Abstract
[11C]N-desmethyl-Loperamide ([11C]dLop) is used in positron emission tomography (PET) to measure the in vivo activity of efflux transporters that block the passage of drugs across the blood-brain barrier. The three most prevalent ATP-binding cassette efflux transporters at the blood-brain barrier are P-glycoprotein (P-gp), multidrug resistance protein 1 (Mrp1), and breast cancer resistance protein (BCRP). We sought to measure the selectivity of dLop among these three transporters. The selectivity of dLop at low concentrations (≤1 nM) was measured both as the accumulation of [3H]dLop in human cells that overexpress each transporter and as the uptake of [11C]dLop in brains of mice that lack genes encoding P-gp, Mrp1, or BCRP. The selectivity of dLop at high concentrations (≥20 μM) was measured as the inhibition of uptake of a fluorescent substrate and the change in cytotoxicity of drugs effluxed at each transporter. Accumulation of [3H]dLop was lowest in cells overexpressing P-gp, and the uptake of [11C]dLop was highest in brains of mice lacking P-gp. At high concentrations, dLop selectively inhibited P-gp function and also decreased the resistance of only the P-gp-expressing cells to cytotoxic agents. dLop is selective for P-gp among these three transporters, but its activity is dependent on concentration. At low concentrations (≤1 nM), dLop acts only as a substrate; at high concentrations (≥20 μM), it acts as both a substrate and an inhibitor (i.e., a competitive substrate). Because low concentrations of radiotracer are used for PET imaging, [11C]dLop acts selectively and only as a substrate for P-gp.
Efflux transporters of the ATP-binding cassette (ABC) family block the entry into cells of many diverse foreign compounds and thereby protect the body from potential toxins (Gottesman, 2002). At the blood-brain barrier, the three most prevalent ABC transporters are P-glycoprotein (P-gp) (encoded by ABCB1), multidrug resistance protein 1 (Mrp1) (encoded by ABCC1), and breast cancer resistance protein (BCRP) (encoded by ABCG2) (Löscher and Potschka, 2005a). They are expressed in the walls of the blood vessels and not only protect the brain from toxins but also impede delivery of therapeutic drugs (Gottesman, 2002; Graff and Pollack, 2004). Among these three transporters, P-gp has been most studied, in part because it probably causes pathological changes in both brain and periphery. At the blood-brain barrier, overexpression of P-gp may contribute to drug-resistant epilepsy (Löscher and Potschka, 2005b), whereas its underexpression may contribute to Alzheimer's disease (Vogelgesang et al., 2004; Cirrito et al., 2005). In the periphery as well as in the brain, overexpression of P-gp in cancer cells is one cause of multidrug resistance to chemotherapy (Gottesman et al., 2002; Szakács et al., 2006).
The in vivo function of P-gp has been quantified using substrates radiolabeled for single photon emission computed tomographic or positron emission tomography (PET) (Kannan et al., 2009). The single photon emission computed tomographic radiotracer [99mTc]sestamibi was the first substrate to image the function of P-gp at the blood-brain barrier and in multidrug-resistant cancers (Piwnica-Worms et al., 1993). However, this radiotracer was later found to be a substrate also for Mrp1, thereby compromising its ability to quantify the function of only P-gp (Hendrikse et al., 1998).
In our search for an improved substrate radiotracer to selectively image P-gp, we evaluated N-desmethyl-loperamide (dLop), the major metabolite of loperamide, a potent opiate agonist used to treat diarrhea. Loperamide (Lop) has no opiate effects on the central nervous system because ABC transporters avidly block its entry into brain (Kurnik et al., 2008). Our PET imaging studies with [11C]dLop in animals suggest, but do not prove, that dLop is selective for P-gp. We found that brain uptake of [11C]dLop is negligible in wild-type mice and in monkeys but is markedly increased in abcb1a/b-knockout mice and in monkeys treated with an inhibitor of P-gp (Lazarova et al., 2008; Zoghbi et al., 2008; Liow et al., 2009). These studies do not definitively prove selectivity for P-gp because we did not test mice with knockout of the other two transporters at the blood-brain barrier (abcc1a and abcg2) and because the inhibitor (tariquidar) used in monkeys is probably not selective at high concentrations for P-gp (Robey et al., 2004).
We used a two-system approach to investigate the selectivity of dLop for P-gp. Because substrate recognition by P-gp is known to differ among species (Syvänen et al., 2009), the first goal of this study was to determine selectivity in human tissues using three pairs of human cell lines that overexpress P-gp, MRP1, or BCRP. However, given that in vitro conditions also differ from those in vivo, the second goal was to determine the selectivity of dLop in three strains of mice that were selectively knocked out for the genes that encode P-gp, Mrp1, and BCRP.
Nomenclature.
For these three efflux transporters, we use upper case italic for the human gene, lower case italic for the mouse gene, and plain font for the expressed protein: 1) ABCB1, abcb1a/b, P-gp; 2) ABCC1, abcc1a, Mrp1; and 3) ABCG2, abcg2, BCRP. The names of the expressed proteins are the same for both species.
Materials and Methods
Chemicals.
[N-Methyl-3H]dLop (American Radiolabeled Chemicals, Inc., St. Louis, MO) was synthesized by [3H3]methylation of di-desmethyl-loperamide (Lazarova et al., 2008) and had a radiochemical purity of 97.7% by high-performance liquid chromatography analysis, a specific activity of 3.0 GBq/μmol, and a concentration of 37 MBq/ml. [11C]dLop was prepared on four separate occasions as a solution in sterile saline (0.9% w/v) containing ascorbic acid (1 mg), as described previously (Lazarova et al., 2008). [11C]dLop was obtained with a radiochemical purity >99% and had a specific radioactivity of 78.3 ± 16.8 GBq/μmol at the time of injection. Unless otherwise specified, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Lines.
Three pairs of cell lines were cultured, each pair consisting of a parental (control) and a resistant (ABC transporter-expressing) line. The three pairs were the human adenocarcinoma cell line KB-3-1 and its ABCB1-expressing variant KB-8-5-11, the human breast cancer cell line MCF-7 and its ABCC1-expressing variant MCF-7/VP16, and the human large cell lung cancer cell line H460 and its ABCG2-expressing variant H460/MX20. The KB and MCF-7 lines were cultured in Dulbecco's modified Eagle's medium, whereas the H460 lines were cultured in RPMI 1640 medium. Resistant cell lines were additionally cultured in the following cytotoxic drugs to maintain ABC transporter expression: colchicine (250 nM; 100 ng/ml) for KB-8-5-11 (Shen et al., 1986); etoposide (4 μM) for MCF-7/VP16 (Schneider et al., 1994); and mitoxantrone (20 nM) for H460/MX20 (Henrich et al., 2007). All culture media were supplemented as reported previously (Lee et al., 1998), and cell lines were grown at 37°C in 5% CO2.
Animals.
Four strains of mice (four animals per strain) were used for imaging studies. Three of the strains were knocked out for the genes encoding either P-gp [26.0 ± 3.4 g; abcb1a/b(−/−]); model 001487), Mrp1 [23.0 ± 1.1 g; abcc1a(−/−); model 001486], or BCRP [25.5 ± 2.6 g; abcg2(−/−); model 002767]; the fourth strain was wild-type [23.6 ± 0.9 g; abcb1a/b(+/+); model FVB]. All mice models were purchased from Taconic Farms (Germantown, NY). Animal experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals (Clark et al., 1996) and were approved by the National Institute of Mental Health Animal Care and Use Committee.
Western Blot Analysis.
The expression of ABC transporter in each cell line was visualized by Western blot analysis. Protein samples were prepared and run on a gel as reported by Brimacombe et al. (2009). In brief, lysed cells were incubated in SDS (5×) buffer, loaded onto a 3 to 8% NuPAGE Novex Tris-acetate gel (Invitrogen, Carlsbad, CA), and transferred to nitrocellulose membranes. Dry blots were blocked in 20% milk for 30 min at 21°C. Blots were then probed for expression of three ABC transporters using three primary antibodies (Supplemental Table 1) for 60 min at 21°C, washed three times for 10 min each, immunoprobed with a secondary antibody (Supplemental Table 1) for 60 min at 21°C, and washed again. To ensure that the same amount of protein was loaded per lane, each blot was immunoprobed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a protein uniformly expressed in cells (Brimacombe et al., 2009).
Cellular Accumulation of [3H]dLop.
The selectivity of dLop (at nanomolar concentrations) for the three ABC transporters was measured in vitro as the accumulation of [3H]dLop in all cell lines. Cells (2.5 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h at 37°C to allow attachment. To ensure stable uptake of [3H]dLop over time, accumulation of [3H]dLop was measured in KB-3-1 cells over the course of 60 min. To initiate the time course assay, [3H]dLop (1 nM) was added to each well. After incubation at 37°C for the appropriate time, the growth media were aspirated, and the wells were washed with ice-cold 1× phosphate-buffered saline (0.5 ml/well). Cells were then lysed with trypsin (0.1 ml/well) by incubation for 90 min at 37°C. Radioactivity in the lysates was measured with liquid scintillation counting. Adsorption of [3H]dLop to the plates was determined by adding [3H]dLop (1 nM) to three wells containing no cells. For the purposes of standardizing accumulation, the number of attached cells was counted in three wells per plate using a Cellometer Automatic Cell Counter (Nexcelcom, Lawrence, MA). Radioactivity was corrected for adsorption, cell counts, and expressed as femtomoles per 106 cells.
To achieve virtually complete inhibition of P-gp, cells from the ABCB1 pair were preincubated with cyclosporin A (10 μM) for 30 min at 37°C before addition of [3H]dLop (1 nM). Cells were then incubated for 45 min at 37°C, washed, lysed, and counted as described above.
Uptake of [11C]dLop in Mice Brains.
The selectivity of dLop was also measured in vivo as the uptake of [11C]dLop in the brains of four strains of mice. Four mice from each strain (16 total) were simultaneously anesthetized with 1.5% isoflurane and injected via the tail vein with [11C]dLop (16.0 ± 4.6 MBq; 0.230 ± 0.084 nmol; 0.1 ml). Mice brains were then scanned using a Focus 220 microPET camera (Siemens Medical Solutions, Knoxville, TN), which has a transaxial field of view of 19 cm and an axial field of view of 7.6 cm. Serial dynamic scans were acquired for 60 min as described previously (Lazarova et al., 2008) and were reconstructed using a Fourier rebinning + 2D ordered subset expectation maximization algorithm, resulting in an image resolution of 1.6 mm at full-width at half-maximum. No attenuation or scatter correction was applied.
Images were analyzed with PMOD (pixelwise modeling computer software; PMOD Group, Zurich, Switzerland). Regions of interest were drawn on coronal slices of the brain. Brain concentration of radioactivity (decay-corrected until injection time) was expressed as the percentage of the standardized uptake value (%SUV), which normalizes for injected activity and body weight: %SUV = (percentage of injected activity/cubic centimeter of brain) × (grams of body weight).
Inhibition of Transporter Function.
Because substrates (at high concentrations) can inhibit ABC transporter function as competitive substrates (Ambudkar et al., 1999), we determined whether dLop could selectively inhibit the function of human P-gp. In addition, we determined the selectivity of loperamide (Lop) because it is administered at pharmacological doses to treat diarrhea. We measured their inhibitory activity by the uptake of a fluorescent substrate (one preferentially effluxed by P-gp, Mrp1, or BCRP) in the presence of high concentrations (micromolar) of dLop or Lop. Four conditions for each transporter were tested: untreated (negative control), inhibitor-treated (positive control), dLop-treated, and Lop-treated. For each condition, 2 × 105 cells were suspended in 1 ml of Iscove's modified Dulbecco's medium supplemented with 5% fetal bovine serum.
The cells were first pretreated with inhibitor, dLop, or Lop for 10 min in a 37°C water bath. Pretreatment conditions were as follows. Untreated cells were incubated in drug-free medium. Inhibitor-treated cells were incubated with one of the following inhibitors of ABC transporters: 10 μM cyclosporin A for P-gp (Kimchi-Sarfaty et al., 2007), 50 μM 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid (MK-571) for Mrp1 (Mitra et al., 2006), or 5 μM fumitremorgin C for BCRP (Wu et al., 2007). dLop- and Lop-treated cells were incubated in media containing 10, 20, or 50 μM.
After pretreatment, cells were isolated by centrifugation and resuspended in 1 ml of Iscove's modified Dulbecco's medium containing the same concentration of inhibitor, dLop, or Lop used during pretreatment. All cells were also incubated with a fluorescent substrate that could detect the activity of each transporter: 4 μM rhodamine 123 for P-gp (Kimchi-Sarfaty et al., 2007), 0.25 μM calcein-AM for Mrp1 (Wu et al., 2007), or 5 μM mitoxantrone for BCRP (Wu et al., 2007). Although calcein-AM is not itself a fluorescent substrate for Mrp1, it is cleaved by cellular esterases to yield calcein, which is a fluorescent substrate effluxed by Mrp1. After addition of the fluorescent substrate, cells were incubated in the dark for 45 min in a 37°C water bath. They were then centrifuged, resuspended in 300 μl of 0.1% bovine serum albumin in 1× phosphate-buffered saline, and kept on ice until analysis. For each cell treatment, fluorescence intensity (cellular uptake of fluorescent substrate) was recorded for a total of 10,000 cells using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) under the FL-2 (rhodamine 123), FL-1 (calcein-AM), and FL-3 (mitoxantrone) channels. Fluorescence-activated cell sorting data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Cytotoxicity Assay.
The ability of dLop and Lop to selectively inhibit the function of each ABC transporter was additionally measured by their effects on the resistance of cells to cytotoxic substrates for each transporter. Cytotoxicity was measured with a colorimetric viability assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Invitrogen). Cytotoxic drugs known to be substrates for each ABC transporter were selected: doxorubicin for P-gp, etoposide for Mrp1, and mitoxantrone for BCRP. Stock solutions of cytotoxic drugs were prepared in Dulbecco's modified Eagle's medium or RPMI 1640 medium containing either 0 or 20 μM dLop or Lop. Cultured cells were seeded and incubated in serial dilutions of the stock solutions as described by Brimacombe et al. (2009). Cytotoxicity (IC50) was defined as the drug concentration that reduced cell viability to 50% of the untreated control and was calculated from three independent experiments. Resistance ratios are also reported for each resistant cell line, determined by dividing the IC50 of the resistant cell line by that of the parental cell line (Brimacombe et al., 2009).
Statistical Analysis.
Data are expressed as mean ± S.D. from three observations for radioactivity accumulation assays, from four observations for mouse imaging studies, from three observations for inhibition assays, and from at least nine observations for cytotoxicity assays. After the data were tested for homogeneity of variance, statistical significance was evaluated for radioactivity accumulation and cytotoxicity assays by the Student's t test (unpaired, two-tailed, α = 0.05) and for imaging studies by a two-way ANOVA followed by the Bonferroni post-t test (α = 0.05).
Results
Each Resistant Cell Line Exclusively Expressed One Functional ABC Transporter.
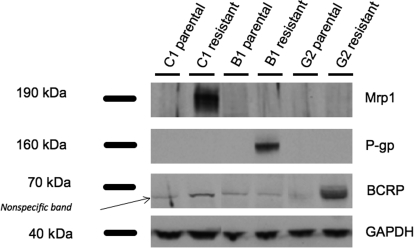
We confirmed that each ABC transporter was exclusively expressed by one of the resistant cell lines using Western analysis: KB-8-5-11 expressed only P-gp; MCF-7/VP16 expressed only Mrp1; and H460/MX20 expressed only BCRP (Fig. 1). None of the parental cell lines had detectable expression of any of these three ABC transporters. Based on band width and intensity, immunoprobing for GAPDH expression showed roughly equal loading of protein in all lanes.
Fig. 1.
Expression of P-gp, Mrp1, and BCRP in three pairs of cell lines as determined by Western blot analysis. The three pairs are (listed as parental and resistant, respectively) as follows: MCF-7 and MCF-7/VP16 (ABCC1); KB-3-1 and KB-8-5-11 (ABCB1); and H460 and H460/MX20 (ABCG2). A protein ladder was loaded with each Western blot to mark the bands for Mrp1 (190 kDa), P-gp (160 kDa), BCRP (70 kDa), and GAPDH (40 kDa). Cell lysates were probed for GAPDH as a control for the amount of protein loaded. A nonspecific band appears in all the lanes when probed against BCRP.
We also confirmed that each ABC transporter was functional: for each cell pair, the resistant cell line had a significantly higher cytotoxicity (IC50) value than its parental line. The ABCB1-expressing cell line was 71-fold more resistant to doxorubicin than its parental line, the ABCC1-expressing cell line was 79-fold more resistant to etoposide than its parental line, and the ABCG2-expressing cell line was 37-fold more resistant to mitoxantrone than its parental line (Table 1).
TABLE 1.
Effect of dLop and Lop on the cytotoxicity of drugs preferentially effluxed by P-gp, Mrp1, and BCRP
IC50 values are mean ± S.D. from three independent experiments.
| Cell Line | Cytotoxic Drug | IC50 | RR | Cytotoxicity |
|||
|---|---|---|---|---|---|---|---|
| dLop (20 μM) |
Lop (20 μM) |
||||||
| IC50 | RR | IC50 | RR | ||||
| B1 resistant | Doxorubicin | 846 ± 68 nM | 71 | 234 ± 57 nM | 20* | 144 ± 15 nM | 12* |
| B1 parental | Doxorubicin | 12 ± 4 nM | |||||
| C1 resistant | Etoposide | 79 ± 20 μM | 79 | 140 ± 41 μM | 140† | 169 ± 73 μM | 169† |
| C1 parental | Etoposide | 1 ± 1 μM | |||||
| G2 resistant | Mitoxantrone | 223 ± 54 nM | 37 | 256 ± 56 nM | 42 | 367 ± 118 nM | 61 |
| G2 parental | Mitoxantrone | 6 ± 1 nM | |||||
RR, resistance ratio, which is the quotient of the IC50 value of the resistant cell line to that of the parental line.
P < 0.001 (α = 0.05, from initial IC50 value of resistant cell line) by Student's two-tailed t-test.
P > 0.18.
At Low Concentrations (≤1 nM), dLop Is a Substrate Selective for Human ABCB1.
[3H]dLop accumulation was stable after 30 min (Fig. 2) and was significantly different between the parental and resistant cells of only the ABCB1 pair (Fig. 3). Parental cells of the ABCB1 pair (210 ± 15 fmol/106 cells) accumulated 4 times more [3H]dLop than ABCB1-expressing cells (42 ± 5 fmol/106 cells; P < 0.001) (Fig. 3). After treatment with cyclosporin A, ABCB1-expressing cells (124 ± 25 fmol/106 cells) accumulated 3 times more [3H]dLop than untreated ones (42 ± 5 fmol/106 cells; P < 0.001) (data not shown). Furthermore, after treatment with cyclosporin A, accumulation of [3H]dLop in ABCB1-expressing cells was equivalent to that in parental cells (P = 0.10). In contrast to these findings for the ABCB1 pair, the parental and resistant cells of the ABCC1 and ABCG2 pairs accumulated the same amount of [3H]dLop (P = 0.86 for ABCC1 and P = 0.18 for ABCG2) (Fig. 3).
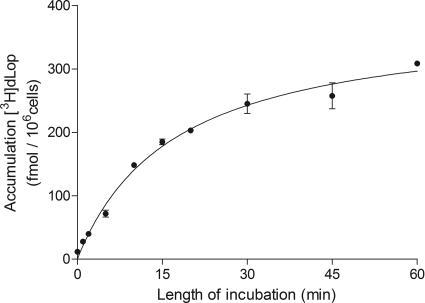
Fig. 2.
Time-dependent accumulation of [3H]dLop in parental cells of the ABCB1 line. Cells were incubated with [3H]dLop (1 nM), lysed at the times indicated, and assayed for radioactivity. Data represent means ± S.D. of three observations.
Fig. 3.
Accumulation of [3H]dLop by three pairs of parental (□) and drug-resistant (■) cells. Accumulation was significantly different between the parental and resistant cells of only the ABCB1 pair. The amount of [3H]dLop in cells was assayed 45 min after the addition of [3H]dLop (1 nM). Data represent means ± S.D. of three observations. ***, P < 0.001 by unpaired Student's two-tailed t test (α = 0.05).
dLop Is Selectively Taken up into Brains of abcb1a/b-Knockout Mice.
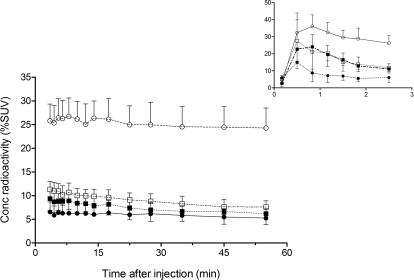
After injection of [11C]dLop into four strains of mice, the brain concentration of radioactivity in abcb1a/b-knockout mice (25.5 ± 4.1% SUV; P < 0.0001) was at least 2.5-fold higher than that in abcc1a-knockout (7.9 ± 2.1% SUV), abcg2-knockout (9.6 ± 1.6% SUV), or wild-type (6.0 ± 2.1% SUV) mice (Fig. 4).
Fig. 4.
Concentration of radioactivity (% SUV) measured by PET in brains of four strains of mice after injection of [11C]dLop. Three strains of mice were selectively knocked out for the gene that encodes either P-gp (○), Mrp1 (□), or BCRP (■); the fourth strain was wild-type (●). Concentration of radioactivity in abcb1a/b-knockout mice was at least 2.5 times higher (P < 0.0001 by two-way ANOVA) than that in the other three strains of mice. Symbols represent mean ± S.D. values from four mice per strain. For clarity, the concentration of radioactivity taken up into brain in the first 3 min is shown as an inset.
At High Concentrations (≥20 μM), dLop and Lop Selectively Inhibit P-gp Function.
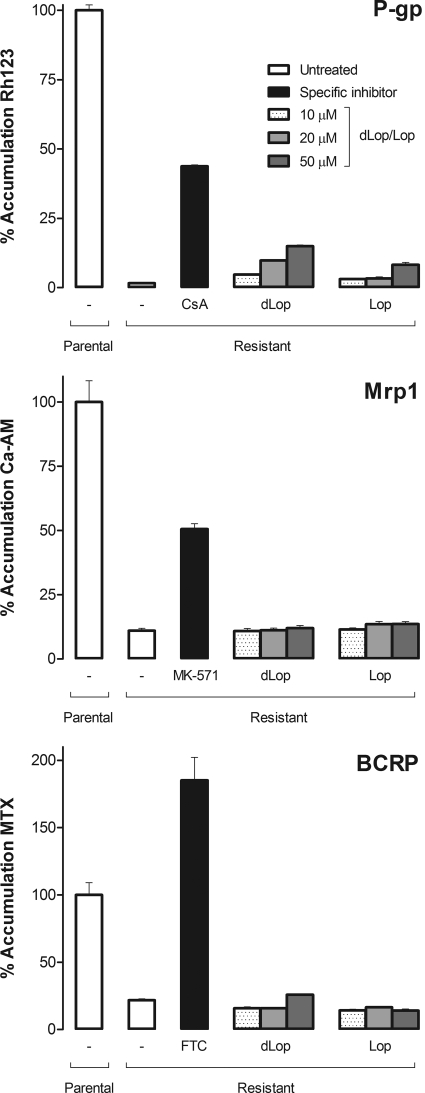
Using inhibition and cytotoxicity assays, we found that both dLop and Lop inhibited P-gp function in a concentration-dependent manner but had no effect on Mrp1 or BCRP. In the inhibition assay, expressed relative to the low uptake of fluorescent substrate in resistant cells, the two highest concentrations (20 and 50 μM) of both dLop and Lop increased uptake by 4- and 12-fold, respectively (Fig. 5). At these same concentrations, neither dLop nor Lop inhibited Mrp1 or BCRP function (Fig. 5). Inhibitors specific for each ABC transporter were used as a positive control to demonstrate inhibition of efflux in each resistant cell line. Cyclosporin A increased accumulation of the P-gp substrate 30-fold; MK-571 increased the accumulation of the Mrp1 substrate 5-fold; and fumitremorgin C increased the accumulation of the BCRP substrate 6-fold. In fact, fumitremorgin C increased uptake of the fluorescent substrate in ABCG2-expressing cells higher than that in untreated parental H460 cells. This result was not anomalous because H460 cells are known to express a small amount of functional BCRP that is not detectable by Western analysis (Henrich et al., 2007). Furthermore, when H460 cells were treated with fumitremorgin C, they accumulated the same amount of fluorescent substrate as the resistant cells (data not shown). Parental cell lines were used as an additional control to show accumulation of each fluorescent substrate without (Fig. 5) and with modulators (data not shown).
Fig. 5.
The ability of dLop and Lop to inhibit (as competitive substrates) the function of P-gp, Mrp1, and BCRP. Both dLop and Lop selectively inhibited only P-gp function. The cellular accumulation of transporter-specific fluorescent substrate is shown as a bar that represents the mean fluorescence intensity normalized to accumulation of untreated parental cells for a total of 10,000 cells. For each cell line pair, accumulation is shown for untreated parental and resistant cells (white bar), inhibitor-treated resistant cells (black bar), and dLop- or Lop-treated resistant cells (shade-graduated bars). Fluorescent substrates with activity for each transporter were used: rhodamine 123 (Rh123) for P-gp, calcein-AM (Ca-AM) for Mrp1, and mitoxantrone (MTX) for BCRP. In addition to substrate, an inhibitor was used for each ABC transporter: cyclosporin A (CsA; 10 μM) for P-gp, MK-571 (50 μM) for Mrp1, and fumitremorgin C (FTC; 5 μM) for BCRP. Bars represent the mean ± S.D. of three observations. Some error bars are smaller than the line thickness of the bars and, therefore, are not visible.
In the cytotoxicity assay, we found that dLop (20 μM) and Lop (20 μM) sensitized the ABCB1-expressing cell line to doxorubicin by ∼4-fold (P < 0.001) and ∼6-fold (P < 0.001), respectively (Table 1). However, neither compound significantly modulated the resistance of the ABCC1- or ABCG2-expressing cell lines to etoposide or to mitoxantrone, respectively. When assayed in the absence of cytotoxic agents, neither dLop nor Lop was toxic (IC50 > 50 μM) by themselves to any cell line (data not shown).
Discussion
In both human and mouse tissues, dLop is selective for P-gp among these three most prevalent efflux transporters at the blood-brain barrier. However, the activity of dLop is dependent on its concentration. At low concentrations, dLop acts as only a substrate; at high concentrations, it acts as both a substrate and an inhibitor (i.e., a competitive substrate). In human cells, the selectivity of dLop for P-gp was demonstrated because accumulation of [3H]dLop significantly differed between the parental and resistant cells for only the ABCB1 pair but not for the ABCC1 or ABCG2 pairs. The selectivity measured in vitro using human cells paralleled that in vivo using transgenic mice, because the concentration of radioactivity in brain after injection of [11C]dLop was at least 2.5-fold higher in abcb1a/b-knockout mice than in abcc1a-knockout, abcg2-knockout, or wild-type mice.
High concentrations of both dLop and Lop selectively inhibit P-gp as competitive substrates but not Mrp1 or BCRP. Selectivity at high concentrations was demonstrated in two ways. First, both dLop and Lop (≥20 μM) inhibited the function of only P-gp but not that of Mrp1 or BCRP. Our results also suggest that Lop is a more avid substrate than dLop because Lop inhibited P-gp function at a lower concentration than dLop. Second, both dLop and Lop (20 μM) significantly decreased the resistance of only the ABCB1-expressing cell line to its cognate cytotoxic substrate. The ability of dLop and Lop to inhibit P-gp function as competitive substrates is consistent with that of other substrates of ABC transporters. For example, the P-gp substrate verapamil also inhibits the function of P-gp as a competitive substrate (Ambudkar et al., 1999). Although high concentrations (micromolar) of both dLop and Lop inhibit P-gp function, the low concentrations (nanomolar) typically used for PET radiotracers would reflect substrate activity and not competitive inhibition of the function of the transporter.
Our method of using both cultured human cells and knockout mice to measure the selectivity of substrates for ABC transporters is valuable for two reasons. First, because selectivity for human ABC transporters was measured using human cells, our results were not confounded by species differences in substrate selectivity that is known for ABC transporters (Syvänen et al., 2009). Second, we showed that our results from the in vitro model extended to an in vivo model, which consisted of knockout mice that selectively lack one of the three ABC transporters. This in vivo model was also advantageous because it avoided the use of pharmacological inhibitors for ABC transporters. Although some inhibitors are reported to be selective for one ABC transporter at low concentrations, they may lose that selectivity at high concentrations. For example, low concentrations (≤100 nM) of tariquidar appear to be selective for P-gp (Martin et al., 1999; Fox and Bates, 2007), but higher concentrations (>1 μM) show cross-reactivity for BCRP (Robey et al., 2004). Therefore, the use of both human cells and knockout mice is advantageous because the two systems allow selective interrogation of a single ABC transporter.
One limitation of our imaging studies is the possibility that the abcc1a- and abcg2-knockout mice had altered peripheral metabolism of [11C]dLop to make it appear that dLop is not a substrate for these two transporters. For example, these mice might have metabolized the radioligand so quickly that little was available for uptake into brain. However, we think this possibility is highly unlikely. We previously studied the metabolism of [11C]dLop in abcb1a/b-knockout and wild-type mice by measuring the metabolic profile of radioactivity in extracts of plasma and brain at 30 min after injection of [11C]dLop (Lazarova et al., 2008). The concentrations of parent radioligand and radiometabolites in plasma were similar for both abcb1a/b-knockout and wild-type mice. However, the concentrations in brain differed, with severalfold higher concentrations of [11C]dLop in brains of abcb1a/b-knockout than of wild-type mice (Lazarova et al., 2008). Because the knockout mice for abcc1a and abcg2 derive from the same genetic background as the other two strains, they are highly unlikely to have unusually fast or altered metabolism to cause a false-negative result.
Will [11C]dLop be useful to measure P-gp in neuropathological conditions associated with both decreased and increased function? [11C]dLop has low brain uptake at baseline, and P-gp inhibition increases brain uptake 2- to 4-fold (Kreisl et al., 2009). These characteristics suggest that [11C]dLop will be useful to measure P-gp in pathophysiological conditions associated with decreased function, such as Alzheimer's disease. That is, decreased function of P-gp in Alzheimer's disease would be predicted to increase brain uptake of [11C]dLop. However, the uptake [11C]dLop into brain at baseline is so low that it may not be useful to measure P-gp in pathological conditions associated with increased function, such as drug-resistant epilepsy. In contrast, a substrate radiotracer, such as [11C]verapamil, which has moderate brain uptake at baseline might be capable of measuring increased P-gp function. However, a recent study by Langer et al. (2007) found no significant decrease of [11C]verapamil uptake in drug-resistant epilepsy. Further studies are required to determine the relative utility of substrates, such as [11C]verapamil, that have moderate brain uptake at baseline compared with those, such as [11C]dLop, that have low brain uptake at baseline.
In conclusion, our results show that dLop is selective for P-gp both in human cells and in live mice and that its activity is dependent on concentration. At low concentrations, dLop acts as only a substrate; at high concentrations, it acts as both a substrate and an inhibitor (i.e., a competitive substrate). Because low concentrations of radiotracer are used for PET imaging, [11C]dLop acts selectively and only as a substrate for P-gp at the blood-brain barrier.
Supplementary Material
Acknowledgments.
This work was a collaboration between the National Institute of Mental Health, the National Cancer Institute, and the Karolinska Institute. We thank Yi Zhang, Elise Luong, and Jinsoo Hong for assisting with the synthesis of [11C]dLop and George Leiman for editorial assistance.
This research was supported in part by the Intramural Research Programs of the National Institutes of Health National Institute of Mental Health [Project Z01-MH002852-04] and the National Cancer Institute [ Project Z01-BC005598].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.031161.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- ABC
- ATP-binding cassette
- P-gp
- P-glycoprotein
- Mrp1
- multidrug resistance protein 1
- BCRP
- breast cancer resistance protein
- PET
- positron emission tomography
- dLop
- N-desmethyl-loperamide
- Lop
- loperamide
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- SUV
- standard uptake value
- MK-571
- 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid.
References
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398 [DOI] [PubMed] [Google Scholar]
- Brimacombe KR, Hall MD, Auld DS, Inglese J, Austin CP, Gottesman MM, Fung KL. (2009) A dual-fluorescence high-throughput cell line system for probing multidrug resistance. Assay Drug Dev Technol 7:233–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. (2005) P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest 115:3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JD, Balwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggerda JA, VandeBer JL. (1996) Guide for the Care and Use of Laboratory Animals, National Academy Press, Washington, DC: [Google Scholar]
- Fox E, Bates SE. (2007) Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther 7:447–459 [DOI] [PubMed] [Google Scholar]
- Gottesman MM. (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627 [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58 [DOI] [PubMed] [Google Scholar]
- Graff CL, Pollack GM. (2004) Drug transport at the blood-brain barrier and the choroid plexus. Curr Drug Metab 5:95–108 [DOI] [PubMed] [Google Scholar]
- Hendrikse NH, Schinkel AH, de Vries EG, Fluks E, Van der Graaf WT, Willemsen AT, Vaalburg W, Franssen EJ. (1998) Complete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. Br J Pharmacol 124:1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich CJ, Robey RW, Bokesch HR, Bates SE, Shukla S, Ambudkar SV, Dean M, McMahon JB. (2007) New inhibitors of ABCG2 identified by high-throughput screening. Mol Cancer Ther 6:3271–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan P, John C, Zoghbi SS, Halldin C, Gottesman MM, Innis RB, Hall MD. (2009) Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther 86:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. (2007) A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315:525–528 [DOI] [PubMed] [Google Scholar]
- Kreisl WC, Liow J-S, Kimura N, Seneca N, Zoghbi SS, Herscovitch P, Pike VW, Innis R. (2009) P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med doi: jnumed.109.070151v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnik D, Sofowora GG, Donahue JP, Nair UB, Wilkinson GR, Wood AJ, Muszkat M. (2008) Tariquidar, a selective P-glycoprotein inhibitor, does not potentiate loperamide's opioid brain effects in humans despite full inhibition of lymphocyte P-glycoprotein. Anesthesiology 109:1092–1099 [DOI] [PubMed] [Google Scholar]
- Langer O, Bauer M, Hammers A, Karch R, Pataraia E, Koepp MJ, Abrahim A, Luurtsema G, Brunner M, Sunder-Plassmann R, et al. (2007) Pharmacoresistance in epilepsy: a pilot PET study with the P-glycoprotein substrate R-[11C]verapamil. Epilepsia 48:1774–1784 [DOI] [PubMed] [Google Scholar]
- Lazarova N, Zoghbi SS, Hong J, Seneca N, Tuan E, Gladding RL, Liow JS, Taku A, Innis RB, Pike VW. (2008) Synthesis and evaluation of [11C]N-desmethyl-loperamide as a new and improved PET radiotracer for imaging P-gp function. J Med Chem 51:6034–6043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang KT, Ambudkar SV, Pastan I, Dey S. (1998) HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 37:3594–3601 [DOI] [PubMed] [Google Scholar]
- Liow JS, Kreisl W, Zoghbi SS, Lazarova N, Seneca N, Gladding RL, Taku A, Herscovitch P, Pike VW, Innis RB. (2009) P-glycoprotein function at the blood-brain barrier imaged using 11C-N-desmethyl-loperamide in monkeys. J Nucl Med 50:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Potschka H. (2005a) Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 6:591–602 [DOI] [PubMed] [Google Scholar]
- Löscher W, Potschka H. (2005b) Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol 76:22–76 [DOI] [PubMed] [Google Scholar]
- Martin C, Berridge G, Mistry P, Higgins C, Charlton P, Callaghan R. (1999) The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol 128:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. (2006) Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA 103:16394–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms D, Chiu ML, Budding M, Kronauge JF, Kramer RA, Croop JM. (1993) Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. Cancer Res 53:977–984 [PubMed] [Google Scholar]
- Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. (2004) Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res 64:1242–1246 [DOI] [PubMed] [Google Scholar]
- Schneider E, Horton JK, Yang CH, Nakagawa M, Cowan KH. (1994) Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res 54:152–158 [PubMed] [Google Scholar]
- Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman MM. (1986) Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem 261:7762–7770 [PubMed] [Google Scholar]
- Syvänen S, Lindhe O, Palner M, Kornum BR, Rahman O, Långström B, Knudsen GM, Hammarlund-Udenaes M. (2009) Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos 37:635–643 [DOI] [PubMed] [Google Scholar]
- Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234 [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Warzok RW, Cascorbi I, Kunert-Keil C, Schroeder E, Kroemer HK, Siegmund W, Walker LC, Pahnke J. (2004) The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer's disease. Curr Alzheimer Res 1:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. (2007) Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol Cancer Ther 6:3287–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi SS, Liow JS, Yasuno F, Hong J, Tuan E, Lazarova N, Gladding RL, Pike VW, Innis RB. (2008) 11C-Loperamide and its N-desmethyl radiometabolite are avid substrates for brain permeability-glycoprotein efflux. J Nucl Med 49:649–656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.