Abstract
In nitrogen-fixing symbiosis, plant sanctions against ineffective bacteria have been demonstrated in previous studies performed on soybean and yellow bush lupin, both developing determinate nodules with Bradyrhizobium sp. strains. In this study, we focused on the widely studied symbiotic association Medicago truncatula–Sinorhizobium meliloti, which forms indeterminate nodules. Using two strains isolated from the same soil and displaying different nitrogen fixation phenotypes on the same fixed plant line, we analysed the existence of both partner choice and plant sanctions by performing split-root experiments. By measuring different parameters such as the nodule number, the nodule biomass per nodule and the number of viable rhizobia per nodule, we showed that M. truncatula is able to select rhizobia based on recognition signals, both before and after the nitrogen fixation step. However, no sanction mechanism, described as a decrease in rhizobia fitness inside the nodules, was detected. Consequently, even if partner choice seems to be widespread among legumes, sanction of non-effective rhizobia might not be universal.
Keywords: partner choice, sanction, symbiosis, nitrogen fixation, Sinorhizobium meliloti, indeterminate nodules
1. Introduction
Many legumes interact with nitrogen-fixing bacteria (called rhizobia) by forming root nodules, inside which, differentiated rhizobia (called bacteroids) have the ability to reduce atmospheric nitrogen into ammonium, making nitrogen available for the plant. In this mutually beneficial interaction, the plant provides in return a protected environment and supplies carbon photosynthates to the bacteria. Mutualisms, such as this symbiosis, can be compared with a ‘biological market’ (Noë & Hammerstein 1994), where the plant can exchange nutrients with several genetically different individuals (soil rhizobia population), thus initiating possible conflict. Strains presenting different fixation levels had previously been described from natural rhizobia populations, e.g. in Sinorhizobium sp. (Miller & Sirois 1982; Rangin et al. 2008). As rhizobia are not transmitted vertically between plant generations, mutualism persistence must partially be explained by the partner choice model (Bull & Rice 1991; Simms & Taylor 2002), where the plant selects for a particular rhizobium based on its symbiotic efficiency. It is not clear why populations of rhizobia contain nitrogen-fixing strains (given that nitrogen fixation is metabolically costly) when non-fixing strains are also capable of nodulating the same plant without making any metabolic concession.
The ‘tragedy of the commons’ theory (Hardin 1968), which describes a conflict between individual and common interests over commodities, can be applied to the legume–rhizobia symbiosis. Rhizobia benefit by getting the highest amount of resources from the plant while providing the least fixed nitrogen, thus increasing their own reproductive rate and reaching the highest fitness and competitiveness in the population, but also by giving more resources to the plant, in order to increase plant fitness and subsequently increasing the probability that the symbiosis will be maintained over subsequent generations by the presence of the appropriate host plant. The evolutionary persistence of this symbiotic cooperation provides evidence that the plant imposes selection on rhizobia by either rewarding the most cooperative genotypes, or sanctioning the cheaters and the less cooperative populations.
Whereas a theoretical model of legume sanctions was first proposed by Denison (2000), Kiers et al. (2003) provided the first experimental evidence of plant sanctions in the soybean–Bradyrhizobium japonicum interaction. By replacing air with a N2-free atmosphere (Ar : O2 mix), they showed that plants could not only reduce resource allocation to rhizobia that failed to fix N2 inside their root nodules, but also evaluate the fixation level of rhizobia inside each nodule independently and apply intermediate sanctions depending on their fixation level (Kiers et al. 2006). This controlling mechanism was inferred by estimating bacterial fitness, which gives a direct estimate of the number of individuals that contribute to producing the next bacterial generation. In fact, bacterial fitness is based on the assumption that the number of bacteria isolated from intact nodules is monotonically related to the number of bacteria that actually survive nodule senescence to reproduce.
Several mechanisms may result from an ineffective (or poorly efficient) rhizobia–host interaction. Partner choice is described as the discrimination by the plant–host against less-cooperative rhizobia based on recognition signals, whereas plant sanction is a differential allocation of resources among nodules. Thus, any selection before the fixation stage would be considered as partner choice, whereas selection after detection of the efficiency level may be either partner choice or sanction.
Legume nodules are usually classified into two groups: determinate nodules inside which bacteroids maintain the ability to reproduce, and indeterminate nodules, where bacteroids lose the ability to replicate (see Denison 2000 for a review), owing to the endoreplication of their genome (Mergaert et al. 2006), and thus cannot contribute to the next rhizobial generation. However, viable rhizobia contained in persistent infection threads and possibly in the infection zone of the nodules maintain the ability to divide. They can be released in the plant rhizosphere after senescence of such indeterminate nodules (Paau et al. 1980) and then have an impact on the evolution of the bacterial population (Heath & Tiffin 2007, 2009). So far, plant sanctions have been detected only on soybean determinate nodules (Kiers et al. 2003) or on yellow bush lupin nodules (Simms et al. 2006), in which bacteroids apparently have the ability to dedifferentiate (Sprent et al. 1987). Consequently, the question of the ubiquity of sanctions remains open, particularly for plants forming ‘true’ indeterminate nodules (Oono et al. 2009). Since bacteroids are non-replicable in indeterminate nodules, the absence of detectable sanctions on the replicable bacteria may seem logical in the case of a mixed nodule. However, with nodules occupied by only one strain, the plant may apply some sanction on the nodule as a whole (as opposed to the bacteroids per se) and thus on any viable rhizobia within it.
The goal of our study was to explore the existence of partner choice and sanctions in a well-studied model of plant–bacteria symbiosis, the Medicago truncatula–Sinorhizobium meliloti association. Such mechanisms have been analysed recently (Heath & Tiffin 2009), but the present data contradict results from published studies (Miller & Sirois 1982), leading to uncertainties about the conclusions provided (Oono et al. 2009). To analyse such mechanisms in this rhizobia–host interaction, we used a split-root experiment system with natural strains.
2. Material and methods
(a). Inoculation and plant growth
Medicago truncatula fixed line F83-005 was grown either with S. meliloti strain STM 5472 and/or strain STM 5480, both isolated from the same soil sample in a previous study (Rangin et al. 2008). Seeds were surface-sterilized and germinated in the dark for 48 h. Plants were grown in tanks containing an aerated nutrient solution, with root systems split into two similar halves, as described in Ruffel et al. (2008). When seedlings were one week old, the bacterial inoculum was added and potassium nitrate was removed. Each half-root system of each plant was inoculated either with the same strain (single-inoculation assay) or with a different strain (mixed inoculation) to test for the reality of sanction when the plant has the choice. Eight and four plant replicates per combination were performed for the single and the mixed assays, respectively.
The two strains were grown in 20 ml yeast mannitol liquid medium for 3 days at 27°C under agitation (Vincent 1970) and washed twice with sterile water. Each seedling was inoculated with 1.5 ml inoculum (5 × 106 colony forming units ml−1) and plants were grown for seven weeks.
The total number of nodules produced on each half-plant system was counted, the total fresh biomass of nodules was measured on each half-plant system and the shoot of each plant was collected, dried for 48 h at 72°C and weighed. The number of total viable bacteria (reproductive offspring) inside all the nodules (pooled together) per half-plant system was also estimated by recording the number of colony-forming units produced from crushing, serially diluting and plating all subsamples (after surface sterilization of nodules). From these measurements, the biomass per nodule and the number of rhizobia per nodule were calculated by dividing the nodule biomass or the number of rhizobia by the number of nodules per half-root system.
(b). Statistical tests
Means comparisons between the two strains were conducted with ANOVAs using Statistica 6 software. Since the rhizobia infection and fitness could be estimated with three different parameters (number of nodules, biomass per nodule and rhizobia per nodule), MANOVA analyses were conducted on these multivariate datasets. Post hoc tests on each measurement were then used to assess significant differences shown on the graphs. When necessary, measurements were log-transformed to normalize residuals and maintain homoscedasticity.
3. Results and discussion
(a). Single-strain inoculations: understanding the symbiotic quality of the strains
The two strains used in this study display different fixation phenotypes on the F83-005 plant line genotype. According to dry aerial biomasses, strain STM 5480 appears efficient in terms of nitrogen fixation (fix+), whereas strain STM 5472 is not (fix−). This contrasting efficiency, when associated with the F83-005 plant, was confirmed by acetylene reduction measurements (data not shown). Fixation polymorphism of different S. meliloti strains when inoculated on the same M. truncatula genotype has been observed previously, and Simsek et al. (2007) suggested that succinoglycan oligosaccharide structure may be involved (at least partially) in the nitrogen fixation variation.
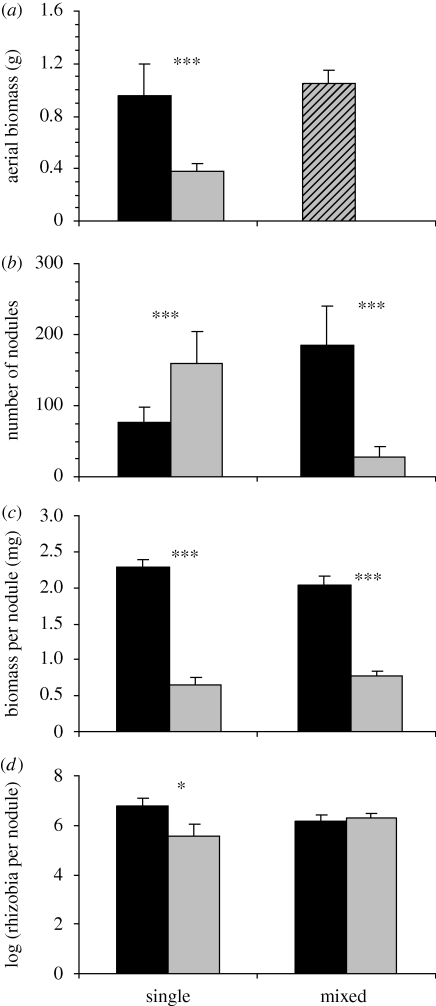
In the single-inoculation assay (figure 1, left part), nodule number was found to be significantly higher in those roots inoculated with the non-fixing strain compared with the N2-fixing strain. Observing a higher nodulation rate by a non-fixing strain is a classical result, because the plant forms abundant nodules in order to find efficient strains in its environment. Single-strain inoculations are generally not pertinent to test for the partner-choice/sanctions hypothesis (Oono et al. 2009). However, there is one possible exception, which is when sanction is absolute (West et al. 2002), i.e. when the plant might choose to deprive resources to poorly efficient strains even in the absence of alternative bacterial choices, because the nodule performs below a certain threshold. Here, both the biomass per nodule and the number of cultivable rhizobia per nodule were found to be significantly higher for the N2-fixing strain compared with the non-fixing strain (figure 1). As the mean biomass per nodule was conversely higher in the N2-fixing strain, M. truncatula plants may have the ability to restrict nodule development when interacting with an inefficient strain, probably to avoid wasting resources. The plant may even restrict the rhizobial fitness in these nodules (measured as the number of viable rhizobia per nodule), suggesting that the plant may sanction less efficient rhizobia when the plant is in contact with a single-strain genotype.
Figure 1.
Measurements of aerial biomass, nodule number, nodule biomass per nodule and rhizobia per nodule obtained in the split-root experiment. ‘Single’ represents values with the same bacterial strain at each plant side, whereas ‘mixed’ represents values with each strain on each plant side. Black and white colours indicated the plant line/strain combination with the strains STM 5480 and STM 5472, respectively. Significant differences by paired post hoc tests: *p < 0.05; ***p < 0.001.
However, this result should be treated with caution for several reasons. First, with one strain inoculated per plant, smaller nodules on smaller plants may just reflect the nitrogen fixation differences between the two strains, hinting at a partner-choice mechanism that actually does not exist. Such uncertainty suggests that several strains may be necessary. Second, for each of the two strains, no significant correlation was found between the biomass per nodule and the number of reproductive rhizobia inside the nodule (data not shown), whereas we have demonstrated a significant positive correlation between the nodule biomass and the nodule size, measured as the projection surface of fresh scanned nodules (C. Gubry-Rangin 2008, unpublished data). These results may therefore contrast with the positive correlation between the size and the viable rhizobia found in M. truncatula (Heath & Tiffin 2007). As discussed by Oono et al. (2009), this relationship may vary among different rhizobia, and Heath & Tiffin (2007) measured the relationship with Sinorhizobium medicae whereas S. meliloti was investigated in the present study. However, even if a positive correlation is a classical result in determinate nodules containing viable bacteroids (Kiers et al. 2006; Simms et al. 2006), complementary investigations are needed on indeterminate nodules containing dimorphic rhizobia. For example, absence of a positive correlation might happen in indeterminate nodules if the infection zone (zone II of the nodule containing reproductive bacteria) would be particularly reduced over the other zones of the nodule.
(b). Pre-infection partner choice
Because single-strain inoculation is not reflecting the natural environment, we analysed partner choice and plant sanction in the case where a plant can choose between different bacterial partners. A split-root treatment with the two different strains was performed with each strain being inoculated only on one half-plant root. This double-inoculation treatment ensures that differences of nodule development or rhizobia fitness are not owing to the overall nitrogen status of the plant. In this mixed inoculation (figure 1, right part), the N2-fixing strain is associated with a greater number of nodules than the non-fixing strain, indicating that it is preferentially selected, suggesting a pre-infection partner choice. Some recognition signals rather than nitrogen fixation should have favoured the number of nodules formed with the efficient strain. Then, both rhizobia seem to exert honest signalling (i.e. the recognition signal reliably reflects partner quality) and the non-fixing strain seems not to mimic the signal of the effective strain (as it is recognized as having a low efficiency). Our results support the findings of Heath & Tiffin (2009), who detected a pre-infection partner choice in M. truncatula–S. meliloti interaction with natural strains. However, Amarger (1981) reported that competition for nodule formation did not vary between effective and ineffective strains of S. meliloti. These ineffective strains are spontaneous antibiotic-resistant mutants of the effective strains, and they may therefore present the same recognition signals and thus may be not detected as cheaters.
Another explanation for the higher number of nodules in the N-rich half-root might be that a root half with better nitrogen nutrition forms more nodules. Furthermore, we can note that the number of nodules observed with the efficient strain is higher in the mixed inoculation than in the single inoculation. As plants regulate their whole nitrogen content, these results suggest that when one side of the plant is N-deficient, genetic mechanisms lead to an upregulation of nodule formation in the non-limiting side of the plant in order to maintain the optimal nitrogen content. Such mechanisms were previously described for plants starved in nitrate on a half-root system (Ruffel et al. 2008).
(c). Post-infection partner choice
In the mixed treatment, a significantly higher biomass per nodule was measured for the N2-fixing strain than for the non-fixing strain, suggesting that M. truncatula is able to exert a post-infection partner choice on nodules containing less efficient rhizobia. In a previous analysis on M. truncatula in interaction with S. meliloti, Heath & Tiffin (2009) showed that a plant could not allocate resources preferentially to nodules containing the most beneficial strains, suggesting that post-infection partner choice cannot be detected in natural occurring strains. They suggested that the fixation level of the different strains did not vary enough and that the plant could not discriminate between them. However, we previously found that even in a single soil, drastic fixation polymorphism can be detected (Rangin et al. 2008), and we observed here that the plant is apparently able to select strains based on this criterion. In fact, our measure of biomass per nodule suggested that plants have the ability to recognize nodules containing the best fixing strains and can favour their development against those containing the less beneficial strains. One major difference between the result of Heath & Tiffin (2009) and the data presented here is the level of efficiency of the strains. One of the two strains we used was a nodulating (nod+) but non-efficient strain (fix- phenotype with acetylene reduction assay), whereas all the strains that Heath & Tiffin (2009) used were nod+/fix+ (production of several pods in interaction with the plant), except for Sals b–Naut 3 interaction (nod−/fix−). Since post-infection partner choice was shown with nod+/fix− rhizobia but not with nod+/fix+ rhizobia presenting different efficiencies, such a partner-choice mechanism might only occur against extreme cheater or non-cooperative rhizobia and not against intermediate or less-cooperative rhizobia. Such a partner-choice hypothesis should be complemented either with several non-fixing strain analyses or by comparing an efficient strain with its isogenic mutant (i.e. carrying a mutation of a gene required for nitrogen fixation). Additionally, some experiments with argon replacing nitrogen (as described in Kiers et al. 2006) might shed some light about the existence of an intermediate partner choice in this model interaction.
(d). Sanctions?
Because a sanction mechanism may often lead to a restriction of a rhizobial population based on their number of its subsequent descendants, we analysed the number of viable rhizobia per nodule in the different associations. In the mixed treatment, no significant differences were shown between the two strains. Thus, M. truncatula cannot decrease rhizobia fitness inside less efficient nodules. It is important to note that in the single-strain inoculations, the non-fixing strain contained a lower number of viable rhizobia per nodule than the fixing strain. Thus the same number of viable rhizobia per nodule observed between the two strains in the mixed inoculation cannot be the result of some host-strain interaction. However, some caution is required regarding this result owing to the absence of correlation between the size of the nodule and the number of reproductive rhizobia inside (as discussed previously). From these results, our experiments did not support the existence of sanction in the M. truncatula–S. meliloti association. To our knowledge, this is the first time that such mechanisms have been demonstrated in indeterminate nodules with rhizobial fitness measurements. We cannot exclude the possibility that the plant truly applied sanctions against the non-fixing strain (we did not compare the two statuses for the same strain since it is not an ‘artificial’ non-fixing status with argon replacing nitrogen like in previous studies). However, even if applied, these sanctions were not enough to result in a lower fitness of the non fixing bacteria compared with the fixing strain. By comparison, Minchin et al. (1983) did show on several legumes (among other Medicago sativa L.) that decreased nodule oxygen permeability is observed in nodules where nodule fixation was blocked, which is also a way to sanction non-effective nodules in soybean (Kiers et al. 2003). Their results suggest that sanctions might exist even in legumes presenting indeterminate nodules. However, Minchin et al. (1983) did not analyse M. truncatula, and as shown by Kiers et al. (2007), legume sanction strength varies even between different cultivars of the same species, suggesting that such a mechanism is not universal.
4. Conclusion
The use of sanctions on non-reproductive bacteroids remains evolutionarily unclear, and no study has been able to demonstrate the existence of such a mechanism. Therefore, sanction mechanisms, which produce differential resource allocation among nodules and subsequently have an impact on the rhizobia inside, may not exist in such a system. However, partner choice, which can occur both at the pre-infection and post-infection stages of the bacteria–host interaction, appears to be a good mechanism for maintaining effective symbiosis and to counterselect less cooperative (or cheater) partners.
Acknowledgements
This work was supported by a programme funded by INRA Santé des Plantes et Environment (Rhizosphere Ecology of Annual Medics programme). C.G.-R. was supported by a PhD fellowship from the French Ministry of Education and Research and by a teaching fellowship from the University Montpellier II. We warmly thank Jean-Marie Prospéri and Magalie Delalande who provided the M. truncatula seeds. We are very grateful to Jean-Claude Cleyet-Marel, Lucette Mauré and Karine Heulin, who provided help for nodule harvest and rhizobia counting. We also warmly thank Marc Lepetit for his help with split-root assays and Gisèle Laguerre for helpful discussions. Finally, we acknowledge Graeme W. Nicol for the language revision of the manuscript and three anonymous reviewers for their comments that much improved this manuscript.
References
- Amarger N.1981Competition for nodule formation between effective and ineffective strains of Rhizobium meliloti. Soil Biol. Biochem. 13, 475–480 (doi:10.1016/0038-0717(81)90037-7) [Google Scholar]
- Bull J. J., Rice W. R.1991Distinguishing mechanisms for the evolution of co-operation. J. Theor. Biol. 149, 63–74 (doi:10.1016/S0022-5193(05)80072-4) [DOI] [PubMed] [Google Scholar]
- Denison R. F.2000Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am. Nat. 156, 567–576 (doi:10.1016/j.femsle.2004.07.013) [DOI] [PubMed] [Google Scholar]
- Hardin G.1968The tragedy of the commons. Science 162, 1243–1248 (doi:10.1126/science.162.3859.1243) [PubMed] [Google Scholar]
- Heath K. D., Tiffin P.2007Context dependence in the coevolution of plant and rhizobial mutualists. Proc. R. Soc. B 274, 1905–1912 (doi:10.1098/rspb.2007.0495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath K. D., Tiffin P.2009Stabilizing mechanisms in a legume–rhizobium mutualism. Evolution 63, 652–662 (doi:10.1111/j.1558-5646.2008.00582.x) [DOI] [PubMed] [Google Scholar]
- Kiers E. T., Rousseau R. A., West S. A., Denison R. F.2003Host sanctions and the legume–rhizobium mutualism. Nature 425, 78–81 (doi:10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- Kiers E. T., Rousseau R. A., Denison R. F.2006Measured sanctions: legume hosts detect quantitative variation in rhizobium cooperation and punish accordingly. Evol. Ecol. Res. 8, 1077–1086 [Google Scholar]
- Kiers E. T., Hutton M. G., Denison R. F.2007Human selection and the relaxation of legumes defences against ineffective rhizobia. Proc. R. Soc. B 274, 3119–3126 (doi:10.1098/rspb.2007.1187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergaert P., et al. 2006Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium–legume symbiosis. Proc. Natl Acad. Sci. USA 103, 5230–5235 (doi:10.1073/pnas.0600912103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. W., Sirois J. C.1982Relative efficacy of different alfalfa cultivar—Rhizobium meliloti strain combinations for symbiotic nitrogen fixation. Appl. Environ. Microbiol. 43, 764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin F. R., Witty J. F., Sheehy J. E., Muller M.1983A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J. Exp. Bot. 34, 641–649 (doi:10.1093/jxb/34.5.641) [Google Scholar]
- Noë R., Hammerstein P.1994Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11 (doi:10.1007/BF00167053) [Google Scholar]
- Oono R., Denison R. F., Kiers E. T.2009Controlling the reproductive fate of rhizobia: how universal are legume sanctions? New Phytol. 183, 967–979 (doi:10.1111/j.1469-8137.2009.02941.x) [DOI] [PubMed] [Google Scholar]
- Paau A. S., Bloch C. B., Brill W. J.1980Developmental fate of Rhizobium meliloti bacteroids in alfalfa nodules. J. Bacteriol. 143, 1480–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangin C., Brunel B., Cleyet-Marel J.-C., Perrineau M.-M., Béna G.2008Effects of Medicago truncatula genetic diversity, rhizobial competition, and strain effectiveness on the diversity of a natural Sinorhizobium species community. Appl. Environ. Microbiol. 74, 5653–5661 (doi:10.1128/AEM.01107-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffel S., et al. 2008Systemic signaling of the plant N status triggers specific transcriptome responses depending on the N source in Medicago truncatula. Plant Physiol. 146, 2020–2035 (doi:/10.1104/pp.107.115667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms E. L., Taylor D. L.2002Partner choice in nitrogen-fixation mutualisms of legumes and rhizobia. Integr. Comp. Biol. 42, 369–380 (doi:10.1093/icb/42.2.369) [DOI] [PubMed] [Google Scholar]
- Simms E. L., Taylor D. L., Povich J., Shefferson R. P., Sachs J. L., Urbina M., Tausczik Y.2006An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proc. R. Soc. B 273, 77–81 (doi:10.1098/rspb.2005.3292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek S., Ojanen-Reuhs T., Stephens S. B., Reuhs B. L.2007Strain-ecotype specificity in Sinorhizobium meliloti–Medicago truncatula symbiosis is correlated to succinoglycan oligosaccharide structure. J. Bacteriol. 189, 7733–7740 (doi:10.1128/JB.00739-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. I., Sutherland J. M., De Faria S. M.1987Some aspects of the biology of nitrogen-fixing organisms. Phil. Trans. R. Soc. Lond. B 317, 111–129 (doi:10.1098/rstb.1987.0051) [Google Scholar]
- Vincent J. M.1970A manual for the practical study of root-nodule bacteria Oxford, UK: Blackwell Scientific Publications Ltd [Google Scholar]
- West S. A., Kiers E. T., Simms E. L., Denison R. F.2002Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc. R. Soc. Lond. B 269, 685–694 (doi:10.1098/rspb.2001.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]