Abstract
Migrating insects use their sensory systems to acquire local and global cues about their surroundings. Previous research on tethered insects suggests that, in addition to vision and cephalic bristles, insects use antennal mechanosensory feedback to maintain their airspeeds. Owing to the large displacements of migratory insects and difficulties inherent in tracking single individuals, the roles of these sensory inputs have never been tested in freely migrating insects. We tracked individual uraniid moths (Urania fulgens) as they migrated diurnally over the Panama Canal, and measured airspeeds and orientation for individuals with either intact or amputated flagella. Consistent with prior observations that antennal input is necessary for flight control, 59 per cent of the experimental moths could not fly after flagella amputation. The remaining fraction (41%) was flight-capable and maintained its prior airspeeds despite severe reduction in antennal input. Thus, maintenance of airspeeds may not involve antennal input alone, and is probably mediated by other modalities. Moths with amputated flagella could not recover their proper migratory orientations, suggesting that antennal integrity is necessary for long-distance navigation.
Keywords: insect migration, antenna, flight
1. Introduction
Long-range navigation places extraordinary demands on the sensorimotor physiology of migrating insects (Dingle 1996). These challenges include the maintenance of migratory orientations and airspeeds in face of variable wind speeds and direction (e.g. Srygley 2001a; Dudley et al. 2002). Because typical migratory movements involve seasonal locomotion between approximately the same geographical regions using limited lipid reserves, researchers have argued that migrating insects detect airspeeds and implement wind-drift correction to optimize energy expenditure (Srygley & Dudley 2008).
Of the modalities involved in flight speed estimation, vision plays a critical role in modulating groundspeed. Experiments on bees (Heran 1955), locusts (Gewecke 1970) and butterflies (Gewecke & Niehaus 1981) showed that flying insects also use antennae to detect airflow and potentially regulate their airspeeds. With their antennae amputated, tethered butterflies altered their wing kinematics to increase their flight speeds (Gewecke & Niehaus 1981). Additionally, cephalic bristles can detect airflow over the head (Weis Fogh 1949). Despite the rich history of studies on insect migration (Williams 1957), our knowledge of how migrating insects regulate their airspeed and orientation remains stymied by the inability to track migrating individuals over large distances (see, however, Wikelski et al. 2006).
To meet these challenges, radio telemetry (Cooke et al. 2004) and radar technology (Smith et al. 1993) can be used to remotely track the position and groundspeed of insects moving through complex habitats. Another method involves using motor boats to track individual insects flying close to water surfaces of lakes (Dudley & Devries 1990). This technique enables direct measurements of migratory airspeeds, a better measure of their physiological performance than groundspeeds (e.g. Srygley et al. 1996). Hence, we used this method to study antennal role in migrating insects.
In crepuscular moths Manduca sexta, proper mechanical loading of basal antennal mechanosensors is crucial for flight control. Moths with amputated flagella lost control of their flight trajectories, but regained it after antennae were reattached (Sane et al. 2007). These studies on moths flown under low-light laboratory conditions do not, however, readily extend to insects flying with natural visual stimuli. Thus, we conducted experiments on the diurnal moth Urania fulgens during its migratory flight over Lake Gatun, part of the Panama Canal. Migrating U. fulgens are highly motivated fliers and offer a convenient system to study antennal role in migratory flight.
2. Material and methods
The neotropical moth U. fulgens migrates diurnally over Lake Gatun on the Panama Canal (figure 1a). Despite suggestions of 4–6-year migration cycles driven by host plant toxicity (Smith 1983), migration patterns in this species are very unpredictable. Experiments reported here were conducted from 4 to 22 June 2006. Solitary U. fulgens individuals migrating over Lake Gatun could be readily tracked because they flew at low heights (less than 2 m above the surface) with relatively straight paths. Details of field measurements of U. fulgens moths appear elsewhere (Dudley & DeVries 1990; Dudley et al. 2002). Briefly, we pursued migrating moths in a 14 feet aluminium dinghy driven such that it remained aligned parallel to the moth's trajectory. We used an anemometer (TSI Model 8347, St Paul, MN, USA) held laterally from the boat and behind the free-flying moth to measure its airspeed. After measuring airspeed, we captured the moth with an insect net (see film in the electronic supplementary material), stopped the boat and measured the moth's track with a hand-held compass.
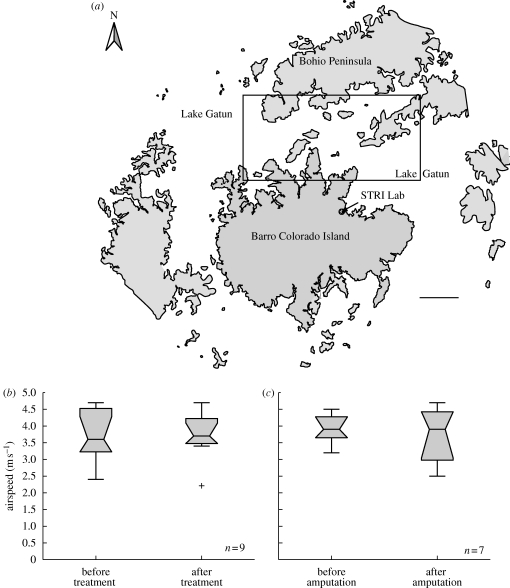
Figure 1.
(a) Rectangle delineates release sites for U. fulgens moths on Lake Gatun, Panama. The lake shore and islands near the site of release are outlined. Migratory airspeeds for (b) sham-treated versus (c) flagella-amputated moths before and after treatment. Airspeed data are plotted as notched, whisker plots. Bottom and top of each box correspond to lower and upper quartile values, respectively. A horizontal black line within each box represents the median. Whiskers represent values up to 1.5 times the interquartile range and plus symbols indicate the outlier points beyond this range. Non-overlapping notches imply different medians at the 5% significance level. (a) Scale bar, 1000 m.
Captured moths were subjected to either flagella amputation or sham treatments. In the former, moths were restrained and both antennal flagella were cut using microscissors, but leaving the basal mechanosensory apparatus intact. In the latter treatment, only the wispy tips of both flagella were cut. Following either treatment, moths were allowed to walk on a dry surface until they voluntarily initiated flight. After moths settled into a regular flight pattern, we again measured their airspeed and track as described above. If moths did not resume normal flight, we tried to induce them to fly by gently blowing on their heads. Typically such moths, if they did indeed take off, flew less than a few metres vertically before rapidly losing height and were deemed to be ‘flight-impaired’.
It was not always possible to measure both direction and airspeed pre- and post-antennectomy in every moth. Thus, although 12/13 sham (12/25 experimental) moths were flight-capable, we recorded airspeeds of only nine sham (seven experimental) moths. If moths flew in a certain direction but could not sustain flight long enough for airspeeds to be measured, we noted only the direction data (13 shams, 16 experimental). Owing to motion of the boat on water, experimental reattachment of cut antennae was not possible (e.g. Sane et al. 2007).
3. Results
(a). Flight initiation in sham-treated versus experimental moths
Sham-treated Urania typically resumed flight within a minute after release, and showed a stereotypic behaviour reminiscent of insect orientation flights. This behaviour may be qualitatively subdivided into two phases. Immediately following release, moths circled slowly in upwardly widening helical trajectories. Following such circling, moths descended in altitude and increased their forward speed until they settled into a constant airspeed and direction 1–2 m from the water's surface in an apparent resumption of migratory flight.
(b). Influence of flagella amputation on migratory flight speeds
After release, 12 of 13 sham-treated moths voluntarily initiated and sustained flight. In contrast, although all flagella-removed individuals voluntarily initiated flight, 13 of 25 moths (one-tailed Fisher's exact test, p = 0.007) were flight-impaired, typically hitting the water within a minute of take-off. The remainder of this sample (12 of 25) voluntarily initiated and sustained flight over longer time periods (more than 3 min), indicating compensation for the absence of antennal mechanosensory input.
The mean airspeed of all untreated moths was 3.82 ± 0.68 m s−1 in agreement with previously reported airspeeds in U. fulgens (Dudley et al. 2002). The mean airspeed of moths after sham treatment (3.71 ± 0.71 m s−1) was not significantly different from that before treatment (3.73 ± 0.84 m s−1; paired-sample t-test, p > 0.9; n = 9; figure 1b). Similarly, the mean airspeed of flight-capable moths following flagella removal (3.75 ± 0.85 m s−1) was not significantly different from that before treatment (3.93 ± 0.45 m s−1, paired-sample t-test, p > 0.4; n = 7; figure 1c). Thus, flagella amputation altered flight performance in the majority of treated moths, but did not influence the airspeeds of those moths that remained flight-capable.
(c). Influence of antennae on migratory orientation
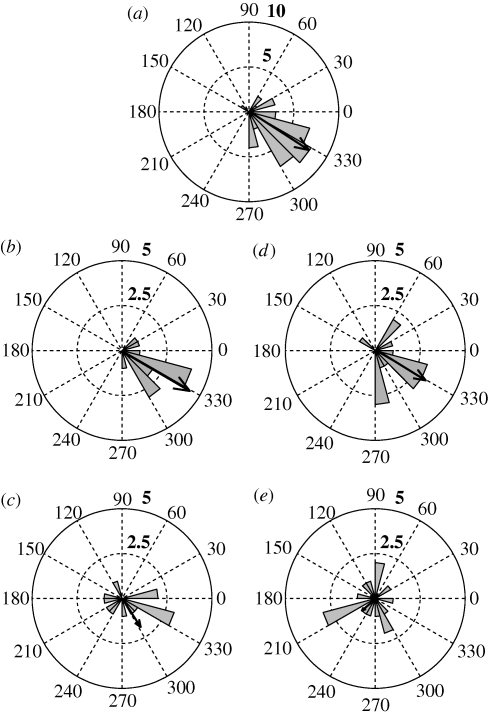
Because the stimuli to antennal mechanosensors may be necessary for flight control, antennal amputation should significantly affect the ability of moths to regain their migratory orientation. We thus compared orientation data before and after antennal treatments for sham-treated and flagella-amputated groups using a circular parametric paired-sample test (Zar 1999, pp. 645–647). For sham-treated moths, we could not reject the hypothesis that the direction of flight before treatment was the same as that after treatment (Ncontrol = 13, Fcontrol = 2.2878, F0.05(1),2,N−2 = 3.98; p > 0.1; figure 2b,c). However, for flagella-amputated moths, we rejected the hypothesis that post-treatment and pre-treatment moths had equivalent flight orientations (Nflagella-amputated = 16, Fflagella-amputated = 5.9564, F0.05(1),2,N−2 = 3.74; p < 0.025; figure 2d,e). Thus, although flagella-amputated moths that were flight-capable flew at normal airspeeds, their migratory orientations were significantly disrupted when compared with sham-treated moths.
Figure 2.
Rose diagrams of natural flight orientation for U. fulgens moths pre-capture and their orientation after treatment and release over the lake. Black arrows within the plots show the direction (α) and magnitude (r) of the mean orientation vector between 0 (no moths orient in that direction) and 1 (all moths orient in that direction). (a) Pooled data for moths from both treatment groups before capture (n = 38, α = −33°, r = 0.78). (b) Sham-treated moths before capture (n = 13, α = −30°, r = 0.86) and (c) after sham treatment (n = 13, α = −57°, r = 0.36). (d) Experimental moths before capture (n = 16, α = −32°, r = 0.65) and (e) after flagella amputation (n = 16, α = −140°, r = 0.18). Normal numbers, compass directions; grey triangles, number of moths in each 15° compass sector; bold numbers, sample size denoted by each concentric circle.
4. Discussion
(a). Maintenance of airspeeds during migratory flight
Previous research on tethered insects has suggested that insects use antennal deflection owing to aerodynamic drag to detect flight speeds (Gewecke 1974). This hypothesis predicts that antennal integrity is necessary for maintenance of insect airspeed. However, a key finding of our study was that airspeeds of sham-treated and flagella-amputated U. fulgens were not significantly different from either each other or from moths with intact flagella. Although moths with amputated flagella were significantly less likely to resume normal flight, the flight-capable individuals maintained pre-treatment airspeeds. Thus, sensory modalities other than antennal mechanosensors regulate airspeed in migrating U. fulgens.
Diverse insects including bees (Srinivasan et al. 1996), flies (David 1979) and moths (Kennedy & Marsh 1974) rely on visual cues to modulate groundspeed during flight. Because large water bodies provide few visual patterns, insects flying over such habitats have difficulty modulating their groundspeed. For example, bees flying over lakes only partially compensate for ambient airflows and show an increased likelihood of crashing into the water (Heran & Lindauer 1963). In contrast, Phoebis sennae butterflies fly readily over large water bodies and probably use optic flow from wave-induced surface references to compensate for cross-wind or tailwind drift over the Caribbean Sea (Srygley 2001a,b). In U. fulgens, the maintenance of airspeeds regardless of ambient winds may reflect the effect of sparse visual cues. Alternatively, this may result from bilaterally symmetric motor output, ensuring rectilinear flight unaffected by sensory feedback.
In addition to vision, other mechanosensors may also be involved in airspeed maintenance. In locusts (Weis Fogh 1949; Arbas 1986), mechanosensory cephalic bristles respond to self-induced flow from wing movements (Sane 2006; Sane & Jacobson 2006) and send this input to motor centres via tritocerebral commissure giant interneurons (Bacon & Mohl 1979). Cephalic bristles probably serve a similar function in moths and could mitigate the absence of antennal mechanosensory feedback.
(b). Role of antennae in migratory orientation
Although the absence of antennal input did not alter airspeeds of antennectomized moths, it significantly disrupted migratory orientation compared with sham-treated animals. The mechanistic role of antennae in migratory orientation is not well understood for the insects in general. In monarch butterflies, the presence of a circadian clock in the flagellum may be involved in compensation for sun's movement, as evident from disruption of migratory orientation in antennectomized butterflies (Merlin et al. 2009). Although our results are consistent with those of Merlin et al. (2009), they are unlikely to result from a disruption of the circadian system given the short time spans over which our experiments were conducted. Lepidopterans are also known to use magnetoreception (e.g. Srygley & Dudley 2008), but there is no evidence for or against the involvement of antennae in this sensory modality. Moreover, the inability of the majority of antennectomized U. fulgens moths to sustain flight suggests that the lack of antennal input directly affects flight stabilization and hence also disrupts aerial manoeuvring, as observed in experiments on butterflies and moths (e.g. Sane et al. 2007). We observed an absence of upward helical flight in those flagella-amputated moths that could fly. Because such helical flight paths may be used by insects to identify the celestial polarization cues required for reorienting the moth in the proper direction, their disruption by antennectomy may underlie their scattered orientations.
Acknowledgements
Journal of Experimental Biology funded travel and stay in Panama (S.P.S.) and Smithsonian Tropical Research Institute provided facilities and logistical support.
Footnotes
One contribution of 11 to a Special feature on ‘Control and dynamics of animal movement’.
References
- Arbas E.1986Control of hindlimb posture by wind-sensitive hairs and antennae during locust flight. J. Comp. Physiol. A 159, 849–857 (doi:10.1007/BF00603738) [DOI] [PubMed] [Google Scholar]
- Bacon J., Mohl B.1979Activity of an identified wind interneuron in a flying locust. Nature 278, 638–640 (doi:10.1038/278638a0) [Google Scholar]
- Cooke S. J., Hinch S. G., Wikelski M., Andrews R. D., Kuchel L. J., Wolcott T. G., Butler P. J.2004Biotelemetry: a mechanistic approach to ecology. Trends Ecol. Evol. 19, 334–343 (doi:10.1016/j.tree.2004.04.003) [DOI] [PubMed] [Google Scholar]
- David C. T.1979Optomotor control of speed and height by free-flying Drosophila. J. Exp. Biol. 82, 389–392 [DOI] [PubMed] [Google Scholar]
- Dingle H.1996Migration: the biology of life on the move. Oxford, UK: Oxford University Press [Google Scholar]
- Dudley R., Devries P. J.1990Flight physiology of migrating Urania fulgens (Uraniidae) moths—kinematics and aerodynamics of natural free flight. J. Comp. Physiol. A 167, 145–154 [Google Scholar]
- Dudley R., Srygley R. B., Oliveira E. G., DeVries P. J.2002Flight speeds, lipid reserves, and predation of the migratory neotropical moth Urania fulgens (Uraniidae). Biotropica 34, 452–458 [Google Scholar]
- Gewecke M.1970Antennae—another wind-sensitive receptor in locusts. Nature 225, 1263 (doi:10.1038/2251263a0) [DOI] [PubMed] [Google Scholar]
- Gewecke M.1974The antennae of insects as air-current sense organs and their relationship to the control of flight. In Experimental analysis of insect behaviour (ed. Barton Browne L.), pp. 100–113 Berlin, Germany: Springer [Google Scholar]
- Gewecke M., Niehaus M.1981Flight an flight control by the antennae in the small tortoiseshell (Aglais Urticae).1. Flight balance experiments. J. Comp. Physiol. 145, 249–256 [Google Scholar]
- Heran H.1955Versuche Uber Die Windkompensation Der Bienen. Naturwissenschaften 42, 132–133 (doi:10.1007/BF00589418) [Google Scholar]
- Heran H., Lindauer M.1963Windkompensation Und Seitenwindkorrektur Der Bienen Beim Flug Uber Wasser. Z. Vergleichende Physiol. 47, 39–55 (doi:10.1007/BF00342890) [Google Scholar]
- Kennedy J. S., Marsh D.1974Pheromone-regulated anemotaxis in flying moths. Science 184, 999–1001 (doi:10.1126/science.184.4140.999) [DOI] [PubMed] [Google Scholar]
- Merlin C., Gegear R. J., Reppert S. M.2009Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325, 1700–1704 (doi:10.1126/science.1176221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane S. P.2006Induced airflow in flying insects—I. A theoretical model of the induced flow. J. Exp. Biol. 209, 32–42 (doi:10.1242/jeb.01957) [DOI] [PubMed] [Google Scholar]
- Sane S. P., Jacobson N. P.2006Induced airflow in flying insects—II. Measurement of induced flow. J. Exp. Biol. 209, 43–56 (doi:10.1242/jeb.01958) [DOI] [PubMed] [Google Scholar]
- Sane S. P., Dieudonne A., Willis M. A., Daniel T. L.2007Antennal mechanosensors mediate flight control in moths. Science 315, 863–866 (doi:10.1126/science.1133598) [DOI] [PubMed] [Google Scholar]
- Smith N. G.1983Host plant toxicity and migration in the dayflying moth Urania. Florida Entomol. 66, 76–85 (doi:10.2307/3494552) [Google Scholar]
- Smith A. D., Riley J. R., Gregory R. D.1993A method for routine monitoring of the aerial migration of insects by using a vertical-looking radar. Phil. Trans. R. Soc. Lond. B 340, 393–404 (doi:10.1098/rstb.1993.0081) [Google Scholar]
- Srinivasan M. V., Zhang S. W., Lehrer M., Collett T. S.1996Honeybee navigation en route to the goal: visual flight control and odometry. J. Exp. Biol. 199, 237–244 [DOI] [PubMed] [Google Scholar]
- Srygley R. B.2001aSexual differences in tailwind drift compensation in Phoebis sennae butterflies (Lepidoptera: Pieridae) migrating over seas. Behav. Ecol. 12, 607–611 (doi:10.1093/beheco/12.5.607) [Google Scholar]
- Srygley R. B.2001bCompensation for fluctuations in crosswind drift without stationary landmarks in butterflies migrating over seas. Anim. Behav. 61, 191–203 (doi:10.1006/anbe.2000.1551) [DOI] [PubMed] [Google Scholar]
- Srygley R. B., Dudley R.2008Optimal strategies for insects migrating in the flight boundary layer: mechanisms and consequences. Integr. Comp. Biol. 48, 119–133 (doi:10.1093/icb/icn011) [DOI] [PubMed] [Google Scholar]
- Srygley R. B., Oliveira E. G., Dudley R.1996Wind drift compensation, flyways, and conservation of diurnal, migrant neotropical Lepidoptera. Proc. R. Soc. Lond. B 263, 1351–1357 (doi:10.1098/rspb.1996.0198) [Google Scholar]
- Weis Fogh T.1949An aerodynamic sense organ stimulating and regulating flight in locusts. Nature 164, 873 (doi:10.1038/164873a0) [DOI] [PubMed] [Google Scholar]
- Wikelski M., Moskowitz D., Adelman J. S., Cochran J., Wilcove D. S., May M. L.2006Simple rules guide dragonfly migration. Biol. Lett. 2, 325–329 (doi:10.1098/rsbl.2006.0487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. B.1957Insect migration. Annu. Rev. Entomol. 2, 163–180 (doi:10.1146/annurev.en.02.010157.001115) [Google Scholar]
- Zar J. H.1999Biostatistical analysis. Upper Saddle River, NJ: Prentice-Hall [Google Scholar]