Abstract
For prey species that rely on learning to recognize their predators, natural selection should favour individuals able to learn as early as possible. The earliest point at which individuals can gather information about the identity of their potential predators is during the embryonic stage. Indeed, recent experiments have demonstrated that amphibians can learn to recognize predators prior to hatching. Here, we conditioned woodfrog embryos to recognize predatory salamander cues either in the morning or in the evening, and subsequently exposed the two-week-old tadpoles to salamander cues either in the morning or in the evening, and recorded the intensity of their antipredator behaviour. The data indicate that amphibians learn to recognize potential predators while still in the egg, and also learn the temporal component of this information, which they use later in life, to adjust the intensity of their antipredator responses throughout the day.
Keywords: predator recognition, temporal learning, embryonic learning
1. Introduction
Being able to distinguish predators from non-predators is a fundamental pre-requisite for prey to avoid being eaten. Numerous species of prey require learning to recognize predators and previous work has highlighted the various types of information that prey can gather about their predators. Some of the most sophisticated examples come from the aquatic literature, with prey learning to adjust their antipredator behaviours according to the predator's identity and relatedness to other known predators, the risk level posed by that predator as well as the predator's size, density, proximity and time at which the predator is most active (reviewed by Ferrari et al. in press).
Because learning appears to be crucial in fine-tuning responses to predators, selection should favour animals that acquire information about potential predators as early in life as possible. For most aquatic species, the earliest time at which an individual can acquire this information is while the embryo is still in the egg, in contact with the aquatic medium. In the emerging new field of embryonic learning, two papers have recently documented that amphibians indeed have the ability to learn predators while still in the egg. Mathis et al. (2008) showed that embryonic woodfrogs, Rana sylvatica, exposed to the odour of injured tadpoles paired with cues from a novel salamander learned to recognize the salamanders as dangerous and responded as tadpoles to salamander cues two weeks after hatching. In a subsequent experiment, Ferrari & Chivers (in press) showed that embryos learn the danger level associated with the predator using the concentration of injured tadpole cues experienced in the egg.
Amphibians have been shown to have a sophisticated ability to learn to match time of day with predator activity. For example, Ferrari & Chivers (2009) demonstrated that tadpoles learn to respond to novel predators at particular times of the day, after a 6-day training period. Tadpoles are preyed upon by many ectotherm predators, such as salamanders, which have clear diel patterns of activity (Holomuzki & Collins 1983). Hence, learning to respond to predators at times when they in fact represent a threat may be adaptive. It follows from time-sensitive learning that there may be a significant fitness cost to responding to predator odours outside of the normal foraging time for that predator. Our preliminary data on woodfrogs indicate that time-specific embryonic conditioning results in tadpoles showing great variation in the intensity of their antipredator responses throughout the day. Consequently, in this study, we tested the hypothesis that, under natural conditions, woodfrog embryos can learn temporal information about their predators, and subsequently, as tadpoles, adjust the intensity of antipredator responses according to ‘time of day’.
2. Material and methods
Information about water, predators and test subjects along with information about the embryonic conditioning treatments are posted in the electronic supplementary material.
(a). Experimental setup and procedures
Each of five freshly laid egg clutches was divided into six groups (or sub-clutches) of approximately 50–75 eggs. The 30 sub-clutches (single masses of eggs with the egg jelly intact) were then transferred into 3.5 l pails filled with 2 l of conditioned well water. All five clutches were at Gosner developmental stages 10–11; at this stage, the neural tube is not yet formed.
The layout of the embryonic conditioning followed a randomized block design, in which each clutch represented a block. Embryonic woodfrogs were exposed to one of three treatments: (i) well water, (ii) salamander odour, or (iii) crushed tadpoles paired with salamander odour at one of two times of the day: (i) from 1100 to 1300 h (hereafter ‘morning’) or (ii) from 1900 to 2100 h (hereafter ‘evening’). The embryos were treated for 7 days, and treatments stopped prior to the embryos hatching. See the electronic supplementary material for more details regarding the embryonic conditioning methodology.
(b). Tadpole testing and behavioural assay
Following the embryonic exposure phase of the experiment, tadpoles were raised for two weeks before being tested. Each group was tested for their responses to (i) water (negative control), (ii) crushed conspecific cues (positive control), or (iii) salamander odour. Tests were conducted over 5 days between 1030 and 1330 h for the morning group and between 1830 and 2130 h for the evening group.
Larval amphibians decrease activity as an antipredator response. Thus, tadpoles were tested using a well-established protocol, consisting of measuring their activity (line crosses) for 4 min prior to and 4 min following injections of stimuli in 0.5 l testing cups (Mathis et al. 2008; Ferrari & Chivers 2009; see the electronic supplementary material for full description). Tadpoles were exposed to 5 ml of well water, 5 ml of salamander odour or 5 ml of crushed conspecific cues. The salamander odour was obtained by soaking two salamanders in 3 l of conditioned well water for 24 h. The salamander odour was frozen and used for both the morning and evening testing. The crushed conspecific cues were obtained by crushing one tadpole in 5 ml of water. The crushed conspecific cues were prepared fresh and used within 2 min of being made. The order of the treatments was randomized within and between days. The observer was blind to the treatments. Overall, 20–25 tadpoles from each sub-clutch were tested.
(c). Statistical analysis
We calculated the change in proportion of line crosses from the pre-stimulus baseline. The responses of tadpoles coming from the same sub-clutch exposed to the same cues were not independent. Hence, these data were averaged so that sub-clutch, not tadpole, represented our sampling unit. Owing to the large number of treatments (two conditioning times × three conditioning cues × two testing times × three testing cues), we analysed the responses of tadpoles for each testing cue, looking at the effects of conditioning times, conditioning cues and testing times on the responses of tadpoles to water, to crushed conspecific cues and to salamander odour separately. We analysed the data using a randomized block design ANOVA, in which we assessed the effect of conditioning time, conditioning cue and testing time (fixed factors) and clutch (block).
3. Results
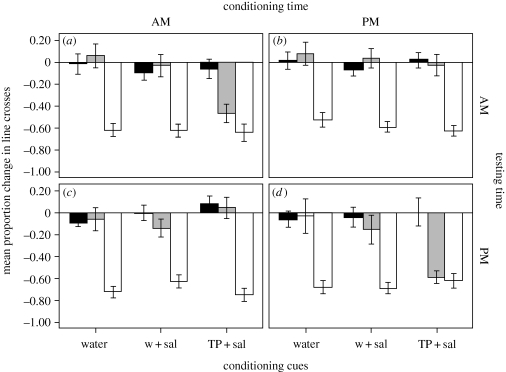
The ANOVA revealed no effect of conditioning time, conditioning cue, testing time, clutch or any two- or three-way interactions on the responses of tadpoles to water and injured conspecific cues (all p > 0.1, figure 1). Tadpoles consistently showed strong responses to injured conspecifics cues but did not respond to water. The ANOVA revealed a three-way interaction between conditioning time, conditioning cue and testing time (F2,6 = 17.9, p = 0.003) on the responses of tadpoles to salamander odour. Time of conditioning and time of testing did not affect the responses of tadpoles to salamander odour, for those tadpoles that were conditioned with water paired with salamander odour as embryos (table 1). However, for tadpoles that were conditioned with crushed conspecific cues paired with salamander odour as embryos, the time at which they were conditioned affected the responses to salamander odour at different times of the day: tadpoles responded to salamander odour only if the time of testing matched the time of conditioning (table 1 and figure 1).
Figure 1.
Mean (±s.e.) proportion change in line crosses from the pre-stimulus baseline for woodfrog tadpoles exposed to water (black bars), salamander odour (grey bars) or crushed tadpole cues (white bars) in the (a,b) morning or (c,d) evening. As embryos, woodfrogs were conditioned for 7 days with water, salamander odour (sal) or crushed tadpoles paired with salamander odour (TP + sal) in the (a,c) morning or in the (b,d) evening.
Table 1.
ANOVA table investigating the effect of testing time (morning versus evening), embryonic conditioning time (morning versus evening) and clutch on the responses of tadpoles to salamander odour. These woodfrogs were exposed to water, salamander odour or crushed tadpole cues paired with salamander odour as embryos. Bold p-value is statistically significant.
| water |
salamander |
TP + salamander |
|||||
|---|---|---|---|---|---|---|---|
| embryonic conditioning cues source | d.f. | F | p | F | p | F | p |
| testing time | 1,3 | 2.03 | 0.25 | 2.59 | 0.21 | 0.06 | 0.83 |
| conditioning time | 1,3 | 0.20 | 0.69 | 0.47 | 0.54 | 1.85 | 0.27 |
| testing time × conditioning time | 1,3 | 0.08 | 0.79 | 0.12 | 0.76 | 155.18 | <0.001 |
| testing time × clutch | 3,3 | 0.37 | 0.78 | 0.65 | 0.63 | 6.06 | 0.09 |
| conditioning time × clutch | 3,3 | 1.24 | 0.43 | 0.15 | 0.93 | 2.53 | 0.23 |
4. Discussion
Our results demonstrate that amphibians can learn both the identity and the temporal foraging pattern of their predators prior to hatching. They subsequently use this information as tadpoles to adjust the time at which they display high intensities of responses to predators. This represents a high level of sophistication of risk assessment by young prey animals, but the exact mechanism that they use to acquire temporal information is unclear, as field conditions do not allow us to distinguish between an internal clock, light intensity or sun position. What we do know, however, is that this temporal learning takes place over a short time period (over a 4 or 5 day period), as the embryos did not have a fully formed neural tube before day 2 or 3. Water temperature during conditioning bouts was highly variable from day to day (over a range of 12°C), which makes it unlikely that the responses are elicited by temperature-specific conditions.
Theoretical models suggest that learning is advantageous in highly variable environments (Ferrari et al. 2007; Stephens 2009). Such would be the case when prey animals, like amphibians, find themselves in environments where predation pressure may vary from extremely low to extremely high (i.e. the variability between years is high). Hence, learning should allow prey to collect information about their environment that will help them make better-informed decisions. However, gathering information about the environment is often costly in terms of both time and energy (Dukas 1998). The temporal learning that we observed implies that the cost of being cautious during a predator's foraging time may be small compared with the probable benefit of reducing mortality. Interestingly, the response to the salamanders disappeared when the exposure time did not match the time when the predator was active. These results imply that individual tadpoles experience predators with highly predictable cycles.
In our experiment, we tested woodfrog larvae two weeks following hatching, and found that the information the tadpoles learned as embryos was used to adjust the intensity of their antipredator responses throughout the day. However, could this temporal information have been used by the embryos themselves? In most cases, embryos cannot display active antipredator behaviour (swimming away from predators), because they are anchored at a specific location. However, embryos may be able to better time their conspicuousness (wriggling movements related to hatching), limiting them, for example, to periods where predators are not foraging. Indeed, numerous species of amphibians show considerable variation in their timing of hatching related to the presence of egg and larval predators (Chivers et al. 2001). Thus, it is possible that temporal learning of information about predation risk is used at an earlier stage than the one we documented.
The window of memory for which learned information is available is often related to the expected value of the information later in life. The information should be available for a longer time if the environment has a low variability (depending on the probability of the information being useful after a given period). If the environment is rapidly changing, the information will be erroneous and potentially costly; thus, the memory window should be shorter (Stephens 2009). In our study, the cues used to mediate learning were crushed tadpole cues paired with salamander cues. It is likely that tadpole-specific predators that tadpoles experienced as embryos will also be present when the embryos in turn reach their larval stage (i.e. the variability within a year is low). Hence, the information still has a high informative potential. This begs the question: would embryos experiencing embryo-specific predators (e.g. leeches feeding on eggs) remember predator-related information as tadpoles? Likewise, it would be fruitful to provide embryos conflicting information about the time at which predators forage and test how such an increase in environmental variability influences learning and memory of predator cues. The field of embryonic learning is at its infancy; further research would bring insights into the evolutionary ecology of learning and forgetting.
Acknowledgements
All work reported was in accordance with the Animal Care Committee Protocol no. 20060014 from University of Saskatchewan. Research funding was provided to D.C. through NSERC.
References
- Chivers D. P., Kiesecker J. M., Marco A., DeVito J., Anderson M. T., Blaustein A. R.2001Predator-induced life-history changes in amphibians: egg predation induces hatching. Oikos 92, 135–142 (doi:10.1034/j.1600-0706.2001.920116.x) [Google Scholar]
- Dukas R.1998Evolutionary ecology of learning. Cognitive ecology: the evolutionary ecology of information processing and decision making (ed. Dukas R.). Chicago, IL: University of Chicago Press [Google Scholar]
- Ferrari M. C. O., Chivers D. P.2009Temporal variability, threat-sensitivity and conflicting information about the nature of risk: understanding the dynamics of tadpole antipredator behaviour. Anim. Behav. 78, 11–16 (doi:10.1016/j.anbehav.2009.03.016) [Google Scholar]
- Ferrari M. C. O., Chivers D. P.In press The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behav. Ecol. Sociobiol. (doi:10.1007/s00265-009-0870-y) [Google Scholar]
- Ferrari M. C. O., Gonzalo A., Messier F., Chivers D. P.2007Generalization of learned predator recognition: an experimental test and framework for future studies. Proc. R. Soc. B. 274, 1853–1859 (doi:10.1098/rspb.2007.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M. C. O., Wisenden B. D., Chivers D. P.In press Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. [Google Scholar]
- Holomuzki J. R., Collins J. P.1983Diel movement of larvae of the tiger salamander Ambystoma tigrinum nebulosum. J. Herpetol. 17, 276–278 (doi:10.2307/1563831) [Google Scholar]
- Mathis A., Ferrari M. C. O., Windel N., Messier F., Chivers D. P.2008Learning by embryos and the ghost of predation future. Proc. R. Soc. B. 275, 2603–2607 (doi:10.1098/rspb.2008.0754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. W.2009Models of information use. In Foraging: behavior and ecology (eds Stephens D. W., Brown J. S., Ydenberg R. C.). Chicago, IL: University of Chicago Press [Google Scholar]