Abstract
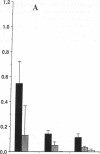
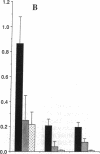
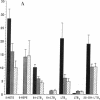
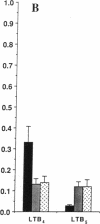
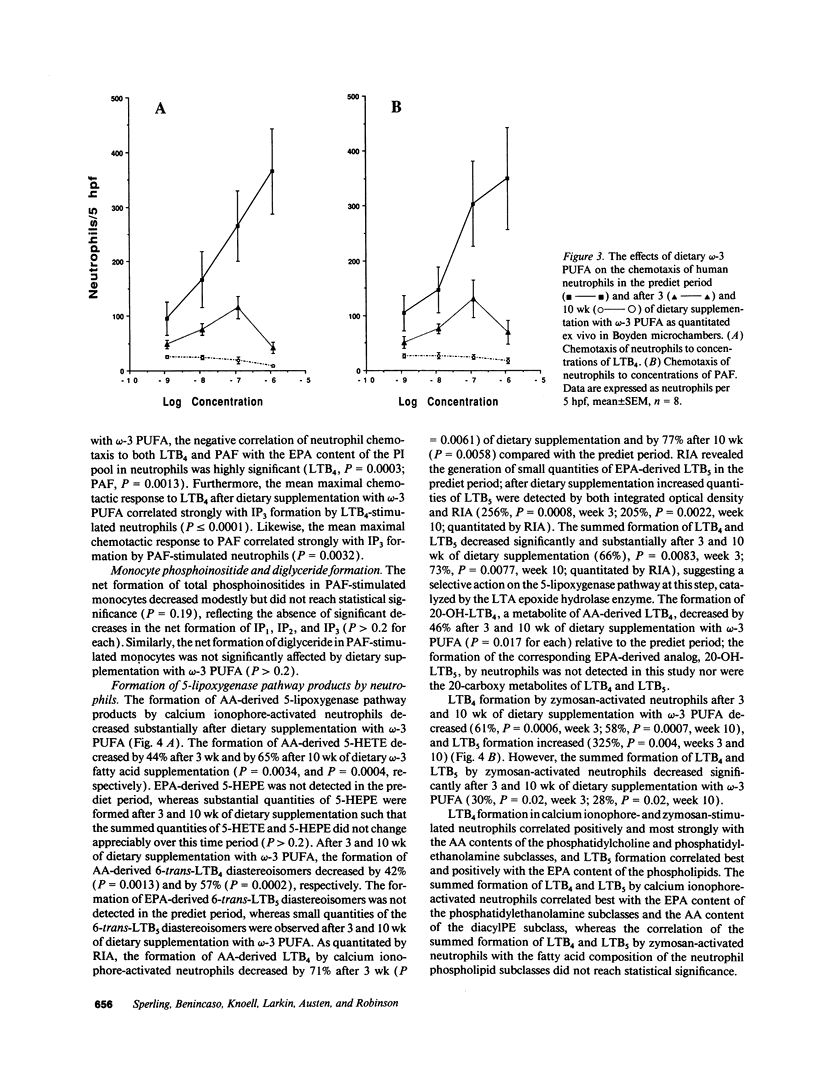
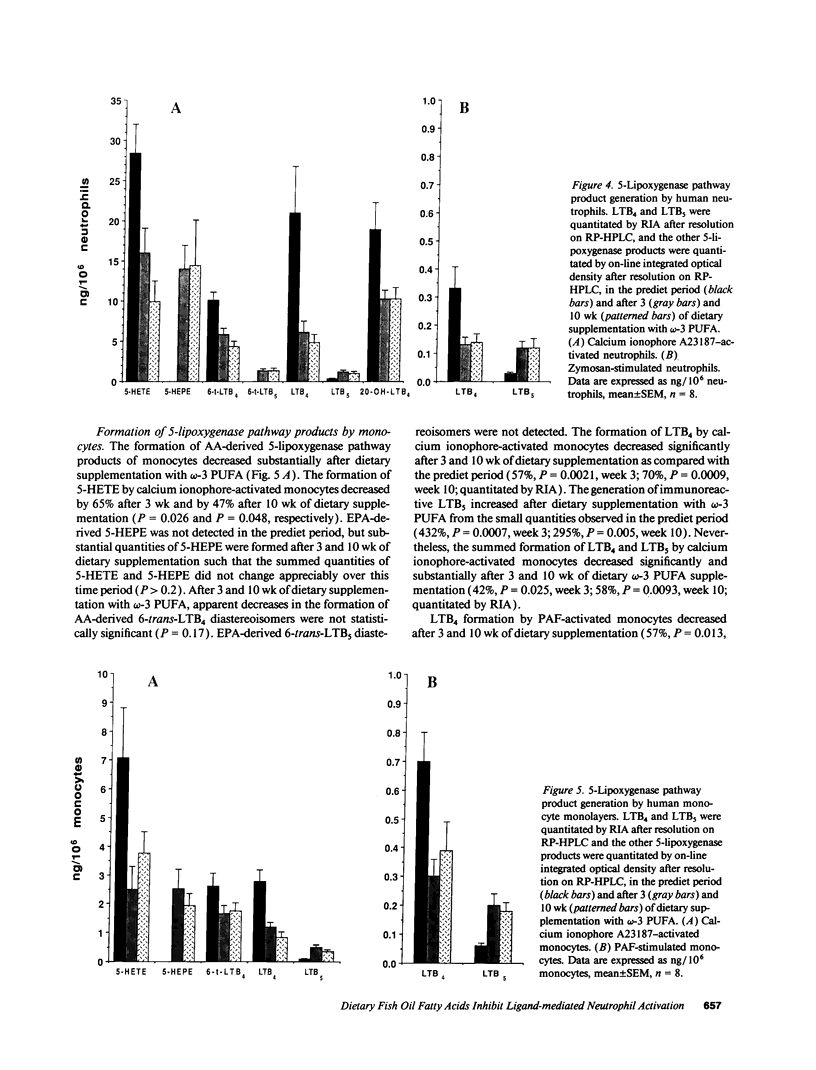
Earlier studies demonstrated that dietary omega-3 polyunsaturated fatty acid (PUFA) supplementation attenuates the chemotactic response of neutrophils and the generation of leukotriene (LT) B4 by neutrophils stimulated with calcium ionophore; however, the mechanisms and relationship of these effects were not examined. Neutrophils and monocytes from eight healthy individuals were examined before and after 3 and 10 wk of dietary supplementation with 20 g SuperEPA daily, which provides 9.4 g eicosapentaenoic acid (EPA) and 5 g docosahexaenoic acid. The maximal neutrophil chemotactic response to LTB4, assessed in Boyden microchambers, decreased by 69% after 3 wk and by 93% after 10 wk from prediet values. The formation of [3H]inositol tris-phosphate (IP3) by [3H]inositol-labeled neutrophils stimulated by LTB4 decreased by 71% after 3 wk (0.033 +/- 0.013% [3H] release, mean +/- SEM) and by 90% after 10 wk (0.011 +/- 0.011%) from predict values (0.114 +/- 0.030%) as quantitated by beta-scintillation counting after resolution on HPLC. LTB4-stimulated neutrophil chemotaxis and IP3 formation correlated significantly (P < 0.0001); each response correlated closely and negatively with the EPA content of the neutrophil phosphatidylinositol (PI) pool (P = 0.0003 and P = 0.0005, respectively). Neither the affinities and densities of the high and low affinity LTB4 receptors on neutrophils nor LTB4-mediated diglyceride formation changed appreciably during the study. Similar results were observed in neutrophils activated with platelet-activating factor (PAF). The summed formation of LTB4 plus LTB5 was selectively inhibited in calcium ionophore-stimulated neutrophils and was also inhibited in zymosan-stimulated neutrophils. The inhibition of the summed formation of LTB4 plus LTB5 in calcium ionophore-stimulated neutrophils and in zymosan-stimulated neutrophils did not correlate significantly with the EPA content of the PI pool. The data indicate that dietary omega-3 PUFA supplementation inhibits the autoamplification of the neutrophil inflammatory response by decreasing LTB4 formation through the inactivation of the LTA epoxide hydrolase and independently by inhibiting LTB4- (and PAF) stimulated chemotaxis by attenuating the formation of IP3 by the PI-selective phospholipase C. This is the initial demonstration that dietary omega-3 PUFA supplementation can suppress signal transduction at the level of the PI-specific phospholipase C in humans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackman R. G. Simplification of analyses of fatty acids in fish lipids and related lipid samples. Acta Med Scand. 1987;222(2):99–103. doi: 10.1111/j.0954-6820.1987.tb10644.x. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989 Oct 15;264(29):17069–17077. [PubMed] [Google Scholar]
- Blank M. L., Cress E. A., Snyder F. Separation and quantitation of phospholipid subclasses as their diradylglycerobenzoate derivatives by normal-phase high-performance liquid chromatography. J Chromatogr. 1987 Apr 17;392:421–425. doi: 10.1016/s0021-9673(01)94286-4. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Characterization of formylmethionyl-leucyl-phenylalanine stimulation of inositol trisphosphate accumulation in rabbit neutrophils. Mol Pharmacol. 1985 Jan;27(1):74–78. [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Quantitative changes in inositol 1,4,5-trisphosphate in chemoattractant-stimulated neutrophils. J Biol Chem. 1986 Nov 25;261(33):15644–15647. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chetty N., Vickers J. D., Kinlough-Rathbone R. L., Packham M. A., Mustard J. F. Eicosapentaenoic acid interferes with U46619-stimulated formation of inositol phosphates in washed rabbit platelets. Thromb Haemost. 1989 Dec 29;62(4):1116–1120. [PubMed] [Google Scholar]
- Cleland L. G., French J. K., Betts W. H., Murphy G. A., Elliott M. J. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J Rheumatol. 1988 Oct;15(10):1471–1475. [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne A. B., Filipowicz B. L., Edwards T. J., Hawkey C. J. High dose eicosapentaenoic acid ethyl ester: effects on lipids and neutrophil leukotriene production in normal volunteers. Br J Clin Pharmacol. 1990 Aug;30(2):187–194. doi: 10.1111/j.1365-2125.1990.tb03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Karnovsky M. J., Austen K. F., Corey E. J., Lewis R. A. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2191–2193. doi: 10.1073/pnas.81.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S., Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol. 1985 Mar;134(3):1914–1919. [PubMed] [Google Scholar]
- Kreisle R. A., Parker C. W. Specific binding of leukotriene B4 to a receptor on human polymorphonuclear leukocytes. J Exp Med. 1983 Feb 1;157(2):628–641. doi: 10.1084/jem.157.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J. M., Bigauoette J., Michalek A. V., Timchalk M. A., Lininger L., Rynes R. I., Huyck C., Zieminski J., Bartholomew L. E. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985 Jan 26;1(8422):184–187. doi: 10.1016/s0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Jubiz W., Michalek A., Rynes R. I., Bartholomew L. E., Bigaouette J., Timchalk M., Beeler D., Lininger L. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987 Apr;106(4):497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Lawrence D. A., Jubiz W., DiGiacomo R., Rynes R., Bartholomew L. E., Sherman M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990 Jun;33(6):810–820. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985 May 9;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Menica-Huerta J. M., Shih C., Corey E. J., Lewis R. A., Austen K. F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984 Feb 25;259(4):2383–2389. [PubMed] [Google Scholar]
- Leslie C. A., Gonnerman W. A., Ullman M. D., Hayes K. C., Franzblau C., Cathcart E. S. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J Exp Med. 1985 Oct 1;162(4):1336–1349. doi: 10.1084/jem.162.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher R., Sachinidis A., Steiner A., Vogt E., Vetter W. Fish oil affects phosphoinositide turnover and thromboxane A metabolism in cultured vascular muscle cells. Biochim Biophys Acta. 1989 Aug 15;1012(3):279–283. doi: 10.1016/0167-4889(89)90109-2. [DOI] [PubMed] [Google Scholar]
- Locher R., Vogt E., Steiner A., Vetter W. The phosphoinositide turnover of vascular smooth muscle cells is influenced by fish oil. J Hypertens Suppl. 1988 Dec;6(4):S222–S224. doi: 10.1097/00004872-198812040-00066. [DOI] [PubMed] [Google Scholar]
- Medini L., Colli S., Mosconi C., Tremoli E., Galli C. Diets rich in n-9, n-6 and n-3 fatty acids differentially affect the generation of inositol phosphates and of thromboxane by stimulated platelets, in the rabbit. Biochem Pharmacol. 1990 Jan 1;39(1):129–133. doi: 10.1016/0006-2952(90)90656-6. [DOI] [PubMed] [Google Scholar]
- Mullmann T. J., Siegel M. I., Egan R. W., Billah M. M. Complement C5a activation of phospholipase D in human neutrophils. A major route to the production of phosphatidates and diglycerides. J Immunol. 1990 Mar 1;144(5):1901–1908. [PubMed] [Google Scholar]
- Nathaniel D. J., Evans J. F., Leblanc Y., Léveillé C., Fitzsimmons B. J., Ford-Hutchinson A. W. Leukotriene A5 is a substrate and an inhibitor of rat and human neutrophil LTA4 hydrolase. Biochem Biophys Res Commun. 1985 Sep 16;131(2):827–835. doi: 10.1016/0006-291x(85)91314-2. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Traynor A. E., Harris A. L., Sklar L. A. LTB4 induced activation signals and responses in neutrophils are short-lived compared to formylpeptide. J Immunol. 1987 Apr 15;138(8):2626–2632. [PubMed] [Google Scholar]
- Patton G. M., Fasulo J. M., Robins S. J. Separation of phospholipids and individual molecular species of phospholipids by high-performance liquid chromatography. J Lipid Res. 1982 Jan;23(1):190–196. [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Prickett J. D., Robinson D. R., Steinberg A. D. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB x NZW F1 mice. J Clin Invest. 1981 Aug;68(2):556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. L., McCullough J. A modified microchamber method for chemotaxis and chemokinesis. Immunol Commun. 1984;13(1):49–62. doi: 10.3109/08820138409025449. [DOI] [PubMed] [Google Scholar]
- Schmidt E. B., Pedersen J. O., Ekelund S., Grunnet N., Jersild C., Dyerberg J. Cod liver oil inhibits neutrophil and monocyte chemotaxis in healthy males. Atherosclerosis. 1989 May;77(1):53–57. doi: 10.1016/0021-9150(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Sperling R. I., Robin J. L., Kylander K. A., Lee T. H., Lewis R. A., Austen K. F. The effects of N-3 polyunsaturated fatty acids on the generation of platelet-activating factor-acether by human monocytes. J Immunol. 1987 Dec 15;139(12):4186–4191. [PubMed] [Google Scholar]
- Sperling R. I., Weinblatt M., Robin J. L., Ravalese J., 3rd, Hoover R. L., House F., Coblyn J. S., Fraser P. A., Spur B. W., Robinson D. R. Effects of dietary supplementation with marine fish oil on leukocyte lipid mediator generation and function in rheumatoid arthritis. Arthritis Rheum. 1987 Sep;30(9):988–997. doi: 10.1002/art.1780300905. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Aepfelbacher M., Lux I., Zimmer B., Weber P. C. Docosahexaenoic acid inhibits PAF and LTD4 stimulated [Ca2+]i-increase in differentiated monocytic U937 cells. Biochim Biophys Acta. 1991 Dec 3;1133(1):38–45. doi: 10.1016/0167-4889(91)90239-t. [DOI] [PubMed] [Google Scholar]
- von Schacky C., Siess W., Fischer S., Weber P. C. A comparative study of eicosapentaenoic acid metabolism by human platelets in vivo and in vitro. J Lipid Res. 1985 Apr;26(4):457–464. [PubMed] [Google Scholar]