Abstract
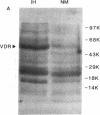
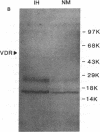
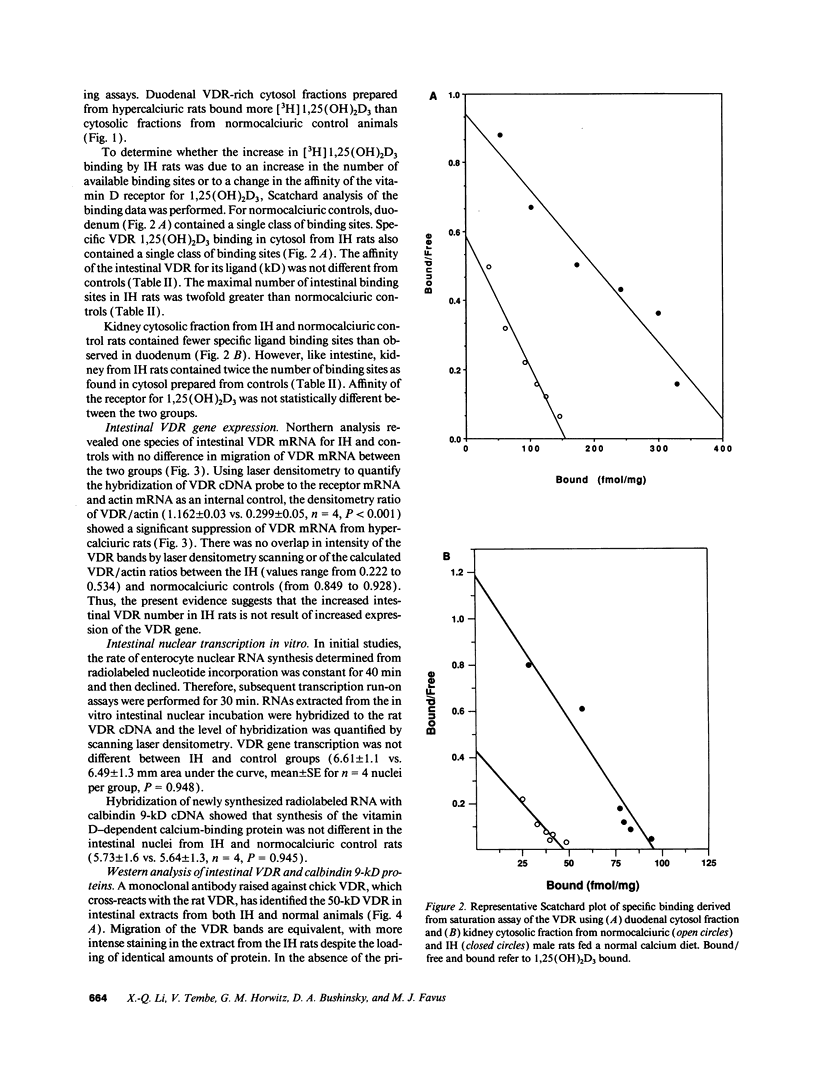
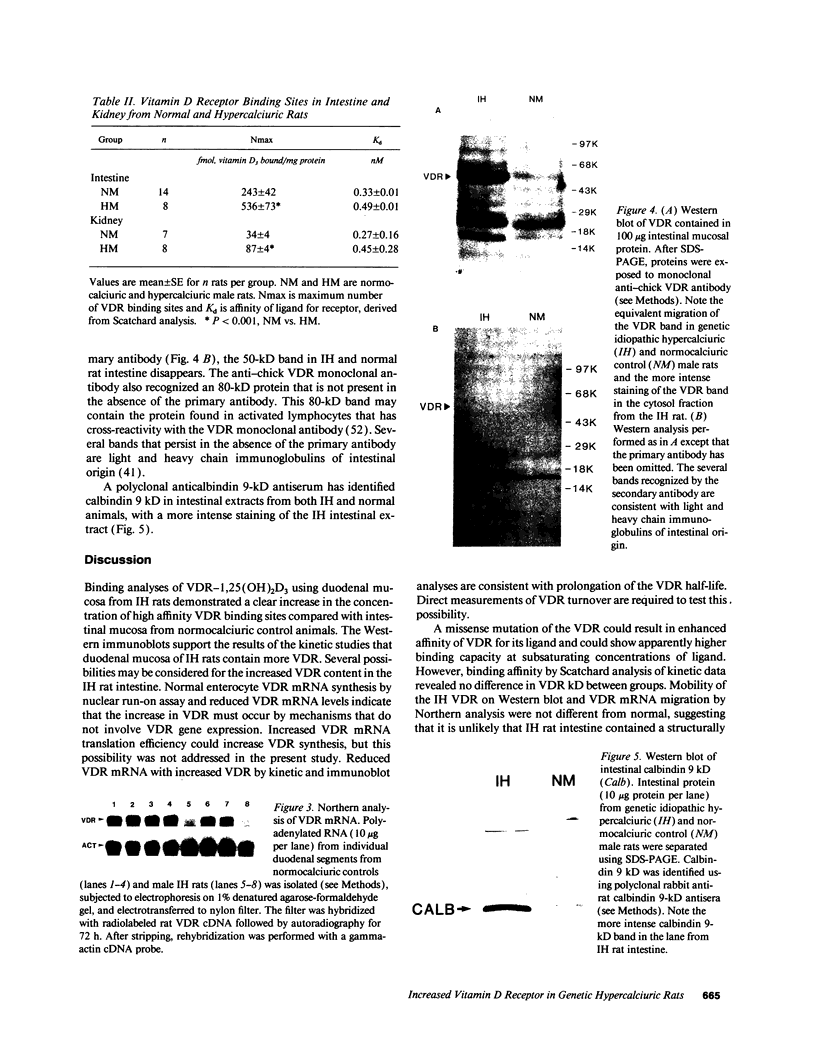
In humans, familial or idiopathic hypercalciuria (IH) is a common cause of hypercalciuria and predisposes to calcium oxalate nephrolithiasis. Intestinal calcium hyperabsorption is a constant feature of IH and may be due to either a vitamin D-independent process in the intestine, a primary overproduction of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], or a defect in renal tubular calcium reabsorption. Selective breeding of spontaneously hypercalciuric male and female Sprague-Dawley rats resulted in offspring with hypercalciuria, increased intestinal calcium absorption, and normal serum 1,25(OH)2D3 levels. The role of the vitamin D receptor (VDR) in the regulation of intestinal calcium absorption was explored in 10th generation male genetic IH rats and normocalciuric controls. Urine calcium excretion was greater in IH rats than controls (2.9 +/- 0.3 vs. 0.7 +/- 0.2 mg/24 h, P < 0.001). IH rat intestine contained twice the abundance of VDR compared with normocalciuric controls (536 +/- 73 vs. 243 +/- 42 nmol/mg protein, P < 0.001), with no difference in the affinity of the receptor for its ligand. Comparable migration of IH and normal intestinal VDR on Western blots and of intestinal VDR mRNA by Northern analysis suggests that the VDR in IH rat intestine is not due to large deletion or addition mutations of the wild-type VDR. IH rat intestine contained greater concentrations of vitamin D-dependent calbindin 9-kD protein. The present studies strongly suggest that increased intestinal VDR number and normal levels of circulating 1,25(OH)2D3 result in increased functional VDR-1,25(OH)2D3 complexes, which exert biological actions in enterocytes to increase intestinal calcium transport. Intestinal calcium hyperabsorption in the IH rat may be the first example of a genetic disorder resulting from a pathologic increase in VDR.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge S. J., Peck W. A., Berman M., Whedon G. D. Study of calcium absorption in man: a kinetic analysis and physiologic model. J Clin Invest. 1969 Sep;48(9):1705–1713. doi: 10.1172/JCI106136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus A. E., Insogna K. L., Lang R., Ellison A. F., Dreyer B. E. Evidence for disordered control of 1,25-dihydroxyvitamin D production in absorptive hypercalciuria. N Engl J Med. 1984 Jul 12;311(2):73–80. doi: 10.1056/NEJM198407123110201. [DOI] [PubMed] [Google Scholar]
- Bruns M. E., Fausto A., Avioli L. V. Placental calcium binding protein in rats. Apparent identity with vitamin D-dependent calcium binding protein from rat intestine. J Biol Chem. 1978 May 10;253(9):3186–3190. [PubMed] [Google Scholar]
- Bushinsky D. A., Favus M. J. Mechanism of hypercalciuria in genetic hypercalciuric rats. Inherited defect in intestinal calcium transport. J Clin Invest. 1988 Nov;82(5):1585–1591. doi: 10.1172/JCI113770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANIGGIA A., GENNARI C., CESARI L. INTESTINAL ABSORPTION OF 45CA IN STONE-FORMING PATIENTS. Br Med J. 1965 Feb 13;1(5432):427–429. doi: 10.1136/bmj.1.5432.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. L., Hauschka P. V., Cabrales S., Feldman D. The effects of 1,25-dihydroxyvitamin D3 and dexamethasone on rat osteoblast-like primary cell cultures: receptor occupancy and functional expression patterns for three different bioresponses. Endocrinology. 1986 Jan;118(1):250–259. doi: 10.1210/endo-118-1-250. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coe F. L., Favus M. J., Crockett T., Strauss A. L., Parks J. H., Porat A., Gantt C. L., Sherwood L. M. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med. 1982 Jan;72(1):25–32. doi: 10.1016/0002-9343(82)90567-8. [DOI] [PubMed] [Google Scholar]
- Coe F. L., Parks J. H., Moore E. S. Familial idiopathic hypercalciuria. N Engl J Med. 1979 Feb 15;300(7):337–340. doi: 10.1056/NEJM197902153000703. [DOI] [PubMed] [Google Scholar]
- Costa E. M., Hirst M. A., Feldman D. Regulation of 1,25-dihydroxyvitamin D3 receptors by vitamin D analogs in cultured mammalian cells. Endocrinology. 1985 Nov;117(5):2203–2210. doi: 10.1210/endo-117-5-2203. [DOI] [PubMed] [Google Scholar]
- Dupret J. M., Brun P., Perret C., Lomri N., Thomasset M., Cuisinier-Gleizes P. Transcriptional and post-transcriptional regulation of vitamin D-dependent calcium-binding protein gene expression in the rat duodenum by 1,25-dihydroxycholecalciferol. J Biol Chem. 1987 Dec 5;262(34):16553–16557. [PubMed] [Google Scholar]
- Favus M. J., Coe F. L., Kathpalia S. C., Porat A., Sen P. K., Sherwood L. M. Effects of chlorothiazide on 1,25-dihydroxyvitamin D3, parathyroid hormone, and intestinal calcium absorption in the rat. Am J Physiol. 1982 Jun;242(6):G575–G581. doi: 10.1152/ajpgi.1982.242.6.G575. [DOI] [PubMed] [Google Scholar]
- Favus M. J. Factors that influence absorption and secretion of calcium in the small intestine and colon. Am J Physiol. 1985 Feb;248(2 Pt 1):G147–G157. doi: 10.1152/ajpgi.1985.248.2.G147. [DOI] [PubMed] [Google Scholar]
- Favus M. J. Familial forms of hypercalciuria. J Urol. 1989 Mar;141(3 Pt 2):719–722. doi: 10.1016/s0022-5347(17)40994-3. [DOI] [PubMed] [Google Scholar]
- Favus M. J., Kathpalia S. C., Coe F. L., Mond A. E. Effects of diet calcium and 1,25-dihydroxyvitamin D3 on colon calcium active transport. Am J Physiol. 1980 Feb;238(2):G75–G78. doi: 10.1152/ajpgi.1980.238.2.G75. [DOI] [PubMed] [Google Scholar]
- Favus M. J., Langman C. B. Evidence for calcium-dependent control of 1,25-dihydroxyvitamin D3 production by rat kidney proximal tubules. J Biol Chem. 1986 Aug 25;261(24):11224–11229. [PubMed] [Google Scholar]
- Favus M. J., Mangelsdorf D. J., Tembe V., Coe B. J., Haussler M. R. Evidence for in vivo upregulation of the intestinal vitamin D receptor during dietary calcium restriction in the rat. J Clin Invest. 1988 Jul;82(1):218–224. doi: 10.1172/JCI113574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., McCain T. A., Hirst M. A., Chen T. L., Colston K. W. Characterization of a cytoplasmic receptor-like binder for 1 alpha, 25-dihydroxycholecalciferol in rat intestinal mucosa. J Biol Chem. 1979 Oct 25;254(20):10378–10384. [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- HODGKINSON A., PYRAH L. N. The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg. 1958 Jul;46(195):10–18. doi: 10.1002/bjs.18004619504. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Norman A. W. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969 Jan;62(1):155–162. doi: 10.1073/pnas.62.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insogna K. L., Broadus A. E., Dreyer B. E., Ellison A. F., Gertner J. M. Elevated production rate of 1,25-dihydroxyvitamin D in patients with absorptive hypercalciuria. J Clin Endocrinol Metab. 1985 Sep;61(3):490–495. doi: 10.1210/jcem-61-3-490. [DOI] [PubMed] [Google Scholar]
- Kaplan R. A., Haussler M. R., Deftos L. J., Bone H., Pak C. Y. The role of 1 alpha, 25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest. 1977 May;59(5):756–760. doi: 10.1172/JCI108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K., Thomas D., Langman C., Eby B. Pathophysiology of spontaneous hypercalciuria in laboratory rats. Role of deranged vitamin D metabolism. J Clin Invest. 1985 Aug;76(2):420–425. doi: 10.1172/JCI111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maierhofer W. J., Gray R. W., Cheung H. S., Lemann J., Jr Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 1983 Oct;24(4):555–560. doi: 10.1038/ki.1983.193. [DOI] [PubMed] [Google Scholar]
- Maierhofer W. J., Lemann J., Jr, Gray R. W., Cheung H. S. Dietary calcium and serum 1,25-(OH)2-vitamin D concentrations as determinants of calcium balance in healthy men. Kidney Int. 1984 Nov;26(5):752–759. doi: 10.1038/ki.1984.212. [DOI] [PubMed] [Google Scholar]
- Malloy P. J., Hochberg Z., Tiosano D., Pike J. W., Hughes M. R., Feldman D. The molecular basis of hereditary 1,25-dihydroxyvitamin D3 resistant rickets in seven related families. J Clin Invest. 1990 Dec;86(6):2071–2079. doi: 10.1172/JCI114944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- McGadey J. A tetrazolium method for non-specific alkaline phosphatase. Histochemie. 1970;23(2):180–184. doi: 10.1007/BF00305851. [DOI] [PubMed] [Google Scholar]
- Méhes K., Szelid Z. Autosomal dominant inheritance of hypercalciuria. Eur J Pediatr. 1980 May;133(3):239–242. doi: 10.1007/BF00496083. [DOI] [PubMed] [Google Scholar]
- Ozono K., Sone T., Pike J. W. The genomic mechanism of action of 1,25-dihydroxyvitamin D3. J Bone Miner Res. 1991 Oct;6(10):1021–1027. doi: 10.1002/jbmr.5650061002. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., East D. A., Sanzenbacher L. J., Delea C. S., Bartter F. C. Gastrointestinal calcium absorption in nephrolithiasis. J Clin Endocrinol Metab. 1972 Aug;35(2):261–270. doi: 10.1210/jcem-35-2-261. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., McGuire J., Peterson R., Britton F., Harrod M. J. Familial absorptive hypercalciuria in a large kindred. J Urol. 1981 Dec;126(6):717–719. doi: 10.1016/s0022-5347(17)54715-1. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., McGuire J., Peterson R., Britton F., Harrod M. J. Familial absorptive hypercalciuria in a large kindred. J Urol. 1981 Dec;126(6):717–719. doi: 10.1016/s0022-5347(17)54715-1. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Oata M., Lawrence E. C., Snyder W. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974 Aug;54(2):387–400. doi: 10.1172/JCI107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C. Y., Waters O., Arnold L., Holt K., Cox C., Barilla D. Mechanism for calcium urolithiasis among patients with hyperuricosuria: supersaturation of urine with respect to monosodium urate. J Clin Invest. 1977 Mar;59(3):426–431. doi: 10.1172/JCI108656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike J. W. Monoclonal antibodies to chick intestinal receptors for 1,25-dihydroxyvitamin D3. Interaction and effects of binding on receptor function. J Biol Chem. 1984 Jan 25;259(2):1167–1173. [PubMed] [Google Scholar]
- Pols H. A., Birkenhäger J. C., Schilte J. P., Visser T. J. Evidence that the self-induced metabolism of 1,25-dihydroxyvitamin D-3 limits the homologous up-regulation of its receptor in rat osteosarcoma cells. Biochim Biophys Acta. 1988 Jun 30;970(2):122–129. doi: 10.1016/0167-4889(88)90170-x. [DOI] [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Norman A. W. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989 Apr 13;320(15):980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L., Orf J. W., Hollis B. W. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984 Jan;58(1):91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L. Self-induction of 1,25-dihydroxyvitamin D3 metabolism limits receptor occupancy and target tissue responsiveness. J Biol Chem. 1989 Sep 25;264(27):15917–15921. [PubMed] [Google Scholar]
- Robertson W. G., Morgan D. B. The distribution of urinary calcium excretions in normal persons and stone-formers. Clin Chim Acta. 1972 Mar;37:503–508. doi: 10.1016/0009-8981(72)90475-5. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren M. E., DeLuca H. F. Serum calcium and vitamin D regulate 1,25-dihydroxyvitamin D3 receptor concentration in rat kidney in vivo. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4312–4314. doi: 10.1073/pnas.87.11.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Shen F. H., Baylink D. J., Nielsen R. L., Sherrard D. J., Ivey J. L., Haussler M. R. Increased serum 1,25-dihydroxyvitamin D in idiopathic hypercalciuria. J Lab Clin Med. 1977 Dec;90(6):955–962. [PubMed] [Google Scholar]
- Strom M., Sandgren M. E., Brown T. A., DeLuca H. F. 1,25-Dihydroxyvitamin D3 up-regulates the 1,25-dihydroxyvitamin D3 receptor in vivo. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9770–9773. doi: 10.1073/pnas.86.24.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf W. E., Sar M., Narbaitz R., Reid F. A., DeLuca H. F., Tanaka Y. Cellular and subcellular localization of 1,25-(OH)2-vitamin D3 in rat kidney: comparison with localization of parathyroid hormone and estradiol. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1149–1153. doi: 10.1073/pnas.77.2.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. A., Walker V. R. Responses to hydrochlorothiazide and acetazolamide in patients with calcium stones. Evidence suggesting a defect in renal tubular function. N Engl J Med. 1980 Mar 27;302(13):709–713. doi: 10.1056/NEJM198003273021302. [DOI] [PubMed] [Google Scholar]
- Vaulont S., Munnich A., Marie J., Reach G., Pichard A. L., Simon M. P., Besmond C., Barbry P., Kahn A. Cyclic AMP as a transcriptional inhibitor of upper eukaryotic gene transcription. Biochem Biophys Res Commun. 1984 Nov 30;125(1):135–141. doi: 10.1016/s0006-291x(84)80345-9. [DOI] [PubMed] [Google Scholar]
- Weber D. V., Coe F. L., Parks J. H., Dunn M. S., Tembe V. Urinary saturation measurements in calcium nephrolithiasis. Ann Intern Med. 1979 Feb;90(2):180–184. doi: 10.7326/0003-4819-90-2-180. [DOI] [PubMed] [Google Scholar]
- Yu X. P., Hustmyer F. G., Garvey W. T., Manolagas S. C. Demonstration of a 1,25-dihydroxyvitamin D3-responsive protein in human lymphocytes: immunologic crossreactivity and inverse regulation with the vitamin D receptor. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8347–8351. doi: 10.1073/pnas.88.19.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwekh J. E., Pak C. Y. Selective effects of thiazide therapy on serum 1 alpha,25-dihydroxyvitamin D and intestinal calcium absorption in renal and absorptive hypercalciurias. Metabolism. 1980 Jan;29(1):13–17. doi: 10.1016/0026-0495(80)90091-8. [DOI] [PubMed] [Google Scholar]