Abstract
Background
Almost all animals, including insects, need to adapt to temperature fluctuations. The molecular basis of thermal adaptation is not well understood, although a number of candidate genes have been proposed. However, a functional link between candidate genes and thermal tolerance has rarely been established. The gene Frost (Fst) was first discovered when Drosophila flies were exposed to cold stress, but the biological function(s) of Fst has so far not been characterized. Because Fst is up-regulated after a cold stress, we tested whether it was essential for chill-coma recovery.
Methodology/Principal Findings
A marked increase in Fst expression was detected (by RT-PCR) during recovery from cold stress, peaking at 42-fold after 2 h. The GAL4/UAS system was used to knock down expression of Fst and recovery ability was assessed in transgenic adults following 12 h of chill coma at 0°C. The ability to recover from cold stress (short-, medium- and long-term) was significantly altered in the transgenic adults that had Fst silenced. These findings show that Fst plays an essential role in the recovery from chill coma in both males and females.
Conclusions/Significance
The Frost gene is essential for cold tolerance in Drosophila melanogaster and may play an important role in thermal adaptation.
Introduction
Insects subjected to seasonally low temperatures have evolved a range of physiological and molecular adaptations to survive [1]. The molecular mechanisms behind cold stress and associated chilling injuries are complex and still poorly understood [2], [3]. Drosophila melanogaster has adapted successfully to diverse thermal environments and provides a useful model system for understanding the molecular basis of thermal adaptation.
While some studies have considered genes that might be involved in cold tolerance in Drosophila [4], the molecular basis of cold stress resistance is poorly understood in comparison to heat resistance. It appears that more genes/proteins are activated during recovery phases following cold stress compared to the actual stress period [2], [5] and these phases need to be differentiated in experimental studies [6]. Recovery from chill-coma is a trait widely studied by evolutionary geneticists (e.g. ref [4], [7]) because it is adaptively significant [4], [8] but its underlying molecular basis is not well-understood.
Frost (Fst) is one of the few candidate genes that have been implicated in cold tolerance in D. melanogaster. This gene was first discovered and characterized by Goto [9] in flies exposed to cold stress. Recent studies have also suggested that Fst might be a good candidate for thermal adaptation [11], [12]. Fst was up-regulated during recovery from cold stress but, unlike heat-shock genes [7], Fst expression was not altered after heat stress [10]. However, a functional relationship between Fst and cold tolerance remains to be established. Fst has also been reported to respond weakly to a range of abiotic stressors, such as dietary shifts, desiccation, chemical toxicity, insecticide exposure and hypoxia [10], [13]–[16]. Fst may also be involved in immune response against virus, bacteria and fungi [17]–[20].
In the present study we showed that the mRNA level of Fst was markedly increased in adults recovering from cold stress. We demonstrated that silencing Fst by transgenic RNA inference impaired the recovery process from chill coma in both sexes. Expression of Fst thus seems to be crucial for developing cold tolerance in D. melanogaster adults.
Methods
Drosophila stocks and breeding conditions
The wild type D. melanogaster strain was derived from about 50 females collected in Innisfail (Australian east coast) in May 2008 (see ref [7] for more details). RNAi-mediated Fst knockdown was achieved using the GAL4/UAS system [21]. The UAS-Fst line was obtained from the Vienna Drosophila RNAi Center (transformant ID: KK102049) [22]. The tubulin-GAL4 (genotype: w*; tubP-GAL4/TM3, Act-GFP JMR2, Ser1, provided by Phil Batterham, University of Melbourne) and the actin5C-GAL4 (Bloomington Drosophila Stock Center, #4414) lines were used separately to drive the expression of the UAS-Fst, both resulted in ubiquitous Fst mRNA knockdown. Progeny were tested in cold recovery assays. To control for genetic background effects, the same GAL4 driver lines were crossed to the w1118 line (from BDRC) and their progeny assayed alongside with their GAL4/UAS-Fst counterparts. Fly stocks were maintained in 250 ml bottles in uncrowded conditions. Bottles were kept at 25°C, 70% relative humidity, and continuous light on a standard fly medium as previously described [23].
Cold stress and recovery conditions
All tests were performed using synchronized 4-day old flies, sexed without CO2 anaesthesia. To establish the Fst mRNA expression during the cold stress and during the recovery period, we used the same method as described in Colinet et al. [7]. Briefly, wild flies were cold stressed at 0°C to induce chill coma, and sampled after 0.25, 3, 6 and 9 h of cold stress (denoted as S025, S3, S6 and S9 respectively). After 9 h of cold stress, flies were allowed to recover at 25°C and Fst mRNA expression was measured after 0.5, 2, 4, and 8 h of recovery (denoted as R05, R2, R4 and R8 respectively). For every sampling time there was a corresponding control, consisted of flies kept at 25°C for the same duration (n = 4×20 flies).
RNA extraction and quantitative real time PCRs
RNA extractions were performed using the RNeasy RNA extraction kit and the RNase-Free DNase Set (Qiagen, Australia) as described in Colinet et al. [7]. cDNA was synthesized using the Superscript III First-Strand Synthesis System (Invitrogen, Australia), according to manufacturer's instructions. Fst primers were designed with the Primer3 module (http://www.angis.org.au) (forward: 5′-GGAACAGAGGTGGAATAGCCAAAATC-3′ and reverse: 5′-GCCTTGATTGTTTCCGTGAGATTG-3′). The qRT-PCRs were performed on the LightCycler® 480 system (Roche Diagnostics, Australia) following the method previously described [7]. Relative expression ratios (i.e., fold change) were calculated using the 2−ΔΔCt method [24]. RpS20 was used as a housekeeping reference gene (see ref [7]). To verify the extent of gene knockdown, Fst mRNA levels were compared between the untreated flies, kept at 25°C (i.e. basal expression) and the treated flies, recovering for 2 h after cold stress (i.e. during Fst up-regulation). Such a comparison in Fst expression was conducted separately in males and females (n = 3×20 flies per line).
Chill-coma recovery assays
Three types of assays were used to measure recovery abilities after 12 h of chill-coma at 0°C. Firstly, ‘short-term recovery’ was assessed by comparing recovery times of both GAL4/UAS-Fst and GAL4/+ lines at 25°C. Flies were considered recovered when they stood up [25]. Recovery curves were compared between lines using Mantel-Cox analysis with a censoring factor for individuals that did not recover at the end of the experiment. Forty-five flies were monitored for each line. To test for ‘long-term recovery’, the mortality of flies after cold stress was assessed when they had been held in food vials at 25°C for 24 h. Chi square contingency tests were used to compare mortality rates between GAL4/UAS-Fst and GAL4/+ lines. Mortality rates were based on 150 flies for each line. Finally, an additional ‘medium-term recovery’ test was performed with flies derived from act-GAL4 crosses. This test was designed to monitor mobility status during 8 h following the cold stress, and represents a modified version of a climbing activity test described elsewhere [26]. Briefly, flies were individually transferred to a 9.5 cm plastic vial. The height flies reached within 7 sec after a mechanical stimulation was noted. Flies were divided into three categories: (a) injured, no climbing; (b) recovering, slow climbing without reaching the top of the vial within 7 sec; (c) fit, fast climbing and reaching the top of the vial within 7 sec. The 7 sec observation time was chosen because preliminary assays showed that all unstressed flies reach the top of a vial within 6 sec (5.1±1.3 sec, n = 50). This test was performed repeatedly on the same individuals after 2, 4, 6 and 8 h of recovery (25°C). Flies were maintained on food during this period. Chi square contingency tests were carried out to compare numbers of flies in the three categories for the act-GAL4/UAS-Fst and act-GAL4/+ lines. Seventy flies were tested for each line. This test was not performed on flies derived from tub-GAL4 crosses which were less vigorous even when they were unstressed (34% did not reach the top of the vial within 10 sec, n = 50). All statistical tests were performed using Prism V 5.01 (GraphPad software, Inc. 2007).
Results
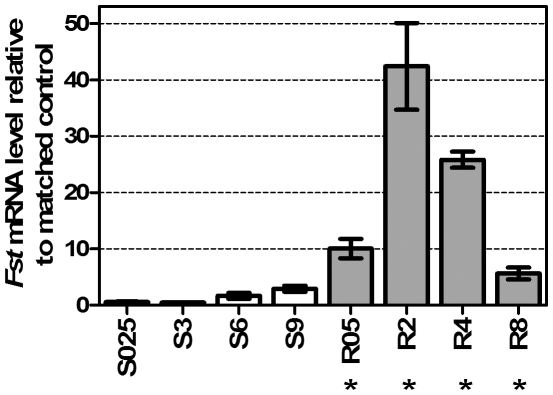
Expression of Fst was not altered during the cold stress period, but Fst was significantly up-regulated during the recovery phase at 25°C. Expression peaked after 2 h of recovery, when there was a maximal 42-fold change relative to controls (Fig. 1). Because of this significant up-regulation during recovery from cold stress, we suspected that Fst may have an essential role in chill-coma recovery.
Figure 1. Upregulation of Fst during cold stress and recovery.
White bars represent cold stressed treatment (S) at 0°C for 0.25 to 9 h and grey bars denote recovery (R) at 25°C for 0.5 to 8 h. Relative expressions are calculated using the 2−ΔΔCt method. Expression levels of Fst are normalized against the housekeeping reference RpS20 and values are expressed as fold change relative to control (mean±SE; n = 4). The symbol (*) indicates when a value is significantly different from untreated controls (t-test).
Lines derived from tubulin-GAL4 driver
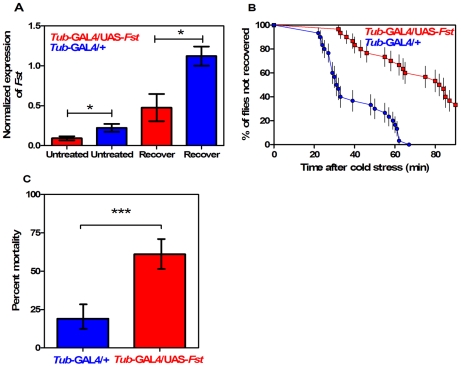
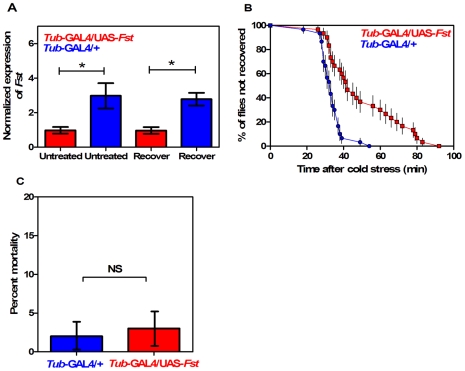
Fst mRNA expression was significantly reduced in tub-GAL4/UAS-Fst females compared to tub-GAL4/+ females, both when flies were untreated (t = 10.11, P<0.001, IC: 0.166−0.094, r2 = 0.962) and when they were recovering from the cold stress (t = 13.36, P<0.001, IC: 0.779−0.511, r2 = 0.978) (Fig. 2A). Fst expression was also significantly repressed in tub-GAL4/UAS-Fst males compared to tub-GAL4/+ males, both when flies were untreated (t = 11.43, P<0.001, IC: 2.490−1.517, r2 = 0.970) and recovering from cold stress (t = 18.78, P<0.001, IC: 2.080 to 1.544, r2 = 0.988) (Fig. 3A). Fst knockdown had a significant effect on short-term recovery in both sexes but particularly in females (Fig. 2B, 3B), resulting in significantly different recovery curves (Mantel-Cox: χ2 = 34.33; df = 1; P<0.001 for females and χ2 = 20.50; df = 1; P<0.001 for males). In females (Fig. 2B) all the tub-GAL4/+ flies recovered within 62 min, while 33% of flies still had not recovered in the tub-GAL4/UAS-Fst group after 90 min. In males (Fig. 3B) all flies recovered within 90 min but recovery time was longer in the tub-GAL4/UAS-Fst group. Nevertheless all flies did eventually recover. For the long-term assay, there was a significant difference in mortality between females from the two lines (Fig. 2C) (χ2 = 37.41; df = 1; P<0.001), with mortality reaching 61% in the tub-GAL4/UAS-Fst flies compared to 19% in the tub-GAL4/+ controls. In males, mortality in the two groups did not differ significantly (χ2 = 0.16, df = 1; P = 0.68) (Fig. 3C).
Figure 2. Silencing the cold-inducible Fst expression impairs chill coma recovery in tub-GAL4-driven females.
(A) Expression of Fst mRNA in untreated (kept at 25°C) and recovering (2 h at 25°C after 12 h at 0°C) females. Expression levels of Fst are normalized against the housekeeping reference RpS20 and values are √1/x transformed (mean±CI; n = 3). The symbol (*) indicates when the level is significantly different in tub-GAL4/UAS-Fst versus tub-GAL4/+ females (t-test). (B) Comparison of temporal recovery curves in tub-GAL4/UAS-Fst (squares) versus tub-GAL4/+ (circles) females. Time to recover from chill coma was monitored in females recovering at 25°C after 12 h of cold stress at 0°C. Each dot represents the mean percentage (±SE); 45 females were tested per line. (C) Mortality rate in tub-GAL4/UAS-Fst versus tub-GAL4/+ females. Mortality was assessed in flies recovering for 24 h at 25°C after 12 h of cold stress at 0°C. Bars represents the percentage (±CI) derived from 150 females in each line. The symbol (*) indicates a significant difference between lines (Chi square test).
Figure 3. Silencing the cold-inducible Fst expression impairs chill coma recovery in tub-GAL4-driven males.
(A) Expression of Fst mRNA in untreated (kept at 25°C) and recovering (2 h at 25°C after 12 h at 0°C) males. Expression levels of Fst are normalized against the housekeeping reference RpS20 and values are √1/x transformed (mean±CI; n = 3). The symbol (*) indicates when the level is significantly different in tub-GAL4/UAS-Fst versus tub-GAL4/+ males (t-test). (B) Comparison of temporal recovery curves in tub-GAL4/UAS-Fst (squares) versus tub-GAL4/+ (circles) males. Time to recover from chill coma was monitored in males recovering at 25°C after 12 h of cold stress at 0°C. Each dot represents the mean percentage (±SE); 45 males were tested per line. (C) Mortality rate in tub-GAL4/UAS-Fst versus tub-GAL4/+ males. Mortality was assessed in flies recovering for 24 h at 25°C after 12 h of cold stress at 0°C. Bars represents the percentage (±CI) derived from 150 males in each line. The symbol (*) indicates a significant difference between lines (Chi square test).
Lines derived from actin-GAL4 driver
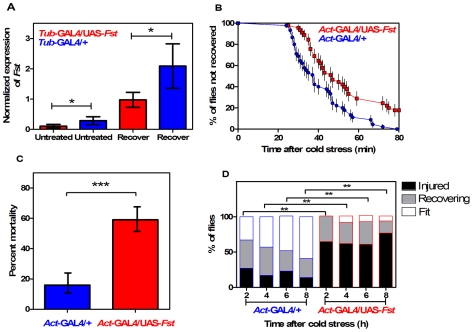
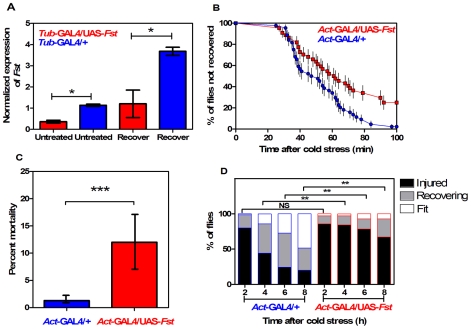
Fst mRNA expression was significantly reduced in act-GAL4/UAS-Fst females compared to act-GAL4/+ females, both when flies were untreated (t = 5.47, P = 0.005, IC: 0.273−0.089, r2 = 0.882) and when they were recovering from the cold stress (t = 6.19, P = 0.003, IC: 1.615−0.615, r2 = 0.905) (Fig. 4A). Fst expression was also significantly suppressed in act-GAL4/UAS-Fst males compared to act-GAL4/+ males, both when flies were untreated (t = 37.60, P<0.001, IC: 0.833−0.719, r2 = 0.997) and recovering from the cold stress (t = 15.78, P<0.001, IC: 2.913−2.041, r2 = 0.984) (Fig. 5A). Short-term recovery was significantly different between lines for both sexes (Fig. 4B, 5B) (Mantel-Cox: χ2 = 12.50; df = 1; P<0.001 for females; χ2 = 9.63; df = 1; P = 0.002 for males). For females (Fig. 4B), all the act-GAL4/+ control flies recovered within 80 min, while 18% of flies had not recovered in the act-GAL4/UAS-Fst group. A similar pattern was observed in males (Fig. 5B) with 25% of flies failing to recover in the act-GAL4/UAS-Fst group after 100 min. All flies eventually recovered. For the long-term recovery assay, a significant difference was observed in females (χ2 = 60.23; df = 1; P<0.001), mortality reached 59% in the act-GAL4/UAS-Fst flies compared to 16% in the act-GAL4/+ controls (Fig. 4C). Males also differed significantly for mortality (χ2 = 0.13; df = 1; P = 0.002), which reached 12% in the act-GAL4/UAS-Fst flies and 1.5% in the act-GAL4/+ flies (Fig. 5C). In addition, the medium-term recovery tests revealed significant differences in movement patterns between the act-GAL4/UAS-Fst and the act-GAL4/+ control flies (Fig. 4D, 5D) (χ2 tests: P<0.05). A high proportion of females were initially injured in the act-GAL4/UAS-Fst group and this proportion remained high during the observation period (Fig. 4D). In contrast, females from the act-GAL4/+ group gradually recovered, with the proportion of designated as ‘fit’ increasing while ‘injured’ flies decreased in proportion (Fig. 4D). A similar pattern was observed in the males (Fig. 5D) where the act-GAL4/+ flies gradually recovered while the majority of the act-GAL4/UAS-Fst flies remained injured.
Figure 4. Silencing the cold-inducible Fst expression impairs chill coma recovery in act-GAL4-driven females.
(A) Expression of Fst mRNA in untreated (kept at 25°C) and recovering (2 h at 25°C after 12 h at 0°C) females. Expression levels of Fst are normalized against the housekeeping reference RpS20 and values are √1/x transformed (mean±CI; n = 3). The symbol (*) indicates when the level is significantly different in act-GAL4/UAS-Fst versus act-GAL4/+ females (t-test). (B) Comparison of temporal recovery curves in act-GAL4/UAS-Fst (squares) versus act-GAL4/+ (circles) females. Time to recover from chill coma was monitored in females recovering at 25°C after 12 h of cold stress at 0°C. Each dot represents the mean percentage (±SE); 45 females were tested per line. (C) Mortality rate in act-GAL4/UAS-Fst versus act-GAL4/+ females. Mortality was assessed in flies recovering for 24 h at 25°C after 12 h of cold stress at 0°C. Bars represents the percentage (±CI) derived from 150 females in each line. The symbol (*) indicates a significant difference between lines (Chi square test). (D) Climbing activity monitored in act-GAL4/UAS-Fst versus act-GAL4/+ females. Measurements were taken in recovering females after 2, 4, 6 and 8 h at 25°C following 12 h at 0°C. Flies were categorized as fit (fast climbing) or recovering (slow climbing) or injured (no climbing). The symbol (*) indicate significant differences between lines (Chi square test, n = 70).
Figure 5. Silencing the cold-inducible Fst expression impairs chill coma recovery in act-GAL4-driven males.
(A) Expression of Fst mRNA in untreated (kept at 25°C) and recovering (2 h at 25°C after 12 h at 0°C) males. Expression levels of Fst are normalized against the housekeeping reference RpS20 and values are √1/x transformed (mean±CI; n = 3). The symbol (*) indicates when the level is significantly different in act-GAL4/UAS-Fst versus act-GAL4/+ males (t-test). (B) Comparison of temporal recovery curves in act-GAL4/UAS-Fst (squares) versus act-GAL4/+ (circles) males. Time to recover from chill coma was monitored in males recovering at 25°C after 12 h of cold stress at 0°C. Each dot represents the mean percentage (±SE); 45 males were tested per line. (C) Mortality rate in act-GAL4/UAS-Fst versus act-GAL4/+ males. Mortality was assessed in flies recovering for 24 h at 25°C after 12 h of cold stress at 0°C. Bars represents the percentage (±CI) derived from 150 males in each line. The symbol (*) indicates a significant difference between lines (Chi square test). (D) Climbing activity monitored in act-GAL4/UAS-Fst versus act-GAL4/+ males. Measurements were taken in recovering males after 2, 4, 6 and 8 h at 25°C following 12 h at 0°C. Flies were categorized as fit (fast climbing) or recovering (slow climbing) or injured (no climbing). The symbol (*) indicate significant differences between lines (Chi square test, n = 70).
Discussion
D. melanogaster is a chill-susceptible species. At 0°C it falls almost instantly into deep chill-coma because of an inability to maintain muscle resting potentials [27]. In addition to this neuromuscular perturbation, chilling injuries accumulate at low temperatures as a result of various physiological dysfunctions (see ref [3] for review). The molecular mechanisms underlying cold stress and recovery from chill-coma are complex and not well understood. Genes involved in heat shock response are known to affect recovery from cold stress in insects [7], [28], [29]. In addition to heat shock genes, the regulation of other genes is presumably important for cold-tolerance. Indeed, multiple genes appear to be up-regulated during recovery from cold stress [30] and Fst is among the candidates suspected to play a role in cold tolerance.
However, the functional relationship between Fst and cold tolerance has not been established prior to this study. Using transgenic gene silencing techniques, the expression of Fst was knocked down. All recovery traits analyzed (i.e. short-, medium- and long-term) were significantly affected in flies where Fst expression was suppressed. Our findings thus show that Fst plays an important role in chill coma recovery in both sexes. This is the first time, to our knowledge, that a biological function has been demonstrated for Fst. QTL and microarrays studies have suggested that Fst might be a candidate for thermal adaptation [11], [12] and our findings indicate that this gene is indeed important for cold recovery.
Although the mechanistic details of how Fst functions as a protein have not been resolved, the primary sequence of Fst suggests that it resembles a mucin-like protein. Frost contains multiple tandem repeats rich in serine, threonine and proline [9], a typical feature of mucins [31]. Like secreted mucins, Frost contains an 18-amino acid signal peptide at the N-terminus [9]. A homology search in annotated protein database (http://www.geneontology.org/) identified two D. melanogaster mucins: Mur18B and Muc11A. Fst mRNA is highly enriched in adult malpighian tubule and midgut [32], [33]. Similarly, Mur18B and Muc11A transcripts are enriched in the tubule of adult flies [34]. The function of insects mucin-like proteins are currently poorly characterized [34] and the relationship between mucins and protection from abiotic stress has not been firmly established. A Drosophila mucin gene (Muc68Ca) was suggested to play an undefined role in heat shock response [35]. There is evidence that mucins protect from oxidative stress [36], [37], which is a typical feature of chilling-injury [38]. Mucins also provide a physical barrier to cells against pathogens and allow homeostasis of local molecular environments with respect to hydration, ionic composition and concentration [31], [39]. This mucin function may be critical because perturbation of ion homeostasis is directly linked to chilling injuries [40], [41] and its reestablishment occurs during recovery [42]. Among the genes up-regulated during cold stress recovery, many encode membrane-related proteins [30]. This is not surprising since the cell membrane is a primary site of chilling or cold-shock injury, as a result of damage to intracellular organelles and the leakage of ions and other solutes across cell membranes [43], [44]. The Fst gene product, presumably a mucin-like protein, may help protect membrane integrity and hence recovery from cold [1]. Silencing Fst might thus impair some protective functions against oxidative stress and/or alter aspects of osmoregulation across membranes in the tubule and midgut. Taken together, this study provides evidence that Fst is essential for chill-coma recovery in adult D. melanogaster and highlights the need to further examine this gene from evolutionary and mechanistic perspectives.
Acknowledgments
The authors are grateful to Anne Kersanté for assistance with data acquisition. We are also grateful to Phillip Daborn and Philip Batterham for providing access to PC2 facility and the GAL4 driver lines (Melbourne University, Australia). Finally we would like to thanks Steve McKechnie (Monash University, Australia) for assisting in the importation of fly lines.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by Fonds de la Recherche Scientifique (FNRS) from Belgium, and the Australian Research Council and the Commonwealth Environmental Research Fund. This paper is number BRC 169 of the Biodiversity Research Centre (UCL-Belgium). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Doucet D, Walker VK, Qin W. The bugs that came in from the cold: molecular adaptations to low temperatures in insects. Cell Mol Life Sci. 2009;66:1404–1418. doi: 10.1007/s00018-009-8320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark MS, Worland MR. How insects survive the cold: molecular mechanisms-a review. J Comp Physiol B. 2008;178:917–933. doi: 10.1007/s00360-008-0286-4. [DOI] [PubMed] [Google Scholar]
- 3.Chown SL, Terblanche JS. Physiological diversity in insects: ecological and evolutionary contexts. Adv in Insect Phys. 2006;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann AA, Sorensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol. 2003;28:175–216. [Google Scholar]
- 5.Colinet H, Nguyen TT, Cloutier C, Michaud D, Hance T. Proteomic profiling of a parasitic wasp exposed to constant and fluctuating cold exposure. Insect Biochem Mol Biol. 2007;37:1177–1188. doi: 10.1016/j.ibmb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Colinet H, Lee SF, Hoffmann A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010;277:174–185. doi: 10.1111/j.1742-4658.2009.07470.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AR, Hoffmann AA, McKechnie SW. Response to selection for rapid chill-coma-recovery in Drosophila melanogaster: physiology and life history traits. Genet Res. 2005;85:15–22. doi: 10.1017/s0016672304007281. [DOI] [PubMed] [Google Scholar]
- 8.Gibert P, Moreteau B, Petavy G, Karan D, David JR. Chill-coma tolerance, a major climatic adaptation among Drosophila species. Evolution. 2001;55:1063–1068. doi: 10.1554/0014-3820(2001)055[1063:cctamc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Goto SG. A novel gene that is up-regulated during recovery from cold shock in Drosophila melanogaster. Gene. 2001;270:259–264. doi: 10.1016/s0378-1119(01)00465-6. [DOI] [PubMed] [Google Scholar]
- 10.Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Mol Biol. 2007;16:435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 11.Morgan TJ, Mackay TFC. Quantitative trait loci for thermotolerance phenotypes in Drosophila melanogaster. Heredity. 2006;96:232–242. doi: 10.1038/sj.hdy.6800786. [DOI] [PubMed] [Google Scholar]
- 12.Laayouni H, García-Franco F, Chávez-Sandoval BE, Trotta V, Beltran S, et al. Thermal evolution of gene expression profiles in Drosophila subobscura. BMC Evol Biol. 2007;7:42. doi: 10.1186/1471-2148-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carsten LD, Watts T, Markow TA. Gene expression patterns accompanying a dietary shift in Drosophila melanogaster. Mol Ecol. 2005;14:3203–3208. doi: 10.1111/j.1365-294X.2005.02654.x. [DOI] [PubMed] [Google Scholar]
- 14.Affleck JG, Neumann K, Wong L, Walker VK. The effects of methotrexate on Drosophila development, female fecundity, and gene expression. Toxicol Sci. 2006;89:495–503. doi: 10.1093/toxsci/kfj036. [DOI] [PubMed] [Google Scholar]
- 15.Jensen HR, Scott IM, Sims SR, Trudeau VL, Arnason JT. The effect of a synergistic concentration of a Piper nigrum extract used in conjunction with pyrethrum upon gene expression in Drosophila melanogaster. Insect Mol Biol. 2006;15:329–339. doi: 10.1111/j.1365-2583.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Roy J, Johnson EA. Identification and function of hypoxia-response genes in Drosophila melanogaster. Physiol Genomics. 2006;25:134–141. doi: 10.1152/physiolgenomics.00262.2005. [DOI] [PubMed] [Google Scholar]
- 17.De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apidianakis Y, Mindrinos MN, Xiao W, Lau GW, Baldini RL, et al. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc Natl Acad Sci USA. 2005;102:2573–2578. doi: 10.1073/pnas.0409588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, et al. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci USA. 2008;105:9367–9372. doi: 10.1073/pnas.0709578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Duffy JB. GAL4 system in Drosophila: a fly geneticist's swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 22.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann AA, Shirriffs J. Geographic variation for wing shape in Drosophila serrata. Evolution. 2002;56:1068–1073. doi: 10.1111/j.0014-3820.2002.tb01418.x. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann AA, Anderson A, Hallas R. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett. 2002;5:614–618. [Google Scholar]
- 26.Minois N, Le Bourg E. Resistance to stress as a function of age in Drosophila melanogaster living in hypergravity. Mech Ageing Dev. 1999;109:53–64. doi: 10.1016/s0047-6374(99)00025-1. [DOI] [PubMed] [Google Scholar]
- 27.Hosler JS, Burns JE, Esch HE. Flight muscle resting potential and species-specific differences in chill coma. J Insect Physiol. 2000;46:621–627. doi: 10.1016/s0022-1910(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 28.Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SA, et al. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci USA. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koštál V, Tollarová-Borovanská M. The 70 kDa heat shock protein assists during the repair of chilling injury in the insect, Pyrrhocoris apterus. PLoS ONE. 2009;4:e4546. doi: 10.1371/journal.pone.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin W, Neal SJ, Robertson RM, Westwood JT, Walker VK. Cold hardening and transcriptional change in Drosophila melanogaster. Insect Mol Biol. 2005;14:607–613. doi: 10.1111/j.1365-2583.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nature Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Kean L, Yang J, Allan A, Davies S, et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004;5:R69. doi: 10.1186/gb-2004-5-9-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 34.Syed ZA, Härd T, Uv A, van Dijk-Härd IF. A Potential Role for Drosophila Mucins in development and physiology. PLoS ONE. 2008;3:e3041. doi: 10.1371/journal.pone.0003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen LT, Nielsen MM, Loeschcke V. New candidate genes for heat resistance in Drosophila melanogaster are regulated by HSF. Cell Stress Chap. 2008;13:177–182. doi: 10.1007/s12192-008-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felton GW, Summers CD. Antioxidant systems in insects. Arch Insect Biochem Physiol. 1995;29:187–197. doi: 10.1002/arch.940290208. [DOI] [PubMed] [Google Scholar]
- 37.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, et al. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- 38.Rojas RR, Leopold RA. Chilling injury in the housefly: Evidence for the role of oxidative stress between pupariation and emergence. Cryobiology. 1996;33:447–458. [Google Scholar]
- 39.Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Ann Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- 40.Kostal V, Vambera J, Bastl J. On the nature of pre-freeze mortality in insects: water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. J Exp Biol. 2004;207:1509–1521. doi: 10.1242/jeb.00923. [DOI] [PubMed] [Google Scholar]
- 41.Kostal V, Yanagimoto M, Bastl J. Chilling-injury and disturbance of ion homeostasis in the coxal muscle of the tropical cockroach (Nauphoeta cinerea). Comp Biochem Physiol B Biochem Mol Biol. 2006;143:171–179. doi: 10.1016/j.cbpb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Kostal V, Renault D, Mehrabianova A, Bastl J. Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: role of ion homeostasis. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:231–238. doi: 10.1016/j.cbpa.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 43.Denlinger DL, Lee RE. Physiology of cold sensitivity. In: Hallman GJ, Denlinger DL, editors. Temperature sensitivity in insects. Application for integrated pest management. Boulder: Westview Press; 1998. pp. 55–95. [Google Scholar]
- 44.Lee JRE, Damodaran K, Yi S-X, Lorigan GA. Rapid cold-hardening increases membrane fluidity and cold tolerance of insect cells. Cryobiology. 2006;52:459–463. doi: 10.1016/j.cryobiol.2006.03.003. [DOI] [PubMed] [Google Scholar]