Abstract
Mammal extinctions are widespread globally, with South Asian species being most threatened. We examine local extinctions of 25 mammals in India. We use historical records to obtain a set of locations at which each species was known to have been present at some time in the last 200 years. We then use occupancy estimation models to draw inferences about current presence at these same locations based on field observations of local experts. We examine predictions about the influence of key factors such as protected areas, forest cover, elevation, human population density and cultural tolerance on species extinction. For all 25 species, estimated local extinction probabilities (referenced to a 100 year time frame) range between 0.14 and 0.96. Time elapsed since the historical occurrence record was an important determinant of extinction probability for 14 species. Protected areas are positively associated with lower extinction of 18 species, although many species occur outside them. We find evidence that higher proportion of forest cover is associated with lower extinction probabilities for seven species. However, for species that prefer open habitats (which have experienced intensive land-use change), forest cover alone appears insufficient to ensure persistence (the complement of extinction). We find that higher altitude is positively associated with lower extinction for eight species. Human population density is positively associated with extinction of 13 species. We find that ‘culturally tolerated’ species do exhibit higher persistence. Overall, large-bodied, rare and habitat specialist mammals tend to have higher extinction probabilities.
Keywords: extinction, India, mammals, occupancy, people, protected areas
1. Introduction
Global extinction of species, driven by anthropogenic factors, is occurring at an unprecedented rate (Pimm et al. 2001; Cardillo et al. 2006; Isaac et al. 2007; Morrison et al. 2007). Large terrestrial mammals are among the most threatened taxa in the world, with 25 per cent of species facing extinction and 50 per cent with declining populations (Channell & Lomolino 2000; Ceballos et al. 2005). Mammals of South Asia are among the most endangered (Schipper et al. 2008). Conservation success will depend on identifying vulnerable species and understanding environmental factors that support their persistence, particularly in human-dominated landscapes such as southern Asia.
We address several key questions relating to mammal extinctions in India, which is recognized as a mega-biodiversity country: do protected areas, forested habitats and higher altitude locations facilitate lower extinction for most large mammals or only for a few species? Can some species persist in areas with high human population densities? Which species can or cannot adapt to human-modified landscapes? How do human cultural attitudes affect species extinctions? Are carnivores more vulnerable to local extinction than herbivores? Are livestock predators and crop-raiders equally vulnerable to extirpation? How does likelihood of extinction vary with body size, diet and geographical rarity among mammalian species?
We examine past and present distributions to assess local extinction vulnerability of 25 Indian mammals. Widespread hunting and land-use changes (deforestation, agricultural expansion), together with rapid economic and demographic growth in the last 100 years, are believed to have severely impacted these species and their habitats (Forest Survey of India 2005; Das et al. 2006). We collected 30 000+ records from natural history, taxidermy and museum observations to identify a sample of sites at which selected species occurred historically across India. We used recent field observations from more than 100 acknowledged wildlife experts to estimate current presence or absence of these species. We use occupancy modelling (MacKenzie et al. 2002, 2006; MacKenzie & Royle 2005) to integrate these historical and current data, to estimate local extinction of species in relation to time of historical record, presence and proportion of protected areas, forest cover, elevation and social factors (human demography and cultural attitudes), as well as species biology. Modelling local species extinction relative to such potential covariates allows us to identify the key variables that have influenced probabilities of local extinction.
2. Material and methods
(a). Sampling approach, species and data sources
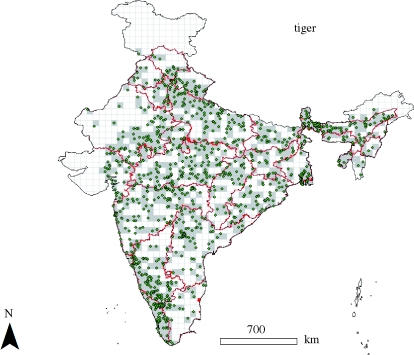
We partitioned India's geographical area into a grid with 1326 cells (average cell size of 2818 km2, range 2461–3059 km2). At this grid cell size, it was feasible for us to get experts to give us their information on species detections on a country-wide scale for multiple species. We obtained geographical location records for mammal species across this region from hunting, taxidermy and museum records. Historic records of species presence were compiled from 50+ museums, libraries and taxidermy firms, British imperial and district gazetteers, hunting journals (150+) and all issues of Journal of Bombay Natural History Society (from 1885–2006) and Indian Forester (1875–2006). Most records were from the years 1860–2000, with few records between the years 1760 and 1860. Overall, we collected 30 000+ records for more than 100 mammal species in India. These historic records provided geographical locations where species had been either observed or hunted. This reconstruction is partial because we can make no statement about historic species occurrence in locations for which there are no records. We selected 25 large mammal species with 64–3606 reliable location records (table 1). We pooled the individual location records for each species to relevant grid cells, to establish its historic presence (table 1 and figure 1). Estimation of current species status (locally extinct or not) for sites of historic occurrence allows us to estimate local extinction for individual species as the fraction of cells in which the species is currently absent.
Table 1.
Estimated local extinction probabilities for large mammals in India. Average estimated extinction probability across India based on extinction probability estimates for each cell (i), 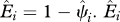 and
and 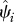 are the estimated extinction and occupancy probabilities for the ith cell. Average extinction across all cells is estimated as
are the estimated extinction and occupancy probabilities for the ith cell. Average extinction across all cells is estimated as 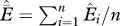 , where n is the total number of cells with historic records.
, where n is the total number of cells with historic records. 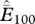 and
and 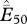 refer to the average estimated proportion of cells showing extinction in the last 100 and 50 years, respectively. The range of estimated probabilities of extinction among all cells is presented in brackets for each species. (Note: these data will be made available to others interested in species-specific collaborations.)
refer to the average estimated proportion of cells showing extinction in the last 100 and 50 years, respectively. The range of estimated probabilities of extinction among all cells is presented in brackets for each species. (Note: these data will be made available to others interested in species-specific collaborations.)
| common name | scientific name | total point records | total cells (n) | average 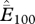
|
average 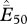
|
IUCN Red List 2009 |
|---|---|---|---|---|---|---|
| chital | Cervus axis | 850 | 322 | 0.45 (0.40–0.52) | 0.45 (0.40–0.52) | LR |
| sambar | Cervus unicolor | 1350 | 448 | 0.34 (0.00–0.91) | 0.28 (0.00–0.89) | LR |
| muntjac | Muntiacus muntjak | 405 | 186 | 0.39 (0.03–0.98) | 0.34 (0.03–0.97) | LR |
| mouse deer | Moschiola meminna | 95 | 54 | 0.81 (0.70–0.92) | 0.74 (0.70–0.88) | LR |
| swamp deer | Cervus duvaucelii | 233 | 83 | 0.90 (0.84–0.96) | 0.87 (0.83–0.95) | VU |
| blackbuck | Antilope cervicapra | 694 | 306 | 0.33 (0.11–0.94) | 0.33 (0.11–0.94) | NT |
| nilgai | Boselaphus tragocamelus | 404 | 217 | 0.29 (0.03–0.80) | 0.27 (0.03–0.80) | LC |
| chinkara | Gazella bennetti | 359 | 197 | 0.58 (0.09–1.00) | 0.45 (0.09–0.99) | LC |
| four-horned antelope | Tetracerus quadricornis | 236 | 135 | 0.51 (0.21–0.93) | 0.37 (0.21–0.90) | VU |
| Nilgiri tahr | Hemitragus hylocrius | 238 | 29 | 0.71 (0.71–0.71) | 0.71 (0.71–0.71) | EN |
| wild pig | Sus scrofa | 743 | 328 | 0.25 (0.24–0.25) | 0.25 (0.24–0.25) | LR |
| gaur | Bos gaurus | 966 | 257 | 0.60 (0.08–0.97) | 0.60 (0.07–0.98) | VU |
| wild buffalo | Bubalus arnee | 403 | 148 | 0.66 (0.58–0.67) | 0.65 (0.58–0.67) | EN |
| elephant | Elephas maximus | 736 | 175 | 0.43 (0.08–0.92) | 0.43 (0.08–0.92) | EN |
| rhino | Rhinoceros unicornis | 267 | 82 | 0.55 (0.21–0.93) | 0.55 (0.21–0.93) | EN |
| black bear | Ursus thibetanus | 166 | 50 | 0.38 (0.07–0.90) | 0.38 (0.14–0.69) | VU |
| brown bear | Ursus arctos | 64 | 9 | 0.35 (0.17–0.57) | 0.35 (0.17–0.57) | VU |
| sloth bear | Melursus ursinus | 919 | 356 | 0.39 (0.17–0.95) | 0.39 (0.08–0.87) | VU |
| hyena | Hyaena hyaena | 267 | 175 | 0.37 (0.34–0.46) | 0.36 (0.34–0.45) | LR |
| jackal | Canis aureus | 373 | 356 | 0.14 (0.09–0.33) | 0.14 (0.09–0.33) | LC |
| wolf | Canis lupus | 277 | 170 | 0.25 (0.10–0.96) | 0.19 (0.10–0.94) | LC |
| wild dog | Cuon alpinus | 434 | 165 | 0.62 (0.05–1.00) | 0.62 (0.08–1.00) | EN |
| tiger | Panthera tigris | 3606 | 572 | 0.67 (0.05–1.00) | 0.58 (0.05–1.00) | EN |
| leopard | Panthera pardus | 2708 | 559 | 0.36 (0.07–0.77) | 0.28 (0.07–0.67) | LC |
| lion | Panthera leo | 231 | 64 | 0.96 (0.55–0.99) | 0.80 (0.38–0.92) | CE |
Figure 1.
Geographical distribution of historic records used to estimate local probability of extinction for tiger (Panthera tigris) in India. Green dots are museum, natural history and hunting records (3606 total) pooled into grey cells, and red lines demarcate State boundaries in India.
We define occupancy, ψi, as the probability that site i is occupied by the species of interest (MacKenzie et al. 2002, 2006). We focus only on grid cells across India for which we have historic records of species occurrence (figure 1). We then estimate the probability that a historically occupied site is still occupied in 2006. Because of this conditioning on cells of historic occurrence, the occupancy parameter is equivalent to the complement of local extinction probability (see the electronic supplementary material for more detailed explanation of extinction probability), i.e. 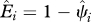 , where ‘^’ denotes estimates, and Ei is local extinction probability or the probability that grid cell i, known to have been occupied by the focal species in the past, is no longer occupied at present. This parameter, Ei, can also be viewed as the expected fraction of cells occupied by the focal species in the past that is still occupied by the species.
, where ‘^’ denotes estimates, and Ei is local extinction probability or the probability that grid cell i, known to have been occupied by the focal species in the past, is no longer occupied at present. This parameter, Ei, can also be viewed as the expected fraction of cells occupied by the focal species in the past that is still occupied by the species.
To determine current (2006) species presence or absence in any grid cell, we surveyed more than 100 ecologists, naturalists and knowledgeable conservation practitioners across India. We used field observations from multiple experts (2–37 per cell) to estimate current probability of extinction for those grid cells with historic records. Specifically, we asked the local experts if they had detected the focal species or not during the previous year. By emphasizing that all reports of presence from these experts had to represent certainty based on direct evidence, we sought to eliminate problems of false presences. We clarified to the experts that we were not going to interpret reports of non-detection to mean absence of the species, but that a reported detection would indeed be interpreted as presence of the species. Multiple detection and non-detection records from individual experts provided us the replicate samples required for estimation of detection probabilities and extinction (MacKenzie et al. 2002, 2006). Detection probability is defined as the probability that a species is detected by an expert, given that the species is actually present in the grid cell. Our approach deals with the critical problem of imperfect detections as well as accommodates unequal sampling effort resulting from varying numbers of experts reporting data from surveyed cells.
(b). Modelling of species occupancy and extinction using covariates
We model occupancy and detection probabilities as functions of covariates using logit link functions (MacKenzie et al. 2002, 2006). We incorporate environmental and social covariates to simultaneously model detection probabilities and estimate occupancy during the year (MacKenzie et al. 2006). We used the maximum-likelihood approach to fit a range of plausible occupancy models (50–115) for each species. These models represent different ecological hypotheses about the factors influencing local probability of extinction for each species (tables S1 and S2 in the electronic supplementary material). We fit various models to the data, and calculated model weights using Akaike's information criterion (AIC) (Burnham & Anderson 2002, 2004; tables S1 and S2 in the electronic supplementary material). We used model averaging to derive weighted averages of occupancy if there were multiple models with substantial weights. We averaged the occupancy estimates themselves, rather than estimated coefficients associated with covariates. The AIC weights were re-scaled to sum to 1 for all models used in the model averaging, and represent the relative appropriateness of a given model relative to others in the model set. These data were analysed using program PRESENCE (v. 2.0, Hines 2006).
We focus on a set of environmental and social covariates that we hypothesize to be important determinants of local species extinction probabilities, and thus represent hypotheses of conservation and ecological interest (table S1 in the electronic supplementary material). Covariates in each cell that we selected included time elapsed (time between the year of the historic record and 2006), presence and proportion of wildlife protected areas, proportion of forest cover, elevation and probable human influences (population density and cultural attitudes towards the species).
Information on protected areas is from the World Database on Protected Areas (WDPA 2003, 2007, 2009). We created two measures: (i) presence or absence of a protected area in a cell and (ii) proportion of the cell occupied by a protected area (table S1 in the electronic supplementary material). We refined and improved the WDPA data using topographic maps, expert opinions, remote sensing and prior knowledge (Joshi et al. 2006; Roy et al. 2006). Proportion of cell covered by a protected area has five categories (0, 1–25, 26–50, 51–75 and 76–100%) numbered 1–5. We determine presence–absence of forest cover from Global Land Cover Facility 2000 (Bartholomé & Belward 2005). Elevation data from CGIAR-CSI SRTM 100 m Digital are used to calculate average elevation in every grid cell (CGIAR 2004). Elevation data were transformed to range between 0 and 10. We developed two covariates to represent human influence on species persistence. Human population density data are derived from the LandScan Global Population Database (Dobson et al. 2000) and log transformed. We use prior knowledge of human hunting patterns, diets and cultural tolerance–reverence for species in different regions across India (Rangarajan 2001; Madhusudan & Karanth 2002; Mishra et al. 2006; Datta 2007; K. K. Karanth & K. U. Karanth 2008, personal observations), to develop a ‘human cultural tolerance’ variable. This variable divided Indian states into three groups, from ‘most tolerant’ to ‘least tolerant’. For the human cultural tolerance variable, we classify the western states of Rajasthan and Gujarat as most tolerant (categorical variable value = 1), the seven northeastern hill states, Chhattisgarh and Jharkhand as least tolerant (variable value = 3) and all other states as intermediate (variable value = 2) in tolerance (Karanth et al. 2009).
For every historic record, we calculate the number of years elapsed between the collection year and 2006. We chose to randomly select one year if records were available for multiple years in a grid cell. We generally expected a positive relationship between time elapsed and the probability that a species was now extinct. If all local extinctions were permanent, we would expect a simple positive relationship between extinction probability and elapsed time. For example, assume the simple case of time-invariant annual extinction probability (E = Pr (locally extinct in year t + 1 | present in year t)). In this case, we could compute the expected probability of extinction for any number of years, T, as Pr (local extinction in T years) = 1 − (1 − E)T. Local extinctions are not always permanent, and probabilities of extinction for any grid cell will depend on rates of both local extinction and re-colonization, and on temporal variation in these parameters. As the available data do not permit estimation of time-specific rates of local extinction or colonization, we assessed the influence of elapsed time, using linear logistic models. Negative estimates of β for elapsed time occurred occasionally, but such estimates are not logically possible, so these models were not considered as candidates for model selection and model averaging.
Our approach yields estimates of occupancy, 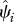 (equivalent to the complement of a local extinction probability), for each cell i, with at least one record of historic species occurrence. As the historic records were collected at different points in time, and because we expect time of the historic record to be relevant to persistence for at least some species, we use coefficient values from top-ranked models to calculate local extinction probabilities at two specific reference times (t = 50 and 100 years ago) for every cell. This standardizes estimates and allows meaningful comparison of grid cells across India, regardless of the year of the historic record. We used model averaging (2–10 top-ranked models, model weights scaled accordingly) for all species. We estimated local extinction (
(equivalent to the complement of a local extinction probability), for each cell i, with at least one record of historic species occurrence. As the historic records were collected at different points in time, and because we expect time of the historic record to be relevant to persistence for at least some species, we use coefficient values from top-ranked models to calculate local extinction probabilities at two specific reference times (t = 50 and 100 years ago) for every cell. This standardizes estimates and allows meaningful comparison of grid cells across India, regardless of the year of the historic record. We used model averaging (2–10 top-ranked models, model weights scaled accordingly) for all species. We estimated local extinction (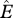 ) as (
) as (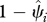 ). These values reflect the probability that in 2006, a species was locally extinct in a given cell i that was occupied by the species at some previous time. Local extinction probability is calculated under each model for each species by cell combination using the estimated β-coefficients and cell covariate values. To calculate local extinction probability for species and cells with multiple supported models, we model-averaged the local extinction probability estimates. These cell-specific estimates are averaged across cells to determine overall species extinction probabilities
). These values reflect the probability that in 2006, a species was locally extinct in a given cell i that was occupied by the species at some previous time. Local extinction probability is calculated under each model for each species by cell combination using the estimated β-coefficients and cell covariate values. To calculate local extinction probability for species and cells with multiple supported models, we model-averaged the local extinction probability estimates. These cell-specific estimates are averaged across cells to determine overall species extinction probabilities 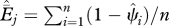 , where
, where 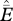 is an estimate of the proportion of cells experiencing a local extinction over the last j years (j = 50 or 100),
is an estimate of the proportion of cells experiencing a local extinction over the last j years (j = 50 or 100), 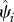 is the estimated occupancy probability (scaled to j years) for the ith cell in 2006, and n is the number of cells with historic records.
is the estimated occupancy probability (scaled to j years) for the ith cell in 2006, and n is the number of cells with historic records.
(c). A priori predictions
We confronted several a priori hypotheses with our data. We expected that the presence of a wildlife protected area in a cell, as well as a higher proportion of the cell being thus protected, would result in lower extinction probabilities for most species (Redford 1992; Newmark 1996; Brashares et al. 2001; Parks & Harcourt 2002). We expected higher proportion of forest cover in a cell to be associated with lower extinction probabilities for 13 species that are associated with forested habitats (chital, sambar, muntjac, mouse deer, Nilgiri tahr, gaur, elephant, black bear, brown bear, sloth bear, wild dog, tiger and leopard). We predicted higher extinction probabilities for grassland and open forest species (rhino, wild buffalo, blackbuck, nilgai, chinkara, four-horned antelope, hyena and wolf) because of earlier and more intensive conversion of such habitats to agriculture historically (Dunbar-Brander 1923; Stebbing 1923; Champion 1934). We expected lower extinction probabilities in montane high-elevation habitats for three species (black bear, brown bear and Nilgiri tahr) because of the relative inaccessibility and the lower probabilities of agricultural conversion (Stebbing 1923).
We expected higher extinction probabilities to be associated with higher human population density (Sanderson et al. 2003; Cardillo et al. 2004) for all large mammals, except five species that were either adaptable to human-dominated landscapes (wild pig, jackal) or culturally tolerated (blackbuck, chinkara and nilgai). We expected these three culturally tolerated species to have lower extinction probabilities, even in areas with high human population densities (Madhusudan & Karanth 2002; Brashares 2003; Corlett 2007; Datta 2007). We expected large-bodied herbivores (elephant, wild buffalo, rhino and gaur), and large carnivores (brown bear, tiger and lion) to be more vulnerable to extinction because they compete directly for space and nutritional needs with human societies or were historically hunted for trophies and bounties (Dunbar-Brander 1923; Champion 1934; Rangarajan 2001). We expected adaptable habitat generalist species (leopard, jackal, wild pig) to be less vulnerable to local extinction, compared with habitat specialists (Nilgiri tahr, brown bear, rhino and swamp deer). We also predicted lower extinction probabilities for herbivores compared with carnivores, and higher extinction probabilities for crop-raiding and livestock-killing species (Woodroffe & Ginsberg 1998). We expected restricted-range endemic species to be more vulnerable compared with widely distributed ones (Ceballos et al. 2005; Morrison et al. 2007; Schipper et al. 2008).
3. Results
(a). Extinction and elapsed time
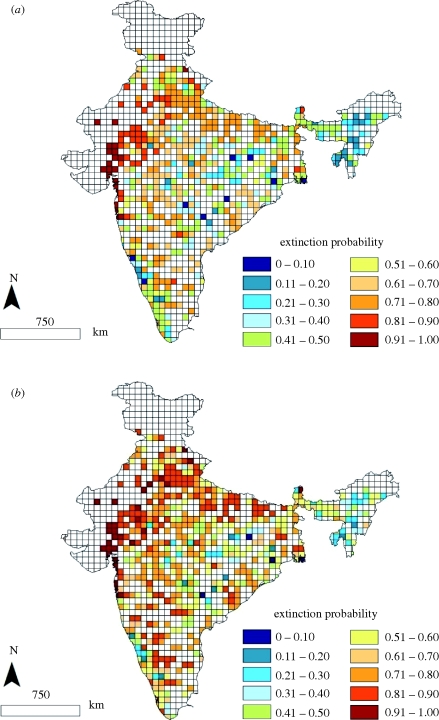
Our local extinction probability estimates for mammals across a 100-year time-frame range between 0.14 and 0.96 (table 1). We expected extinction probability to generally increase with elapsed historical time (the interval between historic recorded year of occurrence and 2006). In fact, elapsed time was present in the top-ranked models (positive relationship with local extinction probability) for nine species (table 2; e.g. muntjac, mouse deer, blackbuck, four-horned antelope, elephant, wolf, tiger (figure 2a,b), leopard and lion) and in lower ranked models with some effect on local extinctions for five additional species.
Table 2.
Estimated β-coefficients (and estimated s.e.) indicating covariate relationships for extinction probability from the top models for all 25 mammal species in India. Note: covariates—park, park presence/absence; park prop, per cent of cell covered; fc, forest cover presence/absence; elv, elevation (transformed); ppl, log (human population density); tol, cultural intolerance; time, time (transformed). For every model additional variations with constant occupancy (ψ (.) p (covariates)) and constant detection (ψ (covariates) p (.)) were also run. Transformation for elevation = covariate × 10/max (covariate).
| species/covariate | constant | forest cover | PA presence | PA prop | elevation | human density | intolerance | time |
|---|---|---|---|---|---|---|---|---|
| chital | −0.79 (0.16) | — | — | — | — | — | — | — |
| sambar | −36.79 (12.07) | 24.39 (11.92) | — | −0.35 (0.23) | −0.54 (0.55) | 0.86 (0.22) | — | 0.05 (0.05) |
| muntjac | 6.48 (1.98) | — | — | −0.58 (0.26) | — | — | −3.65 (0.99) | 0.10 (0.07) |
| mouse deer | 0.48 (0.50) | — | — | — | — | — | — | — |
| swamp deer | 2.40 (0.61) | — | — | — | — | — | — | — |
| blackbuck | −19.66 (7.93) | 19.97 (7.92) | — | — | — | — | 19.27 (7.91) | 0.003 (0.08) |
| nilgai | −13.19 (2.29) | — | — | 0.09 (0.27) | 2.31 (0.57) | 0.58 (0.15) | 1.62 (0.43) | — |
| chinkara | −37.00 (1.84) | 14.01 (2.85) | −0.25 (0.48) | — | 2.25 (0.93) | 1.33 (0.15) | 1.67 (0.59) | — |
| four-horned antelope | 7.39 (10.71) | — | — | −1.67 (1.28) | −13.38 (10.10) | −1.17 (1.15) | 5.69 (3.86) | 0.54 (0.36) |
| Nilgiri tahr | −0.73 (0.89) | — | — | — | — | — | — | — |
| wild pig | −2.82 (0.34) | — | — | — | — | — | — | — |
| gaur | 5.76 (2.07) | — | — | −0.68 (0.22) | — | — | −2.31 (1.01) | — |
| wild buffalo | −0.43 (0.65) | — | — | — | — | — | — | — |
| elephant | 3.78 (1.12) | — | — | −0.17 (0.21) | — | — | −1.88 (0.56) | 0 (0.007) |
| rhino | 55.53 (4.79) | — | — | 8.23 (3.08) | — | −4.70 (0.40) | — | — |
| black bear | 17.86 (5.45) | — | — | — | −6.40 (2.33) | −1.10 (0.37) | — | — |
| brown bear | −0.62 (0.81) | — | — | — | — | — | — | — |
| sloth bear | −9.91 (2.23) | −1.44 (1.70) | — | −0.58 (0.27) | 0.44 (0.20) | 0.78 (0.12) | — | — |
| jackal | −6.34 (1.75) | — | — | −0.32 (0.56) | — | — | 1.77 (0.68) | — |
| hyena | −1.35 (0.25) | — | — | — | — | — | — | — |
| wolf | 25.99 (8.87) | 3.42 (1.45) | −3.42 (1.42) | — | −2.29 (0.78) | −2.25 (0.72) | — | 0.16 (0.21) |
| wild dog | 35.99 (1.35) | — | — | −0.87 (0.29) | 0.33 (0.15) | 0.49 (0.11) | −20.90 (0.60) | — |
| tiger | 19.02 (13.43) | −22.02 (13.71) | — | −1.09 (0.19) | 0.51 (0.17) | 0.36 (0.18) | −0.97 (0.24) | 0.10 (0.04) |
| leopard | −4.73 (2.17) | 0.66 (0.48) | — | −0.37 (0.20) | −1.66 (0.43) | 0.27 (0.15) | −0.13 (0.21) | 0.10 (0.04) |
| lion | −0.22 (1.40) | — | — | −1.45 (0.72) | — | — | — | 0.50 (0.20) |
Figure 2.
Predicted local extinction probability for tiger referenced to (a) 50 years and (b) 100 years. The bluish cells indicate lower estimated extinction probabilities and the redder cells indicate higher estimated extinction probabilities for tigers in India.
(b). Protected areas, forest cover and elevation
Protected areas (in terms of either presence or the proportion of cell covered) were negatively associated with extinction probability for 18 mammal species. For 13 species, β-parameter estimates were negative in their top-ranked models, and for five other species β-parameters were negative in additionally supported models (table 2; table S2 in the electronic supplementary material). Our prediction that protected areas facilitate lower extinction was thus strongly supported by results for 18 species (especially for large carnivores and forest-dwelling mammals).
We expected the presence of forest cover to negatively influence extinction of 13 species. For three of these species, we find negative β-coefficients in the top model, and for four species we find negative β-coefficients in additional supported models (table 2; table S2 in the electronic supplementary material). Our predictions were thus partially supported, with forest cover associated with lower extinction of seven species. However, absence of such evidence (table 2) suggests that forest cover may not be an important determinant of extinction for other species.
We find negative estimates of β-coefficients for the elevation covariate in the top-ranked model for five species (one bear for which we predicted this relationship and four more species), and negative β-coefficient estimates for three other species in additionally supported models (table 2; table S2 in the electronic supplementary material). Our predictions about the importance of high elevation habitats facilitating lower extinction were supported for brown bear, but not for Nilgiri tahr and black bear (all these species are restricted to higher elevations). This apparent inconsistency may be due to either the absence of lower elevation historic records for this species (i.e. there was little variation in the elevation covariate) or use of average elevation values for relatively large grid cells used. Overall, higher elevation appears to facilitate lower extinction probabilities in eight species.
(c). Human population density and cultural tolerance
As expected, human population density was positively associated with local species extinction probabilities. For seven species, estimated β-coefficients were positive in their top-ranked models, and for six other species β-coefficients were positive in additional supported models (table 2; table S2 in the electronic supplementary material). Our expectation of higher extinction probabilities at higher human population densities was thus supported for 13 of the 25 species. We also correctly predicted three adaptable species for which human density was not a relevant covariate affecting extinction (wild pig, jackal, blackbuck). However, similar predictions for chinkara and nilgai were not supported. Overall, we find that human population density does negatively affect species persistence, but some herbivores can persist at higher human densities.
Human cultural tolerance, a feature somewhat unique to southern Asia, did appear to negatively influence local species extinction probabilities. The ‘tolerance covariate’ was coded 1 for tolerant cells, 2 for intermediate cells and 3 for intolerant cells (actually reflected intolerance). For five species, estimated β-coefficients were positive in top-ranked models (table 2; table S2 in the electronic supplementary material) and for two other species estimated β-coefficients were positive in additional models. Thus, our predictions were supported for culturally tolerated species (nilgai, chinkara and blackbuck particularly in Western India), and extended to other species (four-horned antelope, jackal, wild pig, rhino and swamp deer).
We expected large-bodied mammals to be more vulnerable to extinction, which was supported for many species except for elephants, which are culturally tolerated (table 1). However, we also find evidence of higher extinction probabilities for mouse deer and Nilgiri tahr, which are smaller species that occur at inherently low ecological densities. Contrary to our predictions, local extinction probabilities for herbivores (0.25–0.90) and carnivores (0.14–0.96; table 1) were similar. Habitat generalists (leopard, jackal, wild pig) had lower probabilities of extinction than habitat specialists (Nilgiri tahr, brown bear, rhino and swamp deer), supporting our predictions. More specifically, however, culturally tolerated or forest dwelling herbivores, as well as adaptable generalist carnivores, had lower extinction probability estimates (table 1).
4. Discussion
Determining factors that reduce species extinction probabilities is critical to successful conservation efforts. However, most studies of animal distribution and occurrence use methods that do not distinguish true species absence from simple non-detection during surveys. This failure to deal with non-detection underestimates true occupancy, overestimates local extinction and confounds the analyses of spatial covariate data (MacKenzie et al. 2002, 2006) to determine the impact of various environmental and social factors on species extinctions. We combine historic records of past occurrence with current field observations of local wildlife experts to examine extinction patterns for 25 mammals in India. By applying occupancy modelling, we dealt with the issue of imperfect detection, while addressing a priori hypotheses about determinants of local extinction probabilities.
As predicted, the covariate related to elapsed time was important for extinction probabilities of 14 species. Most importantly, all 25 species had relatively high estimated probabilities of local extinction (referenced to 100 years), ranging between 0.14 and 0.96. Our results provide evidence that establishment of protected areas is critically important for low extinction probabilities of at least 18 species. Therefore, species whose current habitat mostly lies outside existing protected areas (mouse deer, blackbuck, nilgai, chinkara, four-horned antelope, sloth bear, jackal, wolf, wild pig), and species for which only a tiny part of their historic range is now in protected areas (swamp deer, wild buffalo, gaur, elephant, Nilgiri tahr, rhino, black bear, lion, wild dog, tiger), will require establishment of new protected areas to ensure their future persistence. We found that forest cover is negatively associated with extinction probabilities of seven species, but this factor alone is not sufficient to ensure persistence of many species. High elevation was negatively associated with extinction probabilities of eight species. Human population density positively influenced local extinction of 13 species, although human cultural tolerance was associated with lower extinction probabilities for seven species. Exceptions to these inferences about cultural tolerance were rare localized endemics (Nilgiri tahr, swamp deer, lion), widely distributed but low-density species (mouse deer), generalist species that adapt to human-dominated landscapes to some extent (wild pig, jackal, wolf, leopard), and culturally tolerated or protected species (blackbuck, nilgai, chinkara). These human cultural factors appear to affect local extinction of both carnivores and herbivores. Overall, most large-bodied animals, habitat specialists and rare species had higher extinction probabilities. It is important to note that our analysis of covariate effects on local species extinction probabilities was conducted at a relatively large spatial scale (cell size of 2800 km2). As human–wildlife conflicts, ecological impacts and conservation interventions often occur at a finer spatial scale, we might expect stronger relationships to exist at these finer scales.
5. Conclusion
Overall, all 25 large mammal species we studied showed substantial probabilities of local (cell level) extinction over the past century. We provide evidence that protected nature reserve areas are critical for reducing the local extinction probabilities of most Indian large mammals. India's current fragmented network of relatively small protected areas (average size less than 300 km2) does have high carrying capacities for large mammals (Karanth et al. 2004). However, given the overall patterns of species extinction estimated in this study, creation of new protected areas and interconnection of existing protected areas will be required through conservation policy and management if many of these mammals are to persist into the future. Our results must be considered in the context of rapid ongoing changes in land use, climate, human demography, cultures and economic growth that are currently occurring in India and southern Asia. Conservation policies must integrate all these factors to ensure the survival of India's large mammals into the future.
Our results have implications beyond conservation, for wider land-use and development policies currently being pursued in India. Regional development plans can incorporate reported occupancy patterns of large mammals by carefully considering the ecological and social processes that led to these patterns. We believe, in the context of India's rapid economic growth (7 per cent per year) and increasing aspirations of its growing human population, adverse impacts of many proposed development projects such as dams, mines, highways and industries can be mitigated substantially by integrating our results to prevent imminent contraction and fragmentation of ranges of many vulnerable mammals.
Acknowledgements
We thank S. L. Pimm and D. L. Urban for their suggestions. We are enormously grateful to the wildlife experts N. Akhtar, R. Ali, S. Amu, A. Aiyadurai, Y. V. Bhatnagar, R. Borges, A. Chandola, S. Chandola, D. Chetry, K. Choudhary, S. Choudhry, H. Dang, S. Dasgupta, S. Dattatri, A. Dutta, P. S. Easa, D. V. Girish, H. Ghuleria, D. Ghose, S. P. Goyal, B. Hegde, Hilaluddin, D. Jathanna, B. Jetva, Y. V. Jhala, A. J. T. JohnSingh, S. Jones, J. Joshua, K. Kakati, Kathju, D. K. Kashyap, R. Kaul, M. Khanduja, J. Kulkarni, A. Kumar, N. S. Kumar, S. Kumar, H. Kumara, A. Lobo, P. Mehta, B. Mohanty, S. Molur, S. Mukherjee, L. Nehemiah, N. Patil, S. Pawar, S. Pradhan, S. Radhakrishna, A. Rahmani, N. Rajamani, S. Rajesh, S. Ram, G. S. Rawat, G. V. Reddy, V. Rishi, A. D. Roy, P. K. Sen, K. Sharma, N. Sharma, K. Sathasivam, J. N. Shah, G. Shahabuddin, D. Sharma, V. Srinivas, G. Sundar, A. Tamim, S. Tiwari, P. Trivedi, H. Tyabji, N. Ved, R. Vyas, R. Wangchuk, T. Wangyal and their associates who completed the surveys. We thank the following institutions Bombay Natural History Society; Van Ingen & Van Ingen Taxidermists; Duke University Library; Harvard Museum of Natural History; American Museum of Natural History; Carnegie Museum; California Academy of Sciences; Field Museum of Natural History; Michigan State Museum; University of Kansas Biodiversity Center; University of Washington Burke Museum; Los Angeles County Museum; Museum of Natural Sciences; Museum of Vertebrate Zoology; Natural History Museum, DC; Museum of Texas Tech University; University of Michigan Museum of Zoology; University of New Mexico Museum of Southwestern Biology; Museum of Natural History Berkley; Yale Peabody Museum; Royal Ontario Museum; New Hancock Museum; University of Manchester Museum; Natural History Museum of Geneva; Hungarian Natural History Museum; National Museum of Wales (Cardiff); New Castle Museum. We thank J. Van Ingen, M. Rangarajan, V. Thapar, S. Kapoor, D. Sinh, J. Terborgh, M. Vale, C. N. Jenkins, P. Karanth, A. Ostrovsky, D. Ostrovsky, R. DeFries, P. M. Kumar, Duke University, Centre for Wildlife Studies, and Wildlife Conservation Society's India Program. K.K.K. received funding from the Conservation, Food and Health Foundation, Forest History Society, Duke International Travel, IDEA Wild, Roger Williams Park Zoo, AZFA Clark Waldram Conservation Fund and Cleveland Zoo.
References
- Bartholomé E., Belward A. S.2005GLC2000: a new approach to global land cover mapping from Earth observation data. Int. J. Remo. Sens. 26, 11 051–11 077 (doi:10.1080/01431160412331291297) [Google Scholar]
- Brashares J. S.2003Correlates of extinction in West Africa. Conserv. Biol. 17, 734–743 (doi:10.1046/j.1523-1739.2003.01592.x) [Google Scholar]
- Brashares J. S., Arcese P., Sam M. K.2001Human demography and reserve size predict wildlife extinction in West Africa. Proc. R. Soc. Lond. B 268, 2473–2478 (doi:10.1098/rspb.2001.1815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multimodal inference: a practical information-theoretic approach New York, NY: Springer [Google Scholar]
- Burnham K. P., Anderson D. R.2004Multimodal inference: understanding AIC and BIC in model selection. Socio. Meth. Rese. 33, 261–304 (doi:10.1177/0049124104268644) [Google Scholar]
- Cardillo M., Purvis A., Sechrest W., Gittleman J. L., Beilby J., Mace G. M.2004Human population density and extinction risk in the world's carnivores. PLoS Biol. 2, 909–914 (doi:10.1371/journal.pbio.0020197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo M., Mace G. M., Gittleman J. L., Purvis A.2006Latent extinction risk and the future battle grounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161 (doi:10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos G., Ehrlich P. R., Soberon J., Salazar I., Fay J. P.2005Global mammal conservation: what must we manage? Science 309, 603–607 (doi:10.1126/science.1114015) [DOI] [PubMed] [Google Scholar]
- CGIAR- CSI 2004. SRTM data V1, International Centre for Tropical Agriculture CIAT. See http://srtm.csi.cgiar.org
- Champion F. W.1934With a camera in tiger land London, UK: Chatto & Windus [Google Scholar]
- Channell R., Lomolino M.2000Dynamic biogeography and the conservation of endangered species. Nature 403, 84–86 (doi:10.1038/47487) [DOI] [PubMed] [Google Scholar]
- Corlett R. T.2007The impact of hunting on the mammalian fauna of tropical Asian forests. Biotropica 39, 292–303 (doi:10.1111/j.1744-7429.2007.00271.x) [Google Scholar]
- Das A., Krishnaswamy J., Bawa K. S., Kiran M. C., Srinivas V., Kumar N. S., Karanth K. U.2006Prioritization of conservation areas in the Western Ghats, India. Biol. Conserv. 133, 16–31 (doi:10.1016/j.biocon.2006.05.023) [Google Scholar]
- Datta A.2007Protecting with people in Namdapha: threatened forests, forgotten people. In Making conservation work (eds Shahabuddin G., Rangarajan M.), pp. 165–209 New Delhi, India: Permanent Black [Google Scholar]
- Dobson J. E., Bright E. A., Coleman P. R., Durfee R. C., Worley B. A.2000LandScan: a global population database for estimating populations at risk. Photogramm. Eng. Remote Sensing 66, 849–857 [Google Scholar]
- Dunbar-Brander A. A.1923Wild animals in central India London, UK: Edward Arnold & Co [Google Scholar]
- Forest Survey of India 2005State of the forest report Dehradun, India: Ministry of Environment and Forests [Google Scholar]
- Hines J. E.2006. Program PRESENCE. See http://www.mbrpwrc.usgs.gov/software/doc/presence/presence.html
- Isaac N. J., Turvey S. T., Collen B., Waterman C., Baillie J. E.2007Mammals on the EDGE: conservation priorities based on threat and phylogeny. PloS ONE 2, e296 (doi:10.1371/journal.pone.0000296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P. K. K., Roy P. S., Singh S., Agrawal S., Yadav D.2006Vegetation cover mapping in India using multi-temporal IRS wide field sensor WiFS data. Remo. Sens. Envmt. 103, 1100–1112 (doi:10.1016/j.rse.2006.04.010) [Google Scholar]
- Karanth K. U., Nichols J. D., Kumar N. S., Link W. A., Hines J. E.2004Tigers and their prey: predicting carnivore densities from prey abundance. Proc. Natl Acad. Sci. USA 101, 4854–4858 (doi:10.1073/pnas.0306210101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth K. K., Nichols J. D., Hines J. E., Karanth U. K., Christensen N. L.2009Patterns and determinants of mammal species occurrence in India. J. Appl. Ecol. 46, 1189–1200 (doi:10.1111/j.1365-2664.2009.01710) [Google Scholar]
- MacKenzie D. I., Royle J. A.2005Designing occupancy studies: general advice and allocating survey effort. J. Appl. Ecol. 42, 1105–1114 (doi:10.1111/j.1365-2664.2005.01098x) [Google Scholar]
- MacKenzie D. I., Nichols J. D., Lachman G. B., Droege S., Royle J. A., Langtimm C. A.2002Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255 (doi:10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2) [Google Scholar]
- MacKenzie D. I., Nichols J. D., Royle J. A., Pollock K. H., Bailey L. A., Hines J. E.2006Occupancy modeling and estimation San Diego, CA: Academic Press [Google Scholar]
- Madhusudan M. D., Karanth K. U.2002Local hunting and the conservation of large mammals in India. Ambio 31, 49–54 (doi:10.1639/0044-7447(2002)031[0049:LHATCO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Mishra C., Madhusudan M. D., Datta A.2006Mammals of the high altitudes of western Arunachal Pradesh, eastern Himalaya: an assessment of threats and conservation needs. Oryx 40, 29–35 (doi:10.1017/S0030605306000032) [Google Scholar]
- Morrison J. M., Sechrest W., Dinerstein E., Wilcove D. S., Lamoreux J. L.2007Persistence of large mammal faunas as indicators of human impact. J. Mammal. 88, 1363–1380 (doi:10.1644/06-MAMM-A-124R2.1) [Google Scholar]
- Newmark W. D.1996Insularization of Tanzanian parks and the local extinction of mammals. Conserv. Biol. 10, 1549–1556 (doi:10.1046/j.1523-1739.1996.10061549.x) [Google Scholar]
- Parks S. A., Harcourt A. H.2002Reserve size, local human density, and mammalian extinctions in U.S protected areas. Conserv. Biol. 16, 800–808 (10.1046/j.1523-1739.2002.00288.x) [Google Scholar]
- Pimm S. L., et al. 2001Can we defy nature's end? Science 233, 2207–2208 (doi:10.1126/science.1061626) [DOI] [PubMed] [Google Scholar]
- Rangarajan M.2001India's wildlife history New Delhi, India: Permanent Black [Google Scholar]
- Redford K. H.1992The empty forest. BioScience 42, 412–422 (doi:10.2307/1311860) [Google Scholar]
- Roy P. S., Joshi P. K., Singh S., Agrawal S., Yadav D., Jegannathan C.2006Biome mapping in India using vegetation type map derived using temporal satellite data and environmental parameters. Ecol. Model. 1107, 148–158 (doi:10.1016/j.ecolmodel.2006.02.045) [Google Scholar]
- Sanderson E. W., Jaiteh M., Levy M. A., Redford K. H., Wannebo A. V., Woolmer G.2003The human footprint and the last of the wild. BioScience 52, 891–904 (doi:10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2) [Google Scholar]
- Schipper J., et al. 2008The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230 (doi:10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- Stebbing E. P.1923The forests of India, vol. I London, UK: John Lane [Google Scholar]
- WDPA 2003, 2007, 2009World Database on Protected Areas. World Conservation Union (IUCN) and UNEP World Monitoring Center (UNEP-WCMC) [Google Scholar]
- Woodroffe R., Ginsberg G. R.1998Edge effects and extinctions of populations inside protected areas. Science 280, 2126–2128 (doi:10.1126/science.280.5372.2126) [DOI] [PubMed] [Google Scholar]