Abstract
Boron plays important roles in many life processes including embryogenesis, bone growth and maintenance, immune function and psychomotor skills. Thus, the delivery of boron by the degradation of borate glass is of special interest in biomedical applications. However, the cytotoxicity of borate glass which arises with the rapid release of boron has to be carefully considered. In this study, it was found that the incorporation of strontium into borate glass can not only moderate the rapid release of boron, but also induce the adhesion of osteoblast-like cells, SaOS-2, thus significantly increasing the cyto-compatibility of borate glass. The formation of multilayers of apatite with porous structure indicates that complete degradation is optimistic, and the spread of SaOS-2 covered by apatite to form a sandwich structure may induce bone-like tissue formation at earlier stages. Therefore, such novel strontium-incorporated borosilicate may act as a new generation of biomaterial for bone regeneration, which not only renders boron as a nutritious element for bone health, but also delivers strontium to stimulate formation of new bones.
Keywords: borate glass, strontium, cytotoxicity, bone regeneration
1. Introduction
Bioactive material serves as an implant to facilitate healing or to compensate for a lack or loss of bone tissue, particularly for osteoporotic fractures, where the conventional metallic reinforcement method for fractured bone is not applicable because of bone fragility and extremely low bone mineral density. The demand for bioactive material is therefore in the need to fill bone defects, augment weak osteoporotic bones and fix fractures. As a result, it requires a highly reactive surface to form a continuous interface with the surrounding bone tissue to induce abundant bone formation, and thus able to be structurally and mechanically compatible with bone tissue, and eventually be replaced by new bones.
The extraordinary performance of bioactive glass (e.g. 45S5 Bioglass) lies in its spontaneous bonding with bone tissues through the formation of a calcium phosphate (Ca–P) layer with a satisfactory biocompatibility and osteoconductivity (Hench & Wilson 1984; Silver et al. 2001; Chen et al. 2006). However, the degradation of this silicon-based glass is highly time-dependent, and the bulk material was reported to remain in the human body upto one year of implantation (Hamadouche et al. 2001). As a result, borate glass, based on a B2O3 network, is now showing potential in bone regeneration, owing to its complete conversion to apatite through a set of dissolution–precipitation reactions similar in nature to those in 45S5 Bioglass (Huang et al. 2006; Yao et al. 2007; Liu et al. 2009b). In particular, the mechanical strength of a scaffold based on a boron network former is significantly higher than that of the silicon network former in 45S5 Bioglass (Liu et al. 2009a).
As a naturally occurring trace element, the role of boron was not well known until recently detected in human diet and found necessary for the optimal health of rats and presumably other mammals (Forrer et al. 2001a,b), e.g. required or beneficial for many life processes including embryogenesis, bone growth and maintenance, immune function and psychomotor skills (Nielsen 2000). Particularly, in post-menopausal women, boron might stimulate hormones, thus mimicking the effects of oestrogen, producing an oestrogen replacement (Nielson et al. 1987; Nielsen 1990). Currently, oestrogen treatment is still one of the most effective methods of preventing post-menopausal bone loss, which can lead to osteoporosis and debilitating fractures. Thus, the low chemical durable glass based on threefold coordination boron network former is of special interest in delivering boron for bone health.
However, until now, the physiological role of boron has been poorly understood. Although detected in daily diet, the actual amount of consumption dramatically varies depending on different sources and regions. However, unfortunately as documented, 2 g of boric acid can kill an infant, and 45 g is lethal to an adult (Garrett 1998). Nevertheless, the related study of biocompatibility of borate glass is very limited, or even overlooked in previous studies. Particularly, the initial degradation rate of borate glass was found to be significantly faster than expected (Huang et al. 2006; Yao et al. 2007). A detailed study and an attempt to control its degradation rate are therefore necessary.
Strontium has recently been suggested as a daily oral supplement for the treatment of osteoporosis, e.g. strontium ranelate (SrR), owing to its dual anti-resorptive and anabolic effects on bone, attributed to enhanced pre-osteoblast differentiation, inhibited osteoclast differentiation and eventually reduced osteoclast function (Buehler et al. 2001; Nielsen 2004; Rizzoli 2005). Thus, bioglass is thought to be an ideal reservoir to deliver strontium, owing to its homogeneous composition and controllable degradation (Lao et al. 2008; Place et al. 2009). In particular, strontium has a larger ion radius (1.13 Å) than that of magnesium ion (0.65 Å) and calcium ion (1.00 Å; Li et al. 2007a). The incorporation of strontium by partial substitution of magnesium and calcium is therefore expected to occupy more space and inhibit the movement and release of other ions in the glass network, and thereby reduce the dissolution rate of borate glass. The aim of this work is therefore to study the chemical structure of borate glass affected by incorporated strontium, and to extensively investigate both its degradation behaviour and biocompatibility. The regeneration of damaged or lost bony tissue is therefore anticipated owing to the dual roles of two important elements: boron and strontium.
2. Experimental
2.1. Fabrication and characterization
Borate glass of composition Na2O–K2O–MgO–CaO–B2O3–SiO2–P2O5 was prepared using a conventional approach by melting reagent-grade chemicals in a platinum crucible at 1200°C for 2 h with stirring, and then quenching in cold stainless steel. The detailed composition is listed in table 1, where strontium was added to partially replace magnesium and calcium. The bulk specimen was sliced into small pieces of size 1 × 1 × 0.5 cm for analysis. The structure and composition were, respectively, characterized by X-ray diffraction (XRD; model D/max 2550V, Rigaku, Tokyo, Japan) and Fourier-transform infrared spectroscopy (FTIR; Perkin-Elmer, USA). The chemical compositions were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Varian Co., USA).
Table 1.
Borate glass composition (mol%) determined by ICP-AES. 2B–6Sr was prepared by 6% MgO substituted by SrO; 2B–12Sr was prepared by 8% MgO and 4% CaO substituted by SrO. A: theoretical value; B: experimental value (mean±SD, n=5). The calculated composition is close to the expected.
| glass | Na2O | K2O | MgO | CaO | SrO | SiO2 | B2O3 | P2O5 |
|---|---|---|---|---|---|---|---|---|
| 2B–0Sr | ||||||||
| A | 6 | 8 | 8 | 22 | 0 | 18 | 36 | 2 |
| B | 6.8 ± 0.9 | 6.9 ± 1.2 | 7.7 ± 0.9 | 21.2 ± 2.7 | 0 | 19 ± 3.3 | 36.4 ± 6.1 | 2 ± 1.1 |
| 2B–6Sr | ||||||||
| A | 6 | 8 | 2 | 22 | 6 | 18 | 36 | 2 |
| B | 6.6 ± 1.7 | 7.1 ± 1.9 | 1.9 ± 0.4 | 21.8 ± 2.6 | 5.8 ± 1.5 | 19.2 ± 2.2 | 35.8 ± 4.7 | 1.8 ± 0.9 |
| 2B–12Sr | ||||||||
| A | 6 | 8 | 0 | 18 | 12 | 18 | 36 | 2 |
| B | 6.7 ± 1.1 | 6.9 ± 1.5 | 0 | 17.7 ± 2.1 | 11.6 ± 2.3 | 18.8 ± 1.7 | 36.4 ± 5.2 | 1.9 ± 0.6 |
2.2. In vitro study
The bioactivity was evaluated by immersing the glass sample in 50 ml of Dulbecco's Modified Eagle's Medium (DMEM) at 37.0 ± 0.1°C, respectively, for 1, 3 and 7 days (degradation and precipitation) and 30 days (cross section). The sample was then separated and rinsed twice in de-ionized water. The surface morphology was observed using scanning electron microscopy (SEM; LEO 1530, Oxford, UK) and the coating was characterized, respectively, by selected-area electron diffraction (SAED; Technai G2 20 STEM; FEI, Hillsboro, OR, USA) and energy-dispersive X-ray spectroscopy (EDX) by scratching from the glass surface. The pH of residual solution was also recorded. The cross section was studied using SEM after 30 days by mounting in epoxy resin.
2.3. Cytotoxicity and cell adhesion
In vitro biocompatibility was evaluated by cytotoxicity through MTT assay based on the requirement of ISO 10993 by L929 cells (International Standards Organization 1992). Firstly, the extraction was prepared by immersing the glass sample into DMEM (Invitrogen, USA) at 37°C for 1 day (0.2 g ml−1). L929 cells were incubated for 24 h at 37°C with 5 per cent CO2, 95 per cent air and complete humidity. Then, the medium in the well was replaced by the prepared solution. After 3 days, 10 µl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) plus 100 µl of DMEM were added into each well. After additional incubation for 4 h, the MTT solution was removed and replaced with 100 µl of dimethylsulphoxide (DMSO). After 10 min of slow shaking, the absorbance was read at wavelengths of 570 and 630 nm. The relative growth rate (RGR) was then calculated. The ion concentration of extraction including boron and strontium was characterized by ICP-AES.
Secondly, the response of osteoblasts was carried out by seeding human osteoblast-like cells, SaOS-2, with a density of 1 × 104 per well on the glass surface. After incubation for 3 days, the glass surface was rinsed with phosphate-buffered saline to remove non-adherent cells. Then, the specimens were fixed using 2.5 per cent glutaraldehyde with cacodylate buffer, washed using cacodylate buffer with sucrose, dehydrated with graded alcohol, and finally observed using SEM (Hitachi S4800 FEG SEM, Horiba, Japan).
2.4. Statistical analysis
All data presented here were expressed as mean ± standard deviation (n = 5) and analysed by one-way ANOVA. Differences were considered statistically significant at p < 0.05.
3. Results and discussion
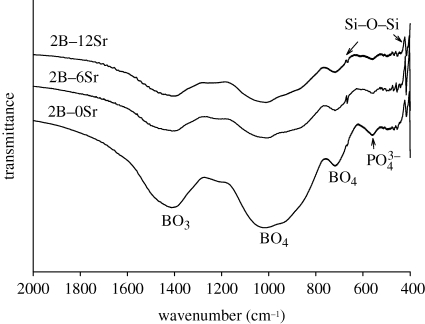
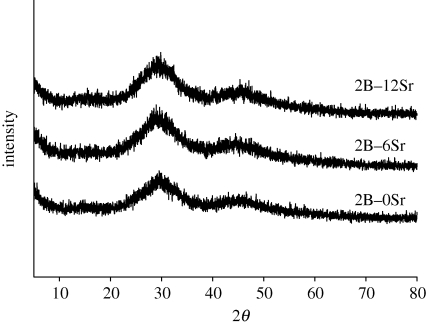
The experimental compositions of glass were close to the expected theoretical values (table 1), indicating that the glass network structure was formed as expected. The incorporation of strontium by (partial) substituting magnesium and calcium can be easily performed owing to chemical similarity. FTIR spectra (figure 1) showed that the glasses were dominated by broad peaks at 600–800 and 800–1200 cm−1, which are the main features of B–O stretching of tetrahedral [BO4] units; meanwhile a strong absorption band at 1200–1500 cm−1 was attributed to the B–O stretching of trigonal [BO3] units; indicating that the main glass network was composed of [BO3] and [BO4]. In addition, the presence of weak peaks at 415 and 668 cm−1 was indicative of Si–O–B linkages, thus verifying the coexistence of silicon units in the boron network structure, and the peak at 560 cm−1 originated from the internal modes of PO43− ion (figure 1). XRD showed that the prepared glass was non-crystalline, although additional strontium was incorporated (figure 2). Thus the added strontium neither affected the glass network structure, nor induced crystallization. The highly reactive surface is therefore formed because of its amorphous nature.
Figure 1.
Characterization of prepared borate glass by FTIR. Strontium was added, designated as 2B–0Sr, 2B–6Sr and 2B–12Sr.
Figure 2.
XRD patterns of prepared strontium-incorporated borate glass, designated as 2B–0Sr, 2B–6Sr and 2B–12Sr. The broad peak indicates non-crystalline characteristic.
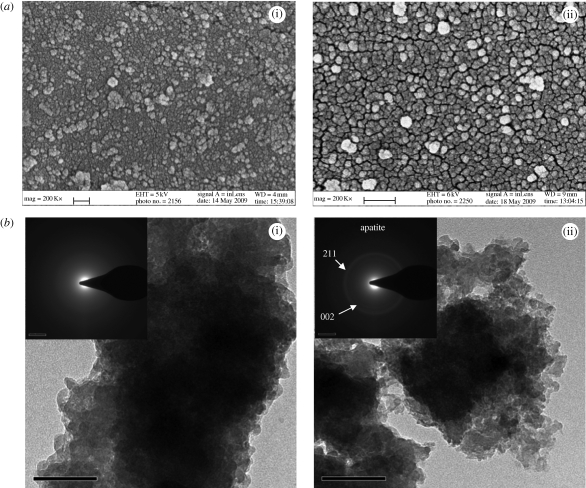
As a result, in the early stage of biomineralization, rapid nucleation could be detected on the glass surface after soaking in DMEM solution for only 1 day (figure 3). Although lacking a long-range order, it gradually converted to a poor-crystalline apatite after 7 days (figure 3), indicated as two broad rings representing (211) and (002) reflections, where the apatite-like material covered the glass surface uniformly. In addition, this was indicated by the change of solution pH that slightly increased from 7.4 to approximately 7.8 after soaking in DMEM solution for 1 day, and further increased to approximately 8.4 after 3 days due to rendering of alkaline elements such as sodium, potassium and magnesium, but slightly decreased to approximtely 8.1 after 7 days typically due to the consumption of OH− ions for the formation of apatite.
Figure 3.
Characterization of the surface (2B–6Sr) after soaking in DMEM solution at 37°C for (i) 1 and (ii) 7 days: (a) surface morphology observed by SEM; (b) composition determined by SEAD. Scale bar: (a) (i) 100 nm, (ii) 200 nm; (b) 100 nm.
Furthermore, the apatite layer was scratched from the glass surface after day 7 and characterized by EDX. It was found that the calculated Ca/P ratio was approximately 1.58, significantly lower than the stoichiometric ratio of pure hydroxyapatite (HA) at 1.67, indicating it as a calcium-deficient apatite. Similar to the result of Lao et al. (2009), the incorporation of both Sr and Mg was detectable, to partially replace the site of Ca (table 2); particularly the content of Sr was up to 4.29 per cent. Bone mineral is an impure form of HA, referred to as ‘biological apatite’. In contrast to synthetic stoichiometric HA, it is basically a calcium-deficient apatite with various substitutions by ions or groups such as Na+, K+ and Mg2+ at regular HA lattice sites (Brown & Constantz 1994). However, whether bone quality will be affected by the incorporation of strontium remains to be answered, since strontium is not a normal constituent of natural human bone (typically, about 320 mg in total in the human body, mostly in bone and connective tissue; Pan et al. 2009).
Table 2.
Element content (Sr, Mg, Ca, P) and calculated Sr/(Ca + Sr), Ca/P and (Ca + Sr)/P ratios of the coatings by soaking in SBF solution with different time, by EDX (mean±SD, n = 5).
| elements |
||||||
|---|---|---|---|---|---|---|
| Sr | Mg | Ca | P | Sr/(Ca + Sr) | Ca/P | (Sr + Ca)/P |
| 1.02 ± 0.72 | 0.33 ± 0.21 | 22.75 ± 4.13 | 14.37 ± 2.68 | 4.29% | 1.58 | 1.65 |
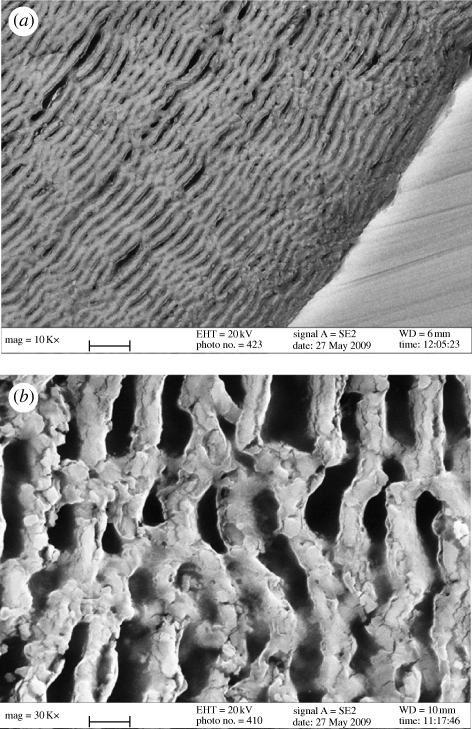
Over a period of time, apatite was formed as a unique multilayer with porous-interconnected structure (figure 4). Essentially, the nucleation of apatite depends on two requirements: supersaturation degree of calcium and phosphate, and pH value. The accumulation of calcium was principally attributed to the rapid release of calcium from the glass surface and the compensation of phosphate was mainly obtained from the DMEM solution since there was limited phosphate in the glass network. Meanwhile, the increased pH of the adjacent solution was due to the rapid release of alkaline elements such as sodium, potassium and magnesium. Therefore, heterogeneous nucleation of apatite spontaneously occurred (figure 3). Then, the surface to nucleate apatite was uniformly moved inwards with the continuous release of calcium from the glass inside, but controlled by the concentration of the reacting ions in solution, particularly phosphate, needed by its consumption mainly from the solution. Thus it appeared that the reaction was hampered until the ionic concentration increased to a value where the reaction could occur again; the regular intervals of the apatite layer were therefore formed. However, the multilayer described in Li et al.'s (2007b) work was mainly attributed to the higher concentration of phosphate in the background solution (250 mM). Thus rapid compensation of phosphate from the solution can be achieved to drive apatite nucleation. Despite facilitating apatite formation, the exaggerated phosphate concentration was a failure in simulating biomineralization in vivo. Therefore, the characteristic of complete degradation as mentioned by Yao et al. (2007) might not only be due to the B–O network being more easily attacked than the Si–O network, but also due to the much easier diffusion of ions through this unique porous structure. Although the degradation rate was unavoidably slowed down with time owing to the thickness of the surrounding apatite layer, the complete conversion to apatite is therefore possible because of its unique multilayer with constant intervals.
Figure 4.
Cross section of borate glass after soaking in DMEM solution for 30 days (2B–6Sr). Apatite was formed as a multilayer with interconnected porous structure. Magnification, (a) ×10 000; (b) ×30 000. Scale bar, (a) 3 µm; (b) 1 µm.
Borate glass was derived from 45S5 Bioglass, with a similar composition, but SiO2 was completely or partially replaced by B2O3 (Huang et al. 2006). As said, the bioactivity was significantly increased judging by the ability of apatite formation (Huang et al. 2006). However, the conversion to HA was reported to be too fast than expected if all SiO2 were substituted by B2O3 (complete conversion to apatite in less than 4 days; Huang et al. 2006; Yao et al. 2007). Therefore, the retention of partial SiO2 (up to 20%) showed significant slow-down of its rapid degradation rate, designated as 2B glass (Yao et al. 2007).
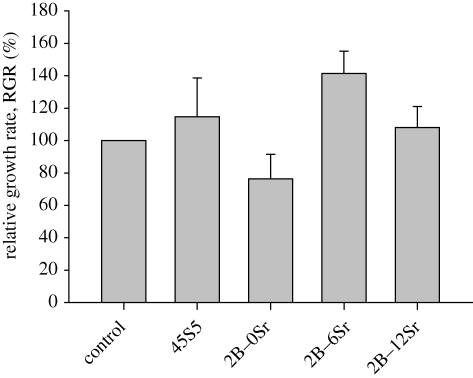
However, the degradation mechanism between borate glass and 45S5 Bioglass is completely different. As reported, the rapid exchange of alkaline ions from 45S5 Bioglass generally resulted in the formation of a porous SiO2-rich gel layer (Hench 1998), which played a key role in transporting ions and precipitating apatite. But determined by solution pH, as was done by Huang et al. (2006), the dissolution of the SiO2-rich layer might be hampered if SiO44− concentration was saturated with respect to the adjacent solution, thus retarding the formation of apatite. However, no SiO2-rich layer was formed during the degradation of borate glass as determined (Huang et al. 2006; Yao et al. 2007), thus generally facilitating the formation of an apatite. However, the biocompatibility of this newly designed borate glass is still debatable, since the related cytotoxicity work is very limited. As a result, the incorporation of strontium to partially replace magnesium was anticipated not only to maintain the stability of the glass network owing to chemical similarity, but also to increase the cyto-compatibility (figure 5).
Figure 5.
Biocompatibility of strontium-incorporated borate glass determined by MTT assay, p < 0.05. Typical 45S5 Bioglass and blank used as control.
Cytotoxicity is a major concern for the design and fabrication of this newly developed bioactive material, to say, the first and most important step. Presently, the L929 cell line is widely used to evaluate the cytotoxicity of materials (Torabinejad et al. 1995; Kaga et al. 2001; Serrano et al. 2004; Scharnweber et al. 2008) based on the requirement of ISO 10993 (International Standards Organization 10993 1992). Testing by this standard, 45S5 Bioglass has been widely reported to possess excellent biocompatibility (Shirtliff & Hench 2003; Wilson et al. 2004; Jones & Hench 2006). As confirmed in this work, MTT result showed that the RGR of L929 cells was significantly more than 100 per cent, thus the corresponding cytotoxicity type was class 0, indicating no cytotoxicity. However, borate glass really showed cytotoxicity and the RGR just passed 75 per cent, thus classified between classes 2 and 1. Indeed, this increased significantly with the incorporation of strontium when the RGR was calculated to be 141 per cent and 108 per cent, both more than 100 per cent, and thus the cytotoxicity was classified as 0 when 6 and 12 per cent strontium were, respectively, added. As a result, similar to 45S5 Bioglass, there was no cytotoxicity.
Boron is an essential element for bone health, but its cytotoxicity depends on its concentration. As seen in table 3, the concentration of boron released from borate glass was nearly twice as high as that released from glass incorporating strontium. It was reported that the radius of strontium ion (1.13 Å) is larger than that of magnesium ion (0.65 Å) and calcium ion (1.00 Å; Li et al. 2007a), thus it occupies more space in the glass network and effectively inhibits the movement and release of other ions and thereby reduces the dissolution rate of borate glass. As a result, the cytotoxicity that arises with the rapid release of boron was eliminated or at least minimized.
Table 3.
Ion (B and Sr) concentration of extraction solution after glass samples soaked for 3 days determined by ICP-AES (mean±SD, n = 5).
| composition | B (ppm) | Sr (ppm) |
|---|---|---|
| 2B–0Sr | 430 ± 17 | 5 ± 2 |
| 2B–6Sr | 210 ± 31 | 19 ± 8 |
| 2B–12Sr | 270 ± 41 | 62 ± 11 |
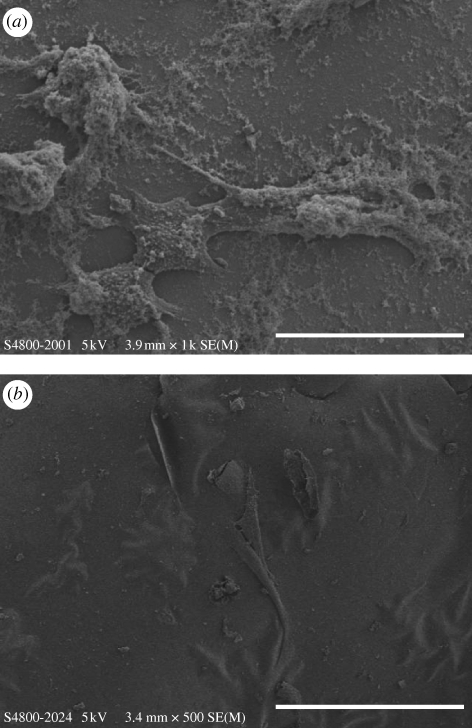
In addition, SaOS-2 cells could spontaneously attach, spread and proliferate well on the glass surface. The nucleation of apatite was not only detected on the glass surface, but also on the cell surface and thus the spread antennae of cells were partially covered by the apatite nucleation (figure 6a). Moreover, some cells were found to be completely covered by the rapid formation of apatite, only leaving contours, but the spreading of cells was still easily detectable. As a result, a sandwich structure was formed containing the apatite and cells (figure 6b). Bone is formed by the mineralization of an organic matrix including cells, osteoid and proteins by the nucleation and growth of apatite. This sandwich structure indicates that strontium-incorporated borate glass could not only stimulate apatite formation, but also induce the adhesion and proliferation of osteoblast cells, which may be the template for new bone formation.
Figure 6.
SEM micrographs showing the morphology of SaOS-2 osteoblast-like cells cultured on 2B–6Sr glass surface after day 3. (a) The spread antennae of cells were observed but partially covered by the formation of apatite. Scale bar, 50 µm. (b) Cells were mostly completely covered by the rapid formation of apatite, only leaving contours, but the spreading of cells was still easily detectable. Scale bar, 100 µm.
Cytotoxicity indicates that although the safety tolerance level of boron in drinking water is estimated to be 40–150 ppm, no acute toxicity was reported even at levels reaching 300 ppm (Garrett 1998). Essentially, boron was found to be rapidly excreted in the urine with a half-life of 21 h and complete elimination in 96 h (Garrett 1998). Thus, there is no accumulated toxicity in the human body. Although the standard MTT assay may tell us the toxic limit, it might not express true safety tolerance due to its unique characteristics. In particular, the co-effects of an interaction between boron and strontium on osteoblast and osteoclast cells are still not known. A biochemical approach and animal trials are therefore necessary and urgent.
4. Conclusion
The incorporation of strontium significantly decreased the cytotoxicity that arises with the rapid dissolution of borate glass leading to exceeding safety tolerance of boron. The complete conversion to apatite is therefore optimistic because of its unique multilayer and porous structure. With the degradation of glass, it will not only render boron as a nutritional element for bone health, but also deliver strontium for new bone formation.
Acknowledgements
This work was funded by Shanghai Committee of Science and Technology (grant no. 08441900500) and Hong Kong RGC HKU7147/07E. The authors are grateful for all the technical support and guidance from the Electron Microscopy Unit of the University of Hong Kong.
References
- Brown P. W., Constantz B. 1994. Hydroxyapatite and related materials. Boca Raton, FL: CRC Press. [Google Scholar]
- Buehler J., Chappuis P., Saffar J. L., Tsouderos Y., Vignery A. 2001. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis). Bone 29, 176–179. ( 10.1016/S8756-3282(01)00484-7) [DOI] [PubMed] [Google Scholar]
- Chen Q. Z., Thompson I. D., Boccaccini A. R. 2006. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 27, 2414–2425. ( 10.1016/j.biomaterials.2005.11.025) [DOI] [PubMed] [Google Scholar]
- Forrer R., Wenker C. H., Cautschi K., Lutz H. 2001a Concentration of 17 trace elements in serum and whole blood of plains viscachas (Lagostomus maximus) by ICP-MS, their reference ranges, and their relation to cataract. Biol. Trace Elem. Res. 81, 47–62. ( 10.1385/BTER:81:1:47) [DOI] [PubMed] [Google Scholar]
- Forrer R., Cautschi K., Lutz H. 2001b Simultaneous measurement of the trace elements Al, As, B, Be, Cd, Co, Cu, Fe, Li, Mn, Mo, Ni, Rb, Se, Sr, and Zn in human serum and their reference ranges by ICP-MS. Biol. Trace Elem. Res. 80, 77–93. ( 10.1385/BTER:80:1:77) [DOI] [PubMed] [Google Scholar]
- Garrett D. H. 1998. Borates. New York, NY: Academic Press. [Google Scholar]
- Hamadouche M., Meunier A., Greenspan D. C., Blanchat C., Zhong J. P., La Torre G. P., Sedel L. 2001. Long-term in vivo bioactivity and degradability of bulk sol-gel bioactive glasses. J. Biomed. Mater. Res. 54, 560–566. () [DOI] [PubMed] [Google Scholar]
- Hench L. L. 1998. Bioceramics. J. Am. Ceram. Soc. 81, 1705–1728. ( 10.1111/j.1151-2916.1998.tb02540.x) [DOI] [Google Scholar]
- Hench L. L., Wilson J. 1984. Surface-active biomaterials. Science 226, 630–636. ( 10.1126/science.6093253) [DOI] [PubMed] [Google Scholar]
- Huang W., Day D. E., Kittiratanapiboon K., Rahaman M. N. 2006. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci.: Mater. Med. 17, 583–596. ( 10.1007/s10856-006-9220-z) [DOI] [PubMed] [Google Scholar]
- International Standards Organization 10993. 1992. Biological evaluation of medical devices: part 5. Tests for cytotoxicity: in vitro methods. Geneva, Switzerland: International Standards Organization. [Google Scholar]
- Jones J. R., Hench L. L. 2006. Biomaterials: bioceramics. In Encyclopedia of medical devices and instrumentation (ed. Webster J. G.), pp. 283–296. Hoboken, NJ: Wiley. [Google Scholar]
- Kaga M., Noda M., Ferracane J. L., Nakamura W., Oguchi H., Sano H. 2001. The in vitro cytotoxicity of eluates from dentin bonding resins and their effect on tyrosine phosphorylation of L929 cells. Dent. Mater. 17, 333–339. ( 10.1016/S0109-5641(00)00091-9) [DOI] [PubMed] [Google Scholar]
- Lao J., Jallot E., Nedelec J. M. 2008. Strontium-delivering glasses with enhanced bioactivity: a new biomaterial for antiosteoporotic applications? Chem. Mater. 20, 4969–4973. ( 10.1021/cm800993s) [DOI] [Google Scholar]
- Lao J., Nedelec J. M., Jallot E. 2009. New strontium-based bioactive glasses: physicochemical reactivity and delivering capability of biologically active dissolution products. J. Mater. Chem. 19, 2940–2949. ( 10.1039/b822214b) [DOI] [Google Scholar]
- Li Z. Y., Lam W. M., Yang C., Xu B., Ni G. X., Abbah S. A., Cheung K. M., Luk K. D., Lu W. W. 2007a Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials 28, 1452–1460. ( 10.1016/j.biomaterials.2006.11.001) [DOI] [PubMed] [Google Scholar]
- Li Y., Rahaman M. N., Fu Q., Bal B. S., Yao A., Day D. E. 2007b Conversion of bioactive borosilicate glass to multilayered hydroxyapatite in dilute phosphate solution. J. Am. Ceram. Soc. 90, 3804–3810. ( 10.1111/j.1551-2916.2007.02057.x) [DOI] [Google Scholar]
- Liu X., Huang W., Fu H., Yao A., Wang D. P., Pan H. B., Lu W. W. 2009a Bioactive borosilicate glass scaffolds: improvement on the strength of glass-based scaffolds for tissue engineering. J. Mater. Sci.: Mater. Med. 20, 365–372. ( 10.1007/s10856-008-3582-3) [DOI] [PubMed] [Google Scholar]
- Liu X., Huang W., Fu H., Yao A., Wang D. P., Pan H. B., Lu W. W., Jiang X., Zhang X. 2009b Bioactive borosilicate glass scaffolds: in vitro degradation and bioactivity behaviors. J. Mater. Sci.: Mater. Med. 20, 1237–1243. ( 10.1007/s10856-009-3691-7) [DOI] [PubMed] [Google Scholar]
- Nielsen F. H. 1990. Studies on the relationship between boron and magnesium, which possibly affects the formation and maintenance of bones. Magnes. Trace Elem. 9, 61–69. [PubMed] [Google Scholar]
- Nielsen F. H. 2000. The emergence of boron as nutritionally important throughout the life cycle. Nutrition 16, 512–514. ( 10.1016/S0899-9007(00)00324-5) [DOI] [PubMed] [Google Scholar]
- Nielsen F. H., Hunt C. D., Mullen L. M., Hunt J. R. 1987. Effect of dietary boron on mineral estrogen and testosterone metabolism in postmenopausal women. FASEB J. 1, 394–397. [PubMed] [Google Scholar]
- Nielsen S. P. 2004. The biological role of strontium. Bone 35, 583–588. ( 10.1016/j.bone.2004.04.026) [DOI] [PubMed] [Google Scholar]
- Pan H. B., Li Z. Y., Lam R., Wong C. T., Darvell B. W., Luk K. D. K., Hu Y., Lu W. W. 2009. Nucleation of strontium-substituted apatite. Cryst. Growth Des. 9, 3342–3345. ( 10.1021/cg900038k) [DOI] [Google Scholar]
- Place E. S., Evans N. D., Stevens M. N. 2009. Complexity in biomaterials for tissue engineering. Nat. Mater. 8, 457–470. ( 10.1038/nmat2441) [DOI] [PubMed] [Google Scholar]
- Rizzoli R. 2005. A new treatment for post-menopausal osteoporosis: strontium ranelate. J. Endocrinol. Invest. 28(Suppl. 8), 50–57. [PubMed] [Google Scholar]
- Scharnweber T., Santos C., Franke R. P., Almeida M. M., Costa M. E. V. 2008. Influence of spray-driedhydroxyapatite-5-fluorouracil granules on cell lines derived from tissues of mesenchymal origin. Molecules 13, 2729–2739. ( 10.3390/molecules13112729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M. C., Pagani R., Vallet-Regí M., Peña J., Rámila A., Izquierdo I., Portolés M. T. 2004. In vitro biocompatibility assessment of poly(ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 25, 5603–5611. ( 10.1016/j.biomaterials.2004.01.037) [DOI] [PubMed] [Google Scholar]
- Shirtliff V. J., Hench L. L. 2003. Bioactive materials for tissue engineering, regeneration and repair. J. Mater. Sci. 38, 4697–4707. ( 10.1023/A:1027414700111) [DOI] [Google Scholar]
- Silver I. A., Deas J., Erecińska M. 2001. Interactions of bioactive glasses with osteoblasts in vitro: effects of 45S5 Bioglass, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials 22, 175–185. ( 10.1016/S0142-9612(00)00173-3) [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Hong C., Pitt Ford T., Kettering J. 1995. Cytotoxicity of four root end filling materials. J. Endodont. 21, 489–492. ( 10.1016/S0099-2399(06)80518-2) [DOI] [PubMed] [Google Scholar]
- Wilson J., Pigott G. H., Schoen F. J., Hench L. L. 2004. Toxicology and biocompatibility of bioglasses. J. Biomed. Mater. Res. 15, 805–817. ( 10.1002/jbm.820150605) [DOI] [PubMed] [Google Scholar]
- Yao A., Wang D. P., Huang W., Rahaman M. N., Day D. E. 2007. In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J. Am. Ceram. Soc. 90, 303–306. ( 10.1111/j.1551-2916.2006.01358.x) [DOI] [Google Scholar]