Abstract
Escape performance is fundamental for survival in fish and most other animals. While previous work has shown that both intrinsic (e.g. size, shape) and extrinsic (e.g. temperature, hypoxia) factors can affect escape performance, the possibility that behavioural asymmetry may affect timing and locomotor performance in startled fish is largely unexplored. Numerous studies have found a relationship between brain lateralization and performance in several cognitive tasks. Here, we tested the hypothesis that behavioural lateralization may affect escape performance in a teleost, the shiner perch Cymatogaster aggregata. Escape responses were elicited by mechanical stimulation and recorded using high-speed video (250 Hz). A number of performance variables were analysed, including directionality, escape latency, turning rate and distance travelled within a fixed time. A lateralization index was obtained by testing the turning preference of each subject in a detour test. While lateralization had no effect on escape directionality, strongly lateralized fish showed higher escape reactivity, i.e. shorter latencies, which were associated with higher turning rates and longer distances travelled. Therefore, lateralization is likely to result in superior ability to escape from predator attacks, since previous work has shown that escape timing, turning rate and distance travelled are among the main determinants of escape success.
Keywords: escape response, fish, lateralization, escape performance, locomotion, anti-predator behaviour
1. Introduction
The ability of fish and many other animals to avoid predation largely depends on their escape performance in terms of timing and locomotion (Ydenberg & Dill 1986; Walker et al. 2005). Fish escape responses are usually mediated by a pair of giant reticulospinal neurons, the Mauthner cells, which ensure short response latencies, of the order of 10–20 ms (Eaton et al. 2001). The typical Mauthner-cell-mediated response consists of a unilateral muscle contraction (stage 1) which bends the body into a ‘C’ (C-start), usually in a direction away from the threat, followed by a contralateral contraction (stage 2, the return flip of the tail) (Eaton et al. 2001; Wakeling 2006).
Escape responses consist of various non-locomotor (e.g. reaction distance, escape latency) and locomotor components (e.g. turning rate, distance-derived performance) (Domenici et al. 2007). Both non-locomotor and locomotor components can affect the survival of fish attacked by predators (Walker et al. 2005). Escape performance can be affected by extrinsic (e.g. environmental factors such as temperature, hypoxia; Wakeling 2006; Domenici et al. 2007) and intrinsic factors (such as body size and shape; Wakeling 2006; Langerhans 2009). However, it is not known whether there are specific functional traits of fish behaviour that may be associated with different levels of escape performance.
One such trait that can be hypothesized as a potential predictor of escape performance is functional lateralization. Once believed to be a unique feature of the human brain, functional lateralization is now recognized to be ubiquitously present among vertebrates (Vallortigara & Bisazza 2002) and other taxa (e.g. arthropods; Ades & Ramires 2002). Recently, various potential selective advantages have been associated with lateralization of cognitive functions (Vallortigara & Rogers 2005). Empirical evidence for advantages of lateralized over non-lateralized individuals has rapidly accumulated in fish, birds and primates in various ecological context, including predator–prey relationships and schooling (McGrew & Marchant 1999; Bisazza & Dadda 2005). Nevertheless, a large proportion of poorly lateralized individuals is commonly observed in animal populations, suggesting potential costs associated with brain lateralization, e.g. strongly lateralized individuals may be at a disadvantage because biologically relevant stimuli are equally likely to appear on either side of the body (Dadda et al. 2009).
Little is known, however, about the possibility that differences in functional asymmetries may be associated with different escape performances. Here, we investigated the escape timing and kinematics in shiner perch (Cymatogaster aggregata) in order to test the hypothesis that behavioural lateralization (assessed using a detour test) may affect escape performance.
2. Material and methods
Escape responses in C. aggregata were elicited by dropping a plastic cylinder onto the water surface (thereby provoking a sudden stimulation which allowed calculation of the escape latency) and recorded using a high-speed camera at 250 frames s−1 (electronic supplementary material). Forty individuals were used for analysis. The following performance variables were measured: escape latency, defined as the time between stimulation and the fish's response; directionality, i.e. whether the C-body bend was oriented ‘towards’ or ‘away’ from the stimulus; turning rate, i.e. the ratio of stage 1 angle/stage 1 duration; distance travelled, i.e. the distance between the fish's centre of mass at the onset of the response and 64 ms later (Domenici et al. 2008; electronic supplementary material), which is proportional to the average speed within that time interval.
Immediately after each escape response trial, fish were subjected to a detour test (Bisazza et al. 1998). Briefly, fish were introduced into a T-maze runway, and their detour to the left or the right at the end of the runway was scored. Ten detour trials were observed for each individual, enabling scoring of the direction and the degree of lateralization of each fish, using a relative lateralization index (LR, from −100 to +100, indicating complete preference for left and right turning, respectively) and an absolute lateralization index (LA, from 0 to 100, i.e. corresponding to the absolute values of LR; electronic supplementary material).
In order to assess whether any of the escape responses observed corresponded (kinematically) to routine turns, fish turning spontaneously while swimming undisturbed were recorded using a high-speed camera at 250 frames s−1 (electronic supplementary material).
3. Results
(a). Detour test
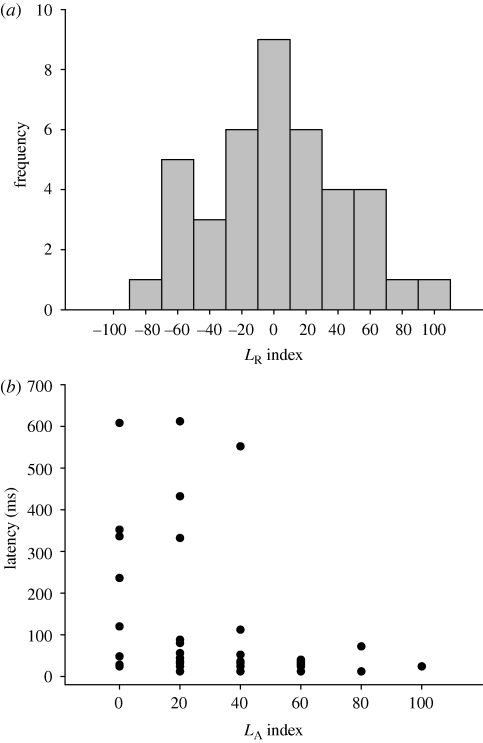
We used LR to analyse turning preference in the detour test. Departures from random choices (0%) were estimated by one-sample two-tailed t-tests performed on the mean values of LR. No statistically significant bias for right or left turns was found (t-test t39 = 0.296, p = 0.769; n = 40). The data distribution was normal (Kolmogorov–Smirnov distribution = 0.119, p = 0.163, n = 40; figure 1a) and its variance did not differ from that of random simulations of left–right turning events (F-test = 0.077, p = 0.783).
Figure 1.
(a) Frequency distribution of LR; (b) the relationship between LA and escape latency.
(b). Escape responses
Although fish tended to respond with a C-bend directed away from the stimulus more often than towards it (62.5% away responses versus 37.5% towards responses), directionality was not statistically biased (binomial test p = 0.154; n = 40). There was no significant correlation between LA and directionality (logistic regression: n = 40, model p = 0.756, LA index p = 0.756, constant p = 0.228, Nagelkerke R2 = 0.003). Escape latency was inversely related to LA (Spearman's correlation coefficient = 0.419, p = 0.007, figure 1b). We conducted a stepwise multiple regression analysis where both LA and stimulus distance were entered as factors into the regression model in order to rule out any possible bias due to stimulus distance (Domenici & Batty 1997). Results showed that the only significant factor was LA (t = 2.359, p = 0.024), suggesting that lateralization directly affects latency, which is not influenced by stimulus distance within the range of distances we used (11.8–37.6 cm).
Locomotor performance (turning rates and distance travelled) could only be determined for 37 individuals, as in three cases the final part of stage 1 was outside the field of view of the camera. Turning rate was significantly related to LA (Spearman's correlation coefficient = 0.387, p = 0.018) and to escape latency (Spearman's correlation coefficient = 0.528, p < 0.001). We conducted a stepwise multiple regression analysis where both LA and latency values were entered as factors into the regression model in order to evaluate their respective relationship with turning rate. Results showed that the only significant factor was latency (t = 6.402, p < 0.001). Moreover, we examined collinearity with the variance inflation factor (VIF). Both values were well below 10 (1 and 1.94 for latency and LA, respectively), indicating that no overlap was present between these factors in the regression model, allowing us to estimate separately their strength of relationship with turning rate (electronic supplementary material). Therefore, LA directly affects latency, and latency directly affects turning rate, so that turning rate is indirectly related to LA. Distance travelled was related to LA (Spearman's correlation coefficient = 0.309, p = 0.032) and escape latency (Spearman's correlation coefficient = 0.384, p = 0.019). As for turning rate, a stepwise multiple regression with LA and latency scores as factors showed that the only significant factor was latency (t = 3.145, p = 0.003). Values of collinearity (VIF) for both factors were well below 10 (1 and 1.09 for latency and LA, respectively), indicating no collinearity. Hence, latency directly affects distance travelled and LA directly affects latency, so that the distance travelled is indirectly related to LA.
(c). Turning rates in spontaneous turns versus escape responses
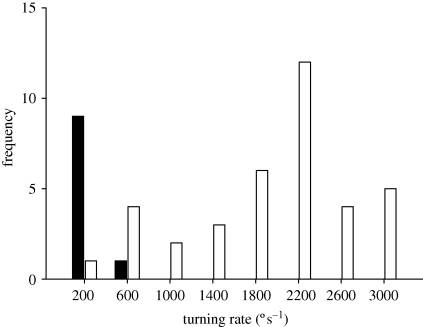
The frequency distributions of turning rates in spontaneous turning and escape responses show a minor overlap (figure 2). The turning rates of spontaneous turns ranged between 68–480° s−1 (277.3 ± 114° s−1, mean ± s.d., n = 10). Only one of the escape responses showed a turning rate within this range, and four others were lower than twice the highest spontaneous turning rate recorded (i.e. 480° s−1). The range of escape turning rates was 389–3139° s−1 (mean 1909 ± 375° s−1, n = 37).
Figure 2.
Frequency distribution of turning rates in spontaneous turns (black bars) and escape responses (empty bars).
4. Discussion
Wild shiner perch shows a normal distribution of LR, with no bias at the sample level, neither in terms of left/right turning preference (in line with Vallortigara & Bisazza 2002), nor with respect to random fluctuations, with 30 per cent of the individuals showing absolute lateralization indices greater than or equal to 60. A potential disadvantage of hemispheric specialization might be that relevant natural stimuli can be located randomly to the animal's left or right side (Vallortigara & Rogers 2005), and more importantly, predators could exploit the predictability of behaviour that arises from the population level lateral bias. Our results show, however, that there are no effects of functional asymmetry (LA) on the directionality of the escape response. On the other hand, if a directional bias overriding stimulation were present in lateralized individuals, we would have expected a higher directionality (i.e. a larger proportion of ‘away’ responses) in non-lateralized individuals. It is possible, however, that escape directionality might be affected by lateralization when predator inspection occurs, since prey may inspect approaching predators from a preferred orientation (Vallortigara & Bisazza 2002). The proportion of away responses (62.5%), although not significantly higher than random, is in line with the data on gregarious fish tested as solitary individuals (Domenici & Batty 1997).
Lateralization enhances cognitive capacity and efficiency of the brain, counteracting potential ecological disadvantages derived from biases in the sensory–motor system (Vallortigara & Rogers 2005). Despite this evidence, little is known about the effect of functional lateralization on escape performance. Our work shows that behavioural lateralization is associated with short escape latencies. This is in line with previous work showing improved brain functions and reduced latencies in several cognitive tasks in lateralized individuals of different species such as birds (Vallortigara & Rogers 2005) and primates (Hopkins & Bennett 1994), but in contrast with results on escape latencies in the teleost G. falcatus (Agrillo et al. 2009). Differences with Agrillo et al. (2009) may be related to differences in time scales (mean latencies 380 ms versus 119 ms in our work), temporal resolution used (25 Hz 2009 versus 250 Hz) and the type of sensory stimulation (visual versus mechanical).
Although escape responses triggered by alternative (non-Mauthner) circuits have been observed, these show longer latencies than Mauthner-cell responses (Eaton et al. 2001). Here, half of the responses in fish with a relatively low LA (i.e. LA < 50) showed relatively long latencies (longer than 50 ms, i.e. slower than typical Mauthner cell responses; Eaton et al. 2001), while only 8 per cent of highly lateralized individuals (i.e. with LA > 50) escaped with such long latencies. Therefore, it is possible that non-lateralized individuals possess a higher sensory threshold for the activation of the Mauthner cells, perhaps due to a generalized higher complexity of neural control for their responses, in line with Levy's suggestion that lateralization might decrease the redundancy of neural operations (Levy 1969).
Turning rate is a relevant measure of muscular performance and manoeuvrability, as high turning rates allow fish to change direction more rapidly and avoid predation (Walker et al. 2005). Responses with slow turning rates are often associated with long latencies (Domenici & Batty 1994). It is therefore not surprising that turning rates decrease with increasing latencies. At the mechanistic level, our results suggest a shift in neural control from fast turning, short latency responses (most probably mediated by the Mauthner cells) to slow turning, long latency responses (most probably mediated by alternative neural circuits) in relation to a decrease in the degree of lateralization. Some of the escape turns observed here are relatively slow, i.e. close to the range of spontaneous turns (figure 2), similar to previous work on herring (Domenici & Batty 1994). While this implies that some of the escapes we observed might be considered spontaneous turns (therefore possibly not triggered by the Mauthner cells or other reticulospinal neurons), at the performance level this makes no difference in terms of potential escape success. Since turning rates are indicative of muscle performance and therefore thrust production, it is not surprising that the results for distance travelled show a similar trend, suggesting that lateralized fish swim faster during escape responses than non-lateralized fish.
At the ecological level, lateralization may increase escape success when facing predator attacks as the variables measured are key factors in avoiding predation (Walker et al. 2005). This does not necessarily imply that lateralized individuals are less vulnerable to predation, because other factors such as prey boldness/shyness affect risk of predation and lateralized individuals may be bolder than non-lateralized ones (Reddon & Hurd 2009). Taken together, these results provide, to our knowledge, the first data on a possible association between brain lateralization and the main performance components of fish escape responses, with important implications in terms of control and dynamics of anti-predator responses in lower vertebrates. This framework is consistent with the previous work on the possible advantages of lateralized individuals in several situations that influence fitness (Bisazza & Dadda 2005; Vallortigara & Rogers 2005).
Acknowledgements
We thank the Friday Harbor Laboratories for financial and logistic support, Prof. John Steffensen and the 565 fish class for useful inputs and Prof. Angelo Bisazza and two anonymous referees for comments.
Footnotes
One contribution of 11 to a Special feature on ‘Control and dynamics of animal movement’.
References
- Ades C., Ramires E. N.2002Asymmetry of leg use during prey handling in the spider Scytodes globula (Scytodidae). J. Insect Behav. 15, 563–570 (doi:10.1023/A:1016337418472) [Google Scholar]
- Agrillo C., Dadda M., Bisazza A.2009Escape behaviour elicited by a visual stimulus. A comparison between lateralised and non-lateralised female topminnows. Laterality 14, 300–314 [DOI] [PubMed] [Google Scholar]
- Bisazza A., Dadda M.2005Enhanced schooling performance in lateralized fishes. Proc. R. Soc. B 272, 1677–1681 (doi:10.1098/rspb.2005.3145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A., Facchin L., Pignatti R., Vallortigara G.1998Lateralization of detour behaviour in poeciliid fish: the effect of species, gender and sexual motivation. Behav. Brain Res. 91, 157–164 (doi:10.1016/S0166-4328(97)00114-9) [DOI] [PubMed] [Google Scholar]
- Dadda M., Zandonà E., Agrillo C., Bisazza A.2009The costs of hemispheric specialization in a fish. Proc. R. Soc. B 276, 4399–4407 (doi:10.1098/rspb.2009.1406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P., Batty R. S.1994Escape manoeuvres of schooling Clupea harengus. J. Fish Biol. 45, 97–110 (doi:10.1111/j.1095-8649.1994.tb01086.x) [Google Scholar]
- Domenici P., Batty R. S.1997Escape behaviour of solitary herring (Clupea harengus) and comparisons with schooling individuals. Mar. Biol. 128, 29–38 (doi:10.1007/s002270050065) [Google Scholar]
- Domenici P., Lefrancois C., Shingles A.2007Hypoxia and the antipredator behaviours of fishes. Phil. Trans. R. Soc. B 362, 2105–2121 (doi:10.1098/rstb.2007.2103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P., Turesson H., Brodersen J., Brönmark C.2008Predator-induced morphology enhances escape locomotion in crucian carp. Proc. R. Soc. B 275, 195–201 (doi:10.1098/rspb.2007.1088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. C., Lee R. K. K., Foreman M. B.2001The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog. Neurobiol. 63, 467–485 (doi:10.1016/S0301-0082(00)00047-2) [DOI] [PubMed] [Google Scholar]
- Hopkins W. D., Bennett A. J.1994Handedness and approach-avoidance behavior in chimpanzees (Pan). J. Exp. Psychol. Anim. Behav. Process. 20, 413–418 (doi:10.1037/0097-7403.20.4.413) [PubMed] [Google Scholar]
- Langerhans R. B.2009Morphology, performance, fitness: Functional insight into a post-Pleistocene radiation of mosquitofish. Biol. Lett. 5, 488–491 (doi:10.1098/rsbl.2009.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J.1969Possible basis for the evolution of lateral specialization of the human brain. Nature 224, 614–615 (doi:10.1038/224614a0) [DOI] [PubMed] [Google Scholar]
- McGrew W. C., Marchant L. F.1999Laterality of hand use pays off in foraging success for wild chimpanzees. Primates 40, 509–513 (doi:10.1007/BF02557586) [Google Scholar]
- Reddon A. R., Hurd P. L.2009Individual differences in cerebral lateralization are associated with shy-bold variation in the convict cichlid. Anim. Behav. 77, 189–193 (doi:10.1016/j.anbehav.2008.09.026) [Google Scholar]
- Vallortigara G., Bisazza A.2002How ancient is brain lateralization? In Comparative vertebrate lateralization (eds Rogers L. J., Andrew R. J.), pp. 9–69 Cambridge, UK: Cambridge University Press [Google Scholar]
- Vallortigara G., Rogers L. J.2005Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–589 [DOI] [PubMed] [Google Scholar]
- Wakeling J. M.2006Fast-start mechanics. In Fish biomechanics: fish physiology, vol. 23 (eds Shadwick R. E., Lauder G. V.), pp. 333–368 San Diego, CA: Academic Press [Google Scholar]
- Walker J. A., Ghalambor C. K., Griset O. L., McKenney D., Reznick D. N.2005Do faster starts increase the probability of evading predators? Funct. Ecol. 19, 808–815 (doi:10.1111/j.1365-2435.2005.01033.x) [Google Scholar]
- Ydenberg R. C., Dill L. M.1986The economics of fleeing from predators. Adv. Stud. Behav. 16, 229–249 (doi:10.1016/S0065-3454(08)60192-8) [Google Scholar]