Abstract
Secondary sexual traits in females are a relatively rare phenomenon. Empirical studies have focused on the role of male mate choice in their evolution; however, recently it has been suggested that secondary sexual traits in females are more likely to be under selection via reproductive competition. We investigated female competition and the influence of female phenotype on fitness in Onthophagus sagittarius, a species of dung beetle that exhibits female-specific horns. We compared reproductive fitness when females were breeding in competition versus breeding alone and found that competition for breeding resources reduced fitness for all females, but that smaller individuals suffered a greater fitness reduction than larger individuals. When females were matched for body size, those with the longest horns gained higher reproductive fitness. The fitness function was positive and linear, favouring increased horn expression. Thus, we present evidence that female body size and horn size in O. sagittarius are under directional selection via competition for reproductive resources. Our study is a rare example of female contest competition selecting for female weaponry.
Keywords: reproductive competition, female horns, weaponry
1. Introduction
Secondary sexual traits are considered hallmarks of sexual selection arising as ornaments via mate choice, or as armaments via competition for access to mates (Darwin 1871; Andersson 1994). In some cases, secondary sexual traits may serve the dual function of ornament and armament (Berglund et al. 1996). Since males are typically the more adorned sex, the majority of studies have focused solely on secondary sexual trait evolution in males. The evolution of such traits in females has generated much less interest and has only very recently begun to be explored (Amundsen 2000; LeBas 2006; Clutton-Brock 2009).
There are numerous examples of independent evolutionary gains of secondary sexual traits in females (Irwin 1994; Burns 1998; Emlen et al. 2005; Ord & Stuart-Fox 2006). In cases in which females express similar or reduced versions of a trait that is also present in males, their existence is typically attributed to genetic correlations existing between the sexes such that selection favouring trait exaggeration in males causes a correlated response in females, whereas where the trait is specific to females, or expressed to a greater extent than in males, direct selection operating on females via male mate choice or female competition is invoked as an explanation (Kraaijeveld et al. 2007). Empirical studies of the functional significance of female secondary sexual traits have focused primarily on the role of male mate choice in driving their evolution, and findings have been mixed; males may prefer high, intermediate or even low levels of secondary sexual trait expression (Amundsen & Forsgren 2001; Nordeide 2002; Chenoweth et al. 2007). As a result, the conditions under which male mate preferences for female ornaments might be expected to drive secondary sexual trait evolution in females are unclear. In conventional mating systems in which females do not compete for males, secondary sexual trait evolution in females has traditionally been viewed as being constrained, since investment in sexual traits may tradeoff against fecundity (Fitzpatrick et al. 1995). However, any potential fecundity costs may be outweighed if trait expression confers a selective benefit.
An often overlooked role for female secondary sexual traits is their function in female contest competition (LeBas 2006). This is surprising given that theoretical considerations have predicted contest competition to be the principal initiating factor in secondary sexual trait evolution in males (Berglund et al. 1996). It is perhaps due to the perceived rarity of these traits in females, or the notion that males are typically the more competitive sex, that competition is often regarded as not having the selective potential to favour secondary sexual trait evolution in females. However, while it may be true that for many systems females do not typically compete for mates, maternally biased investment renders females much more likely than males to experience intense competition for resources important for reproduction (Clutton-Brock 2009). As a result, resource distribution becomes a key determinant of variance in female reproductive success and, thus, the intensity of selection operating on females.
There are examples of female competition for sexual resources (Johnson 1988; Langmore & Davies 1997; Bro-Jørgensen 2002; Heinsohn et al. 2005), and also for non-sexual resources (Wolf 1969). Female secondary sexual traits may be involved in these antagonistic interactions, with their relative expression correlating with aggressiveness (Owens et al. 1994; Jawor et al. 2004), dominance (Johnson 1988; Jones & Hunter 1999) and status (Murphy et al. 2009), suggesting an adaptive value for secondary sexual traits in female contest competition. Traits that function to enhance a female's ability to acquire breeding resources should directly influence female fitness and are therefore expected to be significant targets of sexual selection. However, direct evidence of the fitness advantages for females exhibiting more exaggerated secondary sexual traits is still lacking. Further, our present knowledge about the potential functions of secondary sexual traits in females is still limited to vertebrate taxa, specifically birds and mammals.
Here, we use the dung beetle, Onthophagus sagittarius, to explore reproductive competition in females. Onthophagine dung beetles locate and feed on fresh dung before burrowing below the ground to reproduce. Biparental care is a common feature of the onthophagine mating system, but it is not obligatory; a female may work alone or cooperate with a male to excavate an underground tunnel and drag dung fragments from the surface to an underground chamber to form a brood ball, into which she lays an egg (Hunt & Simmons 2002a,b). One brood ball provides all the available nutrition for the developing larva, and the amount of dung provided determines offspring body size, a trait that correlates with female fecundity and male competitive ability, thus determining individual fitness (Lee & Peng 1981; Emlen 1994, 1997; Hunt & Simmons 1997, 1998, 2000).
Both male and female O. sagittarius express horns, but female horns are morphologically different to those of the male, being different in size and shape, and being expressed in different body locations (Watson & Simmons 2010). Genetic correlations between trait expression in males and females therefore seem an unlikely explanation for the evolution of these female-specific horns. Moreover, previous studies of this species have found no direct evidence for male mating preferences for female horn expression (Watson & Simmons 2010), even though horn expression is a predictor of fecundity in the absence of female competition (Simmons & Emlen 2008). Our aims in this study were to investigate whether female O. sagittarius experience competition for access to dung, a resource vital for brood ball production, and to determine the effects of female body size and horn expression on competitive female fitness.
2. Material and methods
Beetles were reproductively mature, first-generation offspring that bred from individuals collected from Kilcoy, Queensland, Australia, and were reared using established protocols (Hunt & Simmons 2000; Kotiaho & Simmons 2003; Simmons & Emlen 2008).
(a). Fitness consequences of female competition
Body size and horn size measurements of reproductively mature females were obtained using digital callipers and a dissecting microscope with eyepiece graticule, respectively. We measured pronotum width (mm), as this trait reliably measures overall body size (Emlen 1997), and the length of the head horn as a measure of horn size. All measurements were conducted by N.L.W. and are highly repeatable (Watson & Simmons 2010).
Females were first split into three body size categories based on pronotum width: small (4.44–4.99 mm), medium (5.00–5.35 mm) and large (5.37–5.53 mm). The three size classes of females were each divided across two 20 l buckets, three-quarters filled with moist sand and supplied with fresh dung. Males were introduced into these buckets, which were left undisturbed for 7 days to allow mating. Population densities in all mating buckets were the same, with an even sex ratio (40 males : 40 females). One bucket from each female size class was populated with males that had previously undergone sterilizing irradiation, a routine method that has been used previously to sterilize male onthophagines (Hunt & Simmons 2001). Males were sterilized by first anaesthetizing them under nitrogen (5 l min−1) for 10 min and then exposing them to 10 krad of irradiation from a cobalt-60 source. Irradiation induces sublethal mutations in sperm such that irradiated males produce sperm that are functionally competent but result in early embryonic mortality. Eggs fertilized by sperm from irradiated males are not viable, so that brood balls void of larva could be attributed to females mated to irradiated males. The second bucket of females from each size class was provided with normal fertile males, so that any brood balls containing viable eggs/larva could be assigned to these females. Thus, irradiation was used as a marker with which to unambiguously assign maternity to females from a particular size category when those females were breeding together. Females mated to fertile males were used as ‘focal’ females in our competitive breeding trials, whereas females mated to irradiated males were used as background ‘non-focal’ females.
(i). Females breeding alone
We established 60 mated focal females (20 from each size category) individually in breeding chambers (PVC piping 30 cm in length, 9 cm in diameter, three-quarters filled with moist sand and supplied with 25 ml fresh cow dung). Breeding chambers were left for 12 days, after which time they were sieved and brood balls were collected, counted, excess sand was removed and the balls were then weighed. These data provided a baseline expectation for reproductive performance of females in the absence of female contest competition.
(ii). Females breeding in competition
We established 60 focal females (20 replicates for each size category) in breeding chambers, each accompanied by two non-focal females. Thus, each breeding chamber contained three females (one from each size category), but only one female had been mated to a male with viable sperm. Breeding chambers were left for 12 days, after which time they were sieved, brood balls were collected and counted, excess sand was removed, then the balls were weighed and checked for egg viability. The number of broods from the focal female (viable) and her two competitors (inviable) were scored.
(b). Independent effect of horn size on competitive female fitness
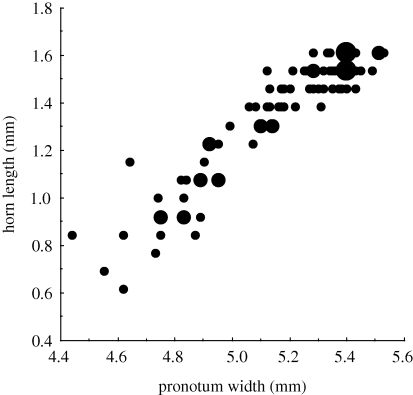
To examine the effects of horn size on competitive success, we controlled for the effects of body size by comparing relative female fitness (the difference in numbers of brood balls produced) between two females that were matched for body size but differed in horn size. Females were selected for competitive trials by plotting the relationship between female body size and horn size (figure 1) and selecting two females that had either a positive or negative residual horn length for a given pronotum width. We measured 188 females from which we selected 31 pairs that were matched for body size. Each of the females from a competitive pair was mated either to a normal male or to an irradiated male, so that we were able to assign maternity based on the viability of the broods produced. Pairs of females were then set up in breeding chambers, with 12 ml of dung to ensure that resource availability was limited. Breeding chambers were left for 12 days, after which time the number of broods produced by each female was assessed.
Figure 1.
Relationship between female body size (pronotum width) and horn size (head horn length). Symbol size represents from 1 to 3 overlapping data points (R2 = 0.87, p < 0.0001).
3. Results
(a). Fitness consequences of female competition
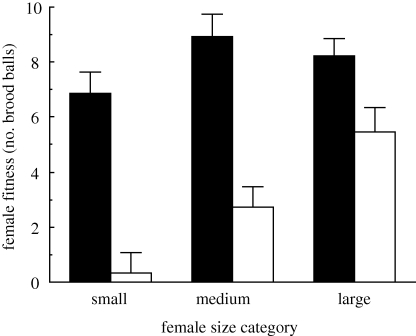
Female size category, presence/absence of competition and the interaction between the two were entered as variables into a general linear model to examine their effects on female fitness (number of brood balls produced by the focal female). The model explained 54 per cent of the variance (F5,83 = 19.77, p < 0.001), and all variables entered were significant: female size category, F2,83 = 9.36, p < 0.001; competition, F1,83 = 66.04, p < 0.001; size category × female competition, F2,83 = 3.64, p = 0.031).
The presence of other individuals during brood ball production reduced female fitness for females of all sizes (figure 2). The extent of the reduction in female fitness was dependent on female body size, such that small females suffered the greatest reduction in brood ball productivity, whereas large females suffered the least.
Figure 2.
Focal female fitness (average number of brood balls produced ± 1 s.e.) when breeding alone (black bars) and in competition (white bars) for all female size categories.
(b). Independent effect of horn size on competitive female fitness
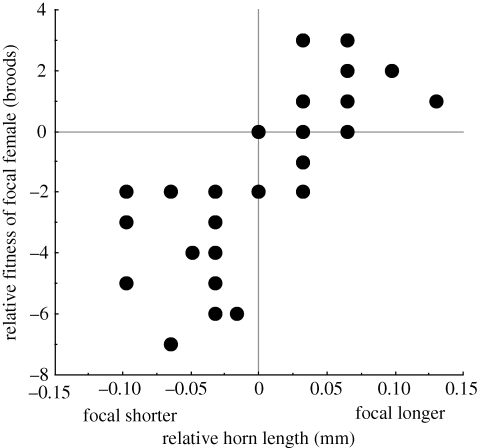
A linear regression was fitted between relative fitness (number of broods produced by focal female − number of broods produced by non-focal female) and relative horn length (horn length of focal female − horn length of non-focal female) of pairs of breeding females that had been matched for body size (R2 = 0.54, p < 0.001, F1,29 = 33.48). The data in figure 3 show that when two females of the same body size were in competition for breeding resources, females with larger horns produced more brood balls, thereby achieving greater reproductive fitness.
Figure 3.
Relationship between relative horn length and relative fitness of competing O. sagittarius females. Positive values on the x- and y-axes represent cases where, between two competing females that had been matched for body size, the focal female had a larger horn and produced more broods relative to her competitor.
4. Discussion
We found that contest competition between female O. sagittarius resulted in a reduction in fitness. Females produced significantly fewer broods when in competition with other breeding females, indicating that competition and/or interference from other females reduced access to dung for brood ball construction. Since one brood ball represents one offspring, the acquisition of dung directly determines female fitness. Females that fail to access dung will not produce offspring; thus, dung is a resource that is vital for reproduction.
It can take a female several hours to construct one brood ball (Hunt & Simmons 2002a), and dung pat quality can decline rapidly depending on climatic conditions, reducing its suitability for use (Moczek & Cochrane 2006). Dung is therefore ephemeral and spatially variable, and competition for access to it, and therefore selection on competitive ability, is likely to be intense. Furthermore, the removal and transport of dung into underground breeding chambers for brood ball construction renders dung a defendable resource within a confined environment in which encounters with other individuals are common. There is evidence that reproductive competition is a feature of female life histories in the genus Onthophagus; females have been found to exhibit intraspecific brood parasitism, raiding other females' brood balls, stealing dung or replacing existing eggs with their own, thereby exploiting a competitor's investment (Moczek & Cochrane 2006). Onthophagine females can also be victims of interspecific parasitism (González Megías & Sánchez-Piñero 2003). A female's probability of success in such encounters, whether it be defence of her own brood or attack of another female's, is likely to be strongly determined by her competitive ability. It has been speculated for the horned beetle Coprophanaeus ensifer, in which both males and females are horned, that female horns might function in defence of nesting resources from rival females, and also against unwanted mating attempts (Otronen 1988). Our data for O. sagittarius illustrate directly the importance of female horns during competition for resources.
We found that the magnitude of the effect imposed by competition on individual female fitness was dependent on female body size. In the presence of competition, small females suffered the greatest reduction in fitness, medium-sized females less so and large females were best able to withstand the effects of competition, suffering the smallest reduction in brood ball productivity. Large females therefore appear to be at a selective advantage during female contest competition. Body size and weapon size have been shown to be important in determining the outcome of contests in many taxa (Andersson 1994). In horned beetles, body size and horn size have both been shown to correlate positively with victory in male contests for access to females (e.g. Eberhard 1979; Otronen 1988; Rasmussen 1994; Pomfret & Knell 2006), with the influence of horn length on competitive success becoming more important when competitors are of similar body sizes (Emlen 1997; Moczek & Emlen 2000). In our study, by matching female competitors for body size, we were able to control for the effect of female body size on competitive ability in order to examine the influence of horn length in isolation, an approach similar to that used by Emlen (1997) in his examination of male competitive ability. We found that when females were matched for body size, competing pairs that exhibited a larger difference in relative horn length had a greater discrepancy in numbers of brood balls produced by each female, such that females with longer horns produced more brood balls, gaining greater resource acquisition and achieving higher fitness. The fitness function we observed in our study was linear and positive, imposing directional selection for increased horn length.
The horns of female O. sagittarius are likely to determine contest outcome via direct physical interaction during combat, although in theory they might also act as signals for non-contact assessment of competitive ability: so-called badges of status (Pärt & Qvarnström 1997; Panhuis & Wilkinson 1999). Behavioural observations suggest that, like other horned beetles (Eberhard 1979), O. sagittarius females use their horns to interact directly with other females; the pronotal and head horns of two rivals ‘interlock’ in a manner such that females can pry and push rivals within confined tunnels to evict resident females, and thereby retain tunnel possession (N. L. Watson 2009, personal observations). As such, the horns of female O. sagittarius function similarly to those of male onthophagines, but instead of being used in contests over mates, they are used for access to resources, a situation that has been found in some birds and mammals (e.g. Heinsohn et al. 2005; Robinson & Kruuk 2007).
Our data show that under competitive situations, body size and horn size in O. sagittarius females are subject to directional sexual selection. In a previous study, we examined the role of male mate choice in female horn evolution. We found little evidence to suggest that males have overt preferences for females based on horn size (Watson & Simmons 2010). Therefore, given the available evidence, the horns of female O. sagittarius are best viewed as weapons, agreeing with previous findings for male onthophagines. However, since the horns are female-specific in O. sagittarius, our findings represent a rare example of a secondary sexual trait that is under intrasexual selection via reproductive competition among females.
The evolution of female weaponry has been explored in other taxa, with the prevailing conclusion being that these traits are under natural selection (Kiltie 1985; Stankowich & Caro 2009). However, this conclusion may be due to that fact that the concept of intrasexual selection is often applied restrictively, only considering competition for ‘mating’ opportunities (Andersson 1994), when in fact selection acts on traits of both sexes involved in competition for ‘reproductive opportunities’, including competition for ‘mating’ and ‘breeding’. Comparisons of weapon function between males and females may fail to recognize the operation of sexual selection on females, simply because competition for mates is not observed. Use of the term ‘social selection’ (West-Eberhard 1983) to refer to competition over resources that are linked to reproduction has further obscured the perception of sexual selection acting on females. Thus, it is important to delineate whether competition is occurring for sexual or non-sexual resources, since it is this that determines whether the evolution of traits that enhance female fitness via competitive ability can be attributed to sexual selection (Clutton-Brock 2007). Inability to do so can result in traits present in females that are functionally similar to those in males being ignored as examples of sexual selection operating in females.
Although it has been voiced numerous times (Amundsen 2000; LeBas 2006; Clutton-Brock 2007), unfortunately it still remains the case that the topic of female competition over reproduction represents a gap in sexual selection theory, and that ‘the fitness consequences of competitive interactions constitute a vast area of ignorance’ (Berglund et al. 1993, p. 186). Our data provide a rare example of sexual selection via female contest competition acting on a female secondary sexual trait, and we hope that our study will stimulate increased effort to document such selection in other taxa.
Acknowledgements
This research was supported by the Australian Research Council. We thank Sean Stankowski for enthusiastic assistance in the field, Doug Emlen for sharing video-recorded behavioural observations, and Ernie Steiner and Jeremy Lindsey at the Department of Agriculture and Food, WA for irradiating beetles.
References
- Amundsen T.2000Female ornaments: genetically correlated or sexually selected? In Animal signals: signalling and signal design in animal communication (eds Espmark Y., Amundsen T., Rosenqvist G.), pp. 133–154 Trondheim, Norway: Tapir Academic Press [Google Scholar]
- Amundsen T., Forsgren E.2001Male mate choice selects for female coloration in a fish. Proc. Natl Acad. Sci. USA 98, 13 155–13 160 (doi:10.1073/pnas.211439298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M.1994Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- Berglund A., Magnhagen C., Bisazza A., Konig B., Huntingford F.1993The adaptive bases of female sexual behavior: reports from a workshop. Behav. Ecol. 4, 184–187 (doi:10.1093/beheco/4.2.184) [Google Scholar]
- Berglund A., Bisazza A., Pilastro A.1996Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399 (doi:10.1111/j.1095-8312.1996.tb01442.x) [Google Scholar]
- Bro-Jørgensen J.2002Overt female mate competition and preference for central males in a lekking antelope. Proc. Natl Acad. Sci. USA 99, 9290–9293 (doi:10.1073/pnas.142125899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K. J.1998A phylogenetic perspective on the evolution of sexual dichromatism in tanagers (Thraupidae): the role of female versus male plumage. Evolution 52, 1219–1224 (doi:10.2307/2411252) [DOI] [PubMed] [Google Scholar]
- Chenoweth S. F., Petfield D., Doughty P., Blows M. W.2007Male choice generates stabilizing sexual selection on a female fecundity correlate. J. Evol. Biol. 20, 1745–1750 (doi:10.1111/j.1420-9101.2007.01390.x) [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.2007Sexual selection in males and females. Science 318, 1882–1885 (doi:10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H.2009Sexual selection in females. Anim. Behav. 77, 3–11 (doi:10.1016/j.anbehav.2008.08.026) [Google Scholar]
- Darwin C.1871The descent of man, and selection in relation to sex. London, UK: Murray [Google Scholar]
- Eberhard W. G.1979The function of horns in Podischnus agenor (Dynastinae) and other beetles. In Sexual selection and reproductive competition in insects (eds Blum M. S., Blum N. A.). New York, NY: Academic Press [Google Scholar]
- Emlen D. J.1994Environmental control of horn length dimorphism in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc. R. Soc. Lond. B 256, 131–136 (doi:10.1098/rspb.1994.0060) [Google Scholar]
- Emlen D. J.1997Alternative reproductive tactics and male dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 41, 335–341 (doi:10.1007/s002650050393) [Google Scholar]
- Emlen D. J., Marangelo J., Ball B., Cunningham C. W.2005Diversity in the weapons of sexual selection: horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae). Evolution 59, 1060–1084 [PubMed] [Google Scholar]
- Fitzpatrick S., Berglund A., Rosenqvist G.1995Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol. J. Linn. Soc. 55, 251–260 (doi:10.1111/j.1095-8312.1995.tb01063.x) [Google Scholar]
- González Megías A., Sánchez-Piñero F.2003Effects of brood parasitism on host reproductive success: evidence from larval interactions among dung beetles. Oecologia 134, 195–202 [DOI] [PubMed] [Google Scholar]
- Heinsohn R., Legge S., Endler J. A.2005Extreme reversed sexual dichromatism in a bird without sex role reversal. Science 309, 617–619 (doi:10.1126/science.1112774) [DOI] [PubMed] [Google Scholar]
- Hunt J., Simmons L. W.1997Patterns of fluctuating asymmetry in beetle horns: an experimental examination of the honest signaling hypothesis. Behav. Ecol. Sociobiol. 41, 109–114 (doi:10.1007/s002650050370) [Google Scholar]
- Hunt J., Simmons L. W.1998Patterns of parental provisioning covary with male morphology in a horned beetle (Onthophagus taurus) (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 42, 447–451 (doi:10.1007/s002650050459) [Google Scholar]
- Hunt J., Simmons L. W.2000Maternal and paternal effects on offspring phenotype in the dung beetle Onthophagus taurus. Evolution 54, 936–941 [DOI] [PubMed] [Google Scholar]
- Hunt J., Simmons L. W.2001Status-dependent selection in the dimorphic beetle Onthophagus taurus. Proc. R. Soc. Lond. B 268, 2409–2414 (doi:10.1098/rspb.2001.1758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J., Simmons L. W.2002aBehavioral dynamics of biparental care in the dung beetle Onthophagus taurus. Anim. Behav. 64, 65–75 (doi:10.1006/anbe.2002.3036) [Google Scholar]
- Hunt J., Simmons L. W.2002bConfidence of paternity and paternal care: covariation revealed through the experimental manipulation of the mating system in the beetle Onthophagus taurus. J. Evol. Biol. 15, 784–795 (doi:10.1046/j.1420-9101.2002.00442.x) [Google Scholar]
- Irwin R. E.1994The evolution of plumage dichromatism in the New World blackbirds: social selection on female brightness? Am. Nat. 144, 890–907 (doi:10.1086/285717) [Google Scholar]
- Jawor J. M., Gray N., Beall S. M., Breitwish R.2004Multiple ornaments correlate with aspects of condition and behaviour in female northern cardinals, Cardinalis cardinalis. Anim. Behav. 67, 875–882 (doi:10.1016/j.anbehav.2003.05.015) [Google Scholar]
- Johnson K.1988Sexual selection in pinyon jays II: male choice and female-female competition. Anim. Behav. 36, 1048–1053 (doi:10.1016/S0003-3472(88)80064-2) [Google Scholar]
- Jones I. L., Hunter F. M.1999Experimental evidence for mutual inter- and intrasexual selection favouring a crested auklet ornament. Anim. Behav. 57, 521–528 (doi:10.1006/anbe.1998.1012) [DOI] [PubMed] [Google Scholar]
- Kiltie R. A.1985Evolution and function of horns and hornlike organs in female ungulates. Biol. J. Linn. Soc. 24, 299–320 (doi:10.1111/j.1095-8312.1985.tb00377.x) [Google Scholar]
- Kotiaho J. S., Simmons L. W.2003Longevity cost of reproduction for males but no longevity cost of mating or courtship for females in the male-dimorphic dung beetle Onthophagus binodis. J. Insect Physiol. 49, 817–822 (doi:10.1016/S0022-1910(03)00117-3) [DOI] [PubMed] [Google Scholar]
- Kraaijeveld K., Kraaijeveld-Smit F. J. L., Komdeur J.2007The evolution of mutual ornamentation. Anim. Behav. 74, 657–677 (doi:10.1016/j.anbehav.2006.12.027) [Google Scholar]
- Langmore N., Davies N. B.1997Female dunnocks use vocalizations to compete for males. Anim. Behav. 53, 881–890 (doi:10.1006/anbe.1996.0306) [Google Scholar]
- LeBas N. R.2006Female finery is not for males. Trends Ecol. Evol. 21, 170–173 (doi:10.1016/j.tree.2006.01.007) [DOI] [PubMed] [Google Scholar]
- Lee J. M., Peng S.1981Influence of adult size of Onthophagus gazella on manure pat degradation, nest construction, and progeny size. Environ. Entomol. 10, 626–630 [Google Scholar]
- Moczek A. P., Cochrane J.2006Intraspecific female brood parasitism in the dung beetle Onthophagus taurus. Ecol. Entomol. 31, 316–321 (doi:10.1111/j.1365-2311.2006.00773.x) [Google Scholar]
- Moczek A. P., Emlen D. J.2000Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim. Behav. 59, 459–466 (doi:10.1006/anbe.1999.1342) [DOI] [PubMed] [Google Scholar]
- Murphy T. G., Hernandez-Mucino D., Osorio-Beristain M., Montgomerie R., Omland K. E.2009Carotenoid-based status signaling by females in the tropical streak-backed oriole. Behav. Ecol. Sociobiol. 20, 1000–1006 [Google Scholar]
- Nordeide J. T.2002Do male sticklebacks prefer females with red ornamentation? Can. J. Zool. 80, 1344–1349 (doi:10.1139/z02-116) [Google Scholar]
- Ord T. J., Stuart-Fox D.2006Ornament evolution in dragon lizards: multiple gains and widespread losses reveal a complex history of evolutionary change. J. Evol. Biol. 19, 797–808 (doi:10.1111/j.1420-9101.2005.01050.x) [DOI] [PubMed] [Google Scholar]
- Otronen M.1988Intra- and intersexual interactions at breeding burrows in the horned beetle, Coprophanaeus ensifer. Anim. Behav. 36, 741–748 (doi:10.1016/S0003-3472(88)80157-X) [Google Scholar]
- Owens I. P. F., Burke T., Thompson D. B. A.1994Extraordinary sex roles in the Eurasian dotterel: female mating arenas, female–female competition, and female mate choice. Am. Nat. 144, 76–100 (doi:10.1086/285662) [Google Scholar]
- Panhuis T. M., Wilkinson G. S.1999Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae). Behav. Ecol. Sociobiol. 46, 221–227 (doi:10.1007/s002650050613) [Google Scholar]
- Pärt T., Qvarnström A.1997Badge size in collared flycatchers predicts outcome of male competition over territories. Anim. Behav. 54, 893–899 (doi:10.1006/anbe.1997.0514) [DOI] [PubMed] [Google Scholar]
- Pomfret J. C., Knell R. J.2006Sexual selection and horn allometry in the dung beetle Euoniticellus intermedius. Anim. Behav. 71, 567–576 (doi:10.1016/j.anbehav.2005.05.023) [Google Scholar]
- Rasmussen J. L.1994The influence of horn and body size on the reproductive behavior of the horned rainbow scarab beetle Phanaeus difformis (Coleoptera: Scarabaeidae). J. Insect Behav. 7, 67–82 (doi:10.1007/BF01989828) [Google Scholar]
- Robinson M. R., Kruuk L. E. B.2007Function of weaponry in females: the use of horns in intrasexual competition for resources in female Soay sheep. Biol. Lett. 3, 651–654 (doi:10.1098/rsbl.2007.0278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W., Emlen D. J.2008No fecundity cost of female secondary sexual trait expression in the horned beetle Onthophagus sagittarius. J. Evol. Biol. 21, 1227–1235 (doi:10.1111/j.1420-9101.2008.01575.x) [DOI] [PubMed] [Google Scholar]
- Stankowich T., Caro T.2009Evolution of weaponry in female bovids. Proc. R. Soc. B 276, 4329–4334 (doi:10.1098/rspb.2009.1256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N. L., Simmons L. W.2010Mate choice in the dung beetle Onthophagus sagittarius: are female horns ornaments? Behav. Ecol. 21, 424–430 (doi:10.1093/beheco/arp207) [Google Scholar]
- West-Eberhard M. J.1983Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (doi:10.1086/413215) [Google Scholar]
- Wolf L. L.1969Female territoriality in a tropical hummingbird. Auk 86, 490–504 [Google Scholar]