Abstract
The lateral line system of larval zebrafish can translate hydrodynamic signals from the environment to guide body movements. Here, I demonstrate a spatial relationship between the organization of afferent neurons in the lateral line ganglion and the innervation of neuromasts along the body. I developed a whole cell patch clamp recording technique to show that afferents innervate multiple direction-sensitive neuromasts, which are sensitive to low fluid velocities. This work lays the foundation to integrate sensory neuroscience and the hydrodynamics of locomotion in a model genetic system.
Keywords: lateral line ganglion, zebrafish, afferent neuron, electrophysiology, neuromast, hydrodynamics
1. Introduction
With few exceptions, animals move according to how they sense their environment. The lateral line system in fishes consists of neuromasts that contain direction-sensitive hair cells, which detect water velocity and acceleration. This information is relayed to bipolar afferent neurons that transfer the signal into the brain, which then generates motor commands to capture prey, avoid predators and orient to current (Coombs & Conley 1997; Montgomery et al. 1997; McHenry et al. 2009). The role of the lateral line as a flow detector has been investigated for decades (Dijkgraaf 1963), yet we still do not have a clear idea of the neurophysiological mechanisms by which the lateral line influences motor behaviours. To achieve this understanding, we must first recognize the principles of lateral line organization and the contribution of its individual components. Investigating the morphology and function of afferent neurons with respect to their neuromasts is challenging for adult fishes, which may have thousands of neuromasts (Coombs et al. 1989). In contrast, larval zebrafish (Danio rerio) are small (approx. 4 mm), transparent and have far fewer afferent neurons and neuromasts (figure 1a), making it possible to label multiple neurons quickly and identify patterns of organization in vivo. Taken together, larval zebrafish are a burgeoning model system that provides powerful new ways of dissecting lateral line function through a combination of optical, genetic and electrophysiological techniques.
Figure 1.
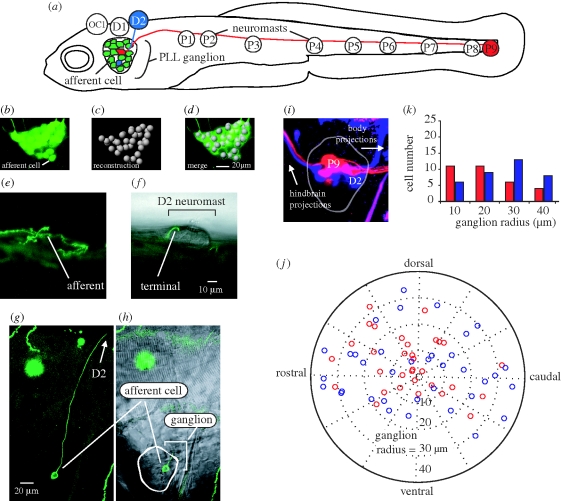
(a) Afferent neurons in the posterior lateral line ganglion of 5 dpf larval zebrafish illustrating connections with D2 and P9 neuromasts (not drawn to scale). Bipolar afferents make projections that terminate on one or more neuromasts labelled OC1, D1-2 and P1-9 (Raible & Kruse 2000; Ledent 2002) as well as to the hindbrain (not shown). (b–d) Afferent cell bodies in a ganglion can be recognized and reconstructed using HUC-GFP transgenic fish. (e,g) DNA injection at the one-celled embryo stage reveals expression of a single afferent innervating the D2 neuromast. (f,h) The position of the afferent in the ganglion and its bulged termini on the neuromast is revealed by merged confocal and Nomarski microscopy images. (i,j) Backfilling dyes into D2 (blue) and P9 (red) neuromasts results in afferent cell body labelling and reveals their relative positions in the ganglion. (k) Graph showing the number of backfilled afferent neurons located in 10 µm concentric ring regions from the centre of the ganglion (n = 14 ganglia). More P9 afferent neurons reside towards the centre of the ganglion while more D2 cells reside in the outer rings of the ganglion.
2. Material and methods
I set out to characterize the organization of the posterior lateral line by first quantifying the number of afferent neurons (herein referred to as afferents) in the ganglion for 5-day post fertilization (dpf) HUC GFP transgenic larval zebrafish (D. rerio; Park et al. 2000). At this stage, it has been established that larvae have a functional lateral line system that is integrated with the motor system (McHenry et al. 2009). Fish were anesthetized with 0.02 per cent solution of MS-222 (Sigma) and embedded in 1.4 per cent low melting point agar (Fisher Scientific). Images of posterior lateral line ganglia (one for each left and right side) were collected with a Zeiss LSM 510 inverted confocal microscope (figure 1b–d) and individual afferents were identified with image recognition software (Neurolucida, MicroBrightField Inc.).
To see if there was a relationship between neuromast body location and afferent position in a ganglion, I electroporated (Axoporator 800A, Molecular Devices) tetramethylrhodamine dye (3000 MW, Molecular Probes Inc.) into individual afferents in 5 dpf larvae. To confirm that electroporation thoroughly labelled afferents and their long processes, I also genetically labelled afferents by injecting a HUC-eGFP construct (25 mg µl−1) into wild-type embryos (pico-injector PLI-100, Harvard Apparatus). Fish were then screened with a Leica MZ 16FA fluorescent microscope to confirm single-cell expression (figure 1e–h). Backfilling neuromasts with rhodamine-loaded glass pipettes provided yet another way to label afferents, since the dyes readily travel from hair cells to afferent soma. This provided the advantage of marking larger numbers of afferents specific to a neuromast and supplemented the other labelling methods that were more time-intensive.
Afferent projections to neuromasts coalesce along the horizontal midline, so it was important to backfill only those neuromasts located at the terminal ends (D2 and P9 neuromast, figure 1a,i). This avoided accidental labelling of other projections and therefore afferents that were not connected to the neuromast of interest. For example, backfilling the P3 neuromast would risk labelling all afferents that innervate neuromasts located caudal to P3 (e.g. P4–P9). In addition, labelling widely spaced neuromasts would most quickly reveal any general pattern of afferent organization in the ganglion. Note that afferents can innervate more than one neuromast (Faucherre et al. 2009), such that backfilling P9 does not indicate that the corresponding afferents project to P9 exclusively. To standardize the X–Y position of neurons in the three-dimensional ganglia across individuals, the distal tip of the cleithrum was chosen as the reference focal depth for each image. Because ganglia varied in shape, I digitized each ganglion outline and bisected it with two lines into equal areas of left/right and top/bottom halves (Matlab v.2007a, Mathworks). I took the centre as the location where these two lines intersected and measured afferent positions relative to this reference point.
Whole cell patch recordings of afferents were conducted in paralysed larvae (1 mg/1 ml α-bungarotoxin, Sigma) to determine changes in their firing rate in response to jets of water directed at specific neuromasts along the body. At the same time, extracellular motor root recordings were performed to be able to evaluate if motor activity, whether spontaneous or elicited by the water jet, was affecting the firing response of the afferents. Both patch and motor root electrodes were pulled from borosilicate glass (model G150-F-3, Warner Instruments) on a Model P97 Flaming/Browning puller (Model P-97, Sutter Instruments). Patch electrodes were pulled to 5–10 MΩ resistances and filled with 125 mM K gluconate, 2.5 mM MgCl2, 10 mM EGTA, 10 mM HEPES buffer, 4 mM Na2ATP, 0.1 per cent sulphorhodamine B, and adjusted to a pH of 7.3 with KOH. Recordings were amplified with a Multiclamp 700A amplifier at a gain of 20 with a low-pass filter set at 30 kHz, with a sampling rate of 63 kHz and converted to digital signals with Digidata 1322A (Axon Instruments). Motor root electrodes were pulled to approximately 30 µm diameter tips, beveled and flame polished with a microforge (MF-830 Narishige USA) and placed on myotomal clefts. Recordings were amplified at a gain of 1000 with a low pass filter set at 5 kHz and a high-pass filter set at 50 Hz. To reveal the sensitivity of afferent neurons to hydrodynamic stimuli, individual neuromasts were deflected with a water micro-jet triggered by a computer-controlled pico-spritzer (Harvard Apparatus). I used a motorized micromanipulator (Siskiyou Co.) to carefully position the pipette to direct the jet orthogonal to the neuromast kinocilia and parallel to the rostrocaudal axis of the body. Water velocity was calibrated by tracking suspended particles (Potters Industries Inc.) ejected from the stimulus pipette (aperture approx. 30 µm, length 3.5 cm) over a range of velocities. At the highest velocities, particles in the jet traveled approximately four neuromast diameters (about 200 µm). All values reported are mean ± standard error.
3. Results
There are 44.8 ± 7.8 afferents in a posterior lateral line ganglion (both left and right side ganglia were used in each of seven fish). Therefore, there are approximately four times as many neurons in the ganglion as there are neuromasts for each side of the body. Backfilled afferents from both D2 and P9 neuromasts showed that 4.4 ± 2.3 afferents contacted each of these neuromasts. There was no significant difference in the number of afferents that innervated D2 versus P9 neuromasts. For afferents that innervated P9 neuromasts, 23 out of 33 (70%) clustered in the centre of the ganglion (i.e. less than or equal to 20 µm radius from the centre). For afferents that contacted D2 neuromasts, 22 out of 36 (61%) were distributed along the outer ring of the ganglion (i.e. more than or equal to 30 µm, figure 1j,k).
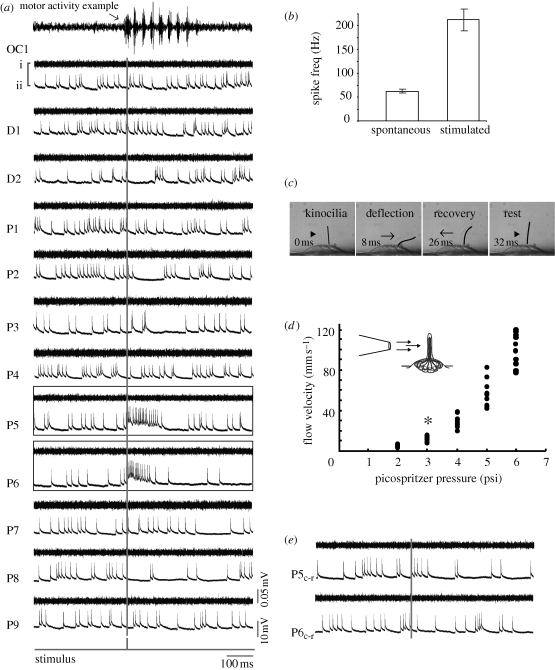
Posterior lateral line afferent neurons fire spontaneously and increased their firing rate from 57.2 ± 5.3 Hz to 214.9 ± 25.8 Hz when their neuromast(s) were stimulated by a water jet (figure 2a–c). Flow velocities above 7 mm s−1 consistently increased firing rate, while lower velocities did not (figure 2d, n = 12 fish). Individual afferents that contacted single or multiple neuromasts were sensitive to deflection from one direction, such that the same magnitude jet directed at neuromasts from the opposite direction did not elicit a response (figure 2e). Ventral motor root recordings show that single neuromast deflections are insufficient to elicit motor behaviours.
Figure 2.
Deflecting a neuromast with a water jet increases firing rate in afferent neurons. (a) Ventral motor root (i) and whole-cell patch clamp recordings of an afferent neuron (ii) while a jet is directed rostrocaudally at each neuromast (rostral is to the left). The stimulus trigger is shown as a grey vertical line. No motor activity occurs during neuromast stimulation. For comparison, an example of swimming activity is shown in the top-most trace. Afferent neurons display spontaneous firing activity even in the absence of a jet. Out of all neuromasts stimulated, only P5 and P6 show an increased firing rate response. (b) All afferents increase their firing rate when the neuromasts that they innervate are stimulated compared to their spontaneous firing rate in the absence of a jet. (c) Deflection of hair cell kinocilia in the D2 neuromast by a jet. The caudal-most kinocilium is traced in black for clarity. (d) Relationship between pressure and ejected water velocity for the stimulus pipette, showing the minimum velocity to elicit afferent firing (asterisk). (e) When the jet is reversed and directed caudorostrally (Pc-r) the afferent neuron does not increase its firing rate. Motor root recordings confirm that no spontaneous firing activity occurs during the experiment, which would decrease neuromast sensitivity due to efferent activity.
4. Discussion
There is an organized, spatial relationship between the position of posterior lateral line afferent neurons in the ganglion and the neuromasts that they innervate. When this pattern is integrated with previous work on afferent projections into the hindbrain, a picture emerges where tail-neuromast afferents are found more centrally in the ganglion and project more dorsal and medial in the hindbrain, while head-neuromast afferents are distributed along the ganglion periphery and project more ventral and lateral into the hindbrain (Alexandre & Ghysen 1999). Since afferents innervating tail neuromasts are more likely to establish multiple-neuromast contacts (Nagiel et al. 2008), this arrangement may have consequences for the sensitivity of the lateral line. This is an example of how morphological data provide functional hypotheses that can be tested with electrophysiology. Using patch and extracellular motor root recordings, we discovered that a small water jet directed at an individual neuromast could be detected by the connected afferent neuron, but overall did not elicit an escape response, as has been shown for larger hydrodynamic stimuli (Liu & Fetcho 1999; McHenry et al. 2009). This leads to the prediction that deflection of multiple neuromasts is required for escapes and that the sensitivity of the system depends on the pattern of multiple versus single neuromast innervation by afferent neurons.
My recordings are the first to demonstrate that afferent neurons make functional contacts with single and sequentially arranged neuromasts in zebrafish. These afferents are sensitive to small scale, direction-specific hydrodynamic perturbations, with several afferents probably coding for directional sensitivity (Nagiel et al. 2008). This supports the idea that afferent neurons are critical for transferring hydrodynamic information to the hindbrain before it is translated into appropriate motor commands. En route to the regions of integration in the hindbrain, afferents also have the potential to modify signals received from neuromasts depending on, for example, differences in intrinsic excitability. This is consistent with the finding that afferents show a decline in response at higher stimulus frequencies, suggesting a contribution from filters that exist beyond the hair cell level (Coombs & Montgomery 1992).
The in vivo patch clamp technique opens up several possibilities to directly uncover the mechanisms of flow sensing in fishes. For example, we now know that afferents can increase their firing rate in response to hydrodynamic deflections of single neuromasts, suggesting that larvae can sense similar flows produced by small invertebrate prey (Catton et al. 2007). Since the time-course of a water jet has an initial acceleration phase followed by a steady velocity phase and then a deceleration phase, the response of an afferent during these distinct phases can be used to investigate the presence of physiologically distinct cell types, which has already been established in adults (Engelmann et al. 2002; Chagnaud et al. 2006). In addition, the progenitors to canal neuromasts lie exposed on the skin before becoming recessed into canals later, providing a unique opportunity to determine whether acceleration sensitivity is due to physiology or to the mechanical filter of the canal arrangement (Coombs et al. 1989; Montgomery et al. 1997). The genetic tractability and accessibility of the larval lateral line system, when combined with the ability to record from afferent neurons with known neuromast connectivity, promises to advance significantly a mechanistic understanding of how vertebrate motor behaviours are initiated and modified through sensory processing.
Acknowledgement
All protocols were approved by the Cornell University Institutional Animal Care and Use Committee.
This research was supported by the National Institutes of Health National Research Service Award (NS051009) and funds from the Whitney Lab for Marine Bioscience (J.C.L) and an NIH grant (NS26539) to Joseph R. Fetcho.
Footnotes
One contribution of 11 to a Special feature on ‘Control and dynamics of animal movement’.
References
- Alexandre D., Ghysen A.1999Somatotopy of the lateral line projection in larval zebrafish. Proc. Natl Acad. Sci. USA 96, 7558–7562 (doi:10.1073/pnas.96.13.7558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catton K. B., Webster D. R., Brown J., Yen J.2007Quantitative analysis of tethered and free-swimming copepodid flow fields. J. Exp. Biol. 210, 299–310 (doi:10.1242/jeb.02633) [DOI] [PubMed] [Google Scholar]
- Chagnaud B. P., Bleckmann H., Engelmann J.2006Neural responses of goldfish lateral line afferents to vortex motions. J. Exp. Biol. 209, 327–342 (doi:10.1242/jeb.01982) [DOI] [PubMed] [Google Scholar]
- Coombs S., Conley R. A.1997Dipole source localization by mottled sculpin. I. Approach strategies. J. Comp. Physiol. A 180, 387–399 (doi:10.1007/s003590050057) [DOI] [PubMed] [Google Scholar]
- Coombs S., Montgomery J. C.1992Fibers innervating different parts of the lateral line system of the Antarctic fish, Trematomus bernacchii, have similar neural responses despite large variations in peripheral morphology. Brain Behav. Evol. 40, 217–233 (doi:10.1159/000113914) [DOI] [PubMed] [Google Scholar]
- Coombs S., Gorner P., Munz H.1989The mechanosensory lateral line: neurobiology and evolution, pp. 1–724 New York, NY: Springer [Google Scholar]
- Dijkgraaf S.1963The functioning and significance of the lateral-line organs. Biol. Rev. Camb. Philos. Soc. 38, 51–105 [DOI] [PubMed] [Google Scholar]
- Engelmann J., Hanke W., Bleckmann H.2002Lateral line reception in still- and running water. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188, 513–526 [DOI] [PubMed] [Google Scholar]
- Faucherre A., Pujol-Marti J., Kawakami K., Lopez-Schier H.2009Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS ONE 4, e4477 (doi:10.1371/journal.pone.0004477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent V.2002Postembryonic development of the posterior lateral line in zebrafish. Development 129, 597–604 [DOI] [PubMed] [Google Scholar]
- Liu K. S., Fetcho J. R.1999Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 23, 325–335 (doi:10.1016/S0896-6273(00)80783-7) [DOI] [PubMed] [Google Scholar]
- McHenry M. J., Feitl K. E., Strother J. A., Van Trump W. J.2009Larval zebrafish rapidly sense the water flow of a predator's strike. Biol. Lett. 5, 477–479 (doi:10.1098/rsbl.2009.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J., Baker C., Carton A.1997The lateral line can mediate rheotaxis in fish. Nature 389, 960–963 (doi:10.1038/40135) [Google Scholar]
- Nagiel A., Andor-Ardo D., Hudspeth A. J.2008Specificity of afferent synapses onto plane-polarized hair cells in the posterior lateral line of the zebrafish. J. Neurosci. 28, 8442–8453 (doi:10.1523/JNEUROSCI.2425-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. C.et al.2000Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev. Biol. 227, 279–293 (doi:10.1006/dbio.2000.9898) [DOI] [PubMed] [Google Scholar]
- Raible D. W., Kruse G. J.2000Organization of the lateral line system in embryonic zebrafish. J. Comp. Neurol. 421, 189–198 (doi:10.1002/(SICI)1096-9861(20000529)421:2<189::AID-CNE5>3.0.CO;2-K) [PubMed] [Google Scholar]