Abstract
The brain activity of a fully awake chimpanzee being presented with her name was investigated. Event-related potentials (ERPs) were measured for each of the following auditory stimuli: the vocal sound of the subject's own name (SON), the vocal sound of a familiar name of another group member, the vocal sound of an unfamiliar name and a non-vocal sound. Some differences in ERP waveforms were detected between kinds of stimuli at latencies at which P3 and Nc components are typically observed in humans. Following stimulus onset, an Nc-like negative shift at approximately 500 ms latency was observed, particularly in response to SON. Such specific ERP patterns suggest that the chimpanzee processes her name differently from other sounds.
Keywords: ERPs, auditory processing, name, self, awake chimpanzee
1. Introduction
The environment is full of sensory stimuli, and it is advantageous to focus on those stimuli that are adaptively significant. An individual's own name is one such significant stimulus. Humans tend to pay much more attention to their own name than to other sounds (Wood & Cowan 1995). An individual's name is unique, in a sense, because it has special meaning and represents the individual. Names are thought to play an important role in helping human infants to recognize themselves as different from other individuals and to develop self-recognition. Chimpanzees are one of the species in which self-recognition is present (e.g. Lin et al. 1992; Bard et al. 2006). Chimpanzees attribute names to individuals, including themselves (e.g. Premack & Premack 1972; Rumbaugh 1977). However, their neural processing of self-relevant stimuli has yet to be explored.
This study investigated name processing in a chimpanzee utilizing event-related potentials (ERPs). To date, only one chimpanzee study exists in which brain activity was investigated in response to the individual's name (Berntson & Boysen 1993). Results from that study demonstrated a late positive ERP component in response to the subject's name. However, only two types of sound stimuli were used: the subject's name and a pure tone. It is possible that the brain activities observed in response to the subject's name were merely responses to vocal sounds, irrespective of the types of syllable streams or meanings. In addition, the subject was lightly sedated during measurements. Since the patterns of ERP components in humans are known to differ depending on the subject's awake/sleep condition (Winter et al. 1995), measurements on awake participants are desirable for deciphering endogenous neural activities.
This study obtained ERPs from a fully awake chimpanzee during the presentation of four types of vocal and non-vocal sound stimuli, including the subject's own name (SON). The study focused on two types of ERP component, P3 and Nc, because it has been well-documented that these components reflect attentional mechanisms in humans. P3, the positive component, has been reported to reflect selective attention and resource allocation at a latency of approximately 300 ms following stimulus onset (Polich 2003 for review). Various types of attention-capturing stimuli, both intrinsically and extrinsically relevant ones, have been reported to elicit P3 in humans. Self-relevant stimuli, such as a SON, enlarge P3 responses predominantly in the parietal area (Berlad & Pratt 1995; Gray et al. 2004). Nc is the negative component observed predominantly in the fronto-central area at a latency of approximately 500 ms following stimulus onset. It is thought to relate to how human infants orient attention to salient stimuli (e.g. Reynolds & Richards 2005). Since the ERP pattern observed in an awake chimpanzee (Ueno et al. 2008) appeared somewhat similar to observations in human infants, the present study focused on components that related to attentional mechanisms in both human adults and human infants. Referring to the previously described typical latencies, we investigated whether and how ERP patterns for SON differed from those for other sound stimuli as the manifestation of differential processing of SON.
2. Material and methods
Mizuki, a 9-year-old female chimpanzee (Pan troglodytes) living in the Great Ape Research Institute, Hayashibara Biochemical Laboratories, Inc., Japan, was the participant. When interacting with human carers and experimenters, she had been called by her name from just after birth.
Three types of vocal stimuli and one non-vocal stimulus were presented. The three vocal stimuli consisted of the subject's own name (‘Mi-zu-ki,’ SON), a familiar name of another group member (‘Tsu-ba-ki,’ FN) and an unfamiliar name (‘A-su-ka,’ UN). The non-vocal stimulus (NV) was white noise that had been adjusted to resemble the sound construction of the SON stimulus. All four stimuli had identical root-mean-square amplitude and similar sound constructions, with an accented sound at the first mora. The duration of all stimuli was 450 ms. The sound intensity measured at the chimpanzee's position averaged 83 dB HL, which was within her audible range (Kojima 1990).
Mizuki participated in four recording sessions, each consisting of four to five blocks. During each block, four types of sound stimuli were presented 80 times, with 1500 ms stimulus onset asynchrony, in pseudo-random order with no consecutive repetition of the same stimulus.
The EEG was recorded at five scalp positions (Fz, Cz, Pz, C3 and C4) according to the International 10–20 system for humans. From EEG recording, the peaks of two types of ERP components were identified in the grand average data. The peak of the P3-like component was defined as the most positive peak in the period 200–450 ms following stimulus onset, and the Nc-like component was defined as the most negative peak in the period 450–600 ms following stimulus onset. The peak amplitudes were calculated separately for each stimulus and electrode. Among the five electrodes, the electrode with the largest positive/negative peak amplitude was selected, and the peak latency of that electrode was used for the following calculations. At the respective peak latencies, the amplitude for each epoch was analysed by two-way analysis of variance with factors of stimulus type and electrode, and then by post hoc test corrected for multiple comparisons. We first examined the differences among the stimuli and/or channels, and we then investigated the specificity of SON among the detected differences. For further details, refer to the electronic supplementary material.
3. Results
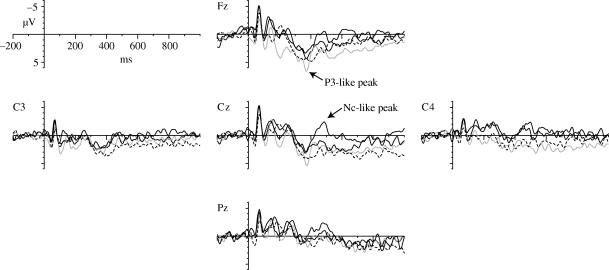
Some positively and negatively shifted ERPs were observed (figure 1). For the amplitude of P3-like components, statistical analysis revealed significance with regard to stimulus type (F3,4780 = 4.93, p < 0.01) and electrode (F4,4780 = 11.68, p < 0.001). A subsequent Tukey's test differed only between subject's name (SON) and group member's name (FN) and between FN and UN (p < 0.01 for both dyads). The P3-like component for FN was significantly greater than for SON and UN. With regard to differences among channels, the P3-like component at Fz was significantly larger than that at C3, C4 and Pz, and the P3-like component at Cz was also significantly larger than that at C4 and Pz (Tukey's test: p < 0.001 for dyads Fz and C4, Fz and Pz and Cz and Pz; p < 0.01 for dyad Cz and C4; p < 0.05 for dyad Fz and C3). No significant differences were observed for any other combination.
Figure 1.
Grand average waveforms of each stimulus at each scalp position. Thick black line, Mizuki (SON); grey line, Tsubaki (FN); thin black line, Asuka (UN); dashed line, white noise (NV).
For the amplitude of Nc-like components, statistical analysis revealed significance with regard to stimulus type (F3,4780 = 9.84, p < 0.001) and electrode (F4,4780 = 3.30, p < 0.05). A subsequent Tukey's test revealed that the negative component for SON was significantly larger than that for any other stimulus (p < 0.001 for dyads SON and FN and SON and non-vocal sound; p < 0.05 for dyad SON and UN). Analysis of differences among channels indicated that the Nc-like component differed only between Fz and Pz (Tukey's test: p < 0.05). The Nc-like component at Pz was significantly larger than that at Fz. No significant differences were detected for any other combination.
No interaction was detected for the P3-like peak (F12,4780 = 0.38, p = 0.97) or for the Nc-like peak (F12,4780 = 0.80, p = 0.65).
4. Discussion
ERPs from a fully awake chimpanzee were measured by presenting four types of sound, which were presumed to differ in meaning and degree of adaptive significance for the subject. In particular, in response to the SON, a negatively shifted ERP was obtained at approximately 500 ms following stimulus onset. To our knowledge, this is the first study in a chimpanzee to elucidate differential neural processing of SON.
The P3-like peaks shifted positively in the fronto-central area in response to all four types of stimuli. Both the stimulus specificity and distribution of the P3-like component differed from that in humans, in which SON typically elicits large P3 response in the parietal area (e.g. Berlad & Pratt 1995). Anatomical discrepancies, such as the brain and skull sizes, distribution of musculature on the skull and its thickness, might contribute to the difference in component distribution (Burrows et al. 2006). It is also possible that neural processing of auditory stimuli differs in some way between the human and chimpanzee. The ERP pattern obtained in the present work was similar to that reported in a previous study (Berntson & Boysen 1993). Taking results from the present and previous studies together, the positive component observed was not considered to reflect specific neural processing of SON. The positive component might reflect the processing of non-monotonous sounds, because all four types of stimuli elicited the component, as did the syllable stream in a previous study (Berntson & Boysen 1993). By contrast, another possibility is that the observed positive component reflected processing of potentially attention-capturing sounds. In the present study, three of the four stimuli were vocal sounds spoken by a very familiar carer, and the remaining sound was a novel non-vocal sound to which the subject had not previously been exposed. Irrespective of the type of sound, all stimuli utilized here could potentially have attracted the subject's attention. The positive component of the group member's name (FN) was greater than that for SON or the UN. Because it is unusual that the name of an absent group member is repeatedly called in an experimental room, the condition could have been strange and attention-capturing for the subject. Such an experimental condition may affect neural responses to FN.
The Nc-like component for SON was significantly larger than for the three remaining stimuli. Such differences in ERP pattern are considered to reflect differential neural processing of SON. Although the distribution of the Nc-like component was not quite similar to that in human (e.g. Reynolds & Richards 2005), it is possible that the observed negative component was the manifestation of an attentional response to salient stimuli with adaptive significance, extreme familiarity or self-relevance for a chimpanzee, as for a human. Mizuki's name may have evoked a specific memory, aroused a mental state (‘emotion’) or evoked a mental representation. The present study cannot determine the details of the cognitive processing that accompanied the observed brain activities. However, compared to the P3-like positive deflection, the Nc-like negative deflection appeared to manifest more selective endogenous neural and cognitive processing in the chimpanzee.
The Nc-like component was not observed in the previous study (Berntson & Boysen 1993). Procedural differences could be responsible for the varying results between the two studies. In the previous study, a single sound was repeatedly presented within a block of time. Therefore, the subject was exposed to a uniform sound stream consisting of her own name. In contrast, in the present study all four stimuli were presented in a pseudo-random order, implying that SON was heard at an unexpected moment each time. The cognitive functions required to process stimuli are thought to differ between these two conditions. The arousal state of the subjects also differed substantially between the two studies. The subject of the previous study was lightly sedated, whereas in the present study, the subject was fully awake during all measurements. Such differences might affect endogenous neural processing of sound stimuli, even though the stimuli potentially share similar adaptive values or meanings.
Acknowledgements
This study was approved by the Animal Welfare and Animal Care Committee of the University of Tokyo and Hayashibara Biochemical Laboratories, Inc.
We thank Dr Naruki Morimura and Asuka Otsuka for their technical support. This study was supported by the Center for Evolutionary Cognitive Science at the University of Tokyo and Grants-in-Aid for Scientific Research (grant nos. 20680015 to S.H., 18200018 to K.H., 19300091 and 20002001 to M.T.) from J.S.P.S.
References
- Bard K. A., Todd B. K., Bernier C., Love C., Leavens D. A.2006Self-awareness in human and chimpanzee infants: what is measured and what is meant by the mark and mirror test? Infancy 9, 191–219 (doi:10.1207/s15327078in0902_6) [Google Scholar]
- Berlad I., Pratt H.1995P300 in response to the subject's own name. Electroencephalogr. Clin. Neurophysiol. 96, 472–474 [DOI] [PubMed] [Google Scholar]
- Berntson G. G., Boysen S. T.1993Vocal perception: brain event-related potentials in a chimpanzee. Dev. Psychobiol. 26, 305–319 (doi:10.1002/dev.420260602) [DOI] [PubMed] [Google Scholar]
- Burrows A. M., Wallers B. M., Parr L. A., Bonar C. J.2006Muscles of facial expression in the chimpanzee (Pan troglodytes): descriptive, comparative and phylogenetic contexts. J. Anat. 208, 62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. M., Ambady N., Lowenthal W. T., Deldin P.2004P300 as an index of station to self-relevant stimuli. J. Exp. Soc. Psychol. 40, 216–224 (doi:10.1016/S0022-1031(03)00092-1) [Google Scholar]
- Kojima S.1990Comparison of auditory functions between chimpanzee and human. Folia Primatol. 55, 62–72 (doi:10.1159/000156501) [DOI] [PubMed] [Google Scholar]
- Lin A. C., Bard K. A., Anderson J. R.1992Development of self-recognition in chimpanzees (Pan troglodytes). J. Compar. Psychol. 106, 120–127 (doi:10.1037/0735-7036.106.2.120) [DOI] [PubMed] [Google Scholar]
- Polich J.2003Theoretical overview of P3a and P3b. In Detection of change: event-related potential and fMRI findings (ed. Polich J.), pp. 83–98 Boston, MA: Kluwer Academic Publishers [Google Scholar]
- Premack A. J., Premack D.1972Teaching language to an ape. Sci. Am. 227, 92–99 [Google Scholar]
- Reynolds G. D., Richards J. E.2005Familiarization, attention, and recognition memory in infancy: an event-related potential and cortical source localization study. Dev. Psychol. 41, 598–615 (doi:10.1037/0012-1649.41.4.598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh D. M.1977Language learning by a chimpanzee: the Lana project New York, NY: Academic Press [Google Scholar]
- Ueno A., et al. 2008Auditory ERPs to stimulus deviance in an awake chimpanzee (Pan troglodytes): towards hominid cognitive neurosciences. PLoS ONE 3, e1442 (doi:10.1371/journal.pone.0001442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter O., Kik A., Kenemans J. L., Elton M.1995Auditory event-related potentials to deviant stimuli during drowsiness and stage 2 sleep. Clin. Neurophysiol. 96, 398–412 [DOI] [PubMed] [Google Scholar]
- Wood N., Cowan N.1995The cocktail party phenomenon revisited: how frequent are attention shifts to one's name in an irrelevant auditory channel? J. Exp. Psychol. Learning Mem. Cognit. 21, 255–260 (doi:10.1037/0278-7393.21.1.255) [DOI] [PubMed] [Google Scholar]