Abstract
Microtubules nucleated from γ-tubulin ring complexes located at the centrosome regulate the localization of organelles, promote vesicular transport and direct cell migration. Although several signaling mechanisms have been identified that regulate microtubule dynamics during interphase, signaling pathways that promote microtubule nucleation remain elusive. We assayed microtubule regrowth following nocodazole washout in human fibroblasts and CHO-K1 cells adhered to fibronectin in either normal serum-free medium or the serum-free, growth-promoting medium, CCM1, which contains IGF1 and androgen, as well as other nutrients. The results indicate that integrin-mediated adhesion is not sufficient to promote rapid microtubule regrowth in either cell type. The addition of androgen, but not IGF1, for 5 minutes was sufficient to promote rapid regrowth and this occurred by a mechanism requiring the androgen receptor. Since Src is a component of the cytoplasmic androgen-receptor-signaling complex, we examined its role using Src siRNA, the Src kinase inhibitor SU6656, and the expression of a constitutively active Src mutant. The data show that Src signaling is both required and sufficient to promote rapid microtubule regrowth in cells adhered to fibronectin. Measurement of the density of microtubules close to the centrosome and the rates of GFP-EB1-labeled microtubules emanating from the centrosome indicated that Src signaling promotes microtubule nucleation. Furthermore, recovery of GFP–γ-tubulin at the centrosome following photobleaching and measurements of endogenous γ-tubulin levels at the centrosome showed that androgen and Src signaling regulate the levels of centrosomal γ-tubulin. Thus, we propose that androgen and Src signaling regulate microtubule nucleation during interphase by promoting the centrosomal localization of γ-tubulin.
Keywords: Microtubules, γ-tubulin, Centrosome, Src, Androgen
Introduction
During interphase, microtubule assembly determines the subcellular localization of organelles, promotes vesicular transport and directs cell migration. The centrosome is an important organizer of microtubules (Doxsey et al., 2005). It comprises two centrioles, mother and daughter, embedded in the pericentriolar material (PCM). Microtubules are nucleated from γ-tubulin ring complexes (γ-TuRCs) present in the PCM (Luders and Stearns, 2007). γ-Tubulin is required for nucleation and associates with other proteins in the cytoplasm to form γ-TuRCs, which then become localized to the PCM (Luders and Stearns, 2007). It is known that the recruitment of γ-tubulin to the centrosome correlates with increased microtubule nucleation as cells enter mitosis, suggesting that this may be an important point of regulation (Khodjakov and Rieder, 1999; Piehl et al., 2004). However, signaling pathways that regulate the centrosomal recruitment of γ-TuRC or microtubule nucleation during interphase have not yet been identified.
Microtubules undergo regulated growth, shrinkage and stabilization in response to intracellular signals. This dynamic nature of microtubules allows cells to rapidly respond to changes in their extracellular environment. The Rho family of GTPases play central roles in controlling microtubule dynamics (Grigoriev et al., 2006). Rho and mDia, a member of the Diaphanous formin family, promote microtubule stabilization by changing the activity of the microtubule-binding proteins EB1 and APC (Palazzo et al., 2001; Palazzo et al., 2004; Wen et al., 2004). Conversely, Rac and Cdc42 promote microtubule growth, at least in part, by inhibiting the activity of the microtubule-destabilizing protein, stathmin (Daub et al., 2001; Wittmann et al., 2004). Interestingly, the regulation of microtubule dynamics has been shown to occur at the cell periphery, whereas microtubules show persistent growth near the centrosome (Komarova et al., 2002).
Accumulating evidence indicates that cell adhesion to the extracellular matrix can regulate centrosome function and microtubule dynamics (Palazzo et al., 2004; Reverte et al., 2006; LaFlamme et al., 2008). Our laboratory previously identified integrins as important regulators of the microtubule cytoskeleton during both interphase and mitosis (Reverte et al., 2006; LaFlamme et al., 2008). Here, we demonstrate that integrin signaling is not sufficient to promote rapid microtubule regrowth and that androgen and Src signaling promote γ-tubulin recruitment to the centrosome and microtubule nucleation.
Results
Integrin signaling is not sufficient to promote rapid microtubule regrowth from the centrosome
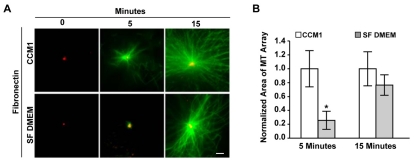
We previously showed that integrins play a major role in regulating the microtubule cytoskeleton, in part by promoting microtubule assembly from the centrosome (Reverte et al., 2006). To determine whether integrin-mediated adhesion is sufficient to promote microtubule assembly, we examined this process in primary human foreskin fibroblasts (HFFs) adhered to fibronectin in either serum-free DMEM or the serum-free, growth-promoting medium, CCM1. The ability of cells to polymerize microtubules from the centrosome was measured using a microtubule regrowth assay, in which microtubules were depolymerized with nocodazole and then allowed to regrow. The results indicate that integrin-mediated adhesion is not sufficient to promote rapid microtubule regrowth from the centrosome, as the size of microtubule arrays are significantly smaller 5 minutes post-nocodazole washout in cells adhered in serum-free DMEM compared with CCM1 (Fig. 1). The size of the microtubule array after 15 minutes of regrowth is similar in serum-free DMEM and CCM1 (Fig. 1). Thus, there is a delay in microtubule regrowth in serum-free DMEM. Others have suggested that a delay in regrowth in this type of assay is due to a defect in microtubule nucleation (Delgehyr et al., 2005).
Fig. 1.
Rapid microtubule regrowth in HFFs is inhibited in serum-free DMEM. (A) Representative images of microtubule regrowth in serum-starved HFFs replated onto fibronectin in CCM1 or serum-free (SF) DMEM, where α-tubulin staining is in green and γ-tubulin staining is in red. (B) Quantification of the area of microtubule regrowth. Plotted is the normalized mean area at 5 and 15 minutes post-nocodazole washout ± s.d. calculated from 150 cells per condition in each of eight independent experiments. *Regrowth in SF DMEM is statistically different from regrowth in CCM1 (P<0.05). Scale bar: 2 μm.
Androgen signaling promotes rapid microtubule regrowth
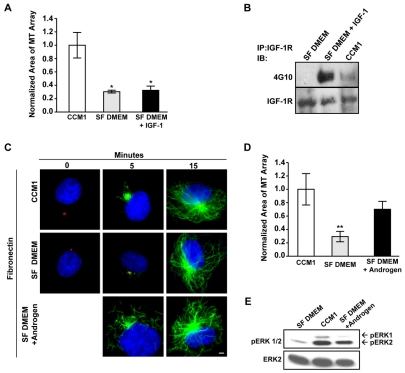
To identify the mechanisms promoting rapid microtubule regrowth, we examined the contribution from individual components of CCM1 not present in serum-free DMEM. CCM1 growth-promoting medium contains insulin-like growth factor-1 (IGF1), androgen, cortisol and transferrin: all of which could regulate microtubule regrowth. We first tested whether IGF1 is sufficient to promote rapid regrowth when added to serum-free DMEM, as previous reports illustrated a crosstalk between the IGF1 receptor (IGF1R) and β1 integrins (Goel et al., 2004). However, IGF1 is not sufficient (Fig. 2A) even though addition of IGF1 activates the IGF1 receptor (Fig. 2B).
Fig. 2.
Androgen promotes microtubule regrowth. (A,B) IGF1 is not sufficient to promote rapid microtubule regrowth. (A) Microtubule regrowth was compared in serum-starved HFFs replated onto fibronectin in CCM1, SF DMEM and in SF DMEM plus IGF1, with IGF1 only added for the regrowth period. Values are the normalized mean area of microtubule arrays at 5 minutes post-nocodazole washout ± s.d. from 150 cells per condition in each of three independent experiments. *Statistically significant different from the CCM1 control (P<0.05). (B) Treating serum-starved HFFs with SF DMEM plus IGF1 or CCM1 triggers the tyrosine phosphorylation of the IGF1 receptor. The IGF1 receptor was immunoprecipitated from cells treated with CCM1, SF DMEM or SF DMEM plus IGF1 and then analyzed by western blot with the phosphotyrosine-specific monoclonal antibody 4G10. Blots were reprobed with antibodies against the IGF1 receptor to control for protein loading. (C,D) Androgen promotes rapid microtubule regrowth. (C) Representative images comparing microtubule regrowth in serum-starved HFFs replated onto fibronectin in CCM1, SF DMEM or SF DMEM plus androgen, with androgen added only during regrowth period. α-Tubulin staining is in green, γ-tubulin staining is in red and DNA staining is in blue. (D) Quantification of the extent of microtubule regrowth 5 minutes post-nocodazole washout. Values are the normalized mean areas ± s.d. calculated from 150 cells per condition from three independent experiments. *Statistically significant difference from both the CCM1 control and SF DMEM plus androgen (P<0.05). (E) Androgen promotes ERK activation. ERK phosphorylation was analyzed by western blotting using an antibody specific for activated ERK. Blots were stripped and reprobed for total ERK as a loading control. Scale bar: 2 μm.
In contrast to IGF1, we found that androgen promotes microtubule regrowth to a similar extent as CCM1 5 minutes post-nocodazole washout (Fig. 2C,D), indicating that androgen is the main component of CCM1 that supports rapid regrowth. CCM1 and androgen appear to also increase the number of microtubules not associated with the centrosome; this is occasionally observed in serum-free DMEM. These microtubules might represent a population nucleated at the Golgi membranes or released from the centrosome (Chabin-Brion et al., 2001; Efimov et al., 2007; Abal et al., 2002). Although the regulation of these microtubules is also interesting, the current study concentrated on centrosomal microtubules by measuring regrowth focused at the centrosome.
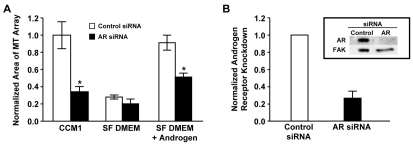
Since androgen regulates cellular processes by binding to its intracellular receptor, we analyzed the role of the androgen receptor in this process by inhibiting its expression with small interference RNA (siRNA). The results indicate that androgen receptor siRNA inhibits rapid microtubule regrowth in cells stimulated with either CCM1 or the addition of androgen to serum-free DMEM (Fig. 3 and supplementary material Fig. S1A). Similar results were obtained with three independent siRNA sequences indicating the results are not due to off target effects (supplementary material Fig. S1B,C). Thus, the androgen receptor is required for androgen to promote rapid microtubule regrowth.
Fig. 3.
Androgen promotes microtubule regrowth by a mechanism requiring the androgen receptor. (A) siRNA targeting the androgen receptor (AR) inhibited microtubule regrowth. HFFs were transfected with either control or AR siRNA, serum starved, and then replated onto fibronectin in either CCM1, SF DMEM or SF DMEM plus androgen, with androgen added only during the 5 minute regrowth period. Values are normalized mean areas ± s.d. calculated from 150 cells per condition in each of three independent experiments. *Values of AR siRNA-treated cells that are statistically different from those of the control siRNA-treated cells (P<0.05). (B) The inhibition of AR expression in each experiment was confirmed by western blotting. Values are AR protein levels ± s.d. in cells treated with control or AR siRNA from the three independent experiments shown in A. Inset: representative western blot.
Androgen promotes rapid microtubule regrowth by a mechanism requiring Src signaling
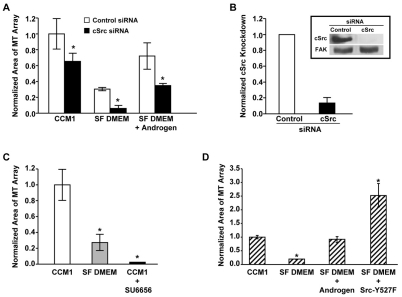
In addition to entering the nucleus to regulate transcription, androgen also activates cytoplasmic signaling cascades, including the activation of ERK, by forming a complex with Src (Baron et al., 2004; Migliaccio et al., 2000; Migliaccio et al., 2002). Since the addition of androgen for only 5 minutes is sufficient to promote rapid microtubule regrowth and to activate ERK (Fig. 2E), we were interested in investigating the contribution of Src signaling in regulating this process. To accomplish this, Src signaling was inhibited by two independent approaches: siRNA targeting Src and the Src-family kinase inhibitor SU6656. Inhibiting the expression of Src by siRNA suppresses rapid microtubule regrowth in CCM1 and in androgen-supplemented serum-free DMEM (Fig. 4A,B and supplementary material Fig. S2A). Inhibiting Src-family kinases with SU6656 suppresses microtubule regrowth even more dramatically (Fig. 4C and supplementary material Fig. S2B). Thus, signaling by Src-family kinases is required for androgen to promote rapid microtubule regrowth.
Fig. 4.
Androgen promotes microtubule regrowth through a mechanism requiring Src-family kinases. (A,B) siRNA targeting Src inhibited microtubule regrowth. HFFs transfected with control or Src siRNA were serum starved and replated onto fibronectin in CCM1, SF DMEM or SF DMEM plus androgen, with androgen added only during the regrowth period. The extent of microtubule regrowth was measured 5 minutes post-nocodazole washout. Values are the normalized mean area ± s.d. calculated from 150 cells per condition in each of three independent experiments. *Values for Src siRNA-treated cells that are statistically different from those of control siRNA-treated cells (P<0.05). (B) Quantification of Src protein levels in cells treated with either control or Src siRNA. Data is from the three independent experiments in A. Inset: representative western blot. (C) The Src kinase inhibitor SU6656 suppresses microtubule regrowth at 5 minutes post-nocodazole washout. HFFs were serum starved and replated onto fibronectin in either CCM1 or SF DMEM. SU6656 was added to CCM1 during the last half hour of nocodazole treatment and in all solutions thereafter. Values are the normalized mean areas ± s.d. calculated from 150 cells per condition in each of three independent experiments. (D) Expression of the constitutively active Src-Y527F mutant promotes microtubule regrowth (for control see supplementary material Fig. S2C). Transfected HFFs were serum-starved and replated onto fibronectin in either CCM1 or SF DMEM and analyzed for regrowth. Values are the normalized mean area ± s.d. calculated from 150 cells per condition in each of three independent experiments. Asterisks in C and D indicate a statistically significant difference from the CCM1 control (P<0.05).
We also asked whether activating Src is sufficient to promote regrowth in cells adhered to fibronectin in serum-free DMEM. Src signaling was activated by expressing a constitutively active Src mutant, containing a tyrosine to phenylalanine substitution at the regulatory tyrosine residue (Hirai and Varmus, 1990) (supplementary material Fig. S2C). We found that the expression of the Src-Y527F mutant is sufficient to promote regrowth in cells plated in serum-free DMEM in the absence of androgen (Fig. 4D and supplementary material Fig. S2D). Together, these data demonstrate the importance of Src signaling in promoting rapid microtubule regrowth.
Microtubule nucleation is promoted by androgen and Src signaling
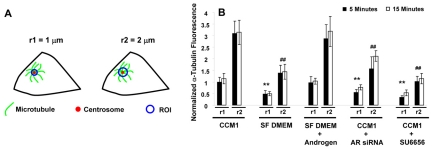
The extent of microtubule regrowth at 5 minutes post nocodazole washout could be affected by mechanisms regulating microtubule nucleation or microtubule dynamics. Since delays in microtubule regrowth have previously been associated with defects in microtubule nucleation (Delgehyr et al., 2005), we focused our experiments on the contribution of microtubule nucleation using two independent approaches. We compared microtubule density close to the centrosome in regrowth assays and the number of new microtubules emanating from the centrosome at steady state.
Since microtubule dynamics are regulated at the cell periphery (Komarova et al., 2002), differences in microtubule density very close to the centrosome should reflect differences in microtubule nucleation. We assayed the density of microtubules by measuring the fluorescence intensity of the α-tubulin signal in concentric circles (radii of 1 and 2 μm) centered at the centrosome (Fig. 5A). The results show that the intensity of the α-tubulin signal at the centrosome is significantly decreased in cells adhered in serum-free DMEM compared with CCM1 (Fig. 5B). Additionally, inhibiting the expression of the androgen receptor by siRNA or the activity of Src family kinases with SU6656 significantly decreased the intensity of the α-tubulin signal compared with CCM1 or androgen-supplemented serum-free DMEM (Fig. 5B). Furthermore, the fluorescence intensity of the α-tubulin signal at the centrosome does not significantly change within each condition between 5 and 15 minutes post-nocodazole washout (Fig. 5B), indicating that the effect on microtubule nucleation does not change within this time frame.
Fig. 5.
Microtubule nucleation is promoted by androgen and Src signaling in microtubule regrowth assays. (A) A schematic representation of the experimental design. The fluorescence intensity of the α-tubulin signal was measured in concentric circles centered on the centrosome with radii (r) of 1 and 2 μm shown here as ‘regions of interest’ or ROIs. (B) Quantification of the microtubule density at the centrosome in regrowth assays performed under the indicated experimental conditions. Values are the normalized mean fluorescence intensities of the α-tubulin signal at 5 and 15 minutes post-nocodazole washout ± s.d. calculated from 50 cells per condition in each of three independent experiments. In these experiments, measurements were normalized to the CCM1 control (radius of 1 μm). *A statistically significant difference from both the CCM1 control and SF DMEM plus androgen using a ROI with a radius of 1 μm at both 5 and 15 minutes; #a statistically significant difference using a ROI with a radius of 2 μm (P<0.05). As a point of reference, the average area of regrowth at 5 minutes is 30.2±7.9 μm2 in CCM1 and 7.7±3.9 μm2 in SF DMEM and at 15 minutes the average area is 311.8±76.4 μm2 in CCM1 and 238.5±46.1 μm2 in SF DMEM.
Together, these data suggest that androgen and Src signaling promote microtubule nucleation. Additionally, the intensity of the α-tubulin signal increases proportionally as the distance from the centrosome increases from 1 to 2 μm in each condition, as microtubules have polymerized beyond this point at the times assayed.
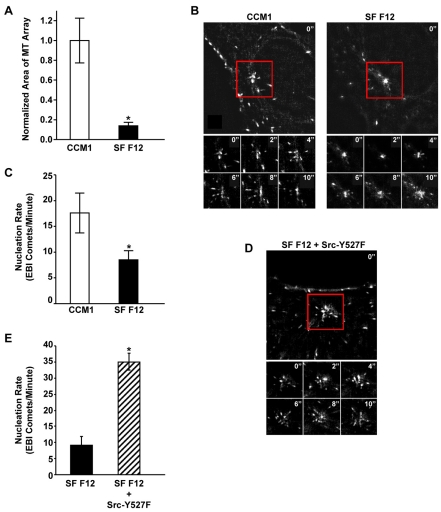
As a second approach, we counted the number of new microtubules emanating from the centrosome under steady state conditions using the microtubule end-binding protein 1 (EB1) to label the plus end of growing microtubules (Akhmanova and Steinmetz, 2008). The number of microtubule plus ends (EB1 comets) leaving the centrosome per unit time has been used to measure nucleation events in real time (Piehl et al., 2004). After confirming that microtubule regrowth is similarly regulated in CHO-K1 cells and human fibroblasts (Fig. 6A), we used CHO-K1 cells stably expressing low levels of GFP-EB1 to demonstrate that the differences in rapid microtubule regrowth described above reflect altered rates in microtubule nucleation at steady state. Using live-cell imaging techniques, we demonstrated that cells adhered to fibronectin in CCM1 have an average nucleation rate of 17.6±3.9 comets/minute, whereas cells adhered in serum-free Ham's F12 have an average nucleation rate of only 8.5±1.8 comets/minute (Fig. 6B,C). Interestingly, both the fluorescence intensity of the α-tubulin signal at the centrosome and the rate at which EB1 comets emanate from the centrosome in CHO-K1 cells are similar in CCM1 and serum-containing media (supplementary material Fig. S3) (Salaycik et al., 2005). Importantly, expression of the constitutively active Src mutant, Src-Y527F, in serum-free Ham's F12 dramatically increases the average nucleation rate to 35.0±3.0 EB1 comets/minute (Fig. 6D,E), confirming the significance of Src signaling in regulating microtubule nucleation.
Fig. 6.
Live-cell imaging of microtubule nucleation promoted by CCM1 and Src signaling. (A) CCM1 promotes rapid microtubule regrowth in CHO-K1 cells. Mitotic cells were collected and replated onto fibronectin in either CCM1 or SF Ham's F12. Values are the normalized mean area of microtubule arrays 5 minutes post-nocodazole washout from 100 cells per condition in three independent experiments. *A statistically significant difference from the CCM1 control (P<0.05). (B-E) Time-lapse imaging was used to track newly nucleated microtubules in CHO-K1 cells stably expressing GFP-EB1. (B) Micrographs comparing EB1 comets emanating from the centrosome of representative cells replated on fibronectin in either CCM1 or SF Ham's F12. Also shown are montages of six images taken every two seconds over a 10 second period for each representative cell. (C) The average rate ± s.d. of newly nucleated microtubules calculated from ten cells per condition in three independent experiments. *A statistically significant difference from the CCM1 control (P<0.05). (D,E) Expression of the constitutively active Src-Y527F mutant promotes microtubule nucleation. (D) Micrograph of EB1 comets emanating from the centrosome of a representative Src-Y527F-transfected cell replated onto fibronectin in SF Ham's F12. Also shown is a montage of six images taken every 2 seconds over a 10 second period. (E) The average rate ± s.d. of newly nucleated microtubules from 10 cells in three independent experiments. *A statistically significant difference from the SF Ham's F12 control (P<0.05).
Regulation of γ-tubulin recruitment to the centrosome
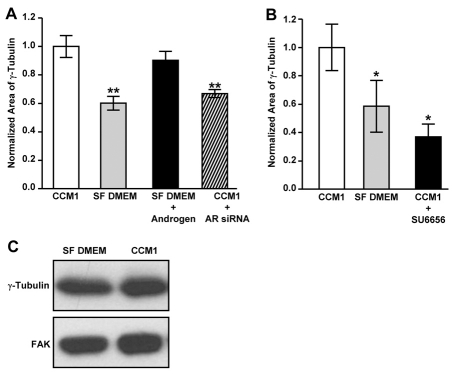
Since microtubule nucleation at the centrosome occurs from γ-tubulin ring complexes (γ-TuRC) located in the pericentriolar material (Luders and Stearns, 2007), we asked whether androgen and Src signaling promote microtubule nucleation by regulating centrosomal γ-tubulin levels. We analyzed centrosomal γ-tubulin HFFs adhered in serum-free DMEM with and without androgen and CCM1 with and without androgen receptor siRNA by measuring the area of γ-tubulin antibody staining in fixed cells (Fig. 7A). The results indicate that androgen stimulation promotes γ-tubulin accumulation at the centrosome, whereas downregulating androgen signaling with androgen receptor siRNA inhibits this process (Fig. 7A). Additionally, inhibiting Src activity with SU6656 also suppresses the centrosomal accumulation of γ-tubulin (Fig. 7B). Importantly, centrosomal levels of γ-tubulin are similarly regulated in CHO-K1 cells (supplementary material Fig. S4A). Furthermore, γ-tubulin protein levels are similar in cells plated in either serum-free DMEM, serum-free Ham's F12 or CCM1 (Fig. 7C and supplementary material Fig. S4B). Thus, our data suggest that androgen and Src signaling regulate microtubule nucleation by promoting the accumulation of γ-tubulin at the centrosome.
Fig. 7.
Androgen and Src signaling promote γ-tubulin accumulation at the centrosome. (A) Androgen signaling promotes γ-tubulin accumulation at the centrosome. HFFs treated with either control or AR siRNA were serum starved and replated onto fibronectin as indicated. The area of γ-tubulin staining at the centrosome was quantified from regrowth assays. (B) The Src kinase inhibitor, SU6656, inhibits γ-tubulin accumulation at the centrosome. The area of γ-tubulin staining at the centrosome was quantified from regrowth assays. (A,B) The mean values ± s.d. calculated from 75 cells per condition in each of three independent experiments. *A statistically significant difference from the CCM1 control (P<0.05). (C) Western blot analysis of γ-tubulin protein levels in cells replated onto fibronectin for 2 hours in either CCM1 or SF DMEM.
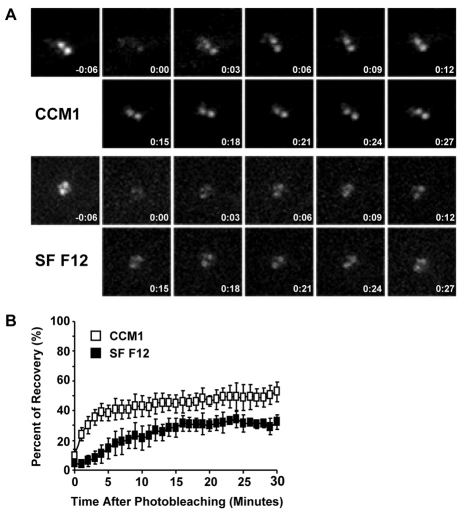
Previous studies have demonstrated that centrosomal γ-tubulin is in dynamic exchange with a cytoplasmic pool and that there are two populations of γ-tubulin at the centrosome: one that is rapidly turned over and another that is more stably bound to the centrosome, presumably associated with the centrioles (Khodjakov and Rieder, 1999). Thus, it seemed possible that the decreased levels of γ-tubulin at the centrosome could be due to a decrease in recruitment of γ-tubulin from the rapidly turned over pool. Therefore, as an independent approach to examine the regulation of γ-tubulin recruitment to the centrosome, we measured fluorescence recovery of GFP–γ-tubulin at the centrosome after photobleaching (FRAP). For these studies, we used CHO-K1 cells stably expressing GFP–γ-tubulin (Khodjakov et al., 2002) and assayed both the initial recovery rate and the percentage of total recovery under different experimental conditions. Consistent with published studies (Khodjakov and Rieder, 1999), we observed a population of γ-tubulin that is rapidly turned over and another that is more stably bound to the centrosome (Fig. 8A,B). Cells adhered in CCM1 recover on average 52±6% of their original signal after 30 minutes, whereas cells adhered in serum-free Ham's F12 recover only 32±5% on average (Fig. 8B). Differences are even greater at 5 minutes: recovery of 38±5% on average for cells adhered in CCM1 and only 15±7% on average for cells adhered in serum-free Ham's F12 (Fig. 8B). The differences between cells adhered in CCM1 and serum-free Ham's F12 are statistically significant at both the 5 and 30 minute time points. Additionally, photobleaching had no effect on cell viability (supplementary material Fig. S5). Together these data suggest that androgen and Src signaling regulate the pool of γ-tubulin that is rapidly turned over at the centrosome.
Fig. 8.
γ-Tubulin turnover at the centrosome is promoted by CCM1. (A,B) FRAP analysis using CHO-K1 cells stably expressing GFP-γ-tubulin. Mitotic cells were collected and replated onto fibronectin in either CCM1 or SF F12. (A) Representative images of pre-bleached and bleached centrosomes for each condition together with images acquired every 3 minutes post-photobleaching. (B) Quantification of the recovery of centrosomal GFP intensity. Values are the mean intensities ± s.d. from five cells per condition in three independent experiments.
Discussion
The assembly of a functional microtubule cytoskeleton is a fundamental cellular process involving microtubule nucleation, anchorage and regulated growth from the centrosome. In this study, we identified androgen and Src signaling as important regulators of nucleation. We also demonstrated that these signaling pathways promote increased levels of γ-tubulin at the centrosome. Thus, we propose that androgen and Src signaling lead to increased microtubule nucleation by promoting the recruitment of γ-tubulin to the centrosome.
Androgen is known to trigger the formation of cytoplasmic signaling complexes containing the androgen receptor and Src-family kinases; the formation of these complexes leads to the activation of Src and other signaling proteins, including ERK and phosphoinositide 3-kinase (Baron et al., 2004; Migliaccio et al., 2000; Sun et al., 2003). Consistent with this mechanism, our data demonstrate that Src and androgen promote γ-tubulin recruitment to the centrosome and microtubule nucleation. We have not formally ruled out the possibility that androgen regulates these processes by activating specific transcriptional programs. However, the effect of androgen is fairly rapid in our assays and occurs within a 5-minute window, suggesting that it regulates microtubule nucleation independent of its transcriptional activity. Microtubule nucleation has also been described to occur at the Golgi complex (Chabin-Brion et al., 2001; Efimov et al., 2007; Miller at al., 2009). It will be interesting to determine whether androgen and Src similarly regulate microtubule nucleation at this site as well.
Other steroid hormones can similarly activate cytoplasmic signaling pathways (Kousteni et al., 2001; Migliaccio et al., 2002) and may regulate microtubule nucleation in other cellular contexts. Additionally, several cell surface receptors, including receptor tyrosine kinases can activate Src signaling and may also promote rapid microtubule nucleation; however, neither the activation of the IGF1 receptor nor integrin engagement was sufficient in our system. Interestingly, cells adhered in serum-containing medium promote microtubule nucleation to similar levels as observed in CCM1; the contributions of androgen and Src signaling in this context are not yet known.
Previous studies have shown that centrosomal levels of γ-tubulin remain relatively constant throughout interphase, but increase dramatically at the onset of mitosis and return to interphase levels as cells exit mitosis (Khodjakov and Rieder, 1999). FRAP analysis of GFP-γ-tubulin indicated that the centrosome contains two populations of γ-tubulin: one that turns over rapidly and another that is more tightly bound (Khodjakov and Rieder, 1999). The dynamic exchange of centrosome-associated γ-tubulin occurs throughout the cell cycle by a microtubule-independent mechanism (Khodjakov and Rieder, 1999). We show that the recruitment of γ-tubulin at the centrosome during interphase can be regulated by androgen and Src signaling. Our data are consistent with a model in which these signaling pathways promote the recruitment of cytoplasmic γ-tubulin to the centrosome and that this ‘charging’ of the centrosome is required for rapid microtubule nucleation. Future studies will determine whether similar pathways regulate enhanced centrosomal recruitment of γ-tubulin at the onset of mitosis, as Src-family kinases are also active at this time in the cell cycle (Roche et al., 1995).
Src could regulate the recruitment of γ-tubulin to the centrosome by several mechanisms. Src may directly promote the assembly of γ-TuRCs and/or the recruitment of γ-TuRCs to the centrosome. γ-Tubulin can form soluble complexes with Src-family kinases and several tyrosine phosphorylated proteins in a Src kinase-dependent manner (Draberova et al., 1999; Kukharskyy et al., 2004; Sulimenko et al., 2006). Thus, Src may phosphorylate a member of the γ-TuRC to promote the assembly of the complex, or may regulate the association or activity of NEDD1/GCP-WD, a protein that is required for the centrosomal recruitment of γ-TuRC (Luders et al., 2006; Haren et al., 2006). Alternatively, Src may indirectly affect this process by regulating the assembly of the PCM or the activity of a centrosomal protein required for the anchorage of γ-TuRC. The identification of Src substrates that promote the recruitment of γ-tubulin to the centrosome and microtubule nucleation will provide important insight into the mechanisms involved.
Cells integrate information from multiple extracellular stimuli to regulate a variety of cellular processes. Current evidence indicates that close collaboration between integrins and other cell surface receptors is important for cells to respond to their extracellular environment (Schwartz and Baron, 1999; Yamada and Even-Ram, 2002). Our previous studies demonstrated that a mutation in the integrin-β subunit cytoplasmic domain inhibits rapid microtubule regrowth in CCM1 (Reverte et al., 2006). Thus, it will be important to determine whether integrins regulate the ability of the androgen receptor and Src to promote γ-tubulin recruitment to the centrosome and microtubule nucleation, or whether integrin and androgen receptors act independently to regulate these processes.
Materials and Methods
Cell culture
Human foreskin fibroblasts (HFFs) from Vec Technologies were cultured in Dulbecco's modiefied Eagle's medium (DMEM) supplemented with 10% FBS, 1% L-glutamine and 1% penicillin-streptomycin (complete DMEM). Chinese hamster ovary (CHO-K1) cells (ATCC) were cultured in Ham's F12 medium supplemented with 10% FBS, 1% L-glutamine and 1% penicillin-streptomycin (complete Ham's F12). Experiments were performed in CCM1 (Hyclone) or serum-free DMEM or serum-free Ham's F12 as indicated. CHO-K1 cells stably expressing GFP–γ-tubulin have been described previously (Khodjakov et al., 2002).
Generation of GFP-EB1-expressing cells
CHO-K1 cells stably expressing EB1-GFP were generated using the Lentiviral Gateway expression system (Invitrogen). EB1-GFP from the pEGFP-N1/EB1 plasmid (Piehl and Cassimeris, 2003) was amplified by PCR with the forward primer 5′-CACCATGGCAGTGAACGTATAC-3′ and reverse primer 5′-TTACTTGTACAGCTCGTCCATGCC-3′ and then inserted into the pENTR/D-TOPO gateway entry vector (Invitrogen). EB1-GFP was then transferred to pLenti6/V5-DEST (Invitrogen) by site-specific recombination as described by the supplier. Successful generation of pLenti6/V5-DEST–EB1-GFP was confirmed by DNA sequence analysis. A lentiviral stock was then produced by co-transfection of the 293FT cell line with the expression construct and the ViraPower packaging mix (Invitrogen) and used to transduce CHO-K1 cells. Cells expressing appropriate levels of GFP-EB1 were isolated by fluorescence-activated cell sorting using the BD-FACS Aria high-speed cell sorter.
Microtubule regrowth
Similar passages of HFFs were serum starved for 24 hours to synchronize cells in G0 and they were then replated onto fibronectin-coated coverslips (see Reverte et al., 2006) in either CCM1, serum-free DMEM or complete DMEM for 2 hours at 37°C. To isolate synchronized CHO-K1 cells, they were grown to ~80% confluence in complete Ham's F12; mitotic cells were then knocked off and replated onto fibronectin-coated coverslips in either CCM1 or serum-free Ham's F12 for 3 hours at 37°C. Cells were then treated at 4°C with 10 μg/ml nocodozole (Calbiochem) for 2 hours (HFFs) or 4 hours (CHO-K1 cells) to depolymerize microtubules. Cells were washed with cold PBS to remove the drug and microtubule regrowth was allowed for 0, 5 and 15 minutes in CCM1 or serum-free DMEM or Ham's F12. Some experiments included the addition of 13 nM IGF1 (Calbiochem) or 10 nM androgen, R1881 (Perkin Elmer) to serum-free DMEM during the regrowth period. Where indicated, 10 μM SU6656 (Calbiochem) was added during the last half hour of nocodazole treatment and to all solutions thereafter. For some experiments, cells were transfected with specific siRNAs or plasmids as described below.
Immunofluorescence microscopy
To visualize microtubules, cells were permeabilized for 30 seconds in 80 mM Pipes, pH 6.8, 5 mM EGTA, pH 8.0, 1 mM MgCl2 and 0.5% Triton X-100, fixed for 10 minutes in the same buffer containing 5% glutaraldehyde, and then incubated for 7 minutes in 1% sodium borohydrate in PBS. Cells were then stained with antibodies against α-tubulin (DM1α; Sigma-Aldrich), γ-tubulin (AK-15; Sigma-Aldrich) and DNA (Hoescht33342; Invitrogen) as previously described (Reverte et al., 2006). Alexa-Flour-488- and Alexa-Flour-594-conjugated secondary antibodies were from Invitrogen. Samples were analyzed using an inverted Nikon TE2000-E microscope equipped with phase contrast and epifluorescence, a digital camera (CoolSNAP HQ; Roper Scientific), a Ludl rotary encoded stage, 37°C incubator, and MetaVue (Molecular Devices) and AutoQuant deconvolution software (AutoQuant Imaging, Inc.). Images were taken on the best centrosomal plane. Exposure time was set and kept constant throughout each independent experiment so that the fluorescent signal from the brightest microtubule array was not overexposed. The perimeter of individual microtubule arrays was outlined and its area calculated. The α-tubulin fluorescent signal near the centrosome was measured in two separate concentric circles centered at the centrosome with radii of 1 and 2 μm. Background fluorescence using circles of corresponding sizes was subtracted from each measurement. The size of γ-tubulin staining was quantified by measuring the area encompassing the fluorescent signal. These different measurements were all made using MetaVue software.
siRNA transfection
HFFs were plated at ~10% confluency in 100 mm dishes for 18 hours in complete DMEM. The cells were then treated with 20 μM siRNA directed against lacZ as a control (control siRNA, Dharmacon), the androgen receptor (AR; Sigma-Aldrich; AR no. 1, 5′-CACCTTTGTGGCCCTCTAT-3′, AR no. 2, 5′-CAGGAATTCCTGTGCATGA-3′, AR no. 3, 5′-CAGAGATCATCTCTGTGCA-3′) or Src (Dharmacon; 5′-TGCCAAATTCCCCATCAAG-3′) using Oligofectamine (Invitrogen). Unless otherwise noted, AR no. 1 siRNA was used. After 3 days, cells were serum starved for 18 hours. One aliquot of cells was replated for microtubule regrowth analysis as described above; a second was lysed with modified RIPA or 2% SDS buffer and the inhibition of AR or Src expression was confirmed by western blotting using antibodies against the androgen receptor (Sigma-Aldrich) or Src (Upstate). Blots were stripped are reprobed for FAK (C-20; Santa Cruz Biotechnology) as a loading control.
Transient transfection
HFFs were plated to ~90% confluency in 60 mm dishes for 18 hours in DMEM supplemented with 10% FBS in the absence of antibiotics. The cells were then transfected using Lipofectamine (Invitrogen) with the following expression vectors: 8 μg GFP-centrin with or without 8 μg Src-Y527F. GFP-centrin was used to identify transfected cells. Four hours after transfection, cells were serum starved for 18 hours and replated to analyze microtubule regrowth as described above. For microtubule nucleation experiments, CHO-K1 cells stably expressing GFP-EB1 were transiently transfected with the pmCherry-1 expression vector (Clontech) with or without the expression vector Src-Y527F using a TransIT CHO transfection kit (Mirus).
Immunoprecipitation and western blotting
To assay IGF1R phosphorylation, cells were lysed with modified RIPA buffer and the IGF1R was immunoprecipitated with antibodies against IGF1Rβ (Cell Signaling) and then analyzed by western blotting with antibodies against tyrosine phosphorylation (4G10, Upstate). Blots were stripped and reprobed with antibodies against IGF1R-β as a loading control. To analyze ERK activation, cells were lysed with 2% SDS buffer and assayed using an antibody against phospho-ERK1/2 (E10; Cell Signaling). Blots were stripped and reprobed for ERK2 (C-14; Santa Cruz Biotechnology) as a loading control. To analyze γ-tubulin levels, cells were lysed with modified RIPA buffer and assayed by western blotting using antibodies against γ-tubulin (AK-15; Sigma-Aldrich). Blots were stripped are reprobed for FAK (C-20; Santa Cruz Biotechnology) as a loading control.
Microtubule nucleation visualized by live-cell imaging
Similar passages of CHO-K1 cells stably expressing GFP-EB1 (described above) were grown to ~80% confluence in complete Ham's F12. Mitotic cells were then knocked off and replated onto glass-bottom tissue culture plates (MatTek Corporation) coated with 20 μg/ml fibronectin in either CCM1 or serum-free Ham's F12 supplemented with 10 mM HEPES, pH 7.4 at 37°C. After 3 hours plates were transferred to a microscope equipped with a heated (37°C) chamber, and GFP images were recorded every 2 seconds for a total of 30 seconds. Images were then compiled, deconvolved and analyzed using ImageJ software. Where indicated, cells were transiently transfected with pmCherry-1 with or without the Src-Y527F expression vector.
Fluorescent recovery after photo-bleaching (FRAP)
Similar passages of CHO-K1 cells stably expressing GFP-γ-tubulin (Khodjakov et al., 2002) were grown to ~80% confluence in complete Ham's F12. Mitotic cells were then knocked off and replated onto 20 μg/ml fibronectin for 3 hours at 37°C in either CCM1 or serum-free Ham's F12 supplemented with 10 mM HEPES, pH 7.4. Coverslips were mounted in Rose chambers and transferred to a confocal microscope equipped with a heated (37°C) chamber for imaging. Photobleaching experiments were conducted on a custom-modified Nikon TE2000-E2 microscope equipped with an electronically controlled X-Y and fast piezo Z-positioner (LUDL). A collimated continuous-wave 488 nm laser beam was steered to the back aperture of the 100× 1.4 NA PlanApoVC objective lens to create a diffraction-limited illuminated spot in the center of the field of view. A more detailed description of the system is available in Magidson et al. (Magidson et al., 2007). Centrosomes were photobleached with four consecutive 300 msecond pulses (1.2 seconds total illumination time). The GFP signal was measured in maximal intensity projections of complete Z-series (4 μm range at 0.25 μm steps) recorded every minute. Photobleaching did not affect cell viability, as cells that were photobleached during G2 were able to complete the cell cycle (supplementary material Fig. S3).
Statistical analysis
Experiments were analyzed by measuring the mean area of the microtubule array, the fluorescence intensity of sub-regions of the array or the area and intensity of γ-tubulin staining at the centrosome as indicated in the figure legends. For each experiment, the mean value of each condition was normalized to the mean value from cells plated in CCM1 as a way to control for independent variation between experiments. The mean normalized values ± s.d. are presented in each graph. Student's t-test was performed when only the CCM1 and serum-free medium conditions were being compared. In all other instances, a test of equal variance, followed by either a 1-way or 2-way ANOVA was performed and comparisons were made using Fisher's LSD test. All P-values <0.05 were deemed as being statistically significant as indicated by an asterisk. All analysis was performed using MiniTab 15 Statistical Software.
Supplementary Material
Acknowledgments
The authors thank Lynne Cassimeris for the GFP-EB1 plasmid, Joan Brugge for the Src expression vector, Jadranka Loncarek for assistance with the FRAP analysis, Bethsaida Nieves for critically reading this manuscript, Debbie Moran for help with the preparation of the figures, and Chang Lim in the flow cytometry core at the Ordway Institute. This work was supported by National Institutes of Health grants GM51540 (S.E.L.) and GM59363 (A.K.). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/12/2094/DC1
References
- Abal M., Piel M., Bouckson-Castaing V., Mogensen M., Sibarita J., Bornens M. (2002). Microtubule release from the centrosome in migrating cells. J. Cell Biol. 159, 731-737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A., Steinmetz M. O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309-322 [DOI] [PubMed] [Google Scholar]
- Baron S., Manin M., Beaudoin C., Leotoing L., Communal Y., Veyssiere G., Morel L. (2004). Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J. Biol. Chem. 279, 14579-14586 [DOI] [PubMed] [Google Scholar]
- Chabin-Brion K., Marceiller J., Perez F., Settegrana C., Drechou A., Durand G., Pous C. (2001). The Golgi complex is a microtubule-organizing organelle. Mol. Biol. Cell 12, 2047-2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H., Gevaert K., Vandekerckhove J., Sobel A., Hall A. (2001). Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J. Biol. Chem. 276, 1677-1680 [DOI] [PubMed] [Google Scholar]
- Delgehyr N., Sillibourne J., Bornens M. (2005). Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118, 1565-1575 [DOI] [PubMed] [Google Scholar]
- Doxsey S., McCollum D., Theurkauf W. (2005). Centrosomes in cellular regulation. Annu. Rev. Cell Dev. Biol. 21, 411-434 [DOI] [PubMed] [Google Scholar]
- Dráberová L., Dráberová E., Surviladze Z., Dráber P., Dráber P. (1999). Protein tyrosine kinase p53/p56(lyn) forms complexes with gamma-tubulin in rat basophilic leukemia cells. Int. Immunol. 11, 1829-1839 [DOI] [PubMed] [Google Scholar]
- Efimov A., Kharitonov A., Efimova N., Loncarek J., Miller P. M., Andreyeva N., Gleeson P., Galjart N., Maia A. R. R., McLeod I. X., et al. (2007). Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-golgi network. Dev. Cell. 12, 917-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel H. L., Fornaro M., Moro L., Teider N., Rhim J. S., King M., Languino L. R. (2004). Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by beta1 integrins. J. Cell Biol. 166, 407-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I., Borisy G., Vorobjev I. (2006). Regulation of microtubule dynamics in 3T3 fibroblasts by Rho family GTPases. Cell Motil. Cytoskeleton 63, 29-40 [DOI] [PubMed] [Google Scholar]
- Haren L., Remy M. H., Bazin I., Callebaut I., Wright M., Merdes A. (2006). NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172, 505-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. (1990). Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol. Cell. Biol. 10, 1307-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C. L. (1999). The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146, 585-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C. L., Sluder G., Cassels G., Sibon O., Wang C. L. (2002). De novo formation of centrosomes in vertebrate cells arrested during S phase. J. Cell Biol. 158, 1171-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y. A., Vorobjev I. A., Borisy G. G. (2002). Life cycle of MTs: persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary. J. Cell Sci. 115, 3527-3539 [DOI] [PubMed] [Google Scholar]
- Kousteni S., Bellido T., Plotkin L. I., O'Brien C. A., Bodenner D. L., Han L., Han K., DiGregorio G. B., Katzenellenbogen J. A., Katzenellenbogen B. S., et al. (2001). Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719-730 [PubMed] [Google Scholar]
- Kukharskyy V., Sulimenko V., Macurek L., Sulimenko T., Draberova E., Draber P. (2004). Complexes of gamma-tubulin with nonreceptor protein tyrosine kinases Src and Fyn in differentiating P19 embryonal carcinoma cells. Exp. Cell Res. 298, 218-228 [DOI] [PubMed] [Google Scholar]
- LaFlamme S. E., Nieves B., Colello D., Reverte C. G. (2008). Integrins as regulators of the mitotic machinery. Curr. Opin. Cell Biol. 20, 576-582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders J., Stearns T. (2007). Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161-167 [DOI] [PubMed] [Google Scholar]
- Luders J., Patel U. K., Stearns T. (2006). GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137-147 [DOI] [PubMed] [Google Scholar]
- Magidson V., Loncarek J., Hergert P., Rieder C. L., Khodjakov A. (2007). Laser microsurgery in the GFP era: A cell biologist's perspective. Methods Cell Biol. 82, 239-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A., Castoria G., Di Domenico M., de Falco A., Bilancio A., Lombardi M., Barone M. V., Ametrano D., Zannini M. S., Abbondanza C., et al. (2000). Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 19, 5406-5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A., Castoria G., Di Domenico M., de Falco A., Bilancio A., Lombardi M., Bottero D., Varricchio L., Nanayakkara M., Rotondi A., et al. (2002). Sex steroid hormones act as growth factors. J. Steroid Biochem. Mol. Biol. 83, 31-35 [DOI] [PubMed] [Google Scholar]
- Miller P. M., Folkmann A. W., Maia A. R. R., Efimova N., Efimov A., Kaverina I. (2009). Golgi-derived CLASP-dependent microtubules control golgi organization and polarized trafficking in motile cells. Nat. Cell Biol. 11, 1069-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A. F., Cook T. A., Alberts A. S., Gundersen G. G. (2001). mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3, 723-729 [DOI] [PubMed] [Google Scholar]
- Palazzo A. F., Eng C. H., Schlaepfer D. D., Marcantonio E. E., Gundersen G. G. (2004). Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303, 836-839 [DOI] [PubMed] [Google Scholar]
- Piehl M., Cassimeris L. (2003). Organization and dynamics of growing microtubule plus ends during early mitosis. Mol. Biol. Cell 14, 916-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl M., Tulu U. S., Wadsworth P., Cassimeris L. (2004). Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. Proc. Natl. Acad. Sci. USA 101, 1584-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverte C. G., Benware A., Jones C. W., LaFlamme S. E. (2006). Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 174, 491-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche S., Fumagalli S., Courtneidge S. A. (1995). Requirement for Src family protein tyrosine kinases in G2 for fibroblast cell division. Science 269, 1567-1569 [DOI] [PubMed] [Google Scholar]
- Salaycik K. J., Fagerstrom C. J., Murthy K., Tulu U. S., Wadsworth P. (2005). Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. J. Cell Sci. 118, 4113-4122 [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Baron V. (1999). Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11, 197-202 [DOI] [PubMed] [Google Scholar]
- Sulimenko V., Draberova E., Sulimenko T., Macurek L., Richterova V., Draber P., Draber P. (2006). Regulation of microtubule formation in activated mast cells by complexes of gamma-tubulin with Fyn and Syk kinases. J. Immunol. 176, 7243-7253 [DOI] [PubMed] [Google Scholar]
- Sun M., Yang L., Feldman R. I., Sun X. M., Bhalla K. N., Jove R., Nicosia S. V., Cheng J. Q. (2003). Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J. Biol. Chem. 278, 42992-3000 [DOI] [PubMed] [Google Scholar]
- Wen Y., Eng C. H., Schmoranzer J., Cabrera-Poch N., Morris E. J., Chen M., Wallar B. J., Alberts A. S., Gundersen G. G. (2004). EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6, 820-830 [DOI] [PubMed] [Google Scholar]
- Wittmann T., Bokoch G. M., Waterman-Storer C. M. (2004). Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J. Biol. Chem. 279, 6196-6203 [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Even-Ram S. (2002). Integrin regulation of growth factor receptors. Nat. Cell Biol. 4, E75-E76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.