Abstract
Three Gyps vulture species are on the brink of extinction in South Asia owing to the veterinary non-steroidal anti-inflammatory drug (NSAID) diclofenac. Carcasses of domesticated ungulates are the main food source for Asia's vultures and birds die from kidney failure after consuming diclofenac-contaminated tissues. Here, we report on the safety testing of the NSAID ketoprofen, which was not reported to cause mortality in clinical treatment of scavenging birds and is rapidly eliminated from livestock tissues. Safety testing was undertaken using captive non-releasable Cape griffon vultures (Gyps coprotheres) and wild-caught African white-backed vultures (G. africanus), both previously identified as susceptible to diclofenac and suitable surrogates. Ketoprofen doses ranged from 0.5 to 5 mg kg−1 vulture body weight, based upon recommended veterinary guidelines and maximum levels of exposure for wild vultures (estimated as 1.54 mg kg−1). Doses were administered by oral gavage or through feeding tissues from cattle dosed with ketoprofen at 6 mg kg−1 cattle body weight, before slaughter. Mortalities occurred at dose levels of 1.5 and 5 mg kg−1 vulture body weight (within the range recommended for clinical treatment) with the same clinical signs as observed for diclofenac. Surveys of livestock carcasses in India indicate that toxic levels of residual ketoprofen are already present in vulture food supplies. Consequently, we strongly recommend that ketoprofen is not used for veterinary treatment of livestock in Asia and in other regions of the world where vultures access livestock carcasses. The only alternative to diclofenac that should be promoted as safe for vultures is the NSAID meloxicam.
Keywords: Gyps, vultures, toxicity, ketoprofen, diclofenac, NSAIDs
1. Introduction
Three species of vulture, endemic to South Asia, are in danger of extinction because their food supply is contaminated with diclofenac, a non-steroidal anti-inflammatory drug (NSAID) used for veterinary treatment of livestock (Oaks et al. 2004). Surveys of livestock carcasses across India indicate that over 10 per cent contained diclofenac residues (Taggart et al. 2009) that occur at sufficient concentrations, in relation to dose-dependent mortality of the Oriental white-backed vulture (Gyps bengalensis), indicating that diclofenac is the sole cause of the population crash (Green et al. 2007). Safety testing has established that the NSAID meloxicam is an effective and vulture-safe alternative to diclofenac (Swan et al. 2006b). However, although meloxicam is now used in South Asia and the manufacture and sale of veterinary diclofenac has been banned, meloxicam remains more expensive and human formulations of diclofenac are being used to treat livestock. In this paper, we describe safety-testing experiments on Gyps vultures for ketoprofen, an NSAID already in use in South Asia, following the protocols recommended by Swan et al. (2006b). Previous data indicated that the clinical treatment of vultures and other scavenging birds with ketoprofen resulted in no confirmed mortality associated with kidney damage (Cuthbert et al. 2006), and that it is rapidly eliminated in livestock (EMEA 1995).
2. Material and methods
Experiments were conducted on non-releasable Cape griffon vultures (G. coprotheres) held captive within their normal aviaries in South Africa, and on wild-caught African white-backed vultures (G. africanus) temporarily held captive in Namibia. Both G. coprotheres and G. africanus are as susceptible to diclofenac poisoning as G. bengalensis (Swan et al. 2006a; Naidoo et al. 2009), and thus are suitable surrogates for safety testing. In order to assess the safety of ketoprofen at a range of doses, via different routes of exposure and to two species of Gyps, the study was undertaken over four phases (table 1). The range of doses (0.5–5 mg kg−1) used included doses below and above the likely maximum level of exposure (MLE) that vultures could ingest in the wild. MLE was based upon residue levels of ketoprofen in kidney tissues of cattle (Bos taurus) slaughtered 1 h after three daily injections of ketoprofen, each at 6 mg kg−1 cattle body weight and a vulture consuming 1.023 kg of kidney (see electronic supplementary material). Ketoprofen doses were within the recommended range for clinical treatment, based upon published guidelines for avian treatment (ranging from 1 to 10 mg kg−1; see electronic supplementary material) and records indicating more than 22 birds (including six individuals from three Gyps species) had been safely treated with ketoprofen at 1–9 mg kg−1 (Cuthbert et al. 2006). Residue levels in kidney and liver tissues of cattle were analysed using LC-ESI/MS (Taggart et al. 2009). Results showed that average tissue concentrations in cattle 1 h after the last of the three doses were 7.13 mg kg−1 in kidney and 0.16 mg kg−1 in liver, resulting in an MLE of 1.54 mg kg−1. Ketoprofen used in the trials was sourced from India (Neoprofin, Ranbaxy) for phases 1 and 2, and from South Africa (Ketofen, Merial) for phases 2–4. Details of the route of dosing (oral gavage or cattle meat), phase of the experiment, dose volume and concentration, and sample sizes are reported in table 1. Birds in phase 2 received tissues from five cattle that had received three daily injections of ketoprofen at 6 mg kg−1 and were slaughtered 1 h after the final dose. The kidneys of these cattle weighed 0.785–0.948 kg per animal. After removing 25 g of tissue for analysis, the remaining kidney was fed to the birds: the difference between the weight of kidney fed and 1 kg being made up with liver. Control birds in phase 2 were fed meat from a butcher and assumed free from NSAIDs. Following treatment, all birds in all phases had access to fresh water, food and shade.
Table 1.
Schedule, dose, route and sample sizes for testing of captive Gyps coprotheres and wild G. africanus vultures.
| species | route of dose | ketoprofen concentration | dose volume/mass | dose rate mg kg−1 | n treated | n control | |
|---|---|---|---|---|---|---|---|
| phase 1 | G. coprotheres | oral gavage | 13 mg ml−1 | 0.8–1 ml | 1.3–1.4 | 5 | 3 |
| phase 2 | G. coprotheres | cattle tissues | kidney 7.13 mg kg−1 | 0.79–0.95 kg | 0.5–1 | 5 | 3 |
| liver 0.16 mg kg−1 | 0.06–0.27 kg | ||||||
| phase 3 | G. coprotheres | oral gavage | 90 mg ml−1 | 0.7–1 ml | 5 | 11 | 0 |
| phase 4 | G. africanus | oral gavage | 10 mg ml−1 | 0.7–0.9 ml | 1.5 and 1.8 | 2 | 2 |
Blood samples were collected from the tarsal or wing vein prior to and at 24 and 48 h after dosing in phase 1, 3 and 4, and 16 h before and 48 h after dosing in phase 2 (to avoid birds regurgitating tissues). Plasma levels of uric acid, albumin, creatinine kinase (CK), alanine aminotransferase (ALT), sodium, potassium and calcium were analysed following standard procedures (see electronic supplementary material). The following observations were made: dose and dose volume of ketoprofen administered, feeding behaviour, time to first signs of unusual behaviour (if any), time to euthanasia (if required) or to mortality. A full necropsy, including gross pathology and histopathology (electronic supplementary material), was undertaken on all vultures that died or were euthanased. Statistical analysis of blood parameters was undertaken by two-way analysis of variance with log10-transformed values for the seven blood parameters as the dependent variables. Two models were fitted, the first considered three treatment–outcome categories as one factor (sham-treated and survived, dosed and survived, dosed and died) and two times of sampling (before and after dosing (at 24 h in phases 1, 3 and 4, and 48 h in phase 2)) as a second factor. Because one dosed bird was given emergency remedial treatment and survived, blood values from this individual were excluded from the analysis. The second model treated log10-transformed dose as a continuous independent variable and two times of sampling as a factor, as before, and the dose × time interaction.
3. Results
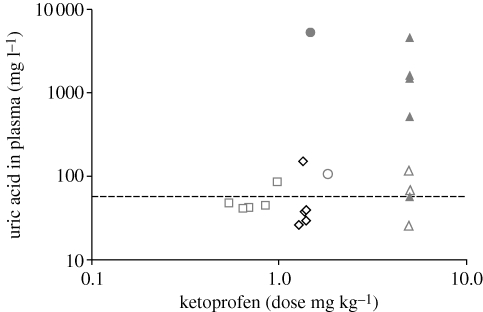
All vultures treated with 0.5–1.4 mg kg−1 of ketoprofen and sham-dosed birds survived and showed no symptoms of toxicity or detectable abnormalities in blood parameters. However, one of the two experimental birds dosed with 1.5 mg kg−1 died, as did 7 of the 11 birds dosed with 5 mg kg−1. Some vultures dosed with 5 mg kg−1 that showed signs of toxicity (prone position) were given remedial treatment with Ringers' lactate (Adcock Ingram, South Africa) and furosemide (Salix) after others in the same experiment had died. One of these birds survived, so the mortality rate at 5 mg kg−1 might have been 8 of 11 birds without intervention. Treatment with Ringers' lactate was also administered to the bird that died after treatment with 1.5 mg kg−1 after it showed signs of toxicity, but it did not recover and was euthanased by the attending veterinarian 29 h after dosing. Signs of toxicity in phases 3 and 4 were noted from 26 to 34 h after dosing, and death occurred after 28–48 h. Analysis of variance (ANOVA) indicated significant changes in three blood parameters (uric acid, ALT and sodium) in relation to treatment-outcome and three (uric acid, ALT and CK) in relation to period alone. Trends in blood concentration with time differed only among treatment-outcome groups for uric acid and potassium, as indicated by significant two-way interaction terms (UA = F2,52 = 23.28, p<0.001; K = F2,46 = 4.31, p<0.02). The second model indicated significant time affects of dose for ALT (F11,16 = 4.38, p<0.01) and CK (F11,16 = 23.25, p<0.001), and significant time × dose interactions for potassium and CK (F10,16 = 3.02, p<0.05 and F11,16 = 13.31, p<0.001, respectively). Following dosing, plasma uric acid levels were up to 40 times higher in birds that died compared with controls or treated birds that survived (figure 1). At necropsy, all birds revealed clinical signs of extensive visceral gout. Histopathology indicated tissue damage primarily in the kidney, with a marked disruption in cell architecture owing to the presence of a large amount of urate tophi. Urate spicules were present in many tubule lumens and were associated with necrosis of adjacent tubule cells. Likewise, spleen, liver and lungs had scattered small tophi throughout.
Figure 1.
Relationship between log10 ketoprofen dose and uric acid concentrations in plasma at 24 h (for phase 1 denotes square symbols, phase 3 triangles and phase 4 denotes circles) and 48 h (phase 2 denotes diamonds). Unfilled symbols represent dosed birds that survived, filled symbols are dosed birds that died, and the horizontal dashed line is the average concentration of uric acid prior to dosing. The mortality in phase 3 with low values of uric acid at 24 h (filled triangle below the control values) subsequently showed a 30-fold increase in uric acid at 33 h post dosing.
4. Discussion
This study demonstrates that ketoprofen is toxic to two species of Gyps vultures at doses that birds could encounter in the wild if they fed upon carcasses of cattle that died within hours of treatment. Moreover, the data raise serious concerns regarding recommended dose levels for ketoprofen for the clinical treatment of birds (currently up to 5–10 mg kg−1), since doses of 1.5 and 5 mg kg−1 produced mortalities. Ketoprofen-related mortality has also been observed in male eider ducks (Mulcahy & Larsen 2003). The timing and symptoms of toxicity, and clinical signs at necropsy (extensive visceral gout and kidney damage) were identical to those found in G. bengalensis, G. afrianus, G. fulvus and G. coprotheres vultures that died after treatment with diclofenac (Oaks et al. 2004; Swan et al. 2006a; Naidoo et al. 2009). Similar findings (gout and/or kidney damage) were also recorded in mortality cases reported in raptors following treatment with the NSAIDs flunixin and carprofen (Cuthbert et al. 2006). This suggests that a common mechanism of toxicity may be responsible for NSAID-related mortality across different orders of birds.
Our initial decision to test the safety of ketoprofen was based partly upon its rapid elimination in livestock (EMEA 1995); since a lower proportion of dosed animals would have residues upon death, than (for example) animals given diclofenac that is eliminated less rapidly. Despite this, results from sampling at carcass dumps across India in 2006 revealed ketoprofen residues in 0.5 per cent of 1488 carcasses (seven cattle and one goat) at tissue concentrations of 0.15–5.6 mg kg−1 (Taggart et al. 2009). Ketoprofen residues are ca 25 times higher in kidney than in cattle liver tissues (present study), suggesting the dose to a vulture consuming kidney tissues from these carcasses is potentially very high and, based on the results of this study, toxic. While the residue prevalence is far lower than that of diclofenac (more than 10%; Taggart et al. 2009), population modelling has demonstrated that just 0.13–0.75 per cent of carcasses need contain a lethal dose in order to cause G. bengalensis populations to decrease at 48 per cent a year (Green et al. 2004). Consequently, the presence of ketoprofen in 0.5 per cent of carcasses represents a potential significant additional source of mortality to that already caused by diclofenac. It is also of concern that several NSAIDs whose safety to vultures has not been tested are in widespread veterinary use within the range of Asian vultures. These include metamizole, phenylbutazone, ibuprofen, naproxen and nimesulide. If, like ketoprofen, these drugs cause mortality in vultures, the response of vulture populations to the eventual removal of diclofenac may be hampered.
To date, meloxicam remains the only NSAID where both safety testing and clinical data demonstrate convincing safety to Gyps vultures and a wider range of avian scavengers (Cuthbert et al. 2006; Swan et al. 2006b). Diclofenac, and now ketoprofen, have been clearly shown to be toxic to Gyps vultures, and carprofen and flunixin are also likely to be toxic (Cuthbert et al. 2006). The veterinary use in livestock of these four NSAIDs must be prohibited and/or very strictly regulated in order to prevent the extinction of Gyps vultures in Asia and potential detrimental impacts to vulture populations in Africa and Europe.
Acknowledgements
The research protocol was approved by the Animal Use and Care Committee of the University of Pretoria and the Research Committee of the Faculty of Veterinary Science, and complied with requirements in the Code for Animal Experimentation. Funding was provided by the Royal Society for the Protection of Birds.
We would like to thank the Ministry of Environment and Tourism in Namibia for permission to undertake safety testing and volunteers from the Rare and Endangered Species Trust Namibia for catching vultures. Comments from Rhys Green and Juliet Vickery improved an earlier version of this manuscript.
References
- Cuthbert R., Parry-Jones J., Green R. E., Pain D. J.2006NSAIDs and scavenging birds: potential impacts beyond Asia's critically endangered vultures. Biol. Lett. 3, 90–93 (doi:10.1098/rsbl.2006.0554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMEA 1995Committee for Veterinary Medicinal Products: Ketoprofen Summary Report. EMEA/MRL/020/95. See http://www.emea.europa.eu/pdfs/vet/mrls/002095en.pdf [Google Scholar]
- Green R. E., Newton I., Shultz S., Cunningham A. A., Gilbert M., Pain D. J., Prakash V.2004Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent. J. Appl. Ecol. 41, 793–800 (doi:10.1111/j.0021-8901.2004.00954.x) [Google Scholar]
- Green R. E., Taggart M. A., Senacha K. R., Raghavan B., Pain D. J., Jhala Y., Cuthbert R.2007Rate of decline of the oriental white-backed vulture population in India estimated from a survey of diclofenac residues in carcasses of ungulates. PLoS ONE 2, e686 (doi:10.1371/journal.pone.0000686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy D. M., Larsen S. R.2003Differential mortality of male spectacled eiders (Somateria fischeri) and king eiders (Somateria spectabilis) subsequent to anesthesia with propofol, bupivacaine, and ketoprofen. J. Avian Med. Surg. 17, 117–123 (doi:10.1647/2001-024) [Google Scholar]
- Naidoo V., Wolter K., Cuthbert R., Duncan N.2009Veterinary diclofenac threatens Africa's endangered vulture species. Regul. Toxicol. Pharm. 53, 205–208 (doi:10.1016/j.yrtph.2009.01.010) [DOI] [PubMed] [Google Scholar]
- Oaks J. L., et al. 2004Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633 (doi:10.1038/nature02317) [DOI] [PubMed] [Google Scholar]
- Swan G., et al. 2006aToxicity of diclofenac to Gyps vultures. Biol. Lett. 2, 279–282 (doi:10.1098/rsbl.2005.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan G., et al. 2006bRemoving the threat of diclofenac to critically endangered Asian vultures. PLoS Biol. 4, 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart M. A., Senacha K., Green R. E., Cuthbert R., Jhala Y., Rahmani A., Meharg A. A., Mateo R., Pain D. J.2009Analysis of nine NSAIDs in ungulate tissues available to critically endangered vultures in India. Environ. Sci. Technol. 43, 4561–4566(doi:10.1021/es9002026) [DOI] [PubMed] [Google Scholar]