Abstract
A recent analysis of brain size evolution reconstructed the plesiomorphic brain–body size allometry for the mammalian order Carnivora, providing an important reference frame for comparative analyses of encephalization (brain volume scaled to body mass). I performed phylogenetically corrected regressions to remove the effects of body mass, calculating correlations between residual values of encephalization with basal metabolic rate (BMR) and six life-history variables (gestation time, neonatal mass, weaning time, weaning mass, litter size, litters per year). No significant correlations were recovered between encephalization and any life-history variable or BMR, arguing against hypotheses relating encephalization to maternal energetic investment. However, after correcting for clade-specific adaptations, I recovered significant correlations for several variables, and further analysis revealed a conserved carnivoran reproductive strategy, linking degree of encephalization to the well-documented mammalian life-history trade-off between neonatal mass and litter size. This strategy of fewer, larger offspring correlating with increased encephalization remains intact even after independent changes in encephalization allometries in the evolutionary history of this clade.
Keywords: brain size, maternal energy hypothesis, Carnivora, Mammalia, likelihood ratios
1. Introduction
The maternal energy hypothesis (MEH) (Martin 1981, 1996) proposes maternal energetic investment in the developing embryo constrains adult brain size. Brains are metabolically expensive (Mink et al. 1981) and maternal basal metabolic rate (BMR) could limit potential energetic investment in expensive embryonic tissues. This should manifest itself in a brain–body size allometry that scales at 0.75 for mammals (Martin 1981), and evolutionary change in encephalization would then require corresponding change in BMR (Martin 1981; Armstrong 1983; Hofman 1983). Therefore, relatively larger brains should be associated with relatively higher BMR.
A BMR-encephalization link has been disputed (e.g. McNab & Eisenberg 1989), although some evidence suggests a relationship between the two variables. Across placental mammals, Isler & Van Schaik (2006) recovered a significant correlation between brain volume and BMR residuals, after regressing each against body mass. At less inclusive clades, however, empirical encephalization allometries often scale significantly lower than 0.75 (e.g. Ashwell 2008; Finarelli 2008b; Finarelli & Flynn 2009). However, BMR is only one potential proxy for maternal energetic investment (Martin 1981); increased encephalization through increased total maternal energy investment in the embryo could also be achieved by lengthening the gestational period (Martin 1996). Pagel & Harvey (1988a) found a relationship between gestation time and neonatal brain volume across placental mammals. Therefore, change in the parameters of reproduction may represent a better measure of maternal investment, and encephalization may be more closely linked with life-history variables (Pagel & Harvey 1988a; Gittleman 1994).
Terrestrial carnivorans provide a model group to test the hypothesized link between maternal energetics and encephalization. Life history, BMR and encephalization are well studied for this group, and the recent reconstruction of the plesiomorphic brain–body size allometry for Carnivora (Finarelli & Flynn 2009) provides a useful baseline for comparison of degree of encephalization. Because this allometry gives the expected plesiomorphic scaling, it relates change in encephalization owing to among-clade change in allometry as well as within-clade variation relative to those allometries. Here, I investigate correlations of encephalization in terrestrial clades in the order Carnivora with BMR and six life-history variables.
2. Material and methods
I compiled data for six reproductive life-history variables: gestation time, neonatal mass, time-to-weaning, weaning mass, litter size and litters per year (table S1 in the electronic supplementary material) and BMR (table S2 in the electronic supplementary material). Because it is necessary to control for correlations induced by general body size allometry (Isler & Van Schaik 2006; Bielby et al. 2007; Sibly & Brown 2007), I calculated encephalization residuals for carnivoran brain volumes relative to the basal Carnivora encephalization allometry (Finarelli & Flynn 2009) (equation S1 in the electronic supplementary material). For BMR and each life-history variable, I calculated phylogenetically corrected regressions (Garland et al. 1992; Garland & Ives 2000) on ln-transformed data using the phenotypic diversity analysis programs module (Midford et al. 2003) for Mesquite (Maddison & Maddison 2007), using a composite carnivoran phylogeny (figure S1 in the electronic supplementary material) (Finarelli & Flynn 2009; Finarelli & Goswami 2009). I then calculated correlation coefficients between the residuals for encephalization and residuals for BMR/life history. I then tested for among-clade differences in correlations by mean-centring the residuals (subtracting mean values from each taxon) by subclade (figure S3 in the electronic supplementary material). After mean-centering residuals, I tested for the presence of significant within-clade correlations that have subsequently been obscured by among-clade adaptation. See the electronic supplementary material, appendix.
3. Results
Body mass and brain volume have previously been shown to be highly correlated with life-history variables for Carnivora (e.g. Gittleman 1986) and BMR (e.g. Martin 1981, 1996; Isler & Van Schaik 2006). Here, most variables correlate significantly with both brain volume and body mass after controlling for phylogeny (Bonferroni correction for seven comparisons), although gestation time is not significantly correlated with either variable, and litter size is only significantly correlated with brain volume (table S3 in the electronic supplementary material). However, brain volume and body mass are themselves highly correlated (r = 0.822, p < 0.001); after controlling for body size, no significant correlations exist between encephalization residuals and life history or BMR residuals (table 1 and figures S4–S10 in the electronic supplementary material). The significant correlations with brain volume reflect mutual correlations with body mass (Pagel & Harvey 1988a).
Table 1.
Correlation of variable residuals with encephalization residuals. (Log L values less than −2 indicate that the observed value of r is significantly different from zero: none of the observed correlations is significant.)
| variable | r | log L |
|---|---|---|
| gestation time | 0.089 | −0.509 |
| newborn mass | 0.194 | −1.880 |
| weaning time | −0.078 | −0.331 |
| weaning mass | 0.202 | −1.085 |
| litter size | −0.075 | −0.410 |
| litters per year | −0.102 | −0.452 |
| BMR | 0.136 | −0.514 |
Testing for differences in correlations with encephalization, mean-centred residuals reconstruct a single correlation across Carnivora for BMR and most life-history variables. That is, within-clade correlations with encephalization are homogeneous across carnivoran clades (table S4 in the electronic supplementary material). Correlations for two variables, gestation time and litters per year differ significantly among clades. Three correlations are reconstructed for gestation time: (i) Musteloidea (skunks, raccoons, weasels, etc.); (ii) Herpestoidea–Viverridae (hyaenas, ‘mongooses,’ civets); and (iii) the remainder of Carnivora (Ursidae (bears), Canidae (wolves, jackals, foxes), Felidae (cats), Nandinia (African palm civet)). The herpestoid–viverrid correlation is not significantly different from zero, whereas musteloids exhibit a strong positive correlation, and Nandinia–Canidae–Felidae–Ursidae a strong negative correlation (table 2). Two correlations are reconstructed for litters per year: (i) Arctoidea (Ursidae + Musteloidea) and (ii) the remaining taxa (Canidae + Feliformia). The Canidae + Feliformia correlation is significantly negative, although the arctoid correlation is insignificant (table 2).
Table 2.
Correlations, sample sizes and log-likelihoods for mean-centred residuals. (Correlation coefficients are given between encephalization and each variable's residuals, re-centred such that the group mean is zero (see the electronic supplementary material for discussion). Gestation time and number of litters per year were reconstructed with significantly different among-group correlations, and each correlation and its log-likelihood is given. All other variables were reconstructed with a single, homogeneous correlation across Carnivora. Correlations that were significantly different from zero (log L < −2) are highlighted in bold.)
| variable | clade | r | n | log L |
|---|---|---|---|---|
| gestation time | Musteloidea | 0.378 | 46 | −3.474 |
| Herpestoids and Viverridae | −0.016 | 27 | −0.003 | |
| Ursidae, Canidae, Felidae, Nandinia | −0.468 | 55 | −6.698 | |
| newborn mass | Carnivora | 0.378 | 99 | −7.562 |
| weaning time | Carnivora | 0.001 | 108 | > −0.001 |
| weaning mass | Carnivora | 0.277 | 53 | −2.081 |
| litter size | Carnivora | −0.299 | 147 | −6.860 |
| litters per year | Arctoidea | 0.244 | 31 | −0.923 |
| Canidae, Feliformia | −0.301 | 57 | −2.655 | |
| BMR | Carnivora | 0.100 | 56 | −0.274 |
Among the variables reconstructed with a single correlation across Carnivora in the mean-centred residuals, several are significantly different from zero: neonatal mass (+), weaning mass (+) and litter size (−) (table 2). None of these was significant when residuals were analysed prior to mean-centring, indicating that wholesale shifting of clades within the plane is responsible for obscuring these correlations in the raw residual data (figures S2c,d and S3 in the electronic supplementary material).
4. Discussion
Although BMR appears to be highly, positively correlated with encephalization across placental mammals (Martin 1981; Isler & Van Schaik 2006), here BMR is not correlated with encephalization within Carnivora. Isler & Van Schaik (2006) also failed to find a significant correlation within Carnivora. This is not surprising, as Carnivora is exceptionally varied ecologically, and BMR has been shown to scale differently across many ecological variables (e.g. diet, habitat, substrate) (McNab 2008). The lack of consistent correlations within constituent mammalian subclades, e.g. Carnivora, argues against a direct link between relative BMR and encephalization. Rather, the apparent relationship appears to connect disparate clades, within which no consistent relationship exists.
That encephalization does not correlate with BMR or reproductive life history would appear to argue against the MEH. However, Martin (1996) noted that increasing total energy investment via prolonging the gestational period may represent a different means to increased encephalization. Yet, no systematic patterns relating encephalization to life history across Carnivora exist. Increased encephalization above the basal allometry (herpestoids and viverrids among living taxa: Finarelli & Flynn 2009) are not accompanied by predictable shifts in life history. For example, both canids and musteloids have significantly higher encephalization residuals than herpestoids/viverrids (Mann–Whitney tests: p < 0.001 and p = 0.002, respectively), yet significantly shorter gestation times than expected for a herpestoid/viverrid of similar mass (p = 0.005 and p = 0.003, respectively). It is therefore unlikely that the MEH sufficiently explains observed changes in carnivoran encephalization allometries, and the causal mechanism behind these changes in allometry remains elusive.
In general, there is little structure between encephalization and the timing of maternal offspring investment across Carnivora. Three significantly different correlations were reconstructed for gestation time. Two of these are significant, but in opposite senses: positive for Musteloidea, negative for Nandinia–Canidae–Felidae–Ursidae. Similarly, with the two correlations for litters per year, the correlation for Canidae + Feliformia was significantly negative, but the arctoid correlation was not significant. Weaning time was not significantly correlated with encephalization in any manner. Thus, altering the tempo of life history does not appear to be reflected in changes in adult encephalization.
However, these results are not necessarily fatal to a concept of maternal investment informing adult encephalization. Several significant correlations exist in the mean-centred residuals (table 2), demonstrating a relationship between reproductive strategy and encephalization, despite the fact that these have subsequently been obscured by among-clade adaptations. Mean-centred residuals for neonatal mass, weaning mass and litter size are significantly correlated with encephalization. The weaning mass correlation appears to reflect the fact that relatively large neonates also tend to be relatively large at weaning: the partial correlation of encephalization with neonatal mass, holding weaning mass constant, is significant (partial r = 0.349, p = 0.011), whereas the partial correlation for weaning mass, controlling for neonatal mass, is not (partial r = −0.014, p = 0.923).
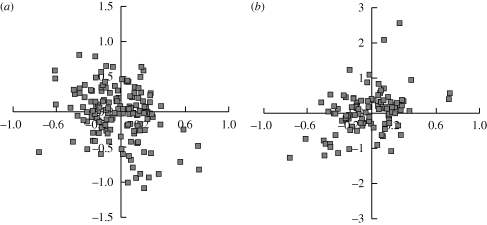
From this, two important correlations emerge: encephalization is correlated positively with neonatal mass and negatively with litter size (figure 1 and table 2). These correlations point to a conserved carnivoran reproductive strategy, taxa with higher encephalization tend to have fewer, larger offspring, a strategy which remains intact even after multiple independent changes in encephalization allometries (Finarelli & Flynn 2009). A trade-off between litter size and neonatal mass after correcting for body mass has been noted in analyses of mammalian life histories, and is sometimes referred to as ‘offspring quality’ (Smith & Fretwell 1974; Bielby et al. 2007). That encephalization correlates significantly with offspring quality demonstrates the importance of maternal energetic investment in the offspring in shaping adult encephalization, even if it is not directly expressed in correlations observed across Carnivora as a whole.
Figure 1.
Bi-plots of mean-centred variable residuals: encephalization (x-axis) is plotted versus (a) litter size and (b) neonate mass. The negative correlation with litter size and positive correlation with neonatal mass point to a conserved reproductive strategy of fewer, larger offspring with increased encephalization, after accounting for among-clade adaptation.
Hierarchical (taxon-level) effects are evident in the relationship between encephalization and both neonatal mass and litter size (Pagel & Harvey 1988b; Jablonski 2007; Finarelli 2008a). Neither neonatal mass nor litter size residuals correlate significantly with encephalization residuals prior to mean-centring. Individual carnivoran clades arrive at different specific relationships between these variables, and these among-clade adaptations shift clades within the residual space, obscuring relationships when analysed across Carnivora. However, conserved within-clade relationships are evident, revealed in the mean-centred residuals. Both musteloids and canids have higher encephalization residuals than herpestoids/viverrids, but both clades are characterized by higher litter size residuals (p = 0.001 and p < 0.001, respectively). This among-clade offset makes it appear as if no correlation exists across Carnivora, despite a consistent, negative within-clade correlation (figure S2 in the electronic supplementary material). Similarly, musteloids have significantly lower neonatal mass residuals (p = 0.001), obscuring the positive correlation within-clade correlation with encephalization and neonatal mass.
Acknowledgements
I would like to thank J. Flynn A. Goswami and two anonymous reviewers for their helpful comments and feedback. This project was funded, in part, by the University of Michigan Society of Fellows.
References
- Armstrong E.1983Brain size and metabolism in mammals. Science 220, 1302–1304 (doi:10.1126/science.6407108) [DOI] [PubMed] [Google Scholar]
- Ashwell K. W. S.2008Encephalization of Australian and New Guinean marsupials. Brain Behav. Evol. 71, 181–199 (doi:10.1159/000114406) [DOI] [PubMed] [Google Scholar]
- Bielby J., Mace G. M., Bininda-Emonds O. R. P., Cardillo M., Gittleman J. L., Jones K. E., Orme C. D. L., Purvis A.2007The fast–slow continuum in mammalian life history: an empirical re-evaluation. Am. Nat. 169, 748–757 (doi:10.1086/516847) [DOI] [PubMed] [Google Scholar]
- Finarelli J. A.2008aHierarchy and the reconstruction of evolutionary trends: evidence for constraints on the evolution of body size in terrestrial caniform carnivorans (Mammalia). Paleobiology 34, 553–562 (doi:10.1666/07078.1) [Google Scholar]
- Finarelli J. A.2008bTesting hypotheses of the evolution of brain-body size scaling in the Canidae (Carnivora, Mammalia). Paleobiology 34, 35–45 (doi:10.1666/07030.1) [Google Scholar]
- Finarelli J. A., Flynn J. J.2009Brain size evolution and sociality in Carnivora. Proc. Natl Acad. Sci. USA 106, 9345–9349 (doi:10.1073/pnas.0901780106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finarelli J. A., Goswami A.2009The evolution of orbit orientation and encephalization in the Carnivora (Mammalia). J. Anat. 214, 671–678 (doi:10.1111/j.1469-7580.2009.01061.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T., Ives A. R.2000Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am. Nat. 155, 346–364 (doi:10.1086/303327) [DOI] [PubMed] [Google Scholar]
- Garland T., Harvey P. H., Ives A. R.1992Procedures for the analysis of comparative data using phyogenetically independent contrasts. Syst. Biol. 41, 18–32 [Google Scholar]
- Gittleman J. L.1986Carnivore life history patterns: allometric, phylogenetic, and ecological associations. Am. Nat. 127, 744–771 (doi:10.1086/284523) [Google Scholar]
- Gittleman J. L.1994Female brain size and parental care in carnivores. Proc. Natl Acad. Sci. USA 91, 5495–5497 (doi:10.1073/pnas.91.12.5495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman M. A.1983Energy metabolism, brain size and longevity in mammals. Q. Rev. Biol. 58, 495–512 (doi:10.1086/413544) [DOI] [PubMed] [Google Scholar]
- Isler K., Van Schaik C. P.2006Metabolic costs of brain size evolution. Biol. Lett. 2, 557–560 (doi:10.1098/rsbl.2006.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski D.2007Scale and hierarchy in macroevolution. Palaeontology 50, 87–109 (doi:10.1111/j.1475-4983.2006.00615.x) [Google Scholar]
- Maddison W. P., Maddison D. R.2007Mesquite: a modular system for evolutionary analysis, v. 2.6. See http://www.mesquiteproject.org [Google Scholar]
- Martin R. D.1981Relative brain size and basal metabolic-rate in terrestrial vertebrates. Nature 293, 57–60 (doi:10.1038/293057a0) [DOI] [PubMed] [Google Scholar]
- Martin R. D.1996Scaling of the mammalian brain: the maternal energy hypothesis. N. Physiol. Sci. 11, 149–156 [Google Scholar]
- McNab B. K.2008An analysis of the factors that influence the level and scaling of mammalian BMR. Comp. Biochem. Physiol. 151, 5–28 (doi:10.1016/j.cbpa.2008.05.008) [DOI] [PubMed] [Google Scholar]
- McNab B. K., Eisenberg J. F.1989Brain size and its relation to the rate of metabolism in mammals. Am. Nat. 133, 157–167 (doi:10.1086/284907) [Google Scholar]
- Midford P., Garland T., Maddison W. P.2003PDAP package (of Mesquite), v. 1.09. [Google Scholar]
- Mink J. W., Blumenschine R. J., Adams D. B.1981Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. 241, R203–R212 [DOI] [PubMed] [Google Scholar]
- Pagel M. D., Harvey P. H.1988aHow mammals produce large-brained offspring. Evolution 42, 948–957 (doi:10.2307/2408910) [DOI] [PubMed] [Google Scholar]
- Pagel M. D., Harvey P. H.1988bThe taxon-level problem in the evolution of mammalian brain size: facts and artifacts. Am. Nat. 132, 344–359 (doi:10.1086/284857) [Google Scholar]
- Sibly R. M., Brown J. H.2007Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl Acad. Sci. USA 104, 17 707–17 712 (doi:10.1073/pnas.0707725104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. C., Fretwell S. D.1974The optimal balance between size and number of offspring. Am. Nat. 108, 499–506 (doi:10.1086/282929) [Google Scholar]