Abstract
Rab GTPases are essential for vesicular transport, whereas adenosine triphosphate (ATP) is the most important and versatile of the activated carriers in the cell. But there are little reports to clarify the connection between ATP and Rab GTPases. A cDNA clone (Rab14) from Bombyx mori was expressed in Escherichia coli as a glutathione S-transferase fusion protein and purified. The protein bound to [3H]-GDP and [35S]-GTPγS. Binding of [35S]-GTPγS was inhibited by guanosine diphosphate (GDP), guanosine triphosphate (GTP) and ATP. Rab14 showed GTP- and ATP-hydrolysis activity. The Km value of Rab14 for ATP was lower than that for GTP. Human Rab14 also showed an ATPase activity. Furthermore, bound [3H]-GDP was exchanged efficiently with GTP and ATP. These results suggest that Rab14 is an ATPase as well as GTPase and gives Rab14 an exciting integrative function between cell metabolic status and membrane trafficking.
Keywords: Bombyx mori, ATP, Rab
1. Introduction
The Rab proteins comprise the largest subgroup of the Ras superfamily of small GTPases. The Rab subgroup includes approximately 70 mammalian members. These proteins act as regulators of trafficking between subcellular compartments in eukaryotic cells (Alvarez et al. 2003; Ali & Seabra 2005).
Like other GTPases, Rab proteins have the capacity to serve as molecular switches by cycling between the guanosine diphosphate-(GDP) and guanosine triphosphate-(GTP) bound forms. The GTP-bound form (active form) of the Rab protein is slowly converted to the GDP-bound form (inactive form) by the intrinsic capacity of the protein to hydrolyse GTP. In turn, activation of the protein involves the replacement of GDP with GTP. On the subcellular membrane, the GTP-bound form of the Rab protein binds many effector proteins and performs a variety of cellular processes (Strick & Elferink 2005; Grosshans et al. 2006; Handley & Burgoyne 2008). After Rab proteins complete their designated functions on the subcellular membrane, they return to the cytosol as the inactive GDP-bound form.
Rab14 is ubiquitously expressed and is localized on the Golgi/trans Golgi network and early endosomes (Junutula et al. 2004; Proikas-Cezanne et al. 2006; Kitt et al. 2008); it has been found on adaptor protein 1A-coated liposomes by using proteomic analysis (Baust et al. 2006). Furthermore, Rab14 is involved in the regulation of glucose transport in muscle cells via the Rab GTPase-activating protein AS160 (Ishikura et al. 2007).
Whereas adenosine triphosphate (ATP) is the most important and versatile of the activated carriers in the cell, it supplies energy for many of the pumps that transport substances into and out of the cell (Kuhlbrandt et al. 1998). It also powers the molecular motors that enable muscle cells to contract (Rayment et al. 1993) and nerve cells to transport materials from one end of their long axons to the other (Kamal & Goldstein 2000). ATP is a signal molecule of protein phosphorylation of protein kinases (Hunter 1987). ATP is also necessary as energy material for membrane transporters (Vergne et al. 2004; Starai et al. 2005; Liu et al. 2007). But there is little report to uncover the relationships between ATP and Rab GTPases.
We previously isolated Rab14 cDNA from Bombyx mori and produced Rab14 proteins in Escherichia coli. The expressed proteins showed [3H]GDP-binding activity (Uno et al. 2004, 2006).
Here, we demonstrate that Rab14 showed ATP binding, was converted from the ATP-bound state to the adenosine diphosphate (ADP)-bound state by intrinsic ATP hydrolysis activity and then returned to the ATP-bound state with the exchange with ATP.
2. Material and methods
(a). Material
Glutathione S-sepharose resin, pGEX6P2, [3H]GDP and [35S]GTPγS came from GE Healthcare UK (Little Chalfont, Buckinghamshire, UK).
(b). Purification of Rab proteins
Cells transformed with pGEX6P2 containing cDNAs of Rab14 were pre-incubated overnight in Luria broth medium, after which the medium was diluted 1 : 100 and incubated for a further 3 h at 37°C. Expression of the glutathione S-transferase (GST)-fusion protein was then induced by adding 0.1 mM isopropyl 1-thio-β-D-galactoside; this was followed by an additional incubation for 24 h at 16°C. Cells were collected by centrifugation and suspended in Tris-buffered saline (TBS; 140 mM NaCl and 50 mM Tris–HCl (pH 7.3)), disrupted by sonication, and then cleared by centrifugation. Aliquots of supernatant were applied to a glutathione S-sepharose column equilibrated with TBS. The column was washed with TBS, and proteins were eluted with 50 mM Tris–HCl (pH 8.0) containing 0.1 M NaCl and 10 mM glutathione. Eluted proteins were dialysed against 50 mM Tris–HCl and 1 mM ethylenediaminetetraacetic acid. The protein concentrations of the eluates were determined in accordance with the method of Lowry et al. (1951). The cDNA fragments containing the entire coding sequences of human Rab14 were amplified by PCR. The protein was expressed and purified as described above. Dominant positive mutant of Rab14 (Q70L) was constructed using site-directed mutagenesis (Kyei et al. 2006).
(c). Determination of [3H]GDP and [35S]GTPγS binding activities
Rab14 (5 µg) was incubated for 2 h at 25°C in a 100 µl mixture containing 50 mM Tris–HCl (pH 8.0), various concentrations of [3H]GDP or [35S]GTPγS, and 5 mM MgCl2. The reaction was stopped by the addition of 1 ml of wash buffer (50 mM Tris–HCl (pH 8.0), 25 mM MgCl2, 0.1 M NaCl and 0.025% bovine serum albumin), followed by rapid transfer to a nitrocellulose membrane (Advantec Toyo, Tokyo, Japan). The nitrocellulose membrane was washed twice with wash buffer and dried. The radioactivity on the nitrocellulose membrane was counted by a liquid scintillation counter (LSC-5100, Aloka, Tokyo, Japan).
(d). Competition for GTPγS binding to GST-Rab14 by nucleotides
A reaction mixture containing 5.0 µg GST-Rab14 and 50 mM Tris–HCl (pH 8.0), was incubated at 25°C for 20 min with 20 µM [35S] GTPγS in the absence or presence of individual nucleotides (GTPγS, GTP, GDP, ATP and ADP) at 20 mM concentrations. The reaction was stopped by the addition of 1 ml of wash buffer, followed by rapid transfer to a nitrocellulose membrane. The radioactivity on the nitrocellulose membrane was counted by an LSC as described above.
(e). Measurement of GTPase or ATPase activity
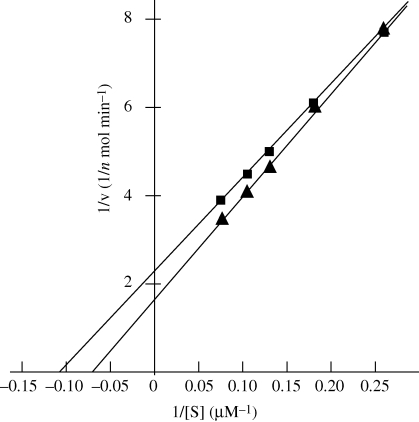
GST-Rab14 (0.5 µg) was incubated at 25°C in 100 µl of reaction mixture (50 mM Tris–HCl (pH 7.8), 1 mM dithiothreitol, 50 µM GTP (or ATP) and 5 mM MgCl2). The reaction was stopped by the addition of 200 µl of Biomol Green Reagent (Biomol Research Laboratories, Pennsylvania, USA). After incubation of the mixture at room temperature for 30 min, the absorbance at a wavelength of 620 nm was measured. The amount of inorganic phosphate liberated was determined by using Na2HPO4 as the standard. As a control, GST was substituted for the GST-Rab14. The amounts of phosphate liberated were calculated by subtracting the values for GST alone from those for GST-Rab14 (figure 1).
Figure 1.
Lineweaver-Burk plots of GTPase activity (filled triangles) and ATPase activity (filled squares).
(f). Exchange of bound [3H]-GDP with nucleotides
[3H]-GDP-bound GST-Rab14 protein was made by incubating GST-Rab14 (5.0 µg) for 1 h at 25°C in a reaction mixture containing 50 mM Tris–HCl (pH 8.0), 5 mM MgCl2 and 20 µM [3H]-GDP. Exchange of [3H]-GDP on GST-Rab14 was initiated by adding a 1000-fold excess of either unlabelled GTP or ATP.
After incubation at 25°C, bound [3H]-GDP was trapped on a nitrocellulose membrane filter and its radioactivity was measured as described for the [3H]-GDP binding assay.
3. Results
(a). GTPγS and GDP binding
First, to determine whether Rab14 bound to GTP and GDP, the binding activity of Rab14 to [35S]GTPγS or [3H]GDP was measured. Rab14 proteins were incubated with [35S]GTPγS or [3H]GDP. The inoculate was passed through a nitrocellulose membrane and the radioactivity of the materials remaining on the membrane was measured, revealing that Rab14 protein bound to GTPγS and GDP. The dissociation constants (Kd) of Rab14 for GTPγS and GDP were calculated to be 20.8 and 5.7 µM, respectively.
Next we examined competitive inhibitions with various nucleotides (table 1). GTP binding was inhibited by GTP, GDP and ATP. These results indicate that Rab14 bound to GTP, GDP and ATP.
Table 1.
Competition for GTPγS binding to GST-Rab14 by nucleotides.
| competitor | GTPγS | GTP | GDP | ATP | ADP |
|---|---|---|---|---|---|
| inhibition (%) | |||||
| Rab14 | 99.0 | 98.6 | 100.0 | 94.5 | 93.4 |
(b). GTP and ATP hydrolysis with Rab
To determine whether Rab14 hydrolysed other nucleotides besides GTP, ATPase and GTPase activities were also measured. Rab14 was incubated with GTP or ATP at 30°C in 25 µl aliquots of reaction mixture. The inorganic phosphate released was calculated by measuring the absorbance at a wavelength of 660 nm.
Hydrolysis reactions proceeded linearly for at least 180 min. The Km values of Rab14 for ATPase and GTPase were 9.71 and 14.5 µM, respectively. These results indicate that Rab14 has GTPase and ATPase activity.
Further, to ascertain whether mammalian Rab14 protein has similar properties or not, activities of human Rab14 were examined. The Km values of human Rab14 for ATPase and GTPase were 7.34 and 20.1 µM, respectively.
Dominant positive mutant (Q70L), which does not show GTPase activity and is a constitutively active mutant, was constructed and expressed in E. coli. This mutant had little activity of GTPase and ATPase (0.1% and 0.1% to wild Rab14 activities, respectively).
This is the first report, to our knowledge, to show that insect Rab proteins is an ATPase as well as a GTPase.
(c). Exchange of [3H]GDP
Next, exchange of [3H]GDP with ATP or GTP was measured. Rates of exchanges of [3H]-GDP with ATP and GTP for 1 min were 79.6 and 69 per cent. This result indicates that Rab14 protein efficiently exchanged GTP with ATP or GTP.
4. Discussion
Guanine nucleotide is a major regulator of Rab proteins in their cycling between the active and inactive forms, similar to the other small GTP-binding proteins. The GTP-bound form (active form) is slowly converted to the GDP-bound form (inactive form) by the intrinsic capacity to hydrolyse GTP. In turn, activation involves the replacement of GDP with GTP.
We found that Rab14 proteins of B. mori bound to GTP and GDP in vitro. Furthermore, Rab14 proteins showed ATPase activity as well as GTPase activity. Comparison of Km values revealed that Rab14 had a stronger affinity for ATP than for GTP. Furthermore, the human Rab14 protein had similar properties.
GDP bound with Rab14 was efficiently exchanged with ATP and with GTP. Rab1, another Rab protein in B. mori, also bound ATP but did not exchange GDP with ATP (exchange rates: GTP 12.6% and ATP 0% for 30 min; data not shown). These results suggest that, like GTP, ATP is a regulatory nucleotide for Rab14.
Dominant positive mutants (Kyei et al. 2006), which do not show GTPase activity, had little activity of GTPase and ATPase. These results suggest that ATP fits the binding pocket of Rab14 proteins. GAP (GTPase activating protein) accelerates the intrinsic GTP hydrolysis rate of Ras superfamily members (Bernards 2003; Bos et al. 2007). It is important to examine whether GAP protein accelerates ATPase activity or not.
ATP is energy material for many of the pumps such as ion transporters and the molecular motors such as myosin. ATP is also necessary to protein transport related to Rab proteins (Vergne et al. 2004; Starai et al. 2005; Liu et al. 2007). But there is little report to clarify the relationship between ATP and Rabs. These results give Rab14 an exciting integrative function between cell metabolic status and membrane trafficking. We are conducting further studies to clarify the importance of ATP in the functions of Rab proteins.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (C), no. 20580053, from the Japan Society for the Promotion of Science.
References
- Ali B. R., Seabra M. C.2005Targeting of Rab GTPases to cellular membranes. Biochem. Soc. Trans. 33, 652–656 (doi:10.1042/BST0330652) [DOI] [PubMed] [Google Scholar]
- Alvarez C., Garcia-Mata R., Brandon E., Sztul E.2003COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol. Biol. Cell. 14, 2116–2127 (doi:10.1091/mbc.E02-09-0625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baust T., Czupalla C., Krause E., Bourel-Bonnet L., Hoflack B.2006Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes. Proc. Natl Acad. Sci. USA 103, 3159–3164 (doi:10.1073/pnas.0511062103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A.2003GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603, 47–82 [DOI] [PubMed] [Google Scholar]
- Bos J. L., Rehmann H., Wittinghofer A.2007GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (doi:10.1016/j.cell.2007.05.018) [DOI] [PubMed] [Google Scholar]
- Grosshans B. L., Ortiz D., Novick P.2006Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl Acad. Sci. USA 103, 11 821–11 827 (doi:10.1073/pnas.0601617103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley M. T., Burgoyne R. D.2008The Rab27 effector Rabphilin, unlike Granuphilin and Noc2, rapidly exchanges between secretory granules and cytosol in PC12 cells. Biochem. Biophys. Res. Commun. 373, 275–281 (doi:10.1016/j.bbrc.2008.06.043) [DOI] [PubMed] [Google Scholar]
- Hunter T.1987A thousand and one protein kinases. Cell 50, 823–829 (doi:10.1016/0092-8674(87)90509-5) [DOI] [PubMed] [Google Scholar]
- Ishikura S., Bilan P. J., Klip A.2007Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem. Biophys. Res. Commun. 353, 1074–1079 (doi:10.1016/j.bbrc.2006.12.140) [DOI] [PubMed] [Google Scholar]
- Junutula J. R., De Maziere A. M., Peden A. A., Ervin K. E., Advani R. J., Van Dijk S. M., Klumperman J., Scheller R. H.2004Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol. Biol. Cell. 15, 2218–2229 (doi:10.1091/mbc.E03-10-0777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A., Goldstein L. S.2000Connecting vesicle transport to the cytoskeleton. Curr. Opin. Cell. Biol. 12, 503–508 (doi:10.1016/S0955-0674(00)00123-X) [DOI] [PubMed] [Google Scholar]
- Kitt K. N., Hernandez-Deviez D., Ballantyne S. D., Spiliotis E. T., Casanova J. E., Wilson J. M.2008Rab14 regulates apical targeting in polarized epithelial cells. Traffic 9, 1218–1231 (doi:10.1111/j.1600-0854.2008.00752.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrandt W., Auer M., Scarborough G. A.1998Structure of the P-type ATPases. Curr. Opin. Struct. Biol. 8, 510–516 (doi:10.1016/S0959-440X(98)80130-9) [DOI] [PubMed] [Google Scholar]
- Kyei G. B., Vergne I., Chua J., Roberts E., Harris J., Junutula J. R., Deretic V.2006Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. EMBO J. 25, 5250–5259 (doi:10.1038/sj.emboj.7601407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Bartz R., Zehmer J. K., Ying Y. S., Zhu M., Serrero G., Anderson R. G.2007Rab-regulated interaction of early endosomes with lipid droplets. Biochim. Biophys. Acta 1773, 784–793 (doi:10.1016/j.bbamcr.2007.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J.1951Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- Proikas-Cezanne T., Gaugel A., Frickey T., Nordheim A.2006Rab14 is part of the early endosomal clathrin-coated TGN microdomain. FEBS Lett. 580, 5241–5246 (doi:10.1016/j.febslet.2006.08.053) [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Base K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M.1993Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261, 50–58 (doi:10.1126/science.8316857) [DOI] [PubMed] [Google Scholar]
- Starai V. J., Thorngren N., Fratti R. A., Wickner W.2005Ion regulation of homotypic vacuole fusion in Saccharomyces cerevisiae. J. Biol. Chem. 280, 16 754–16 762 (doi:10.1074/jbc.M500421200) [DOI] [PubMed] [Google Scholar]
- Strick D. J., Elferink L. A.2005Rab15 effector protein: a novel protein for receptor recycling from the endocytic recycling compartment. Mol. Biol. Cell 16, 5699–5709 (doi:10.1091/mbc.E05-03-0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno T., Nakao A., Katsurauma C.2004Phosphorylation of Rab proteins from the brain of Bombyx mori. Arch Insect Biochem. Physiol. 57, 68–77 (doi:10.1002/arch.20014) [DOI] [PubMed] [Google Scholar]
- Uno T., Nakao A., Fujiwara Y., Katsurauma C., Nakada T., Itoh O.2006Molecular cloning and expression of protein kinase C from Bombyx mori. Arch Insect Biochem. Physiol. 61, 65–76 (doi:10.1002/arch.20098) [DOI] [PubMed] [Google Scholar]
- Vergne I., Fratti R. A., Hill P. J., Chua J., Belisle J., Deretic V.2004Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell 15, 751–760 (doi:10.1091/mbc.E03-05-0307) [DOI] [PMC free article] [PubMed] [Google Scholar]