Abstract
Frogs that build foam nests floating on water face the problems of over-dispersion of the secretions used and eggs being dangerously exposed at the foam : air interface. Nest construction behaviour of túngara frogs, Engystomops pustulosus, has features that may circumvent these problems. Pairs build nests in periodic bursts of foam production and egg deposition, three discrete phases being discernible. The first is characterized by a bubble raft without egg deposition and an approximately linear increase in duration of mixing events with time. This phase may reduce initial over-dispersion of foam precursor materials until a critical concentration is achieved. The main building phase is marked by mixing events and start-to-start intervals being nearly constant in duration. During the final phase, mixing events do not change in duration but intervals between them increase in an exponential-like fashion. Pairs joining a colonial nesting abbreviate their initial phase, presumably by exploiting a pioneer pair's bubble raft, thereby reducing energy and material expenditure, and time exposed to predators. Finally, eggs are deposited only in the centre of nests with a continuously produced, approximately 1 cm deep egg-free cortex that protectively encloses hatched larvae in stranded nests.
Keywords: Amphibia, túngara frog, Engystomops pustulosus, foam nest, nest construction behaviour
1. Introduction
Animals create foams for many purposes, the most widespread being in reproduction or protection of juvenile forms. The largest such constructions produced by land animals are the nests of species of frogs that form foam enclosures for their eggs and refuges for their hatched larvae. These nests are remarkable for their stability under harsh environmental conditions, and the diversity of sites in which they are produced, such as on the surface of water, in burrows or suspended in vegetation.
Foams need mechanical processing for the incorporation of air bubbles, and the processes and materials required may differ between types of foam. Those produced by frogs that build nests floating on, and with, water face particular biophysical challenges; the foams must contain surfactant(s) to reduce the surface tension of water, and anti-microbial factors, all of which must be compatible with naked eggs and sperm (Cooper et al. 2004; Fleming et al. 2009; Mackenzie et al. 2009). Also, the construction behaviour must avoid over-dispersal of the secretions used in water, and the eggs must be deposited so that they are not dangerously exposed at the foam : air interface, yet have sufficient access to oxygen should oxygen storage in foam be limited (Seymour 1999).
Túngara frogs, Engystomops pustulosus Cope, 1864 Leiuperidae, nest on the surface of small, often temporary, ponds (Ryan 1985). The males collect eggs and foam precursor fluid from the female's cloaca with their feet, and create a roughly hemispherical mound of foam incorporating pond water by a mixing action of their legs. Nest construction proceeds as a series of short mixing events, the whole process taking about an hour (Breder 1946; Heyer & Rand 1977). Nest construction appears to be a simple process of regular, periodic bouts of egg release and foam production that should result in eggs being disorderedly distributed throughout the foam. But, as shown here, the nesting process is sophisticated and divisible into three distinct phases that are characterized by progressive changes in the duration of, and intervals between, mixing events, and the nests have a differentiated internal structure.
2. Material and methods
Amplexing pairs of E. pustulosus were collected at night after heavy rain around village areas in the Northern Range of Trinidad, West Indies, in July 2002, 2004 and 2008, returned to the laboratory and placed into tanks of fresh dechlorinated water and allowed to nest. Digital video films were made under low artificial light conditions, and analysed frame by frame at critical events. The beginning and end time points for every mixing event were recorded throughout 16 complete nestings to a resolution of 0.04 s. Plotting and curve fitting were carried out using standard procedures using Origin software. Collections were approved by the Wildlife Division of Trinidad and Tobago, and all frogs were returned to their collection areas after observation.
3. Results
(a). Three-phase nest construction
A semi-dispersed raft of bubbles is produced initially, during which no eggs are released, followed by the appearance of a growing mound into which eggs are incorporated. The males keep their lungs inflated throughout the nesting process, and arch their spines considerably to accommodate the height of the nest as it enlarges (electronic supplementary material, video S1). Time lapse filming reveals that they move regularly from side to side, presumably to ensure proper shaping of a hemisphere (electronic supplementary material, video S2).
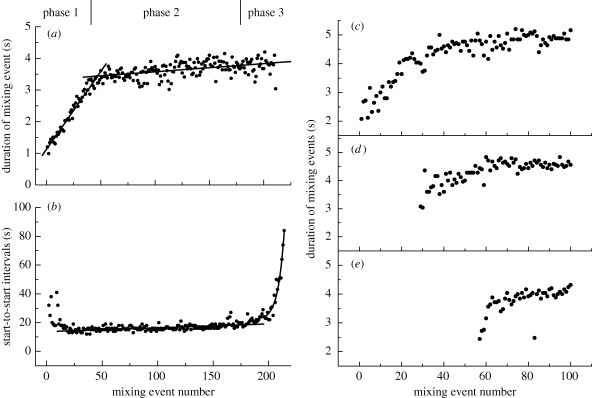
The duration of each mixing event was measured throughout 16 nestings, revealing that mixings are initially short, but increase in duration linearly from about 1 s to a plateau of approximately 4.5 s which is then sustained with little variation until nest completion (figure 1a).
Figure 1.
Three-phase nest building in singleton and colonial nestings. (a) Duration of mixing events throughout a typical nesting, the linear rising phase defining phase 1 corresponding to the initiation, bubble raft phase merging into the main building phase 2. Linear regression lines fitted from event numbers 0 to 50, and from event 50 to termination. (b) Time intervals between the beginnings of each mixing event for the same nesting. Phase 3—termination phase. The fitted lines are a linear regression from event 25 to 180, and an exponential fit from point 180 to the end. (c–e) Duration of mixing events in colonial nesting. (c) Pioneer pair; (d,e) follower pairs. Event numbers given from the beginning of nesting by the pioneer pair. All times are expressed in seconds (s).
The time between the beginnings of successive mixing events is irregular at first, but then settles to regular intervals of about 14 s that are maintained throughout the main building phase (figure 1b). Towards the end of nesting, the time between mixings increases in an exponential-like manner until cessation, though the duration of mixings does not change.
When the pairs are disturbed (in the wild or in the laboratory) and leave the nest, they tend to return to it, reversing into their original position to resume (n = 10). When disturbance occurs, the periods between mixing event beginnings are irregular but soon return approximately to the original periodicity (electronic supplementary material, figure S1).
Thus, nesting is divisible into three discrete overlapping phases. Phase 1 is characterized by the production of a bubble raft with no egg release and mixing events increasing in duration. Phase 2 is the main building phase, marked by both event duration and start-to-start intervals being roughly constant as the foam hemisphere develops. Phase 3 is the termination phase, when duration of mixings does not change but the time between them rises in an exponential-like fashion to cessation.
(b). Colonial nesting
Túngara frogs frequently nest colonially, where many nests are laid in contact with one another, but not co-mixed (Ryan 1985; Zina 2006). Repeating the above analysis for colonial nestings (n = 3) showed that a pioneer pair exhibits the expected gradual increase in duration of mixing events, but the follower pairs abbreviate their initiation phase, beginning with mixings of longer duration, and reach the plateau phase after fewer events (figure 1c–e and electronic supplementary material, figure S2a). When pairs begin a colonial nest simultaneously, there is no such effect (electronic supplementary material, figure S2b).
(c). Nest structure and larval refuge
Vertical sections show eggs concentrated close to the base in the core of the nest, with a distinct cortex of egg-free foam approximately 1 cm deep (figure 2 and electronic supplementary material, figure S3). When nests were constructed against glass, or examined early in building, the eggs were seen to have been placed exclusively in the core, continuously from the outset (not shown). When a completed nest is placed on a dry surface, mimicking post-spawning drying of the natal pond, hatching tadpoles descend to the base of the nest but remain confined to its core, and do not breach the egg-free cortex despite the core's foam becoming fluidized (figure 2c,d). Interestingly, larvae can communicate between the cores of two nests in contact without breaching the protective cortex (electronic supplementary material, video S3).
Figure 2.
Nest structure and refuge. (a) Singleton nest built in the laboratory. (b) Nest sectioned vertically through its centre, lit from below showing egg-free cortex. (c) Laboratory and (d) wild-collected nests placed on a dry Petri dish, incubated for three days until hatching is complete, viewed from below.
4. Discussion
Nest construction by túngara frogs is a sophisticated process in terms of nest architecture and construction process. The initial bubble raft phase seems wasteful of material, energy and delayed oviposition, but may be essential to the production of a correctly proportioned mixture of the female's secretions and water. Mixing precursor fluid directly with pond water would result in rapid dilution of the surfactant proteins required to create foam, so the local concentration of foam components would first need to be raised to an appropriate level before the main building phase can begin. The gradual increase in the duration of mixing events in this phase may solve this problem; prolonged mixings initially may disperse and dilute the fluid excessively, whereas a gradual increase would progressively lead to a coalescent bubble raft until a critical concentration is reached for full quality building foam. The gradual increase in duration might also result from a progressive reduction in viscosity of the medium being processed; the males initially mix foam fluid directly with water, and then increasingly with air as the foam develops. So, the increasing duration of mixings could be functionally important in progressing to a critical concentration of foam materials, or a result of the changing effort required, or both. The fact that pairs return to a nest after disturbance may mean that once females begin to release eggs, they cannot regress to the bubble raft phase without wastefully releasing eggs.
A key element in the production of correct proportions of nest foam precursor fluid and its mixing with water will be the positioning of the pair relative to the water surface. The males were always observed to keep their lungs inflated throughout nesting (electronic supplementary material, video S1), which will have the effect of providing buoyancy and lateral stability for correct positioning at the water surface, and accommodate differing depths of water.
The advantages to the egg-free cortex may be manifold. First, protecting the eggs from dehydration by distancing them from exposure to air. Second, shielding the eggs from light damage; the nests are exposed to direct sunlight but the white foam will be scattering light of all visible wavelengths effectively. Ultraviolet light should be similarly scattered or absorbed by protein in the foam. Túngara frogs have unpigmented or faintly pigmented eggs, so the depth of the cortex through which any stray light must pass would be important for light scatter to be adequately protective. Third, the depth of the double cortices of adjacent nests in a communal nest will probably limit cross-fertilization of eggs deposited in the cores. In relation to this, we have noticed that, during initiation of communal nestings, the male of one pair will issue a call that induces an encroaching pair to move away (M. W. Kennedy 2002, 2004, 2008, unpublished data). Lastly, a cortex would reduce access to the eggs by predators or parasites, which would be given added effect by surfactants in the foam as anti-insect defence (Rostás & Blassmann 2009).
Colonial nesting appears to be preferred by túngara frogs and allied species (Ryan 1985; Zina 2006), but how do the advantages counterbalance the risks? Colonial nests may be more attractive to specialist nest parasites such as frog flies (Downie et al. 1995; Vonesh 2000; Menin & Giaretta 2003; Giaretta & Menin 2004) because such nests are probably easier to locate, and infestation of one nest could permit access to all. Advantages to the frogs may include a decreased aggregate surface : volume ratio, and hence reduced evaporative water loss (Zina 2006). Additionally, joining a nesting already in progress allows exploitation of a pre-existing bubble raft and consequent saving of nest material, energy expenditure and time exposed to predators.
Acknowledgements
This study was supported by the Carnegie Trust for the Universities of Scotland and the Wellcome Trust. Thanks go to Margaret Nutley, Rachel Fleming, Cameron Mackenzie, Gail McLeary and Mary Kennedy for assistance with field collections and filming, to Ross Culloch, Claire Paterson and David Brown for assistance with video analysis, Roger Downie for advice on frog biology and Alan Cooper on biophysics.
References
- Breder C. M.1946Amphibians and reptiles of the Rio Chucunaque Drainage, Darien, Panama, with notes on their life histories and habits. Bull. Am. Mus. Nat. Hist. 86, 381–435 [Google Scholar]
- Cooper A., Kennedy M. W., Fleming R. I., Wilson E. H., Videler H., Wokosin D. L., Su T.-J., Green R. J., Lu J. R.2004Adsorption of frog foam nest proteins at the air–water interface. Biophys. J. 88, 2114–2125 (doi:10.1529/biophysj.104.046268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. R., Disney R. H. L., Collins L., Hancock E. G.1995A new species of Megaselia (Diptera, Phoridae) whose larvae prey upon the eggs of Leptodactylus fuscus (Anura, Leptodactylidae). J. Nat. Hist. 29, 993–1003 (doi:10.1080/00222939500770371) [Google Scholar]
- Fleming R. I., Mackenzie C. D., Cooper A., Kennedy M. W.2009Foam nest components of the túngara frog: a cocktail of proteins conferring physical and biological resilience. Proc. R. Soc. B 276, 1787–1795 (doi:10.1098/rspb.2008.1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaretta A. A., Menin M.2004Reproduction, phenology and mortality sources of a species of Physalaemus (Anura: Leptodactylidae). J. Nat. Hist. 38, 1711–1722 (doi:10.1080/00222930310001597286) [Google Scholar]
- Heyer W. R., Rand A. S.1977Foam nest construction in leptodactylid frogs Leptodactylus pentadactylus and Physalaemus pustulosus (Amphibia, Anura, Leptodactylidae). J. Herpitol. 11, 225–228 (doi:10.2307/1563148) [Google Scholar]
- Mackenzie C. D., Smith B. O., Meister A., Blume A., Zhao X., Lu J. R., Kennedy M. W., Cooper A.2009Ranaspumin-2: structure and function of a surfactant protein from the foam nests of a tropical frog. Biophys. J. 96, 4984–4992 (doi:10.1016/j.bpj.2009.03.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menin M., Giaretta A. A.2003Predation on foam nests of leptodactyline frogs (Anura: Leptodactylidae) by larvae of Beckeriella niger (Diptera: Ephydridae). J. Zool. 261, 239–243 (doi:10.1017/S0952836903004138) [Google Scholar]
- Rostás R., Blassmann K.2009Insects had it first: surfactants as a defence against predators. Proc. R. Soc. B 276, 633–638 (doi:10.1098/rspb.2008.1281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. J.1985The Túngara frog Chicago, IL: The University of Chicago Press [Google Scholar]
- Seymour R. S.1999Respiration of aquatic and terrestrial amphibian embryos. Am. Zool. 39, 261–270 [Google Scholar]
- Vonesh J. R.2000Dipteran predation on the arboreal eggs of four Hyperolius frog species in western Uganda. Copeia 2000, 560–566 (doi:10.1643/0045-8511(2000)000[0560:DPOTAE]2.0.CO;2) [Google Scholar]
- Zina J.2006Communal nests in Physalaemus pustulosus (Amphibia: Leptodactylidae): experimental evidence for female oviposition preferences and protection against desiccation. Amphibia–Reptilia 27, 148–150 (doi:10.1163/156853806776052092) [Google Scholar]