Abstract
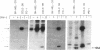
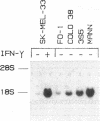
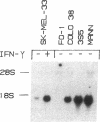
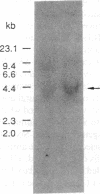
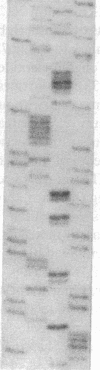
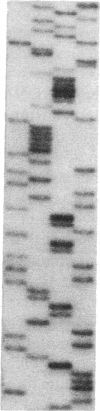
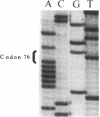
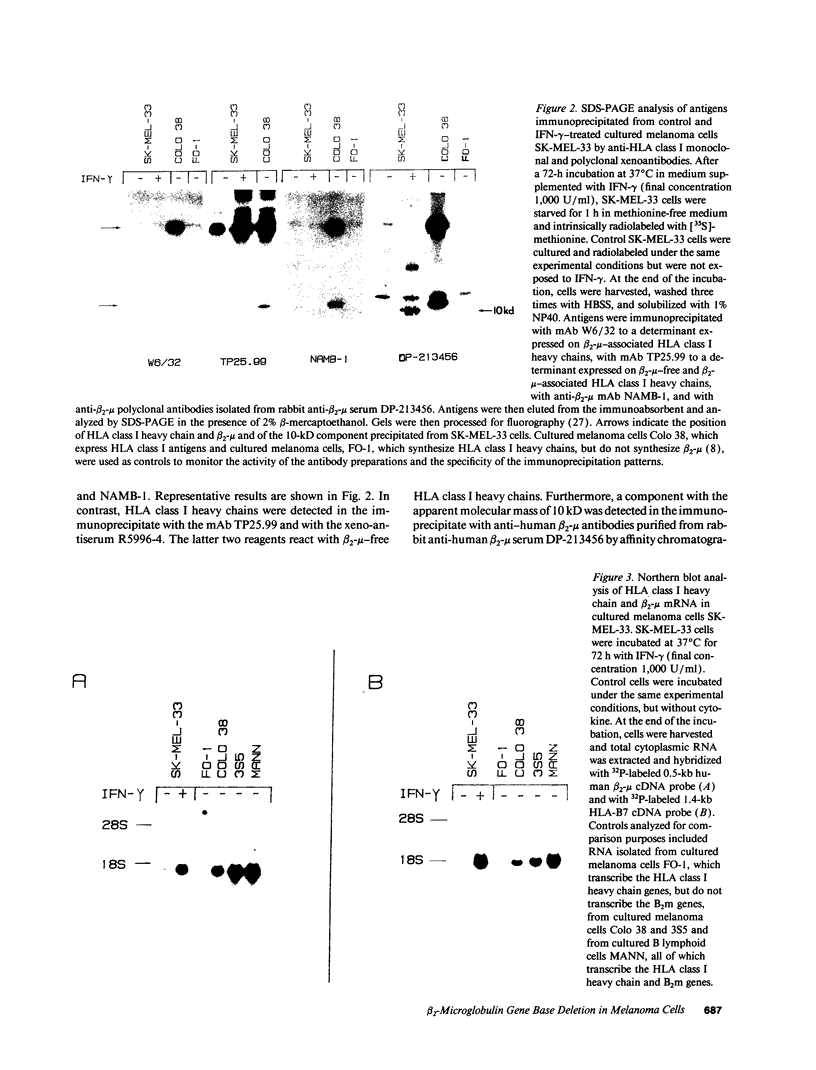
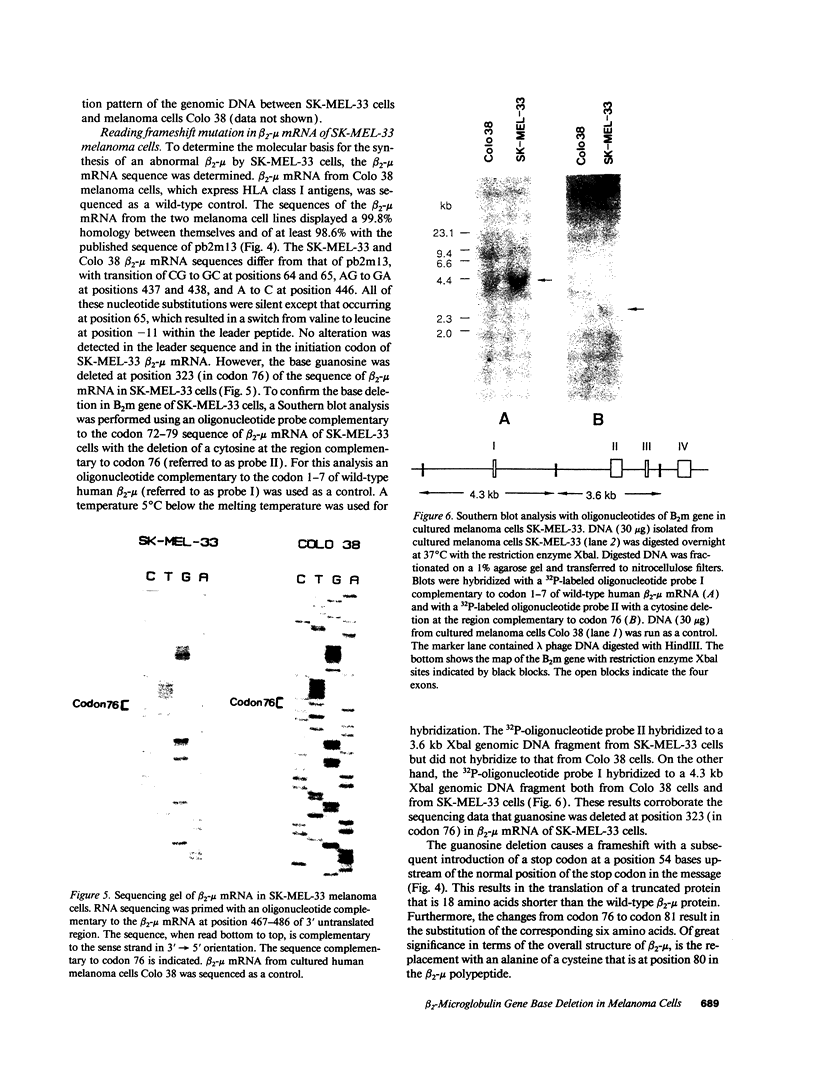
The lack of HLA class I antigen expression by the melanoma cell line SK-MEL-33 is caused by a unique lesion in beta 2-microglobulin (beta 2-mu). Sequencing of beta 2-mu mRNA detected a guanosine deletion at position 323 in codon 76 that causes a frameshift with a subsequent introduction of a stop codon at a position 54 base upstream of the normal position of the stop codon in the message. The loss of 18 amino acids and the change of 6 amino acids, including a cysteine at position 80 in the carboxy terminus of beta 2-mu, are likely to cause marked changes in the structure of the polypeptide. The latter may account for the inability of beta 2-mu to associate with HLA class I heavy chains and for its lack of reactivity with the anti-beta 2-mu mAb tested. HLA class I antigen expression on SK-MEL-33 cells was reconstituted after transfection with a wild-type B2m gene, therefore indicating that the abnormality of endogenous B2m gene is the only mechanism underlying lack of HLA class I antigen expression by SK-MEL-33 cells. The guanosine deletion in B2m gene was detected also in the melanoma tissue from which SK-MEL-33 cells had originated. Therefore, the molecular lesion identified in the SK-MEL-33 melanoma cell line is not caused by a mutation acquired during growth in vitro but is likely to reflect a somatic mutation during tumor progression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- D'Urso C. M., Wang Z. G., Cao Y., Tatake R., Zeff R. A., Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J Clin Invest. 1991 Jan;87(1):284–292. doi: 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R., Rudersdorf R., Chang C., Petersen J., Strandtmann J., Korn N., Sidwell B., Orr H. T. Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8183–8187. doi: 10.1073/pnas.82.23.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Bale S. J., Housman D. E., Dracopoli N. C. Genetics of melanoma. Cancer Surv. 1990;9(4):645–671. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gates F. T., 3rd, Coligan J. E., Kindt T. J. Complete amino acid sequence of murine beta 2-microglobulin: structural evidence for strain-related polymorphism. Proc Natl Acad Sci U S A. 1981 Jan;78(1):554–558. doi: 10.1073/pnas.78.1.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Schulze D. H., Pease L. R., Weiss E. H., Mellor A. L., Flavell R. A., Nathenson S. G. Interaction between Kb and Q4 gene sequences generates the Kbm6 mutation. Mol Cell Biol. 1986 Feb;6(2):645–652. doi: 10.1128/mcb.6.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Güssow D., Rein R., Ginjaar I., Hochstenbach F., Seemann G., Kottman A., Ploegh H. L. The human beta 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987 Nov 1;139(9):3132–3138. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lindmo T., Boven E., Cuttitta F., Fedorko J., Bunn P. A., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984 Aug 3;72(1):77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Matsui M., Temponi M., Ferrone S. Characterization of a monoclonal antibody-defined human melanoma-associated antigen susceptible to induction by immune interferon. J Immunol. 1987 Sep 15;139(6):2088–2095. [PubMed] [Google Scholar]
- Mukherji B., Chakraborty N. G., Sivanandham M. T-cell clones that react against autologous human tumors. Immunol Rev. 1990 Aug;116:33–62. doi: 10.1111/j.1600-065x.1990.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Nakamuro K., Tanigaki N., Pressman Common antigenic structures of HL-A antigens. VI. Common antigenic determinants located on the 33,000 Dalton alloantigenic fragment portion of papain-solubilized HL-A molecules. Immunology. 1975 Dec;29(6):1119–1132. [PMC free article] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Sizer K. C., Seidman J. G., Stallings V., Hyman R. A mutational hot-spot within an intron of the mouse beta 2-microglobulin gene. EMBO J. 1986 Jan;5(1):103–111. doi: 10.1002/j.1460-2075.1986.tb04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Ng A. K., Russo C., Ferrone S. Heterogeneous distribution of the determinants defined by monoclonal antibodies on HLA-A and B antigens bearing molecules. Transplantation. 1982 Jul;34(1):18–23. doi: 10.1097/00007890-198207000-00004. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Poulik M. D., Bloom A. D. Beta 2 -microglobulin production and secretion by lymphocytes in culture. J Immunol. 1973 May;110(5):1430–1433. [PubMed] [Google Scholar]
- Poulik M. D., Ferrone S., Pellegrino M. A., Sevier D. E., Oh S. K., Reisfeld R. A. Association of HL-A antigens and beta 2-microglobulin: concepts and questions. Transplant Rev. 1974;21(0):106–125. doi: 10.1111/j.1600-065x.1974.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Rosa F., Berissi H., Weissenbach J., Maroteaux L., Fellous M., Revel M. The beta2-microglobulin mRNA in human Daudi cells has a mutated initiation codon but is still inducible by interferon. EMBO J. 1983;2(2):239–243. doi: 10.1002/j.1460-2075.1983.tb01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiter D. J., Mattijssen V., Broecker E. B., Ferrone S. MHC antigens in human melanomas. Semin Cancer Biol. 1991 Feb;2(1):35–45. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Arnheim N. Analysis of DNA sequences in forty-year-old paraffin-embedded thin-tissue sections: a bridge between molecular biology and classical histology. Cancer Res. 1988 Aug 15;48(16):4564–4566. [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday M. E., Isselbacher K. J., Gattoni-Celli S., Willett C. G. Altered growth of a human neuroendocrine carcinoma line after transfection of a major histocompatibility complex class I gene. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4700–4704. doi: 10.1073/pnas.86.12.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. S., Nowell P. C., Kornbluth J. Functional role of HLA class I cell-surface molecules in human T-lymphocyte activation and proliferation. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4446–4450. doi: 10.1073/pnas.83.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temponi M., Kageshita T., Perosa F., Ono R., Okada H., Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma. 1989 Feb;8(1):85–95. doi: 10.1089/hyb.1989.8.85. [DOI] [PubMed] [Google Scholar]
- Turco M. C., De Felice M., Corbo L., Morrone G., Mertelsmann R., Ferrone S., Venuta S. Regulatory role of a monomorphic determinant of HLA Class I antigens in T cell proliferation. J Immunol. 1985 Oct;135(4):2268–2273. [PubMed] [Google Scholar]
- Zeff R. A., Nakagawa M., Mashimo H., Gopas J., Nathenson S. G. Failure of cell surface expression of a class I major histocompatibility antigen caused by somatic point mutation. Transplantation. 1990 Apr;49(4):803–808. doi: 10.1097/00007890-199004000-00029. [DOI] [PubMed] [Google Scholar]
- Zweig S. E., Shevach E. M. Production and properties of monoclonal antibodies to guinea pig Ia antigens. Methods Enzymol. 1983;92:66–85. doi: 10.1016/0076-6879(83)92010-4. [DOI] [PubMed] [Google Scholar]
- de Préval C., Mach B. The absence of beta 2-microglobulin in Daudi cells: active gene but inactive messenger RNA. Immunogenetics. 1983;17(2):133–140. doi: 10.1007/BF00364753. [DOI] [PubMed] [Google Scholar]
- van Duinen S. G., Ruiter D. J., Broecker E. B., van der Velde E. A., Sorg C., Welvaart K., Ferrone S. Level of HLA antigens in locoregional metastases and clinical course of the disease in patients with melanoma. Cancer Res. 1988 Feb 15;48(4):1019–1025. [PubMed] [Google Scholar]