Abstract
In extant birds, the hand is permanently abducted towards the ulna, and the wrist joint can bend extensively in this direction to fold the wing when not in use. Anatomically, this asymmetric mobility of the wrist results from the wedge-like shape of one carpal bone, the radiale, and from the well-developed convexity of the trochlea at the proximal end of the carpometacarpus. Among the theropod precursors of birds, a strongly convex trochlea is characteristic of Coelurosauria, a clade including the highly derived Maniraptora in addition to tyrannosaurs and compsognathids. The shape of the radiale can be quantified using a ‘radiale angle’ between the proximal and distal articular surfaces. Measurement of the radiale angle and reconstruction of ancestral states using squared-change parsimony shows that the angle was small (15°) in primitive coelurosaurs but considerably larger (25°) in primitive maniraptorans, indicating that the radiale was more wedge-shaped and the carpal joint more asymmetric. The radiale angle progressively increased still further within Maniraptora, with concurrent elongation of the forelimb feathers and the forelimb itself. Carpal asymmetry would have permitted avian-like folding of the forelimb in order to protect the plumage, an early advantage of the flexible, asymmetric wrist inherited by birds.
Keywords: Theropoda, Maniraptora, Aves, carpus, radiale, feathers
1. Introduction
Extant volant birds possess a highly specialized wrist joint, in which two proximal carpals articulate with a fused carpometacarpus. The proximal part of the carpometacarpus forms an articular trochlea, comprising two convex ridges separated by a transverse groove, and is largely homologous to the structurally similar semilunate carpal (SLC) of derived non-avian theropods (Ostrom 1976). Taxa with a well-developed SLC would have been distinguished by a wrist with enhanced flexibility in the plane of the radius and ulna, a characteristic inherited by crown-group birds (following Gauthier (1986) we use Aves for this crown-group, and Avialae for the larger, stem-based clade containing all maniraptorans closer to Aves than to dromaeosaurids). The mobility of the wrist contributes to the ability of a flapping bird to partly fold the wing during the upstroke, greatly improving flight efficiency (Vazquez 1992), and also permits the wing to be completely folded when not in use. Keeping the wing folded can make a bird less conspicuous, protect the feathers from damage, and prevent the wing from interfering with terrestrial locomotion.
Avian wing-folding is a biologically important behaviour that depends on a highly flexible wrist joint. However, this flexibility is exclusively in the ulnar direction (figure 1), and the typical avian manus is permanently abducted towards the ulna (Ostrom 1976). A similar asymmetry in the flexibility of the wrist has been explicitly inferred in at least some advanced theropods, including the dromaeosaurid Deinonychus (Senter 2006), and has often been tacitly assumed in discussions of forelimb evolution on the line to birds. For example, Padian (e.g. 1985, fig. 3a) has repeatedly presented reconstructions of the predatory strike of a dromaeosaurid theropod that begin with the manus in a position of considerable abduction and end with the manus and antebrachium aligned. However, as acknowledged by Padian (2001), the functional advantage of beginning the predatory strike with the wrist deflected in this manner has never been fully elucidated. By contrast, Sereno & Rao (1992) argued that Archaeopteryx and non-avialan theropods were in fact limited in their ability to abduct the wrist joint, at least in comparison with extant birds and some derived Mesozoic ones. Despite these differing opinions, the anatomical basis of wrist asymmetry in derived theropods has been little discussed.
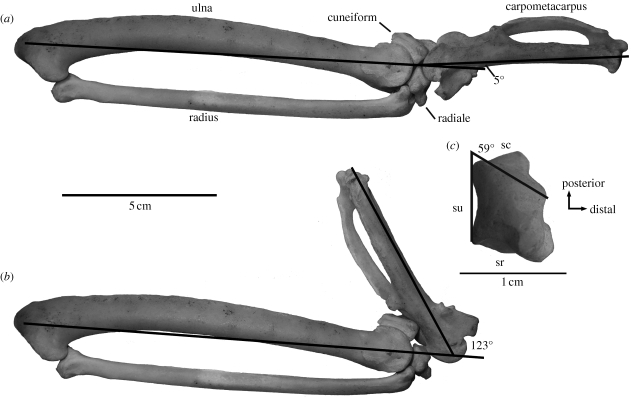
Figure 1.
Left distal forelimb of a turkey, Meleagris gallopavo (IVPP 1222): (a) wrist in minimum abduction; (b) wrist in maximum abduction; and (c) radiale. All views are dorsal. sc, articular surface for carpometacarpus; sr, articular surface for radius; su, articular surface for ulna. Angle of abduction shown in (a,b), radiale angle between ulnar surface and dorsal part of carpometacarpal surface shown in (c). Note that measuring with respect to the palmar part of carpometacarpal surface would result in even larger radiale angle.
The issue of wrist asymmetry has become particularly pertinent following the discovery of numerous non-avialan dinosaur specimens, mainly from the Jehol Group of China (Xu & Norell 2006) that preserve feathers or feather-like integumentary structures. For brevity, all such structures are referred to as feathers in this paper. Some dromaeosaurid, troodontid, oviraptorosaur and therizinosaur specimens show the presence of what we term a ‘pennibrachium’, a forelimb bearing long feathers that form a planar, wing-like surface but are not necessarily used in aerial locomotion. For example, a pennibrachium could have been used by a non-volant theropod in wing-assisted incline running (Dial 2003) or as a display structure. It is possible that pennibrachia were widespread or even predominant among derived non-avialan theropods, despite the fact that direct fossil evidence is limited to a few Lagerstatte. Any theropod with both a pennibrachium and an avian-like capacity for extensive wrist abduction would have been able to fold the pennibrachium during terrestrial locomotion, presumably realizing benefits analogous to those of wing-folding in modern birds.
In the present paper we review the features of the avian carpus that facilitate a large range of abduction, and investigate the distribution of similar features among non-avialan theropods. Finally, we place these results in phylogenetic context to trace the evolution of the distinctive avian carpal configuration through incipient stages prior to the origin of birds, and discuss implications for the evolution of the forelimb and of powered flight.
2. Material and methods
The structure of the carpus was examined in several maniraptoran and non-maniraptoran tetanuran theropods. Some specimens were studied firsthand, but in other cases we relied on photographs or published descriptions (table 1).
Table 1.
Radiale angles in various theropods. (Bold indicates specimens that we examined directly.)
| taxon | angle | specimen/source |
|---|---|---|
| Allosaurus fragilis | 2° | Chure (2001, fig. 2c) |
| Huaxiagnathus orientalis | 18° | Hwang et al. (2004, fig. 8a) |
| Sinosauropteryx prima | 6° | Currie & Chen (2001, fig. 8a) |
| Guanlong wucaii | 8° | IVPP V14531 |
| Alxasaurus elesitaiensis | 39° | IVPP RV93001 |
| Falcarius utahensis | 26° | Utah Museum of Natural History, Salt Lake City, USA (UMNH) VP 12294 |
| Caudipteryx sp. | 76° | IVPP V12430 |
| Haplocheirus | 15° | IVPP V15988 |
| Sinovenator changii | 35° | IVPP V14009 |
| Deinonychus antirrhopus | 31° | YPM 5208 |
| Eoconfuciusornis zhengi | 55° | IVPP V11977 |
| Meleagris gallopavo | 59° | IVPP 1222 |
We use the terms adduction and abduction rather than extension and flexion to describe deflection of the manus in the radial and ulnar directions, respectively. The flexor and extensor surfaces of the manus are, respectively, referred to as palmar and dorsal. Although the wrist of a tetrapod contains multiple synovial articulations, we use carpal joint and wrist joint interchangeably to refer specifically to the joint between the proximal and distal carpal elements, where flexibility of the complex of carpal bones and articulations as a whole (the wrist or carpus) was almost certainly concentrated in non-avian theropods as in modern birds.
We define the radiale angle as the angle between the proximal face of the radiale (in avians, the facet for the ulna; in other theropods, the facet for the radius) and the facet that articulates with the SLC or carpometacarpus. Radiale angles were measured for representatives of all the major clades of coleurosaurian theropods, in each case by visually superimposing lines representing the orientations of the articular surfaces on a photo of the radiale in dorsal or palmar view (see examples in figure 2). For reasons explained below, a large radiale angle is an osteological correlate of a wrist that is asymmetric in the sense that the range of abduction is greater than the range of adduction, based on a reference position in which the long axes of the metacarpus and antebrachium are aligned. Actual estimation of the range of wrist movement would not have been possible for most of the taxa covered in our study, because of a lack of available well-preserved, disarticulated distal forelimb material. However, we measured minimum and maximum angles of abduction in an extant bird (see below) by estimating axial lines for the antebrachium and carpometacarpus as shown in photos of the articulated distal forelimb skeleton (figure 1a,b). Although the range of motion could have been further restricted in life by ligaments and other soft tissues, the range permitted by the skeleton is appropriate for comparison with fossil theropods.
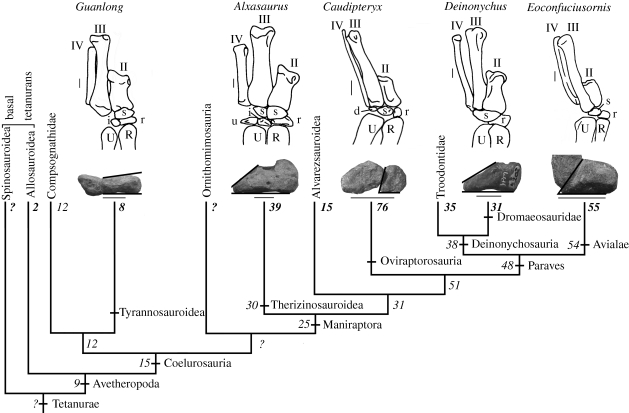
Figure 2.
Evolution of wrist structure and the radiale angle in tetanuran theropods, based on simplified tetanuran phylogeny after Smith et al. (2007) and Zanno et al. (2009). Numbers indicate values of radiale angle between proximal and distal articular surfaces of radiale, in degrees. Values in bold are direct measurements of individual taxa; values in normal italic type are reconstructed ancestral states. See table 1 and electronic supplementary material, figure S1 for full list of measurements used as the basis for reconstructing ancestral states. Wrist drawings show positions close to zero abduction (Guanlong, Alxasaurus, Deinonychus) or minimum abduction (Caudipteryx, Eoconfuciusornis). II–IV, metacarpals II–IV (numbering convention follows extant birds); d, distal carpal; i, intermedium; R, radius; r, radiale; s, semilunate carpal; U, ulna; u, ulnare. Scale bars: 0.25 cm in Eoconfuciusornis, 0.50 cm in Caudipteryx, 1.00 cm in all other taxa.
We reconstructed ancestral states of the radiale angle in Mesquite (Maddison & Maddison 2009), using the squared-change parsimony option.
3. Asymmetry of the avian carpus
In avians, the functional wrist joint is the articulation between the two proximal carpals, the scapholunar and cuneiform, and the trochlea of the carpometacarpus. The scapholunar is a slab-like bone homologous to the radiale plus intermedium of non-avialan tetrapods (Kundrát 2009), but for convenience is referred to as the radiale hereafter. The cuneiform is probably a neomorphic element rather than a homologue of the primitive ulnare (Gishlick 2007; Kundrát 2009).
A forelimb skeleton of the turkey, Meleagris gallopavo (Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China (IVPP) 1222), exemplifies the avian condition (figure 1). The proximal surface of the radiale is divided into two separate, inclined facets for articulation with the radius and ulna (figure 1c: sr, su). The dorsal part of the surface for the carpometacarpus (figure 1c: sc) faces distally and in the ulnar direction, and is set at an angle of about 59° to the facet for the ulna, whereas the palmar part of the surface is even more deflected towards the ulnar side of the wrist. The trochlea of the carpometacarpus, which lies approximately in the radioulnar plane, rotates freely against the radiale. Adduction of the manus is prevented by contact between the extensor process of the carpometacarpus and the radiale, which in fact occurs at a minimum angle of abduction of about 5° (figure 1a).
The cuneiform lies palmar rather than distal to the ulna, and the distal surface of the cuneiform is deeply incised to accommodate an extension of the palmar ridge of the carpometacarpal trochlea. The ridge can rotate within the incision of the cuneiform to a maximum angle of abduction of about 123°, at which point the carpometacarpal shaft contacts the distal surface of the cuneiform dorsal to the incision (figure 1b). It is clear that the great capacity of the avian carpus for abduction involves three principal factors: (i) the large radiale angle, reflecting the ulnar–distal rather than distal orientation of the carpometacarpal facet; (ii) the strong convexity of the proximal trochlea of the carpometacarpus; and (iii) the proximal position and incised topology of the cuneiform. In turn, the large range of abduction permitted by the carpal joint is necessary for folding the wing.
The three factors listed above represent osteological correlates of an asymmetrically mobile wrist with a large range of abduction. The third correlate is not applicable to non-avian theropods, which lack a bone homologous or structurally equivalent to the avian cuneiform. However, the first two correlates can be used to assess carpal function and pennibrachium-folding ability in fossil theropods, because the contact between the SLC and radiale corresponds to the avian condition. Accordingly, a convex SLC in a fossil non-avialan theropod indicates a large range of wrist motion in the radioulnar plane, whereas a large radiale angle gives the radiale a wedge-like shape and indicates that the range of motion was asymmetrically distributed with a bias towards the ulnar side of the wrist. The presence of both features implies that the wrist was capable of the extensive abduction needed to fold the carpus in an avian-like manner. Although there are relatively few taxa for which adequate carpal material is presently available, the evolution of wrist mobility and asymmetry on the line to birds can be broadly traced in the fossil record.
4. Carpal morphology in derived non-avialan theropods
(a). Non-maniraptoran tetanurans
A trochlear SLC broadly resembling the proximal-most part of the avian carpometacarpus is present in nearly all tetanuran theropods, although the degree of proximal convexity is sometimes small (Chure 2001; Rauhut 2003). It is possible that the SLC is not homologous throughout Tetanurae, in that different distal carpal elements may contribute to forming the SLC in different taxa or at least contribute to varying degrees (Chure 2001). For purposes of this study, however, the morphology of the SLC and its effect on the range of motion of the wrist are more important than its homologies.
Among basal (i.e. non-coelurosaurian; see figure 2) tetanurans, Allosaurus has an SLC with a definite trochlea (Chure 2001) that is only slightly convex, and probably permitted little abduction or adduction at the wrist. In coelurosaurs, by contrast, an SLC with a more strongly convex trochlea is a widespread feature, and is well exemplified by the basal tyrannosauroid Guanlong (IVPP V14531). The strong convexity of the palmar ridge of the trochlea, in particular, implies that this type of SLC permitted the wrist joint to rotate through a large range of motion in the radioulnar plane.
The wedge-shaped radiale has a more restricted phylogenetic distribution (figure 2). In Allosaurus, the articular surface formed by the radiale for the SLC is approximately distally directed, resulting in a low radiale angle (Chure 2001; see table 1). Although relatively few basal tetanuran radialia are known, a distally directed surface for the SLC probably represents the primitive tetanuran condition, since this configuration also occurs in the non-tetanuran theropod Coelophysis (Colbert 1989). The low radiale angle would have ensured that the limited flexibility of the basal tetanuran carpus was almost symmetrically distributed between abduction and adduction.
In at least some non-maniraptoran coelurosaurs, the SLC facet of the radiale is also approximately distal rather than ulnar–distal. In Guanlong, the radiale is partially fused to an element that presumably represents the intermedium (figure 2), and the two together form a transversely concave distal facet for articulation with the SLC. The radiale is minimally wedge-shaped, with a radiale angle of only 8° (figure 2 and table 1).
Among compsognathids, the radiale is at least somewhat wedge-shaped in Huaxiagnathus from the Lower Cretaceous of China. The left radiale of a specimen illustrated by Hwang et al. (2004, fig. 8) has a carpal angle of 18° (table 1); the angle appears even greater in the right radiale, but this element may have been rotated out of its natural orientation. In Sinosauropteryx (Currie & Chen 2001), the carpal angle is much smaller (table 1).
In ornithomimids, up to six carpals are present (Makovicky et al. 2004) but there is little consensus as to their identification. It seems clear that the ornithomimid wrist displays an unusual, autapomorphic architecture, best documented in Struthiomimus (Nicholls & Russell 1985). In this taxon the radiale angle is very small, and the wrist probably had little capacity for radial or ulnar deflection. Variation in carpal structure clearly exists among non-maniraptoran coelurosaurs, but indications of wrist asymmetry are incipient at best.
(b). Therizinosauroidea
Therizinosauroids are probably the most basal maniraptoran theropods (Zanno et al. 2009). In a well-preserved left carpus of Alxasaurus (IVPP, RV93001), the SLC is strongly convex and distinctly trochlear, despite being made up of two separate ossifications (figure 2). The radiale, in contrast to that of Guanlong, is distinctly wedge-shaped. The distal surface is divided into two portions, a non-articular area on the radial side and an inclined facet for the SLC on the ulnar side. The surface for the SLC is set at a radiale angle of 39° to the proximal surface for the radius, so that abduction would have been favoured over adduction. However, the extent of the asymmetry was probably less than in avians, because of the smaller radiale angle in Alxasaurus. An ossified intermedium and ulnare are also present in Alxasaurus (Russell & Dong 1993), although these elements cannot presently be located. They are proximodistally thinner than the radiale (figure 2) and are unlikely to have greatly restricted abduction.
The Early Cretaceous therizinosaur Falcarius has an SLC similar in form to that of Alxasaurus (Zanno 2006). The radiale (UMNH VP 12294) is wedge-like in shape but extends further in the ulnar direction than in Alxasaurus, with a radiale angle of about 26°.
(c). Alvarezsauroidea
In the derived parvicursorine alvarezsauroid Mononykus, the SLC is incorporated into a fused carpometacarpal block, as in avians (Perle et al. 1994). A proximal trochlea is present, but the ulnar portion of the proximal surface of the carpometacarpus forms relatively flat articular surfaces that might have severely limited the range of motion at the carpal joint. No parvicursorine radiale has so far been described, but the carpus clearly resembles the rest of the highly reduced parvicursorine forelimb in showing a distinctive, specialized morphology.
The distal articular facet of the radiale is slightly deflected in the ulnar direction in the basal Jurassic alvarezsauroid Haplocheirus (IVPP V15988). The radiale angle is 15° (table 1), comparable to Huaxiagnathus but considerably less than in Alxasaurus and Falcarius. The SLC has a highly convex trochlea, indicating a considerable range of motion in the radioulnar plane, but the carpal joint is evidently less asymmetric than in therizinosaurs.
(d). Oviraptorosauria
A number of well-preserved oviraptorosaur specimens show that a convex, trochlear SLC is present. In the relatively derived taxa Hagryphus (Zanno & Sampson 2005) and Heyuannia (Lü 2002), the SLC facet of the radiale faces almost in the ulnar direction, and this is also true of the well-preserved left radiale of the basal oviraptorosaur Caudipteryx (IVPP V12430). The radiale angle in this specimen is 76° (figure 2 and table 1), greater than in Meleagris (figure 1c) or the Early Cretaceous confuciusornithid Eoconfuciusornis (figure 2). The extreme deflection of the SLC facet in oviraptorosaurs strongly suggests that the carpal joint was asymmetric to a degree comparable to that seen in avians, with great capacity for abduction and very little for adduction.
(e). Dromaeosauridae
The carpus is well known in dromaeosaurids, and particularly in the large dromaeosaurid Deinonychus (Ostrom 1969). As in Alxasaurus, the distal surface of the radiale is divided into a non-articular surface on the radial side and an inclined SLC facet on the ulnar side. In a well-preserved left radiale of Deinonychus (Yale Peabody Museum, New Haven, USA (YPM) 5208), the radiale angle is 31° (figure 2 and table 1).
The proximal face of the SLC is strongly convex and trochlear. This morphology, and the ulnar–distal orientation of the SLC facet on the radiale, ensure a large, asymmetric range of motion in the radioulnar plane. Senter (2006), manipulating casts of Deinonychus forelimb material, estimated that the carpal joint was virtually incapable of adduction but capable of 62° of abduction. He obtained similar results for the dromaeosaurid Bambiraptor.
(f). Troodontidae
Two available specimens of the troodontid Sinovenator from the Lower Cretaceous of China (IVPP V14009, V12583) each display a well-preserved SLC, and the former specimen also includes a disarticulated left radiale. The radiale (figure 2) is very similar to that of Deinonychus, although in Sinovenator the facet for the SLC is more transversely concave. The overall radiale angle is approximately 35° (table 1). The proximal surface of the SLC is trochlear and strongly convex, and the carpal range of motion was probably similar to that seen in Deinonychus. A convex SLC is also present in Sinornithoides (IVPP V9612), and the radiale of this taxon is wedge-shaped as in other maniraptorans.
5. Discussion
An increase in the ability of the wrist to undergo both adduction and abduction almost certainly accompanied the appearance of the SLC at an early stage of tetanuran evolution. The original selective advantage of this enhanced mobility is not clear, but cannot have related to pennibrachial folding unless relatively basal tetanurans had elongated feathers on the forelimb. Such a possibility should not be dismissed entirely. Specimens representing this grade of evolution have not been recovered from sediments that preserve extensive soft tissue, and filamentous integumentary structures have recently been reported in a basal ornithischian (Zheng et al. 2009). However, it is likely that mobility of the wrist was initially associated with other functions, such as predation (Padian 2001).
Squared-change parsimony reconstruction of the ancestral states of the radiale angle (figure 2) suggests that an angle of 15° was primitive for Coelurosauria, implying slight asymmetry of the wrist joint comparable to the compsognathid Huaxiagnathus. By contrast, the ancestral value for maniraptorans was 25°, only slightly less than in Deinonychus. Although a degree of secondary reduction in the asymmetry of the wrist apparently took place in alvarezsauroids, assuming they are indeed as deeply nested within Maniraptora as shown in figure 2, a wedge-shaped radiale and a bias towards abduction is clearly the norm within Maniraptora as a whole. Furthermore, reconstructed ancestral states demonstrate a general trend towards larger radiale angles in more derived maniraptorans, culminating in the avian condition. However, the measured value of 76° in Caudipteryx suggests that the oviraptorosaur wrist may have independently evolved an even greater abductor bias than that existing in avialans.
The evolution of greater abductor mobility proceeded in association with two separate trends affecting the theropod forelimb (electronic supplementary material, table S1): progressive elongation of the forelimb, and elaboration of the plumage. The most basal theropods known to possess feathers are compsognathids (e.g. Huaxiagnathus: Hwang et al. 2004) and the tyrannosauroid Dilong (Xu et al. 2004), but in these taxa the feathers are typically very short. In the therizinosauroid Beipiaosaurus the feathers on the forearm and manus are elongated relative to the rest of the plumage (with the exception of some unusual elongate filaments along the spine: Xu et al. 2009) and relative to the feathers of non-maniraptoran theropods, initiating a trend that continues in more derived taxa. The oviraptorosaur Caudipteryx, while showing evidence of plumage over much of the body, has distinctly elongated feathers on the manus as well as at the tip of the tail. A similar condition occurs in Protarchaeopteryx, in troodontids (e.g. Anchiornis) and in dromaeosaurids (e.g. Microraptor).
Whether the pennibrachium was used primarily for display, wing-assisted incline running (Dial 2003) or some other function, it was clearly a biologically significant structure. Damage to the pennibrachium would probably have been costly. Wrist abduction offers a means of protecting a pennibrachium from damage (such as abrasion on the substrate or snagging on vegetation). Additional potential benefits include preventing the pennibrachium from interfering with locomotion and other activities, and permitting the animal to reduce its profile. However, it is an open question whether the development of longer feathers originally necessitated an abducted wrist to protect the pennibrachium, or the evolution of a deflected wrist in response to some other functional need created an opportunity for the subsequent development of longer pennibrachial feathers on a longer arm.
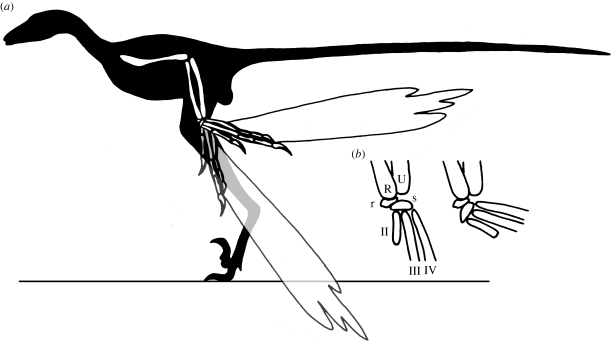
Unless the fossil record eventually reveals that wrist asymmetry definitely preceded or definitely followed the evolution of longer feathers in theropods, determining whether protection of the pennibrachium or some other functional advantage drove the evolution of the asymmetric wrist may be very difficult and will require careful analysis and modelling of the alternative scenarios. Such an analysis would have to encompass the relatively basal tetanurans in which carpal asymmetry first appeared, and not focus solely on paravians or even maniraptorans. Nevertheless, it is clear that carpal asymmetry would have permitted pennibrachial folding, even if asymmetry initially evolved for some other reason. With the humerus retracted and the elbow flexed, the ability to rotate the wrist through even the 60° of abduction that was probably possible in dromaeosaurids (Senter 2006) would have changed the orientation of the primary feathers from approximately ventral (i.e. towards the ground) to nearly posterior (figure 3; electronic supplementary material).
Figure 3.
Effect of abduction through angle of 60° in the dromaeosaurid Microraptor gui: (a) whole-body reconstruction showing the position of the left-hand relative to the body in straight (black) and abducted (grey) configurations; (b) left wrist in anatomically dorsal view, showing carpus in straight (left) and abducted (right) configurations. Abbreviations as in figure 2. Image modified from Hu et al. (2009).
Partial folding of the wing during the upstroke in extant birds, which requires significant abduction of the wrist (Vazquez 1992) could then be seen as an exaptation of a capability that originally evolved in non-volant, non-avialan maniraptorans. In any case it should be recognized that wing-folding ranks alongside flapping and gliding as a specialized capability of the avian forelimb, and the evolution of wing-folding should not be omitted from discussions of the transition from non-volant tetanurans to birds.
Acknowledgements
We thank J. Choiniere, M. Kundrát, Z. Zhou and R. Close for insightful discussion. L. Zanno provided images of the carpi of Falcarius and Hagryphus. F. Zheng and D. Brinkman facilitated our access to specimens at the IVPP and YPM, respectively. We thank the editors and two anonymous referees for their helpful comments. This research was supported by the Chinese Academy of Sciences, the National Natural Science Foundation of China, and the Chinese Ministry of Science and Technology.
References
- Chure D. J.2001The wrist of Allosaurus (Saurischia: Theropoda), with observations on the carpus in theropods. In New perspectives on the origin and early evolution of birds: Proc. Int. Symp. in Honor of John H. Ostrom (eds Gauthier J., Gall L. F.), pp. 97–121 New Haven, CT: Peabody Museum of Natural History [Google Scholar]
- Colbert E. H.1989The Triassic dinosaur Coelophysis. Bull. Mus. N. Arizona 57, 1–160 [Google Scholar]
- Currie P. J., Chen J.2001Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can. J. Earth Sci. 38, 1705–1727 (doi:10.1139/cjes-38-12-1705) [Google Scholar]
- Dial K. P.2003Wing-assisted incline running and the evolution of flight. Science 299, 402–404 (doi:10.1126/science.1078237) [DOI] [PubMed] [Google Scholar]
- Gauthier J.1986Saurischian monophyly and the origin of birds. Mem. Calif. Acad. Sci. 8, 1–55 [Google Scholar]
- Gishlick A.2007Developmental pattern of wrist elements in paleognaths and its bearing on the evolution of theropod carpals. J. Vertebr. Paleontol. 27, 81A–82A [Google Scholar]
- Hu D., Hou L., Zhang L., Xu X.2009A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature 461, 640–643 (doi:10.1038/nature08322) [DOI] [PubMed] [Google Scholar]
- Hwang S. H., Norell M. A., Ji Q., Gao K.2004A large compsognathid from the Early Cretaceous Yixian Formation of China. J. Syst. Palaeontol. 2, 13–30 (doi:10.1017/S1477201903001081) [Google Scholar]
- Kundrát M.2009Primary chondrification foci in the wing basipodium of Struthio camelus with comments on interpretation of autopodial elements in Crocodilia and Aves. J. Exp. Zool. (Mol. Dev. Evol.) 312B, 30–41 [DOI] [PubMed] [Google Scholar]
- Lü J.2002A new oviraptorosaurid (Theropoda: Oviraptorosauria) from the Late Cretaceous of southern China. J. Vertebr. Paleontol. 22, 871–875 (doi:10.1671/0272-4634(2002)022[0871:ANOTOF]2.0.CO;2) [Google Scholar]
- Maddison W. P., Maddison D. R.2009Mesquite: a modular system for evolutionary analysis, v. 2.71. See http://mesquiteproject.org [Google Scholar]
- Makovicky P. J., Kobayashi Y., Currie P. J.2004Ornithomimosauria. In The Dinosauria, 2nd edn (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 137–150 Berkeley, CA: University of California Press [Google Scholar]
- Nicholls E. L., Russell A. P.1985Structure and function of the pectoral girdle and forelimb of Struthiomimus altus (Theropoda: Ornithomimidae). Palaeontology 28, 638–677 [Google Scholar]
- Ostrom J. H.1969Osteology of Deinonychus antirrhopus, an unusual theropod from the Lower Cretaceous of Montana. Bull. Peabody Mus. Nat. Hist. 30, 1–165 [Google Scholar]
- Ostrom J. H.1976Some hypothetical stages in the evolution of avian flight. Smithson. Contrib. Paleobiol. 27, 1–21 [Google Scholar]
- Padian K.1985The origins and aerodynamics of flight in extinct vertebrates. Palaeontology 28, 423–433 [Google Scholar]
- Padian K.2001Stages in the origin of bird flight: beyond the cursorial-arboreal dichotomy. In New perspectives on the origin and early evolution of birds: Proc. Int. Symp. in Honor of John H. Ostrom (eds Gauthier J., Gall L. F.), pp. 255–272 New Haven, CT: Peabody Museum of Natural History [Google Scholar]
- Perle A., Chiappe L. M., Barsbold R., Clark J. M., Norell M. A.1994Skeletal morphology of Mononykus olecranus (Theropoda: Avialae) from the Late Cretaceous of Mongolia. Am. Mus. Novit. 3105, 1–29 [Google Scholar]
- Rauhut O. W. M.2003The interrelationships and evolution of basal theropod dinosaurs. Spec. Papers Palaeontol. 69, 1–215 [Google Scholar]
- Russell D. A., Dong M.1993The affinities of a new theropod from the Alxa Desert, Inner Mongolia, People's Republic of China. Can. J. Earth Sci. 30, 2107–2127 [Google Scholar]
- Senter P.2006Comparison of forelimb function between Deinonychus and Bambiraptor (Theropoda: Dromaeosauridae). J. Vertebr. Paleontol. 26, 897–906 (doi:10.1671/0272-4634(2006)26[897:COFFBD]2.0.CO;2) [Google Scholar]
- Sereno P. C., Rao C.1992Early evolution of avian flight and perching: new evidence from the Lower Cretaceous of China. Science 255, 845–848 (doi:10.1126/science.255.5046.845) [DOI] [PubMed] [Google Scholar]
- Smith N. D., Makovicky P. J., Hammer W. R., Currie P. J.2007Osteology of Cryolophosaurus ellioti (Dinosauria: Theropoda) from the Early Jurassic of Antarctica and implications for early theropod evolution. Zool. J. Linn. Soc. 151, 377–421 (doi:10.1111/j.1096-3642.2007.00325.x) [Google Scholar]
- Xu X., Norell M. A.2006Non-avian dinosaurs from the Lower Cretaceous Jehol Group of western Liaoning, China. Geol. J. 41, 419–437 [Google Scholar]
- Xu X., Norell M. A., Kuang X., Wang X., Zhao Q., Jia C.2004Basal tyrannosauroids from China and evolution of protofeathers in tyrannosauroids. Nature 431, 680–684 (doi:10.1038/nature02855) [DOI] [PubMed] [Google Scholar]
- Xu X., Zheng X., You H.2009A new feather type in a nonavian theropod and the early evolution of feathers. Proc. Natl Acad. Sci. USA 106, 832–834 (doi:10.1073/pnas.0810055106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez R. J.1992Functional morphology of the avian wrist and the evolution of flapping flight. J. Morph. 211, 259–268 (doi:10.1002/jmor.1052110303) [DOI] [PubMed] [Google Scholar]
- Zanno L.2006The pectoral girdle and forelimb of the primitive therizinosauroid Falcarius utahensis (Theropoda: Maniraptora): analyzing evolutionary trends within Therizinosauroidea. J. Vertebr. Paleontol. 26, 636–650 (doi:10.1671/0272-4634(2006)26[636:TPGAFO]2.0.CO;2) [Google Scholar]
- Zanno L. E., Sampson S. D.2005A new oviraptorosaur (Theropoda, Maniraptora) from the Late Cretaceous (Campanian) of Utah. J. Vertebr. Paleontol. 25, 897–904 (doi:10.1671/0272-4634(2005)025[0897:ANOTMF]2.0.CO;2) [Google Scholar]
- Zanno L. E., Gillette D. D., Albright L. B., Titus A. L.2009A new North American therizinosaurid and the role of herbivory in ‘predatory’ dinosaur evolution. Proc. R. Soc. B 276, 3505–3511 (doi:10.1098/rspb.2009.1029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.-Y., You H.-L., Xu X., Dong M.2009An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature 458, 333–336 (doi:10.1038/nature07856) [DOI] [PubMed] [Google Scholar]