kerr et al., 1994), verapamil (Tracy et al., 1999), zopiclone (Becquemont et al., 1999), and buprenorphine (Chang et al., 2006). Furthermore, some substrates, such as amiodarone, amitriptyline, quinine, and triazolam, which are metabolized completely or in part by CYP3A, caused >50% inhibition of CYP2C8 activity at concentrations that corresponded to their CYP3A Km values, suggesting similar affinities for both enzymes (Ong et al., 2000).
In conclusion, livers with the CYP2C8*1/*3 or CYP2C8*1/*4 genotypes did not differ in mRNA or protein expression levels from livers with CYP2C8*1/*1 genotype. However, there was a trend in livers expressing the CYP2C8*1/*4 genotype to have decreased CYP2C8 content. The hepatic protein expression of CYP2C8 did not differ between females and males. Furthermore, age had no apparent effect on CYP2C8 protein expression. Further work needs to be done to characterize and reconcile in vitro and in vivo differences in the metabolic activity of CYP2C8 variants.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL078888]; the National Institutes of Health National Institute of General Medical Sciences [Grant P01-GM32165]; and the National Institutes of Health National Center for Research Resources [Grant 1KL2-RR025015] (to Y.S.L.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.031542.
- P450
- cytochrome P450
- SNPs
- single nucleotide polymorphisms
- HLM
- human liver microsomes
- IRDye
- infrared dye
- PVDF
- polyvinylidene fluoride
- PCR
- polymerase chain reaction.
References
- Bahadur N, Leathart JB, Mutch E, Steimel-Crespi D, Dunn SA, Gilissen R, Houdt JV, Hendrickx J, Mannens G, Bohets H, et al. (2002) CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochem Pharmacol 64:1579–1589 [DOI] [PubMed] [Google Scholar]
- Becquemont L, Mouajjah S, Escaffre O, Beaune P, Funck-Brentano C, Jaillon P. (1999) Cytochrome P-450 3A4 and 2C8 are involved in zopiclone metabolism. Drug Metab Dispos 27:1068–1073 [PubMed] [Google Scholar]
- Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. (2003) CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol 56:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg M, Angerbauer R, Fey P, Kanhai WK, Karl W, Kern A, Ploschke J, Radtke M. (1997) Metabolism of cerivastatin by human liver microsomes in vitro. Characterization of primary metabolic pathways and of cytochrome P450 isozymes involved. Drug Metab Dispos 25:321–331 [PubMed] [Google Scholar]
- Chang SY, Li W, Traeger SC, Wang B, Cui D, Zhang H, Wen B, Rodrigues AD. (2008) Confirmation that cytochrome P450 2C8 (CYP2C8) plays a minor role in (S)-(+)- and (R)-(−)-ibuprofen hydroxylation in vitro. Drug Metab Dispos 36:2513–2522 [DOI] [PubMed] [Google Scholar]
- Chang Y, Moody DE, McCance-Katz EF. (2006) Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos 34:440–448 [DOI] [PubMed] [Google Scholar]
- Dai D, Zeldin DC, Blaisdell JA, Chanas B, Coulter SJ, Ghanayem BI, Goldstein JA. (2001) Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 11:597–607 [DOI] [PubMed] [Google Scholar]
- Daily EB, Aquilante CL. (2009) Cytochrome P450 2C8 pharmacogenetics: a review of clinical studies. Pharmacogenomics 10:1489–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Chen Y, LeCluyse EL, Negishi M, Goldstein JA. (2005) Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha. Mol Pharmacol 68:747–757 [DOI] [PubMed] [Google Scholar]
- García-Martín E, Martínez C, Tabarés B, Frías J, Agúndez JA. (2004) Interindividual variability in ibuprofen pharmacokinetics is related to interaction of cytochrome P450 2C8 and 2C9 amino acid polymorphisms. Clin Pharmacol Ther 76:119–127 [DOI] [PubMed] [Google Scholar]
- Kerr BM, Thummel KE, Wurden CJ, Klein SM, Kroetz DL, Gonzalez FJ, Levy RH. (1994) Human liver carbamazepine metabolism. Role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem Pharmacol 47:1969–1979 [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Thomas S, Bauer S, Tomalik-Scharte D, Hering U, Doroshyenko O, Jetter A, Stehle S, Tsahuridu M, Meineke I, et al. (2006) Pharmacokinetics and pharmacodynamics of rosiglitazone in relation to CYP2C8 genotype. Clin Pharmacol Ther 80:657–667 [DOI] [PubMed] [Google Scholar]
- Lin YS, Dowling AL, Quigley SD, Farin FM, Zhang J, Lamba J, Schuetz EG, Thummel KE. (2002) Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol 62:162–172 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- McSorley LC, Daly AK. (2000) Identification of human cytochrome P450 isoforms that contribute to all-trans-retinoic acid 4-hydroxylation. Biochem Pharmacol 60:517–526 [DOI] [PubMed] [Google Scholar]
- Mück W. (2000) Clinical pharmacokinetics of cerivastatin. Clin. Pharmacokinet 39:99–116 [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, Eichelbaum M, Kivistö KT, Neuvonen PJ. (2005) Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther 77:468–478 [DOI] [PubMed] [Google Scholar]
- Niemi M, Leathart JB, Neuvonen M, Backman JT, Daly AK, Neuvonen PJ. (2003) Polymorphism in CYP2C8 is associated with reduced plasma concentrations of repaglinide. Clin Pharmacol Ther 74:380–387 [DOI] [PubMed] [Google Scholar]
- Ong CE, Coulter S, Birkett DJ, Bhasker CR, Miners JO. (2000) The xenobiotic inhibitor profile of cytochrome P4502C8. Br J Clin Pharmacol 50:573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine MF, Khalighi M, Fisher JM, Shen DD, Kunze KL, Marsh CL, Perkins JD, Thummel KE. (1997) Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 283:1552–1562 [PubMed] [Google Scholar]
- Parikh S, Ouedraogo JB, Goldstein JA, Rosenthal PJ, Kroetz DL. (2007) Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin Pharmacol Ther 82:197–203 [DOI] [PubMed] [Google Scholar]
- Raucy JL, Mueller L, Duan K, Allen SW, Strom S, Lasker JM. (2002) Expression and induction of CYP2C P450 enzymes in primary cultures of human hepatocytes. J Pharmacol Exp Ther 302:475–482 [DOI] [PubMed] [Google Scholar]
- Rendic S, Di Carlo FJ. (1997) Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev 29:413–580 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Antona C, Niemi M, Backman JT, Kajosaari LI, Neuvonen PJ, Robledo M, Ingelman-Sundberg M. (2008) Characterization of novel CYP2C8 haplotypes and their contribution to paclitaxel and repaglinide metabolism. Pharmacogenomics J 8:268–277 [DOI] [PubMed] [Google Scholar]
- Sonnichsen DS, Liu Q, Schuetz EG, Schuetz JD, Pappo A, Relling MV. (1995) Variability in human cytochrome P450 paclitaxel metabolism. J Pharmacol Exp Ther 275:566–575 [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. (1994) Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270:414–423 [PubMed] [Google Scholar]
- Tornio A, Niemi M, Neuvonen PJ, Backman JT. (2008) Trimethoprim and the CYP2C8*3 allele have opposite effects on the pharmacokinetics of pioglitazone. Drug Metab Dispos 36:73–80 [DOI] [PubMed] [Google Scholar]
- Totah RA, Rettie AE. (2005) Cytochrome P450 2C8: substrates, inhibitors, pharmacogenetics, and clinical relevance. Clin Pharmacol Ther 77:341–352 [DOI] [PubMed] [Google Scholar]
- Tracy TS, Korzekwa KR, Gonzalez FJ, Wainer IW. (1999) Cytochrome P450 isoforms involved in metabolism of the enantiomers of verapamil and norverapamil. Br J Clin Pharmacol 47:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM. (2003) Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 38:978–988 [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Moomaw CR, Jesse N, Tomer KB, Beetham J, Hammock BD, Wu S. (1996) Biochemical characterization of the human liver cytochrome P450 arachidonic acid epoxygenase pathway. Arch Biochem Biophys 330:87–96 [DOI] [PubMed] [Google Scholar]