Abstract
The adaptive hypothesis invoked to explain why parasites harm their hosts is known as the trade-off hypothesis, which states that increased parasite transmission comes at the cost of shorter infection duration. This correlation arises because both transmission and disease-induced mortality (i.e. virulence) are increasing functions of parasite within-host density. There is, however, a glaring lack of empirical data to support this hypothesis. Here, we review empirical investigations reporting to what extent within-host viral accumulation determines the transmission rate and the virulence of vector-borne plant viruses. Studies suggest that the correlation between within-plant viral accumulation and transmission rate of natural isolates is positive. Unfortunately, results on the correlation between viral accumulation and virulence are very scarce. We found only very few appropriate studies testing such a correlation, themselves limited by the fact that they use symptoms as a proxy for virulence and are based on very few viral genotypes. Overall, the available evidence does not allow us to confirm or refute the existence of a transmission–virulence trade-off for vector-borne plant viruses. We discuss the type of data that should be collected and how theoretical models can help us refine testable predictions of virulence evolution.
Keywords: evolution, disease, viral accumulation, virulence, transmission
1. Introduction
Although the availability of genomic and molecular details about specific host–parasite interactions is increasing exponentially owing to new molecular biotechnologies, our understanding of the evolution of virulence (i.e. decrease in host fitness due to parasite infection) remains fragmentary. Some pathogens cause noticeable damage to their host, and understanding the parameters underlying virulence evolution is critical for controlling human pathogens and agricultural pests and for the conservation of biodiversity. Moreover, determining the key parameters involved in virulence evolution becomes a priority when ecosystems are submitted to profound modifications such as global climatic change or increased commercial exchanges that spread parasites rapidly to new environments. More simply put, a long-standing question in evolutionary biology is ‘Why do parasites harm their hosts?’ An adaptive answer to this question states that while a high level of parasite replication increases the possibilities for between-host transmission, rapid replication is also likely to be detrimental to the host (i.e. to be associated with high virulence) owing to, for instance, over-exploitation of host resources by the parasite. Many models thus predict the existence of an optimal level of virulence that integrates the benefits of transmission and the cost of killing the host, which is known as the ‘trade-off’ hypothesis (Anderson & May 1982; Ewald 1983; Frank 1996). Although much debated, this hypothesis still awaits sufficient empirical data in order to be properly evaluated (Alizon et al. 2009). Here, we discuss the evolution of virulence of plant viruses, a group of parasites ignored by most reviews on the subject.
Viruses represent one of the most threatening types of parasites for human health and agriculture because they tend to exhibit high evolutionary rates (Froissart et al. 2005; Duffy et al. 2008). This rapid evolution, combined with large-scale dispersal, largely explains our limited success in controlling and eradicating viruses, with a few notable exceptions (e.g. small pox virus). Despite their importance in crop yield losses and ecosystem modification, few empirical studies have focused explicitly on the virulence of plant viruses from an evolutionary point of view. Notable exceptions are two recent reviews that address this question but put more emphasis on the evolution of parasite infectivity, i.e. the ability to cause disease (Jarosz 2002; Sacristan & Garcia-Arenal 2008). We focus on the evolution of virulence, which, following the conventions adopted by the American Phytopathological Society (D'Arcy et al. 2001), is defined as the degree of damage caused to a host and is usually assumed to correlate negatively with host fitness.
The biological cycles of plant viruses, whose evolution was reviewed by Garcia-Arenal et al. (2001), are subject to two specific peculiarities: (i) hosts are not mobile during their vegetative stage and (ii) contact between hosts is rare. Since plant viruses cannot penetrate the intact plant cuticle and the cellulose cell wall, they are transmitted either vertically, or horizontally by vectors (Hull 2001). The latter is by far the most common transmission mode, thus our focus is on vector-borne viruses. The question we ask is the following: ‘To what extent does the level of virus replication within a plant determine the virus transmission rate and the virulence to the host?’ This question is important because it underlies most of the transmission–virulence trade-off hypothesis.
2. Theoretical prediction of evolution of virulence in vector-borne diseases
Natural selection operates on the rate of parasite spread within and between hosts. In most cases, natural selection thus tends to maximize the so-called parasitic basic reproduction ratio, R0, which corresponds to the number of new infections generated by a single infected individual in a wholly susceptible population over the duration of the infection (but see Roberts (2007) for exceptions). In other words, R0 is an expression of the fitness of a pathogen. In the classical Susceptible-Infected model for directly transmitted pathogens, it is given by R0 = βS/(μ + α), where S is the number of susceptible individuals, β is the transmission rate of the parasite from host to host, μ is the natural host mortality rate and α is the additional host mortality rate caused by the infection—usually called virulence (Anderson & May 1982; Frank 1996; Levin 1996).
In the case of a vector-borne disease, however, the expression of R0 is slightly different. If we assume that the population dynamics of the vector are rapid and at equilibrium, and if we ignore clearance from the host, we have (Anderson & May 1991; Mideo & Day 2008):
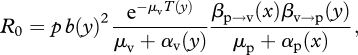 |
2.1 |
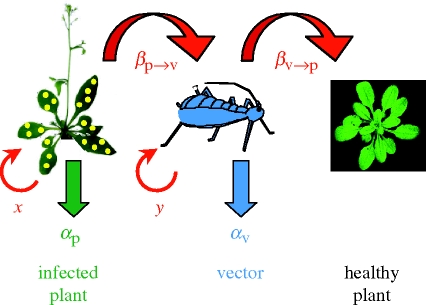
where p is the average number of vectors per plant in the population, b is the vector feeding rate (or probing rate in the case of vectors of plant viruses) per day, T is the incubation time of the parasite in the vector (i.e. the time between vector infection and the time the vector becomes infective), μp and μv are the baseline mortality of the plant and of the vector, respectively, αp and αv are the extra mortality (i.e. the virulence) induced by the parasite on the plant and on the vector, respectively, βp→v is the transmission rate from the infected plant to the vector and βv→p is the transmission rate from the viruliferous (i.e. carrying virus) vector to a healthy plant. Finally, y is viral accumulation in the vector and x the viral accumulation in the plant. We present a relatively general case where many components of R0 depend on viral accumulation, but this is not necessarily the case. This is summarized in figure 1. In this formalism it is assumed that viral accumulation is at least partly determined by viral genotype.
Figure 1.
Effect of within-host viral accumulation on transmission rate and virulence of vector-borne plant viruses. The transmission rate can be decomposed into two steps: from infected plant to vector (βp→v) and from viruliferous vector to healthy plant (βv→p). Within-plant (x) and within-vector (y) viral accumulation are thought to have an effect on mortality of the plant (αp) and vector (αv), respectively.
The trade-off hypothesis rests on two key assumptions: (i) that increasing viral accumulation increases virulence and (ii) that increasing viral accumulation increases virus transmission to another host. This dependency is indicated in equation (2.1) with an x or a y in brackets. The direct consequence of these two assumptions is that a virus genotype with high accumulation rates will benefit from increased transmission (βp→v × βv→p), but will suffer from a shorter infection period owing to rapid host and vector death (αp and αv). In other words, the pleiotropic effect of the accumulation rate generates a transmission–virulence trade-off such that it is not possible to maximize transmission rate and the duration of the infection simultaneously. In the following, we present the evidence for such correlations between accumulation rate and virulence and transmission.
If we assume that parasite strains vary with respect to their accumulation rates (x and y), then this implies that these strains will also have different R0, i.e. different fitnesses at the between-host level. Theory predicts that the strain with the highest R0 is evolutionarily stable and will invade the population (Anderson & May 1982). It can be shown mathematically that an optimal strategy associated with a finite non-zero virulence exists if virulence increases more rapidly than transmission with viral accumulation (Anderson & May 1982; Ewald 1983; Alizon et al. 2009). Note that, in the case of vector-borne diseases, other components of R0, such as the vector feeding rate or the incubation time, may depend on viral accumulation in the vector. This means that the trade-off and the existence of an optimal strategy could also occur through other pleiotropic effects of the viral accumulation rate. More generally, the optimal level of virulence is determined by how the variables affecting R0 depend on the accumulation rate.
Notwithstanding that the trade-off hypothesis is a cornerstone of the theory of virulence evolution, empirical data are scarce. Arguably the major difficulty that could explain the lack of evidence originates from the estimation of the transmission rate. This is maybe why several of the studies that manage to measure the correlation between parasite density and virulence and parasite density and transmission are based on vector-borne diseases, because transmission success to the vector can be estimated experimentally. Studies on rodent malaria (Mackinnon & Read 1999) or on myxoma virus in rabbits (Dwyer et al. 1990) found that parasite density was positively correlated to both virulence and transmission. Analyses by Dwyer et al. (1990) and Bolker et al. (2010) also indicate that there is an optimal level of virulence for the myxoma virus. However, studies on rodent malaria have failed to go beyond a mere correlation between virulence and transmission and find an optimal virulence (see Mackinnon & Read 1999 for a review). Maybe this is because the second part of the parasite life cycle (in the vector) plays an important role. Another possibility to bypass the difficulty to estimate transmission is to consider spore-producing pathogens. De Roode et al. (2008) studied a protozoa infecting the monarch butterfly and showed that parasite load was positively correlated with transmission to the offspring and with virulence. They also showed that there is a spore load that maximizes the infection fitness (or R0), which indicates an optimal virulence. Jensen et al. (2006) showed that the total number of spores produced during an infection of Daphnia magna by the bacteria Pasteuria ramosa is maximized by intermediate virulence. However, because they did not explicitly investigate the correlations between parasite load and virulence or transmission, their result cannot be viewed as a direct test of the trade-off hypothesis. Finally, as far as we are aware, the only directly transmitted parasite for which these relationships have been addressed is HIV: Fraser et al. (2007) found that set-point viral load was positively correlated with virulence and with transmission and that there was an optimal virulence (which corresponds to what is observed in vivo). Several reasons can explain that some studies ‘only’ showed that virulent genotypes tend to be more transmissible but were unable to reach a conclusion regarding the existence of an optimal virulence (e.g. Mackinnon & Read 1999). For instance, even if variation in virulence is obtained by considering different parasite genotypes, host variability can blur the trade-off relationship making it difficult to determine the optimal virulence. Also, important variations in virulence can occur during the growing phase of an epidemic, which further contributes to blur the trade-off relationship for emerging pathogens (Bolker et al. 2010). Finally, the trade-off hypothesis is often criticized through some cases where virulence is clearly not adaptive for the parasite (Levin & Bull 1994). However, these cases can usually be understood through within-host competition (a virulent strain is favoured within a host but disfavoured between hosts). The fact that other processes, in addition to the trade-off, govern virulence evolution does not invalidate the trade-off hypothesis in itself. Overall, the scarcity of empirical data seems to be the main problem and it should be taken as a motivation for the collection of further evidence.
3. Viral accumulation versus transmission rate
Transmission rate is measured as the percentage of plants that become infected after inoculation of viral particles by vector(s) that have fed previously on infected plants. Typically, experimentalists measuring transmission rates allow vectors to acquire viruses from an infected plant before placing one (or a group) of viruliferous vectors on healthy plants. Viral accumulation is measured as viral load in infected leaf extracts, quantified mainly through techniques involving (i) antibodies targeting viral proteins (ELISA, western blot, etc) or (ii) detection of viral nucleic acids (real-time PCR, northern or Southern blot, etc). In order to obtain a correlation between viral accumulation and transmission rate, researchers need to vary the viral quantity acquired by vectors. To our knowledge, this has been achieved by four procedures. In a first procedure (denoted ‘in vitro’ in table 2), virions are purified from infected plants and diluted in an artificial diet medium. Vectors trapped in a feeding cage covered at one end by a stretched parafilm membrane are then fed with different dilutions of virions (Pirone & Megahed 1966; Gera et al. 1979; Pirone 1981; van den Heuvel et al. 1991; Ng et al. 2004; Ng & Falk 2006). Following acquisition feeding, vectors are transferred to uninfected plants for subsequent inoculation. The remaining procedures are denoted as ‘in planta’ in table 2. In a second procedure, vectors are allowed to acquire viral particles on different host genotypes varying in their resistance levels to the virus (Romanow et al. 1986; Gray et al. 1993, 1994; Lapidot et al. 2001; Wintermantel et al. 2008). A third procedure consists of allowing vectors to feed on infected leaves of different ages harboring different levels of virus accumulation (Gray et al. 1991; van den Heuvel et al. 1991). In a fourth procedure, vectors are allowed to acquire viral particles on plants harboring different levels of viruses owing to differential growth conditions (De Bokx et al. 1978; Dusi & Peters 1999) or viral genotypes differing in their accumulation (Banik & Zitter 1990).
Table 2.
Correlation between viral accumulation and transmission rate of vector-borne plant viruses.
| transmission rateb |
|||||||
|---|---|---|---|---|---|---|---|
| virus | mode of transmissiona | βp→v | βv→p | (βp→v × βv→p) | assay | origin of variation | references |
| CMV | NC | + | in vitro | dilution | Pirone & Megahed (1966); Gera et al. (1979) | ||
| CMV | NC | + | in planta | differential viral accumulation in planta | Banik & Zitter (1990) | ||
| PVYN | NC | + | in planta | growing conditions | De Bokx et al. (1978) | ||
| PVY | NC | + | in vitro | dilution | Pirone (1981) | ||
| WMV2 | NC | + | in planta | levels of host resistance | Romanow et al. (1986) | ||
| BtMV | NC | + | in planta | season | Dusi & Peters (1999) | ||
| LIYV | NC | + | in vitro | dilution | Ng & Falk 2006; Ng et al. (2004) | ||
| TICV or ToCV | NC | + | in planta | levels of host resistance | Wintermantel et al. (2008) | ||
| PLRV | C | + | + | in vitro | dilution | van den Heuvel et al. (1991) | |
| PLRV | C | + | + | in planta | leaf age | van den Heuvel et al. (1991) | |
| BYDV | C | + | + | in planta | levels of host resistance | Gray et al. (1991, 1993, 1994) | |
| TSWV | P | + | in planta | accumulation in vector | Rotenberg et al. (2009) | ||
| TYLCV | P | + | in planta | levels of host resistance | Lapidot et al. (2001) | ||
aViruses transmitted in a non-circulative (NC), circulative (C) or propagative (P) manner.
bA (+) indicates a positive correlation between the measured trait and viral accumulation. Empty cells indicate that the relationship has not been measured.
A substantial part of the phytopathological literature deals with studies on the phenotypic expression of point mutation(s) introduced in vitro into infectious viral clones or isolated randomly from a viral population (for a review, see Blanc 2008). Results from such ‘engineered’ viruses provide us mostly with information concerning molecular mechanisms involved in transmission. They also reveal the phenotypic effects of mutations prior to any action of natural selection in the wild. They can thus inform us on whether a particular type of relationship between, e.g. viral accumulation and transmission is ‘hardwired’ and arises necessarily owing to genomic constraints. Interestingly, studies reporting the effect of one or few mutation(s) do not show a positive correlation between transmission and viral accumulation. For instance, different mutations induce a loss of transmission associated with a decrease, an increase or no modification in viral accumulation (Gera et al. 1979; Bruyère et al. 1997; Escriu et al. 2000a,b; Gal-On 2007). We now turn to studies based on viral populations collected in the field.
Transmission of vector-borne plant viruses is achieved through different routes depending on virus genera. Modes of transmission are briefly summarized below and in table 1 and figure 2 (for reviews, see Gray & Banerjee 1999; Ng & Falk 2006; Hogenhout et al. 2008). The studies reviewed here on the relationship between viral accumulation and transmission are summarized in table 2. We discuss modes of transmission because these are likely to impact the overall transmission rate (βp→v and βv→p) and thus potentially affect the evolution of virulence.
Table 1.
Mode of transmission of plant viruses.
| mode of transmission | interaction with the vector | viral genera examples | transmission from infected plant to vector (βp→v) | transmission from viruliferous vector to healthy plant (βv→p) | time during which vectors are viruliferous | example references |
|---|---|---|---|---|---|---|
| non-circulative | restricted to mouth parts | Potyvirus, Cucumovirus | minutes | minutes to hoursa | minutes to hoursa | Palacios et al. (2002); Kalleshwaraswamy & Kumar (2008) |
| circulative | interaction with haemocoel | Luteovirus | minutes to a few hours | days to weeks | days to weeks | Gray et al. (1991) |
| propagative | replication within the vector | Tospovirus, Tenuivirus | minutes to a few hours | days to weeks | vector lifespan | Mehta et al. (1994) |
aException: Nepovirus.
Figure 2.
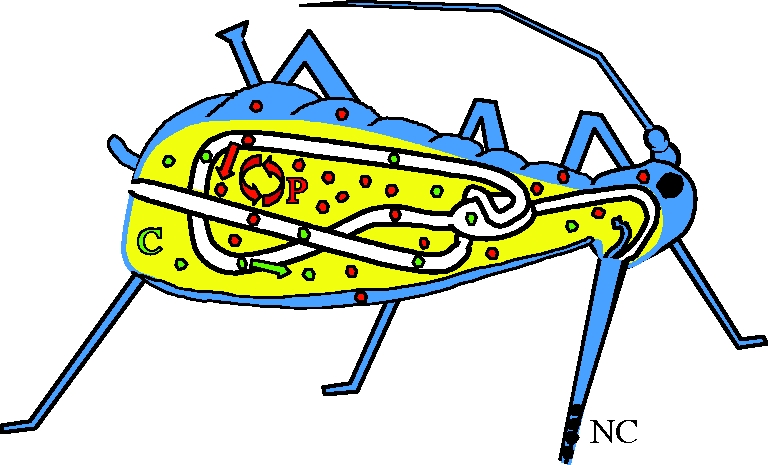
Schematic representation of modes of transmission by vectors. Viruses transmitted in a non-circulative (NC) manner (black) are restricted to the mouth parts. Viruses transmitted in a circulative (C) manner (green) enter the haemocoel (yellow) by endocytosis–exocytosis from the gut, do not replicate in the vector and usually enter the salivary glands from the haemolymph. Viruses transmitted in a propagative (P) manner (red) replicate in different organs of the vectors and may enter the salivary glands either from the haemolymph or from other connecting tissues, e.g. the nervous system or trachea (reviewed in Ng & Falk 2006; Hogenhout et al. 2008). Figure modified from Brault et al. (in press).
Some viruses interact only with the vector's exterior mouth parts (e.g. stylets—the outermost needle-like mouth parts of aphids and other piercing–sucking insects); in such cases, transmission is termed ‘non-circulative’ (Harris 1977). The time between the acquisition of such a virus by a vector from an infected plant and inoculation to another plant ranges from minutes (e.g. CMV) to hours (e.g. BtMV) and even, exceptionally, years (nepoviruses are retained in nematode mouth parts; Demangeat et al. 2005). No transmission occurs after viruliferous vectors have transmitted all the viral particles retained in their mouth parts (Yuan & Ullman 1996; Kalleshwaraswamy & Kumar 2008). The period during which vectors remain viruliferous is very short. This is due partly to the fact that most viral particles are lost (and thus never transmitted) a few minutes after the acquisition period even without feeding behaviour (Palacios et al. 2002; Kalleshwaraswamy & Kumar 2008). These findings also indicate that few viral particles are retained and inoculated by vectors in the case of non-circulating viruses (Ali et al. 2006; Moury et al. 2007; Betancourt et al. 2008).
Another group of viruses ‘circulate’ through the insect haemocoel and reach the salivary glands, allowing them to be inoculated to new plants without reproducing within the vector. Such viruses seem to hijack a constitutive endocytosis–exocytosis mechanism without greatly perturbing cell metabolism (Hogenhout et al. 2008; Brault et al. 2010). To be transmitted, these viruses require a relatively long acquisition time compared with non-circulative viruses (ranging from hours to days) and a latent time, i.e. the time necessary for viral particles to enter the haemocoel from the gut and migrate to the salivary glands, ranging from hours to days. Contrary to viruses transmitted in a non-circulative manner, the time period during which vectors remain viruliferous depends on the vector lifespan and on the amount of viral particles ingested, which is positively correlated to acquisition time (van den Heuvel et al. 1991; Reynaud & Peterschmitt 1992) and viral accumulation in planta (Chen & Gilbertson 2008).
Finally, a variant of the previous mode of transmission is ‘circulative–propagative’ transmission, where the virus replicates in the vector (e.g. TSWV). For some of these viruses, transovarial transmission to vector offspring has been described (e.g. MStV; Ammar et al. 1995). Acquisition of viral particles by vectors can be achieved after a few minutes of feeding on an infected host plant (TYLCV; Mehta et al. 1994; Czosnek et al. 2001). However, inoculation of ingested viruses to another plant will occur only after a latent period, which, depending on virus genus, varies from 24 h (TYLCV; Mehta et al. 1994) to more than one week (MStV; Ammar et al. 1995). As with viruses transmitted in a circulative manner, the period during which vectors remain viruliferous depends on vector lifespan. The main difference between circulative and propagative viruses is that, in the latter case, virus load in the vector increases during the first weeks following infection because of virus replication in the vector. Finally, the transmission rate tends to decrease as vectors get older, probably owing to modification of their feeding behaviour (TYLCV; Czosnek et al. 2001). Notably, TYLCV represents a special case in classification, since it is usually considered to be transmitted in a circulative manner (Hogenhout et al. 2008) but has been shown to replicate within its vector (Czosnek et al. 2001).
Inspection of table 2 shows that, in all cases, the correlation between viral accumulation and transmission, be it βp→v, βv→p or the overall transmission rate (βp→v × βv→p), is positive. This contrasts with the results on ‘engineered’ viruses where no correlation between viral accumulation and transmission rate arises, indicating that the positive correlation observed in field samples does not result from genomic constraints. Clearly more relevant studies are needed, especially for the circulative and propagative transmission modes.
4. Viral accumulation versus virulence
In the case of vector-borne diseases, parasite density can affect virulence in both the host αp(x) and the vector αv(y). In the following section, we report empirical evidence on the correlation between viral accumulation and virulence in both plant and vector. Note that virulence here is estimated through disease-induced mortality. In this, we follow the majority of studies, because fitness components other than survival do not directly affect the R0 of a horizontally transmitted parasite. Some of the studies we discuss report the effects of virus infection on a host fitness component that might be correlated with host survival, and therefore these studies should be interpreted with caution.
(a). Effect of viral accumulation on components of plant fitness
We searched for empirical studies reporting both viral accumulation and accurate measures of components of virulence (i.e. related to survival rate) for several viral genotypes infecting (at least) one host genotype. As for transmission phenotypes, a large number of studies consider the phenotype resulting from point mutation(s) introduced in vitro or isolated randomly from viral populations. Reports yield conflicting results. Viral clones differing by only one point mutation present contrasting phenotypes: some studies report a negative correlation between viral accumulation and the dry weight of the host plant (Poulicard et al. 2010). For some other viral mutants, higher accumulation is associated with phenotypes with more attenuated symptoms (Rodriguez-Cerezo et al. 1991), or with symptoms similar to (Bruyère et al. 1997) or more severe than (Gal-On 2007) those of the wild-type. Note that scientists and plant breeders often use symptoms as an indicator of the effect of viruses on host fitness. Although this can actually be the case when infections provoke host stunting, or nanism or other leaf disorders (Jarosz & Davelos 1995; Zaitlin & Hull 2003), it should be remembered that symptoms might not always be a good proxy, especially when host species harbour genes involved in parasite tolerance (Hoffman & Kolb 1998). Even if no general trend arises from results concerning viral mutant genotypes, such studies demonstrate the existence of variation in the relationships between viral accumulation and virulence or symptom severity.
Unfortunately, studies that reported the phenotype of natural isolates often measured only virulence (Yahara & Oyama 1993; Lapidot et al. 1997; Maskell et al. 1999; Funayama-Noguchi 2001; Malmstrom et al. 2005, 2006) or viral accumulation (Fargette et al. 1987; Llamas-Llamas et al. 1998; Thurston et al. 2001; Untiveros et al. 2007; Wintermantel et al. 2008) of one viral isolate in one or several host genotypes in different environments. To our knowledge, only a handful of studies have measured both phenotypes concomitantly for several viral natural isolates after individual inoculation of one host genotype. These studies estimated within-host viral accumulation induced by several natural isolates, some inducing mild, some intermediate and some severe symptoms. Several host–virus interactions exhibited positive correlations: PNRSV–Rosa indica (Moury et al. 2001), RYMV–Oryza sativa japonica cv Azucena (Fargette et al. 2002), CMV infecting Nicotiana glutinosa or N. tabacum (Shi et al. 2002) or Cucumis sativus (Banik & Zitter 1990), PRSV infecting Cucurbita pepo or Citrullus lanatus (Pacheco et al. 2003), BSV–Musa accuminata (Dahal et al. 1998). The relative limitation of these studies comes from the fact that only a few isolates are tested, and, as mentioned previously, symptoms may not be a good proxy for host survival. One of the reasons explaining the scarcity of suitable studies is that sometimes the relationship was not investigated at the level of the relevant virulence components for horizontally transmitted parasites, e.g. those linked to duration of infection. For instance, several studies looked at the relationship between fecundity and viral accumulation.
Moreover, the relationship between viral accumulation and virulence may be obscured by potential heterogeneity of the hosts with respect to interactions with pathogens. Indeed, plants can be resistant, tolerant and/or susceptible to parasite infection. Resistance is the ability of the host to limit parasite burden, and tolerance is the ability to limit the harm caused by a given burden (Cooper & Jones 1983). Heterogeneity in host plants with respect to tolerance/resistance may blur the correlations between viral accumulation and either virulence or transmission. For instance, interactions between PNRSV–Prunus spp. (Moury et al. 2001) and PRSV–Cucurbita mochata (Pacheco et al. 2003) did not lead to any correlation, probably because some viral isolates accumulated to a high titre in plants, but induced only few or no symptoms. This poses an important methodological problem, as discussed further in the last section.
Empirical data supporting the existence of a correlation between within-host viral accumulation and virulence are scarce. The few studies mentioned above indicate the existence of a positive correlation between virulence and viral accumulation, but of course many more studies, as well as studies looking more directly at the relevant virulence parameters, are needed before a general conclusion can be reached. The effect of host heterogeneity on resistance/tolerance mechanisms and the nature of the observed relationship between viral accumulation and virulence deserve further study.
(b). Effect of viral infection on the vector
The only studies we found on this subject report the qualitative effects of viral infection on transmission through modification of vector behaviour/physiology.
Vector mobility certainly plays a role in the variation of the contact rate between vector and host, thus affecting the probability of virus transmission (Fereres & Moreno 2009). Such variation in mobility has been reported for vectors infected by a circulative virus: these insects exhibit more movement around experimental arenas than uninfected controls, causing a slight increase in the number of infected host plants (Hodge & Powell 2008). Interestingly, enhanced plant penetration by aphid stylets was observed on symptomatic virus-infected plants compared with healthy plants (Alvarez et al. 2007). Moreover, it has long been known that aphids are significantly attracted by yellow (Doring et al. 2009) and that some plant viruses induce symptoms resulting in chlorosis, mosaics or other modifications that lead to yellowing of the leaves. Aphids have been shown to prefer to land and settle on virus-infected plants with yellowing leaves, possibly owing to the fact that insect vectors use visual cues to choose suitable plants (Fereres et al. 1999; Hodge & Powell 2008). Insect vectors also choose plants in response to olfactory cues, and the composition of the volatile organic compounds emitted by the leaves of some plants has been shown to change when the plant is infected by viruses (Ngumbi et al. 2007; Medina-Ortega et al. 2009). These changes can be more or less pronounced. For instance, plants infected by a circulative virus tend to attract their aphid vectors more than plants infected either by non-circulative viruses or by non-vectored viruses or healthy plants (Eigenbrode et al. 2002). However, this pattern seems to depend on the host–virus interaction, since no such differences were observed on other plants and virus species (Castle et al. 1998; Hodge & Powell 2008).
For several plant virus vectors reared on infected plants, studies have shown an increase in components of vector fitness such as survival or fecundity (Hunt & Nault 1990; Blua et al. 1994; Mayer et al. 2002; Belliure et al. 2005; Maris et al. 2007; Sisterson 2008). Such increases result from various mechanisms. In one case, they were due to reduced developmental time of larvae reared on infected plants, which resulted in a shorter period of vulnerability to predation (Belliure et al. 2008). In other cases, the increase was due to the fact that infected plants had increased amino acid contents in phloem sap and decreased honeydew production compared with healthy plants (BYDV, Ajayi 1986; ZYMV, Blua et al. 1994). The latter interpretation is reinforced by the fact that vectors exhibited increased fitness when reared on plants infected by non-vector-borne viruses compared with when reared on healthy plants (Hare & Dodds 1987; Musser et al. 2003). Increased performance of insects reared on some virus-infected plants might thus be due to better availability of nutrients in infected plants. Such effects, however, are not universal, and decreases in vector fitness owing to infection have also been reported (Ellsbury et al. 1985; Power 1992; Sinisterra et al. 2005; Inoue & Sakurai 2006; Hodge & Powell 2008; Matsuura & Hoshino 2009).
Previous studies indicate that vectors settle preferentially on infected plants because of visual or olfactory cues expressed by these plants. Some virus–host interactions lead to an increase in some components of vector fitness. Moreover, infected vectors tend to exhibit increased mobility, which should lead to increase feeding rates. All these processes enhance vector-borne virus transmission, leading to the modification of the basic reproduction ratio R0 (see McElhany et al. (1995) for discussion). Unfortunately, data currently available do not allow us to detect the potential differential effects of viral transmission modes.
5. Concluding remarks
Understanding why parasites harm their hosts is an old question in evolutionary biology. Arguably, one of the most appealing answers comes from the trade-off hypothesis, which states that parasite transmission and virulence are correlated, such that increased transmission leads to shorter infection times (Anderson & May 1982; Ewald 1983). This correlation arises because both transmission and virulence are increasing functions of parasite within-host density.
In the present review, our aim was to investigate whether existing empirical evidence supports the hypothesis that virulence and transmission of vector-borne plant viruses are linked through a common variable, i.e. within-plant viral accumulation. On the one hand, all studies that have looked at the correlation between within-plant viral accumulation and transmission rate, or one of its components, found a positive relationship (table 2). Undoubtedly, more studies are needed on this issue, especially on circulative and propagative viruses; however, the unanimity of positive correlations suggests that this relationship will most probably be confirmed. We were surprised by the paucity of studies on the relationship between viral accumulation and virulence components relevant for testing the trade-off hypothesis. The few studies we identified suffer from their own limitations: disease symptoms were used as a proxy for virulence, and the number of isolates studied was small. The existence, or not, of this correlation thus remains unresolved. At present, we can say only that the existing evidence supports the first part of the trade-off hypothesis, i.e. the correlation between transmission and within-host accumulation, but is inconclusive as to the second part, the virulence–within-host accumulation correlation. Consequently, we cannot conclude whether the trade-off hypothesis holds or not in plant viruses.
It is important to realize that testing the trade-off hypothesis implies more than ‘simply’ establishing the two correlations discussed throughout this paper. One must also show that an optimal virulence level indeed exists. Theory predicts that if both virulence and transmission increase linearly with viral accumulation, virus populations should evolve towards ever higher levels of virulence. As mentioned in §2, an optimal virulence level is evolutionarily stable only if the rate of increase of virulence with viral accumulation is faster than the rate of increase of transmission with viral accumulation. This is difficult to validate in most host–parasite systems (Alizon et al. 2009). In the case of vector-borne plant viruses, a promising option would be to try to show that the transmission rate saturates for high virus accumulation rates within the plant because of the limitation introduced by the vector (Alizon & van Baalen 2008). Such a saturating transmission function combined with, for example, linearly increasing virulence would lead to an intermediate optimal level of viral accumulation.
When addressing this issue, it is thus important to measure all three key parameters (viral accumulation, virulence and transmission) concomitantly for several viral isolates on the same host plant genotype. We feel it is important to clarify two points. The first relates to the relevant virulence components one needs to measure, and the second to the potential effects of host heterogeneity.
Virulence components relevant for testing the trade-off hypothesis are components that directly affect parasite fitness. For horizontally transmitted parasites, such components should typically be related to the duration of the infection, as this determines the time during which the parasite can be transmitted. Host survival is an obvious trait relevant to the duration of infection. In contrast, host fecundity is, a priori, not directly relevant, except if it is linked to survival through a survival–reproduction trade-off. Obviously, fecundity would be a trait directly relevant for vertically transmitted parasites.
Host heterogeneity for tolerance/resistance may strongly affect the virulence–accumulation correlation. Resistance (by definition) affects viral accumulation: if a parasite replicates less in one host genotype than in another, then the former can be said to be more resistant. Tolerance affects the relationship between virulence and accumulation: one host genotype is said to be more tolerant than another if it suffers less from infection, i.e. if virulence is lower, for a given parasite load (Raberg et al. 2009). Comparisons of the correlations between virulence, accumulation and transmission across hosts differing in resistance do not pose any specific problem for testing the trade-off hypothesis: resistance should affect directly only accumulation, and, if the trade-off hypothesis holds, more resistant plants should have lower viral loads, lower transmission and lower virulence than less resistant plants. Variation in tolerance across plants would not pose a problem in measuring the accumulation–transmission correlation, assuming that transmission depends only on accumulation (and not on virulence, for example). It would pose a problem for measuring the accumulation–virulence correlation, however, since, by definition, tolerance affects this relationship. Thus, ignoring such variations in tolerance may mask positive correlations. Therefore, it should be realized that measuring this correlation across plants of different tolerance levels would not be an appropriate test of the trade-off hypothesis. The existence of resistance/tolerance presents no challenge to the trade-off hypothesis per se. When such variation exists, it should be acknowledged. The trade-off could be tested by controlling for the level of resistance/tolerance. For example, one could compare the virulence of different viral isolates that reach different accumulation levels on hosts with a known level of tolerance.
The correlation between viral accumulation, transmission rate and virulence also calls for more theoretical approaches. One possibility would be to investigate how the mode of vector-borne transmission (propagative, circulative or non-circulative) can affect the evolution of virulence. For instance, a key difference would be that, in the case of a virus transmitted in a propagative manner, once a vector is infected, it remains viruliferous until it dies. In the case of a virus transmitted in a circulative manner, however, the period during which a vector remains infected is likely to be correlated with the amount of virus it ingests. Finally, for viruses transmitted in a non-circulative manner, very few viral particles are retained in vector mouth parts, and particles are lost very quickly. These biological facts can be expressed mathematically by varying the transmission function. We believe that such differences in life cycles should lead to different expressions of R0 and thus to different predictions in terms of optimal virulence levels.
Transmission modes of vector-borne animal viruses share some similarities with plant viruses. In fact, vector transmission of animal viruses falls into two classes: ‘mechanical’ and ‘biological’. Mechanical transmission does not involve any replication inside the vector and is exemplified by, for instance, myxoma virus (Barcena et al. 2000) or lumpy skin disease virus (Chihota et al. 2003). Transmission success seems to be vector-specific as some vector species failed to transmit lumpy skin disease virus in recent studies (Chihota et al. 2003), although they were able to transmit other pox viruses (Mellor et al. 1987). This specificity for successful transmission strikingly resembles the case of non-circulative transmission in plant viruses. Biological transmission implies reproduction inside the vector, which applies to the vast majority of vector-borne vertebrate-infecting viruses (arboviruses), which are responsible for a number of severe diseases in humans (yellow fever, dengue, various encephalitides, etc.) and livestock (West Nile encephalomyelitis, Rift Valley fever, vesicular stomatitis, etc.). When the characteristics of these viruses are compared with those of plant viruses, several common features appear between all the latter viruses and plant viruses with a circulative–propagative mode of transmission. Further investigations of the parallels between vector-borne animal and plant viruses concerning the correlation between viral accumulation, transmission rate and virulence could be particularly helpful in revealing general patterns for such diseases. Finally, the circulative transmission mode seems to be a distinctive feature of plant viruses. Understanding why this is the case may help us understand how viruses colonize and exploit vectors to increase their transmission.
List of viral acronyms: banana streak virus (BSV, Badnavirus), barley yellow dwarf virus (BYDV, Luteovirus), beet mild curly top virus (BMCTV, Curtovirus), beet mosaic virus (BtMV, Potyvirus), beet severe curly top virus (BSCTV, Curtovirus), cucumber mosaic virus (CMV, Cucumovirus), lettuce infectious yellows virus (LIYV, Crinivirus), maize streak virus (MSV, Mastrevirus), maize stripe virus (MStV, Tenuivirus), papaya ringspot virus (PRSV, Potyvirus), potato leafroll virus (PLRV, Polerovirus), potato virus Y (PVY, Potyvirus), prunus necrotic ringspot virus (PNRSV, Ilarvirus), ribgrass mosaic virus (RMV, Tobamovirus), rice yellow mottle virus (RYMV, Sobemovirus), tomato chlorosis virus (ToCV, Crinivirus), ryegrass mosaic virus (RMV, Rymovirus), tomato infectious chlorosis virus (TiCV, Crinivirus), tomato spotted wild virus (TSWV, Tospovirus), tomato yellow leaf curl virus (TYLCV, Begomovirus), turnip mosaic virus (TuMV, Potyvirus), watermelon mosaic virus (WMV2, Potyvirus).
Footnotes
One contribution of 14 to a Theme Issue ‘New experimental and theoretical approaches towards the understanding of the emergence of viral infections’.
References
- Ajayi O.1986The effect of Barley yellow dwarf virus on the amino acid composition of spring wheat. Ann. Appl. Biol. 108, 145–149 (doi:10.1111/j.1744-7348.1986.tb01975.x) [Google Scholar]
- Ali A., Li H., Schneider W. L., Sherman D. J., Gray S., Smith D., Roossinck M. J.2006Analysis of genetic bottlenecks during horizontal transmission of Cucumber mosaic virus. J. Virol. 80, 8345–8350 (doi:10.1128/JVI.00568-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizon S., Van Baalen M.2008Transmission–virulence trade-offs in vector-borne diseases. Theor. Popul. Biol. 74, 6–15 (doi:10.1016/j.tpb.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Alizon S., Hurford A., Mideo N., Van Baalen M.2009Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22, 245–259 (doi:10.1111/j.1420-9101.2008.01658.x) [DOI] [PubMed] [Google Scholar]
- Alvarez A. E., Garzo E., Verbeek M., Volman B., Dicke M., Tjallingii W. F.2007Infection of potato plants with Potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomol. Exp. Appl. 125, 135–144 (doi:10.1111/j.1570-7458.2007.00607.x) [Google Scholar]
- Ammar E. D., Gingery R. E., Madden L. V.1995Transmission efficiency of three isolates of Maize stripe tenuivirus in relation to virus titer in the planthopper vector. Plant Pathol. 44, 239–243 (doi:10.1111/j.1365-3059.1995.tb02774.x) [Google Scholar]
- Anderson R. M., May R. M.1982Coevolution of hosts and parasites. Parasitology 85, 411–426 (doi:10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M.1991Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press [Google Scholar]
- Banik M. T., Zitter T. A.1990Determination of Cucumber mosaic virus titer in muskmelon by enzyme-linked immunosorbent assay and correlation with aphid transmission. Plant Dis. 74, 857–859 (doi:10.1094/PD-74-0857) [Google Scholar]
- Barcena J., et al. 2000Horizontal transmissible protection against myxomatosis and rabbit hemorrhagic disease by using a recombinant myxoma virus. J. Virol. 74, 1114–1123 (doi:10.1128/JVI.74.3.1114-1123.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliure B., Janssen A., Maris P. C., Peters D., Sabelis M. W.2005Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70–79 (doi:10.1111/j.1461-0248.2004.00699.x) [Google Scholar]
- Belliure B., Janssen A., Sabelis M.2008Herbivore benefits from vectoring plant virus through reduction of period of vulnerability to predation. Oecologia 156, 797–806 (doi:10.1007/s00442-008-1027-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt M., Fereres A., Fraile A., Garcia-Arenal F.2008Estimation of the effective number of founders that initiate an infection after aphid transmission of a multipartite plant virus. J. Virol. 82, 12 416–12 421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc S.2008Vector transmission of plant viruses. In Encyclopedia of virology (eds Mahy B. W. J., Regenmortel M. H. V. V.). New York, NY: Academic Press [Google Scholar]
- Blua M. J., Perring T. M., Madore M. A.1994Plant virus-induced changes in aphid population development and temporal fluctuations in plant nutrients. J. Chem. Ecol. 20, 691–707 (doi:10.1007/BF02059607) [DOI] [PubMed] [Google Scholar]
- Bolker B. M., Nanda A., Shah D.2010Transient virulence of emerging pathogens. J. R. Soc. Interface 7, 811–822 (doi:10.1098/rsif.2009.0384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V., Tanguy S., Reinbold C., Le Trionnaire G., Arneodo J., Jaubert-Possamai S., Guernec G., Tagu D.2010Transcriptomic analysis of intestinal genes following acquisition of Pea enation mosaic virus by the pea aphid Acyrthosiphon pisum. J. Gen. Virol. 91, 802–808 (doi:10.1099/vir.0.012856-0) [DOI] [PubMed] [Google Scholar]
- Brault V., Uzest M., Monsion B., Jacquot E., Blanc S.In press Aphids as transport devices for plant viruses. C. R. Acad. Sci. Biologie. [DOI] [PubMed] [Google Scholar]
- Bruyère A., Brault V., Ziegler-Graff V., Simonis M. T., Van Den Heuvel J. F. J. M., Richards K., Guilley H., Jonard G., Herrbach E.1997Effects of mutations in the Beet western yellows virus readthrough protein on its expression and packaging and on virus accumulation, symptoms, and aphid transmission. Virology 230, 323–334 (doi:10.1006/viro.1997.8476) [DOI] [PubMed] [Google Scholar]
- Castle S. J., Mowry T. M., Berger P. H.1998Differential settling by Myzus persicae (Homoptera: Aphididae) on various virus infected host plants. Ann. Entomol. Soc. Am. 91, 661–667 [Google Scholar]
- Chen L.-F., Gilbertson R. L.2008Curtovirus–Cucurbit interaction: acquisition host plays a role in leafhopper transmission in a host-dependent manner. Phytopathology 99, 101–108 (doi:10.1094/PHYTO-99-1-0101) [DOI] [PubMed] [Google Scholar]
- Chihota C. M., Rennie L. F., Kitching R. P., Mellor P. S.2003Attempted mechanical transmission of lumpy skin disease virus by biting insects. Med. Vet. Entomol. 17, 294–300 (doi:10.1046/j.1365-2915.2003.00445.x) [DOI] [PubMed] [Google Scholar]
- Cooper J. I., Jones A. T.1983Responses of plants to viruses: proposals for the use of terms. Phytopathology 73, 127–128 (doi:10.1094/Phyto-73-127) [Google Scholar]
- Czosnek H., Ghanim M., Morin S., Rubinstein G., Fridman V., Zeidan M.2001Whiteflies: vectors, and victims (?), of geminiviruses. Adv. Virus Res. 57, 291–322 (doi:10.1016/S0065-3527(01)57006-2) [DOI] [PubMed] [Google Scholar]
- Dahal G., Pasberg-Gauhl C., Gauhl F., Thottappilly G., Hughes J. D. A.1998Studies on a Nigerian isolate of Banana streak badnavirus: II. Effect of intraplant variation on virus accumulation and reliability of diagnosis by ELISA. Ann. Appl. Biol. 132, 263–275 (doi:10.1111/j.1744-7348.1998.tb05202.x) [Google Scholar]
- D'arcy C. J., Eastburn D. M., Schumann G. L.2001Illustrated glossary of plant pathology. The plant health instructor. http://www.apsnet.org/education/IllustratedGlossary/ (doi:10.1094/PHI-I-2001-0219-01) [Google Scholar]
- De Bokx J. A., Van Hoof H. A., Piron P. G. M.1978Relation between concentration of potato virus Y(N) and its availability to Myzus persicae. Eur. J. Plant Pathol. 84, 95–100 (doi:10.1007/BF01981535) [Google Scholar]
- Demangeat G., Voisin R., Minot J.-C., Bosselut N., Fuchs M., Esmenjaud D.2005Survival of Xiphinema index in vineyard soil and retention of Grapevine fanleaf virus over extended time in the absence of host plants. Phytopathology 95, 1151–1156 (doi:10.1094/PHYTO-95-1151) [DOI] [PubMed] [Google Scholar]
- De Roode J. C., Yates A. J., Altizer S.2008Virulence–transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proc. Natl Acad. Sci. USA 105, 7489–7494 (doi:10.1073/pnas.0710909105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring T. F., Archetti M., Hardie J.2009Autumn leaves seen through herbivore eyes. Proc. Biol. Sci. 276, 121–127 (doi:10.1098/rspb.2008.0858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S., Shackelton L. A., Holmes E. C.2008Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genet. 9, 267–276 (doi:10.1038/nrg2323) [DOI] [PubMed] [Google Scholar]
- Dusi A. N., Peters D.1999Beet mosaic virus: its vector and host relationships. J. Phytopathol. 147, 293–298 (doi:10.1111/j.1439-0434.1999.tb03833.x) [Google Scholar]
- Dwyer G., Levin S. A., Buttel L.1990A simulation model of the population dynamics and evolution of myxomatosis. Ecol. Monogr. 60, 423–447 (doi:10.2307/1943014) [Google Scholar]
- Eigenbrode S. D., Ding H., Shiel P., Berger P. H.2002Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. B 269, 455–460 (doi:10.1098/rspb.2001.1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsbury M. M., Pratt R. G., Knight W. E.1985Effects of single and combined infection of arrowleaf clover with Bean yellow mosaic virus and a Phytophthora sp. on reproduction and colonization by pea aphids (Homoptera: Aphididae). Environ. Entomol. 14, 356–359 [Google Scholar]
- Escriu F., Fraile A., Garcia-Arenal F.2000aEvolution of virulence in natural populations of the satellite RNA of Cucumber mosaic virus.. Phytopathology 90, 480–485 (doi:10.1094/PHYTO.2000.90.5.480) [DOI] [PubMed] [Google Scholar]
- Escriu F., Perry K. L., Garcia-Arenal F.2000bTransmissibility of Cucumber mosaic virus by Aphis gossypii correlates with viral accumulation and is affected by the presence of its satellite RNA. Phytopathology 90, 1068–1072 (doi:10.1094/PHYTO.2000.90.10.1068) [DOI] [PubMed] [Google Scholar]
- Ewald P. W.1983Host–parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Evol. Syst. 14, 465–485 (doi:10.1146/annurev.es.14.110183.002341) [Google Scholar]
- Fargette D., Thouvenel J.-C., Fauquet C.1987Virus content of leaves of cassava infected by African cassava mosaic virus. Ann. Appl. Biol. 110, 65–73 (doi:org/10.1111/j.1744-7348.1987.tb03233.x) [Google Scholar]
- Fargette D., Pinel A., Traoré O., Ghesquière A., Konaté G.2002Emergence of resistance-breaking isolates of Rice yellow mottle virus during serial inoculations. Eur. J. Plant Pathol. 108, 585–591 (doi:10.1023/A:1019952907105) [Google Scholar]
- Fereres A., Moreno A.2009Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 141, 158–168 (doi:10.1016/j.virusres.2008.10.020) [DOI] [PubMed] [Google Scholar]
- Fereres A., Kampmeier G. E., Irwin M. E.1999Aphid attraction and preference for soybean and pepper plants infected with Potyviridae. Ann. Entomol. Soc. Am. 92, 542–548 [Google Scholar]
- Frank S. A.1996Models of parasite virulence. Q. Rev. Biol. 71, 37–78 (doi:10.1086/419267) [DOI] [PubMed] [Google Scholar]
- Fraser C., Hollingsworth T. D., Chapman R., De Wolf F., Hanage W. P.2007Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc. Natl Acad. Sci. USA 104, 17 441–17 446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froissart R., Roze D., Uzest M., Galibert L., Blanc S., Michalakis Y.2005Recombination every day: abundant recombination in a virus during a single multi-cellular host infection. PLoS Biol. 3, e89 (doi:10.1371/journal.pbio.0030089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama-Noguchi S.2001Ecophysiology of virus-infected plants: a case study of Eupatorium makinoi infected by geminivirus. Plant Biol. 3, 251–262 (doi:10.1055/s-2001-15199) [Google Scholar]
- Gal-on A.2007Zucchini yellow mosaic virus: insect transmission and pathogenicity: the tails of two proteins. Mol. Plant Pathol. 8, 139–150 (doi:10.1111/j.1364-3703.2007.00381.x) [DOI] [PubMed] [Google Scholar]
- Garcia-Arenal F., Fraile A., Malpica J. M.2001Variability and genetic structure of plant virus populations. Annu. Rev. Phytopathol. 39, 157–186 (doi:10.1146/annurev.phyto.39.1.157) [DOI] [PubMed] [Google Scholar]
- Gera A., Loebenstein G., Raccah B.1979Protein coats of two strains of Cucumber mosaic virus affect transmission by Aphis gossypii. Phytopathology 69, 396–399 (doi:10.1094/Phyto-69-396) [Google Scholar]
- Gray S. M., Banerjee N.1999Mechanisms of arthropod transmission of plant and animal viruses. Microbiol. Mol. Biol. Rev. 63, 128–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S. M., Power A. G., Smith D. M., Seaman A. J., Altman N. S.1991Aphid transmission of Barley yellow dwarf virus: acquisition access periods and virus concentration requirements. Phytopathology 81, 539–545 (doi:10.1094/Phyto-81-539) [Google Scholar]
- Gray S. M., Smith D., Altman N. S.1993Barley yellow dwarf virus isolate-specific resistance in spring oats reduced virus accumulation and aphid transmission. Phytopathology 83, 716–720 (doi:10.1094/Phyto-83-716) [Google Scholar]
- Gray S. M., Smith D., Sorrells M.1994Reduction of disease incidence in small field plots by isolate-specific resistance to Barley yellow dwarf virus. Phytopathology 84, 713–718 (doi:10.1094/Phyto-84-713) [Google Scholar]
- Hare D. J., Dodds J. A.1987Survival of the Colorado potato beetle on virus-infected tomato in relation to plant nitrogen and alkaloid content. Entomol. Exp. Appl. 44, 31–35 (doi:10.1007/BF00361306) [Google Scholar]
- Harris K. F.1977An ingestion–egestion hypothesis of non circulative virus transmission. In Aphids as virus vectors (eds Harris K. F., Maramorosch K.). New York, NY, Academic Press [Google Scholar]
- Hodge S., Powell G.2008Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environ. Entomol. 37, 1573–1581 (doi:10.1603/0046-225X-37.6.1573) [DOI] [PubMed] [Google Scholar]
- Hoffman T. K., Kolb F. L.1998Effects of Barley yellow dwarf virus on yield and yield components of drilled winter wheat. Plant Dis. 82, 620–624 (doi:10.1094/PDIS.1998.82.6.620) [DOI] [PubMed] [Google Scholar]
- Hogenhout S. A., Ammar E.-D., Whitfield A. E., Redinbaugh M. G.2008Insect–vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46, 327–359 (doi:10.1146/annurev.phyto.022508.092135) [DOI] [PubMed] [Google Scholar]
- Hull R.2001Matthews' plant virology San Diego, CA: Academic Press [Google Scholar]
- Hunt R. E., Nault L. R.1990Influence of life history of grasses and Maize chlorotic dwarf virus on the biotic potential of the leafhopper Graminella nigrifrons (Homoptera: Cicadellidae). Environ. Entomol. 19, 76–84 [Google Scholar]
- Inoue T., Sakurai T.2006Infection of Tomato spotted wilt virus (TSWV) shortens the life span of thelytokous Thrips tabaci (Thysanoptera: Thripidae). Appl. Entomol. Zool. 41, 239–246 (doi:10.1303/aez.2006.239) [Google Scholar]
- Jarosz A. M.2002Virulence management in plant–pathogen interactions. In Adaptive dynamics of infectious diseases: in pursuit of virulence management (eds Dieckmann U., Metz J. A., Sabelis M. W., Sigmund K.). Cambridge, UK: Cambridge University Press [Google Scholar]
- Jarosz A. M., Davelos A. L.1995Effects of disease in wild plant populations and the evolution of pathogen aggressiveness. New Phytol. 129, 371–387 (doi:10.1111/j.1469-8137.1995.tb04308.x) [Google Scholar]
- Jensen K. H., Little T. J., Skorping A., Ebert D.2006Empirical support for optimal virulence in a castrating parasite. PLoS Biol. 4, e197 (doi:10.1371/journal.pbio.0040197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalleshwaraswamy C. M., Kumar N. K. K.2008Transmission efficiency of Papaya ringspot virus by three aphid species. Phytopathology 98, 541–546 [DOI] [PubMed] [Google Scholar]
- Lapidot M., Friedmann M., Lachman O., Yehezkel A., Nahon S., Cohen S., Pilowsky M.1997Comparison of resistance level to Tomato yellow leaf curl virus among commercial cultivars and breeding lines. Plant Dis. 81, 1425–1428 (doi:10.1094/PDIS.1997.81.12.1425) [DOI] [PubMed] [Google Scholar]
- Lapidot M., Friedmann M., Pilowsky M., Ben-Joseph R., Cohen S.2001Effect of host plant resistance to Tomato yellow leaf curl virus (TYLCV) on virus acquisition and transmission by its whitefly vector. Phytopathology 91, 1209–1213 (doi:10.1094/PHYTO.2001.91.12.1209) [DOI] [PubMed] [Google Scholar]
- Levin B. R.1996The evolution and maintenance of virulence in microparasites. Emerge. Infect. Dis. 2, 93–102 (doi:10.3201/eid0202.960203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. R., Bull J. J.1994Short-sighted evolution and the virulence of pathogenic microorganisms. Trends Microbiol. 2, 76–81 (doi:10.1016/0966-842X(94)90538-X) [DOI] [PubMed] [Google Scholar]
- Llamas-Llamas M. E., Zavaleta-Mejia E., Gonzalez-Hernandez V. A., Cervantes-Diaz L., Santizo-Rincon J. A., Ochoa-Martinez D. L.1998Effect of temperature on symptom expression and accumulation of Tomato spotted wilt virus in different host species. Plant Pathol. 47, 341–347 (doi:10.1046/j.1365-3059.1998.00249.x) [Google Scholar]
- Mackinnon M. J., Read A. F.1999Selection for high and low virulence in the malaria parasite Plasmodium chabaudi. Proc. R. Soc. B 266, 741–748 (doi:10.1098/rspb.1999.0699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom C. M., Hughes C. C., Newton L. A., Stoner C. J.2005Virus infection in remnant native bunchgrasses from invaded California grasslands. New Phytol. 168, 217–230 (doi:10.1111/j.1469-8137.2005.01479.x) [DOI] [PubMed] [Google Scholar]
- Malmstrom C., Stoner C., Brandenburg S., Newton L.2006Virus infection and grazing exert counteracting influences on survivorship of native bunchgrass seedlings competing with invasive exotics. J. Ecol. 94, 264–275 (doi:10.1111/j.1365-2745.2006.01101.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris P. C., Joosten N. N., Goldbach R. W., Peters D.2007Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 94, 706–711 (doi:10.1094/PHYTO.2004.94.7.706) [DOI] [PubMed] [Google Scholar]
- Maskell L. C., Raybould A. F., Cooper J. I., Edwards M.-L., Gray A. J.1999Effects of Turnip mosaic virus and Turnip yellow mosaic virus on the survival, growth and reproduction of wild cabbage (Brassica oleracea). Ann. Appl. BioI. 135, 401–407 (doi:10.1111/j.1744-7348.1999.tb00867.x) [Google Scholar]
- Matsuura S., Hoshino S.2009Effect of Tomato yellow leaf curl disease on reproduction of Bemisia tabaci Q biotype (Hemiptera: Aleyrodidae) on tomato plants. Appl. Entomol. Zool. 44, 143–148 (doi:10.1303/aez.2009.143) [Google Scholar]
- Mayer R. T., Inbar M., Mckenzie C. L., Shatters R., Borowicz V., Albrecht U., Powell C. A., Doostdar H.2002Multitrophic interactions of the silverleaf whitefly, host plants, competing herbivores, and phytopathogens. Arch. Insect. Biochem. Physiol. 51, 151–169 (doi:10.1002/arch.10065) [DOI] [PubMed] [Google Scholar]
- Mcelhany P., Real L. A., Power A. G.1995Vector preference and disease dynamics: a study of Barley yellow dwarf virus. Ecology 76, 444–457 (doi:10.2307/1941203) [Google Scholar]
- Medina-Ortega K. J., Bosque-Perez N. A., Ngumbi E., Jimenez-Martinez E. S., Eigenbrode S. D.2009Rhopalosiphum padi (Hemiptera: Aphididae) responses to volatile cues from Barley yellow dwarf virus-infected wheat. Environ. Entomol. 38, 836–845 (doi:10.1603/022.038.0337) [DOI] [PubMed] [Google Scholar]
- Mehta P., Wyman J. A., Nakhla M. K., Maxwell D. P.1994Transmission of Tomato yellow leaf curl geminivirus by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 87, 1291–1297 [DOI] [PubMed] [Google Scholar]
- Mellor P. S., Kitching R. P., Wilkinson P. J.1987Mechanical transmission of Capripox virus and African swine fever virus by Stomoxys calcitrans. Res. Vet. Sci. 43, 109–112 [PubMed] [Google Scholar]
- Mideo N., Day T.2008On the evolution of reproductive restraint in malaria. Proc. R. Soc. B 275, 1217–1224 (doi:10.1098/rspb.2007.1545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moury B., Cardin L., Onesto J. P., Candresse T., Poupet A.2001Survey of Prunus necrotic ringspot virus in rose and its variability in rose and Prunus spp. Phytopathology 91, 84–91 (doi:10.1094/PHYTO.2001.91.1.84) [DOI] [PubMed] [Google Scholar]
- Moury B., Fabre F., Senoussi R.2007Estimation of the number of virus particles transmitted by an insect vector. Proc. Natl Acad. Sci. USA 104, 17 891–17 896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser R. O., Hum-Musser S. M., Felton G. W., Gergerich R. C.2003Increased larval growth and preference for virus-infected leaves by the Mexican bean beetle, Epilachna varivestis mulsant, a plant virus vector. J. Insect Behav. 16, 247–256 (doi:10.1023/A:1023919902976) [Google Scholar]
- Ng J. C., Falk B. W.2006Virus–vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 44, 183–212 (doi:10.1146/annurev.phyto.44.070505.143325) [DOI] [PubMed] [Google Scholar]
- Ng J. C. K., Tian T., Falk B. W.2004Quantitative parameters determining whitefly (Bemisia tabaci) transmission of Lettuce infectious yellows virus and an engineered defective RNA. J. Gen. Virol. 85, 2697–2707 (doi:10.1099/vir.0.80189-0) [DOI] [PubMed] [Google Scholar]
- Ngumbi E., Eigenbrode S. D., Bosque-Perez N. A., Ding H., Rodriguez A.2007Myzus persicae is arrested more by blends than by individual compounds elevated in headspace of PLRV-infected potato. J. Chem. Ecol. 33, 1733–1747 (doi:10.1007/s10886-007-9340-z) [DOI] [PubMed] [Google Scholar]
- Pacheco D. A., Rezende J. A. M., Piedade S. M. D. S.2003Biomass, virus concentration, and symptomatology of cucurbits infected by mild and severe strains of Papaya ringspot virus. Scientia Agricola 60, 691–698 [Google Scholar]
- Palacios I., Drucker M., Blanc S., Leite S., Moreno A., Fereres A.2002Cauliflower mosaic virus is preferentially acquired from the phloem by its aphid vectors. J. Gen. Virol. 83, 3163–3171 [DOI] [PubMed] [Google Scholar]
- Pirone T. P.1981Efficiency and selectivity of the helper-component-mediated aphid transmission of purified potyviruses. Phytopathology 71, 922–924 (doi:10.1094/Phyto-71-922) [Google Scholar]
- Pirone T. P., Megahed E. S.1966Aphid transmissibility of some purified viruses and viral RNAs. Virology 30, 631–637 (doi:10.1016/0042-6822(66)90168-1) [DOI] [PubMed] [Google Scholar]
- Poulicard N., Pinel-Galzi A., Hebrard E., Fargette D.2010Why Rice yellow mottle virus, a rapidly evolving RNA plant virus, is not efficient at breaking rymv1-2 resistance. Mol. Plant Pathol. 11, 145–154 (doi:10.1111/j.1364-3703.2009.00582.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power A. G.1992Patterns of virulence and benevolence in insect-borne pathogens of plants. Crit. Rev. Plant Sci. 11, 351–372 [Google Scholar]
- Raberg L., Graham A. L., Read A. F.2009Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49 (doi:10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud B., Peterschmitt M.1992A study of the mode of transmission of Maize streak virus by Cicadulina mbila using an enzyme-linked immunosorbent assay. Ann. Appl. Biol. 121, 85–94 (doi:10.1111/j.1744-7348.1992.tb03989.x) [Google Scholar]
- Roberts M. G.2007The pluses and minuses of 0. J. R. Soc. Interface 4, 949–961 (doi:10.1098/rsif.2007.1031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cerezo E., Klein P. G., Shaw J. G.1991A determinant of disease symptom severity is located in the 3′-terminal noncoding region of the RNA of a plant virus. Proc. Natl Acad. Sci. USA 88, 9863–9867 (doi:10.1073/pnas.88.21.9863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanow L. R., Moyer J. W., Kennedy G. G.1986Alteration of efficiencies of acquisition and inoculation of Watermelon mosaic virus 2 by plant resistance to the virus and to an aphid vector. Phytopathology 76, 1276–1281 (doi:10.1094/Phyto-76-1276) [Google Scholar]
- Rotenberg D., Kumar K. N. K., Ullman D. E., Montero-Astùa M., Willis D. K., German T. L., Whitfield A. E.2009Variation in tomato spotted wilt virus titer in Frankliniella occidentalis and its association with frequency of transmission. Phytopathology 99, 404–410 (http://dx.doi.org/10.1094/PHYTO-99-4-0404) [DOI] [PubMed] [Google Scholar]
- Sacristan S., Garcia-Arenal F.2008The evolution of virulence and pathogenicity in plant pathogen populations. Mol. Plant Pathol. 9, 369–384 (doi:10.1111/j.1364-3703.2007.00460.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B.-J., Palukaitis P., Symons R. H.2002Differential virulence by strains of Cucumber mosaic virus is mediated by the 2b gene. Mol. plant Microbe Interact. 15, 947–955 (doi:10.1094/MPMI.2002.15.9.947) [DOI] [PubMed] [Google Scholar]
- Sinisterra X. H., Mckenzie C. L., Hunter W. B., Powell C. A., Shatters R. G., Jr2005Differential transcriptional activity of plant-pathogenic begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyrodidae). J. Gen. Virol. 86, 1525–1532 (doi:10.1099/vir.0.80665-0) [DOI] [PubMed] [Google Scholar]
- Sisterson M. S.2008Effects of insect–vector preference for healthy or infected plants on pathogen spread: insights from a model. J. Econ. Entomol. 101, 1–8 (doi:10.1603/0022-0493(2008)101[1:EOIPFH]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Thurston M. I., Pallett D. W., Cortina-Borja M., Edwards M. L., Raybould A. F., Cooper J. I.2001The incidence of viruses in wild Brassica nigra in Dorset (UK). Ann. Appl. Biol. 139, 277–284 (doi:10.1111/j.1744-7348.2001.tb00140.x) [Google Scholar]
- Untiveros M., Fuentes S., Salazar L. F.2007Synergistic interaction of Sweet potato chlorotic stunt virus (Crinivirus) with carla-, cucumo-, ipomo-, and potyviruses infecting Sweet Potato. Plant Dis. 91, 669–676 (doi:10.1094/PDIS-91-6-0669) [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel J. F. J. M., Boerma T. M., Peters D.1991Transmission of Potato leafroll virus from plants and artificial diets by Myzus persicae. Phytopathology 81, 150–154 [Google Scholar]
- Wintermantel W. M., Cortez A. A., Anchieta A. G., Gulati-Sakhuja A., Hladky L. L.2008Co-infection by two criniviruses alters accumulation of each virus in a host-specific manner and influences efficiency of virus transmission. Phytopathology 98, 1340–1345 (doi:10.1094/PHYTO-98-12-1340) [DOI] [PubMed] [Google Scholar]
- Yahara T., Oyama K.1993Effects of virus infection on demographic traits of an agamospermous population of Eupatorium chinense (Asteraceae). Oecologia 96, 310–315 (doi:10.1007/BF00317499) [DOI] [PubMed] [Google Scholar]
- Yuan C., Ullman D. E.1996Comparison of efficiency and propensity as measures of vector importance in Zucchini yellow mosaic potyvirus transmission by Aphis gossypii and A. craccivora.. Phytopathology 86, 698–703 (doi:10.1094/Phyto-86-698) [Google Scholar]
- Zaitlin M., Hull R.2003Plant virus–host interactions. Annu. Rev. Plant Physiol. 38, 291–315 (doi:10.1146/annurev.pp.38.060187.001451) [Google Scholar]