Abstract
Current methods of assessing climate-induced shifts of species distributions rarely account for species interactions and usually ignore potential differences in response times of interacting taxa to climate change. Here, we used species-richness data from 1005 breeding bird and 1417 woody plant species in Kenya and employed model-averaged coefficients from regression models and median climatic forecasts assembled across 15 climate-change scenarios to predict bird species richness under climate change. Forecasts assuming an instantaneous response of woody plants and birds to climate change suggested increases in future bird species richness across most of Kenya whereas forecasts assuming strongly lagged woody plant responses to climate change indicated a reversed trend, i.e. reduced bird species richness. Uncertainties in predictions of future bird species richness were geographically structured, mainly owing to uncertainties in projected precipitation changes. We conclude that assessments of future species responses to climate change are very sensitive to current uncertainties in regional climate-change projections, and to the inclusion or not of time-lagged interacting taxa. We expect even stronger effects for more specialized plant–animal associations. Given the slow response time of woody plant distributions to climate change, current estimates of future biodiversity of many animal taxa may be both biased and too optimistic.
Keywords: biodiversity change, biotic interactions, climate-change impacts, general circulation model, predictive model, uncertainty
1. Introduction
Climate change is thought to be among the major current threats to biodiversity (Sala et al. 2000; Walther et al. 2002; Parmesan 2006; Jetz et al. 2007), and by the end of this century large portions of the Earth's surface may experience climates not found at present (Williams et al. 2007). Recent species- and community-level approaches to assessing biodiversity changes and shifts in species' distributions under future climate-change scenarios employ forecasting techniques (e.g. species distribution models or species-richness forecasts) to estimate the relationship between current patterns of species distributions and climatic variables, and to project future biodiversity consequences under climate change (e.g. Thomas et al. 2004; Thuiller et al. 2005; Lemoine et al. 2007; La Sorte et al. 2009). These models usually assume that species interactions and biotic associations play a relatively minor role at broad geographical scales (Davis et al. 1998; Pearson & Dawson 2003), regardless of whether individual species' distributions or more integrated attributes of ecological communities, such as species richness, are modelled. It has therefore been questioned whether these approaches accurately forecast changes in biodiversity under climate change (Davis et al. 1998; Araújo & Rahbek 2006; Araújo & Luoto 2007).
To date, little is known about the effect of species interactions on spatial distribution of biodiversity under climate change. For instance, for two monophagous butterfly species and their food plants in Europe, it has been suggested that climate change has the potential to further limit the ranges of trophically linked species because butterflies and their food plants do not necessarily react in a similar manner to global change (Araújo & Luoto 2007; Schweiger et al. 2008). Overall, however, it remains largely unclear whether and how different responses of interacting species to climate change might influence future species distributions. Different time lags may be particularly relevant for many animal groups (such as birds, small mammals, or insects) if associated species (particularly woody plants) have relatively low dispersal rates and long generation times, and therefore relatively slow distributional responses. Different response times of interacting species to climate change could thus lead to a spatial mismatch between suitable climate niche space and the presence of the associated species on which the focal organisms depend (Araújo & Luoto 2007; Schweiger et al. 2008). Species associations between plants and animals involving little specialization, including non-trophic relationships (e.g. between birds or mammals and woody plants), are more widespread than specialized relationships (e.g. between monophagous butterflies and host plants). However, whether such little-specialized biotic associations can affect distributional shifts of interacting species under climate change has not yet been evaluated.
Here, we explore the implications of including plant–bird associations and potential time lags of woody plants into forecasts of bird species richness in response to climate change. Birds and plants are suitable groups for such analyses because biotic associations play an important role in shaping species-richness patterns at broad geographical scales (Lee & Rotenberry 2005; Kissling et al. 2007, 2008; Qian 2007). Although reciprocal specialization between individual bird and plant species is rare (Zamora 2000), plants are at the base of terrestrial food webs and provide a great variety of food resources relevant for bird consumers (Hutchinson 1959; Cody 1985; Kissling et al. 2007). Moreover, woody plants are key structural elements of terrestrial ecosystems, determining habitat configuration and providing foraging substrates and nesting sites for many bird species (Cody 1985). Empirical field studies suggest that the effect of plant diversity on bird diversity is largely driven by an increase in horizontal and vertical heterogeneity of vegetation cover the more woody plant species are present (MacArthur & MacArthur 1961). Consequently, the dependence of birds on woody plants results in the spatial congruence of the richness patterns of both groups not only at local but also at broad geographical scales (Lee & Rotenberry 2005; Kissling et al. 2007, 2008; Qian 2007). Despite their importance, biotic associations between birds and woody plants have so far been disregarded when forecasting climate-change impacts on bird diversity (Thomas et al. 2004; Jetz et al. 2007; Lemoine et al. 2007; Huntley et al. 2008).
Our analysis is based on an exhaustive dataset summarizing the geographical distributions of all native breeding bird and woody plant species across Kenya at a spatial resolution of ca 55 km (0.5° grid cells). Previous work with this dataset used structural equation modelling to disentangle direct and indirect effects of climatic factors and woody plant species richness on bird species richness (Kissling et al. 2008). Results indicated that bird and woody plant species richness in Kenya at this spatial scale are predominantly linked via functional relationships, probably driven by vegetation structural complexity, and that effects of climatic variables on bird species richness are largely indirect via effects on woody plants. Here, we extend this reasoning and develop predictive regression models for bird and woody plant species richness. We use 15 climate-change scenarios (Solomon et al. 2007) to assess likely changes in bird diversity across Kenya, including interactions between birds and woody plant species and potential time lags of the latter group. We compare two forecasting scenarios. In Forecast 1 (instantaneous change of woody plants), we assume that woody plant species richness responds instantaneously to climate change (based on future climate surfaces) and that bird species richness responds directly to future climatic conditions and future woody plant richness. In Forecast 2 (no change of woody plants), we assume the same as in Forecast 1, except that woody plant species richness shows a strong time lag in its response to climate change, not changing over the time period concerned. Time lags in the response of woody plants to climate change are realistic, given the dispersal limitation, long generation time and longevity of woody plant species. Thus, we test the hypothesis that even relatively unspecialized biotic associations affect forecasts of future biodiversity change and ask how variable and assumption-sensitive these predictions are.
2. Material and methods
(a). Species-richness data
We used comprehensive species distribution data on all breeding birds (Lewis & Pomeroy 1989) and all woody plants (Beentje 1994) across Kenya. This bioinformatic database has recently been compiled (Kissling et al. 2008) at a spatial resolution of 0.5°× 0.5° cells (approx. 55.5 km) and contains 228 grid cells, of which 160 cells are included here as these are known to provide reasonable estimates of bird and woody plant species richness (Field et al. 2005; Kissling et al. 2008). Distribution information on 1005 breeding bird species and 1417 woody plant species was included, and species richness of both taxa was estimated from this information for each grid cell. Vagrant bird species and species represented only by anecdotal records (n = 60 species) were not included (Kissling et al. 2008). Similarly, plants that are non-native, 2.5 m or less in height at maturity, or that are not truly woody were excluded (Field et al. 2005). To our knowledge, this database currently contains the most comprehensive information on bird and woody plant distributions at a regional scale in tropical Africa.
(b). Environmental variables
We focused on three environmental variables (PREC, mean annual precipitation in mm; TEMP, mean annual temperature in °C; TOPO, topographic heterogeneity in m) as predictors of bird species richness in our models. All three variables have previously been shown to be important determinants of species richness of birds and woody plants across Kenya (Kissling et al. 2008), and more generally at broad spatial scales (Jetz & Rahbek 2002; Hawkins et al. 2003; Field et al. 2005; Luoto & Heikkinen 2008). Mean values of PREC and TEMP were calculated at 0.5° resolution from the CRU TS 2.1 dataset for the period 1960–1989 (Mitchell & Jones 2005). This time period fits well with the period of data collection for the species distribution data (Lewis & Pomeroy 1989; Beentje 1994). PREC was used to characterize water availability (O'Brien et al. 1998), and, as with the southern African dataset of O'Brien et al. (1998), is almost identical to rainfall in Kenya, where non-liquid precipitation only falls on the very highest mountain peaks. Thus, it measures water that is directly available to plants. TEMP was used to measure ambient energy input (Hawkins et al. 2003), and in Kenya correlates almost perfectly with Thornthwaite's formulation of potential evapotranspiration, which is used in the interim general models of O'Brien (1998) and Field et al. (2005). TOPO was measured as altitudinal range (maximum minus minimum elevation per grid cell) and included to characterize habitat heterogeneity and within-cell climatic variability (O'Brien et al. 2000; Luoto & Heikkinen 2008). Values were extracted from the 30 arc-second SRTM-GTOPO30 dataset provided by The Global Land Cover Facility (available at http://glcf.umiacs.umd.edu/data/srtm/). We initially tested seasonality in precipitation (calculated as the coefficient of variation of the monthly precipitation values; Kissling et al. 2008) as a predictor variable, but models were not improved by this variable and we thus omitted it. We did not use other variables included in Kissling et al. (2008), such as potential evapotranspiration and land-cover diversity, because these are not yet available at the appropriate scale and accuracy for future climate-change scenarios (see next section).
(c). Climate-change scenarios
To characterize potential future climate surfaces, we calculated PREC and TEMP from predicted monthly values over the period 2069–2098. The values were derived from five widely established general circulation models (GCMs) (table 1). All of these models (hereafter referred to as GFDL, ECHAM, HadCM3, MIROC and NCAR) have been used in the World Climate Research Programme's (WCRP's) Coupled Model Intercomparison Project phase 3 (CMIP3) (available from https://esg.llnl.gov:8443/home/publicHomePage.do) and cover a wide variety of different model types, projections and sensitivities. For each model, we included climate-change projections under forcing from three contrasting emission scenarios, provided by the Special Report on Emission Scenarios of the Intergovernmental Panel on Climate Change (Nakicenovic & Swart 2000; Solomon et al. 2007): the pessimistic A2, the balanced A1B and the optimistic B1 emission scenario. All climate projections were spatially interpolated to 0.5° resolution and bias-corrected for the reference time period (1960–1989) with modified Climatic Research Unit (CRU) climate data (Oesterle et al. 2003; Mitchell & Jones 2005), using additive correction of TEMP and multiplicative correction of PREC. For all 15 GCM/emission scenario combinations (GFDL-A2, GFDL-B1, GFDL-A1B, ECHAM5-A2, ECHAM5-B1, ECHAM5-A1B, etc.), we calculated climate anomalies of PREC and TEMP for the years 2069–2098 with respect to the reference period (1960–1989).
Table 1.
Summary characteristics of 15 climate-change scenarios across Kenya for precipitation and temperature. Presented are empirical input values for present-day climate (1960–1989, note the slight variation in input values for present-day precipitation), potential future climate surfaces (2069–2098) and median changes in climate between the two periods. The final column gives the median change across 160 grid cells of 0.5° × 0.5° size. The final rows give the median values across the 15 climate-change scenarios. Codes of climate-change scenarios are: GFDL, Geophysical Fluid Dynamics Laboratory Coupled Model; ECHAM, the Max Planck Institute for Meteorology's ECHAM GCM; HadCM3, Hadley Center Coupled Model; MIROC, Model for Interdisciplinary Research on Climate; NCAR, National Center for Atmospheric Research model; A2, emission scenario assuming a strong increase in fossil fuel consumption and related global CO2 emissions; A1B, emission scenario assuming a balanced use of fossil fuel consumption; B1, emission scenario assuming lower levels of fossil fuel consumption and related global CO2 emissions.
| present-day climate (1960–1989) |
potential future climate (2069–2098) |
||||||
|---|---|---|---|---|---|---|---|
| climate scenario | min | median | max | min | median | max | median change in climate (n = 160) |
| mean annual precipitation (mm) | |||||||
| GFDL-A2 | 189 | 677 | 1838 | 145 | 565 | 1566 | −115 |
| GFDL-A1B | 189 | 677 | 1838 | 155 | 597 | 1548 | −87 |
| GFDL-B1 | 189 | 677 | 1838 | 176 | 649 | 1620 | −39 |
| ECHAM-A2 | 189 | 676 | 1841 | 343 | 2120 | 5124 | 1475 |
| ECHAM-A1B | 189 | 676 | 1841 | 247 | 1116 | 3359 | 464 |
| ECHAM-B1 | 189 | 676 | 1841 | 218 | 590 | 2035 | −22 |
| HadCM3-A2 | 188 | 674 | 1839 | 291 | 3164 | 9821 | 2605 |
| HadCM3-A1B | 188 | 674 | 1839 | 247 | 1376 | 5870 | 753 |
| HadCM3-B1 | 188 | 674 | 1839 | 231 | 1738 | 6727 | 1023 |
| MIROC-A2 | 188 | 673 | 1838 | 326 | 1155 | 2941 | 487 |
| MIROC-A1B | 188 | 673 | 1838 | 311 | 1145 | 2965 | 481 |
| MIROC-B1 | 188 | 673 | 1838 | 284 | 990 | 2449 | 317 |
| NCAR-A2 | 189 | 674 | 1838 | 289 | 924 | 2439 | 245 |
| NCAR-A1B | 189 | 674 | 1838 | 268 | 908 | 2575 | 228 |
| NCAR-B1 | 189 | 674 | 1838 | 232 | 780 | 2100 | 106 |
| median | 189 | 674 | 1838 | 247 | 990 | 2575 | 317 |
| annual mean temperature (°C) | |||||||
| GFDL-A2 | 12.7 | 25.6 | 28.9 | 15.6 | 28.4 | 31.7 | 2.9 |
| GFDL-A1B | 12.7 | 25.6 | 28.9 | 15.2 | 28.0 | 31.3 | 2.4 |
| GFDL-B1 | 12.7 | 25.6 | 28.9 | 14.3 | 27.1 | 30.4 | 1.6 |
| ECHAM-A2 | 12.7 | 25.6 | 28.9 | 15.9 | 28.7 | 32.1 | 3.2 |
| ECHAM-A1B | 12.7 | 25.6 | 28.9 | 15.8 | 28.6 | 32.0 | 3.1 |
| ECHAM-B1 | 12.7 | 25.6 | 28.9 | 14.8 | 27.7 | 31.0 | 2.1 |
| HadCM3-A2 | 12.7 | 25.6 | 28.9 | 15.6 | 28.3 | 31.9 | 2.8 |
| HadCM3-A1B | 12.7 | 25.6 | 28.9 | 15.3 | 28.0 | 31.5 | 2.5 |
| HadCM3-B1 | 12.7 | 25.6 | 28.9 | 14.7 | 27.3 | 30.9 | 1.9 |
| MIROC-A2 | 12.7 | 25.6 | 28.9 | 15.5 | 28.6 | 32.4 | 3.0 |
| MIROC-A1B | 12.7 | 25.6 | 28.9 | 15.2 | 28.2 | 31.9 | 2.6 |
| MIROC-B1 | 12.7 | 25.6 | 28.9 | 14.4 | 27.4 | 31.0 | 1.8 |
| NCAR-A2 | 12.7 | 25.6 | 28.9 | 15.0 | 27.8 | 31.1 | 2.2 |
| NCAR-A1B | 12.7 | 25.6 | 28.9 | 14.6 | 27.4 | 30.8 | 1.9 |
| NCAR-B1 | 12.7 | 25.6 | 28.9 | 14.0 | 26.8 | 30.1 | 1.2 |
| median | 12.7 | 25.6 | 28.9 | 15.2 | 27.9 | 31.3 | 2.4 |
(d). Model selection and validation
We tested and evaluated all possible one predictor and multiple predictor regression models (general linear models, GLMs) with Gaussian error distribution and identity link (McCullagh & Nelder 1989) to relate current bird species richness to current climatic variables (PREC, TEMP), topography (TOPO), and current woody plant species richness (WOODRICH; electronic supplementary material, table S1). We evaluated similar regression models to relate current woody plant species richness to current climatic variables and topography (electronic supplementary material, table S2). Model selection was based on explained variance (R2), Akaike information criterion (AIC) values and results from cross-validation (see next paragraph). The differences in AIC values (i.e. ΔAIC) between the focal model and the model with the lowest AIC were used to rank models from best to worst (Burnham & Anderson 2002). We later used the best GLMs (§2e) for both bird and woody plant species richness and derived average parameter estimates (i.e. regression coefficients) to predict future patterns of bird and woody plant species richness across Kenya. To improve normality and linearity in the relationship between variables, we square-root transformed woody plant richness in all analyses but left other variables untransformed (see also Kissling et al. 2008). The inclusion of second-order polynomials of explanatory variables to account for potential nonlinear relationships did not significantly improve AIC values, so we report results only from the linear fits.
To evaluate the predictive performance of our GLMs, we performed cross-validation (Guisan & Zimmermann 2000) across the 160 grid cells within our Kenyan dataset. We calibrated each GLM with a 50 per cent random sample (n = 80 grid cells) of the dataset and evaluated it against the remaining 50 per cent (n = 80 grid cells). This cross-validation was done 1000 times per GLM. In each of the 1000 subsamples, the prediction accuracy of the GLM was measured by predicting the response variable (i.e. bird or woody plant species richness) for the remaining 50 per cent of the grid cells, and then calculating Spearman's rank correlations between observed and predicted species-richness values. We also report the slopes of the best-fit lines from single-predictor GLMs between observed and predicted species richness as a measure of prediction accuracy. Our approach allowed us to test the predictive performance and accuracy of models within our dataset and is an appropriate method for model validation when data from other spatial or temporal domains are not available against which model predictions can be evaluated (Guisan & Zimmermann 2000; Araújo & Rahbek 2006).
Spatial autocorrelation is a frequent phenomenon in geographical ecology and can affect estimates of model coefficients and inference from statistical models (Legendre 1993; Bini et al. 2009). We therefore compared all GLMs with spatial linear models (SLMs; here ‘spatial simultaneous autoregressive error models’—see Kissling & Carl 2008), which can account for spatial patterns in the response variable that are not predicted by explanatory variables. The spatial weights matrix in SLMs was calculated with two neighbours (only one for the cross-validation) and a row standardized coding style (for details on model selection of SLMs, see Kissling & Carl 2008). To quantify spatial autocorrelation in our dataset, we calculated Moran's I values (Legendre 1993) on the residuals of our GLMs and SLMs using the same neighbourhood definitions. All statistical analyses were done with the software R (available at http://www.R-project.org). Moran's I values and SLMs were calculated using the R library ‘spdep’, v. 0.4–6, provided by Roger Bivand (http://cran.r-project.org/web/packages/).
(e). Forecasts of bird species richness
To predict future patterns of bird and woody plant species richness across Kenya, we averaged parameter estimates (i.e. partial regression coefficients) from the best models (evaluated by ΔAIC) for each response variable (i.e. bird and woody plant species richness). We refer to them as averaged bird and plant GLMs. We used model averaging for linear regression models as implemented in the R library ‘BMA’, v. 3.05 (available at http://cran.r-project.org/web/packages/) to derive these parameter estimates. This model-averaging approach is based on Bayesian inference and weights parameter estimates of predictor variables according to their probabilities of occurring in the candidate set of best models (Raftery 1995). We adopted this approach to include several similar best models rather than choosing only one, but we note that individual models from the set of best models gave qualitative similar results of predicted future bird and plant species richness. We additionally provide standardized partial regression coefficients for each predictor variable to indicate their relative importance.
We compared two forecast models (Forecasts 1 and 2) to estimate the potential future bird species richness across Kenya. In Forecast 1 (instantaneous change of woody plants), we predicted future bird species richness with the averaged bird GLM from projected future climate and an instantaneous response of woody plant species richness to climate change. To model the instantaneous response of woody plant species richness to climate change, we predicted future woody plant species richness with the averaged plant GLM and future climatic variables (see below for more details). Forecast 2 (no change of woody plants) predicted future bird species richness from climatic variables (future values) and present-day patterns of woody plant species richness. Thus, both forecasts used the same averaged bird GLM, the difference being that future WOODRICH was used in Forecast 1, whereas present-day WOODRICH was used in Forecast 2.
To model the instantaneous response of woody plant species richness to climate change in Forecast 1, we first modelled current woody plant species richness with the averaged plant GLM (using present-day climatic variables, i.e. 1960–1989 climate, for each of the 15 climate models separately) and then modelled future woody plant species richness with the same plant GLM (using future climatic variables, i.e. 2069–2098 climate, again for all climate models separately). We then extracted for each grid cell the change between modelled present-day and modelled future woody plant species richness. We added this change in modelled woody plant species richness to the observed present-day WOODRICH values to estimate future WOODRICH (assuming an instantaneous response of woody plant species richness to climate change). In Forecast 2, we used the observed present-day WOODRICH without adding the change in modelled woody plant species richness. So, both forecasts used observed present-day WOODRICH, but in Forecast 1, the change between modelled present-day and modelled future woody plant species richness was added to the observed present-day plant richness. In all cases, predicted negative values of WOODRICH were set to zero, under the assumption that they indicate unsuitability of the environment to plants. TOPO values were assumed to remain constant until 2098.
For each of the two forecasts, we provide 15 predictions of the potential changes in bird species richness across Kenya, based on the 15 climate-change scenarios and WOODRICH as explained above. We calculated the expected future changes in bird species richness (for each of these 15 predictions per forecast) as the difference between modelled current bird species richness (modelled with 1960–1989 climate) and projected future bird species richness (modelled with 2069–2098 climate), similar to those of woody plants. Using modelled current bird species richness instead of observed species richness makes results more comparable between predictive models. In all cases, predicted negative values of bird species richness were also set to zero assuming that they indicate unsuitability of the environment to birds. We finally combined information from all 15 climate-change projections to account for intermodel variations and climate model uncertainties. We provide average values of future changes in bird species richness across Kenya for each forecast by calculating, for each grid cell, the median value of predicted changes in bird species richness across the 15 climate-change scenarios. As a measure of uncertainty, we calculate the interquartile range (IQR) of predicted values for each grid cell across the 15 climate-change scenarios for each of the two forecasts.
3. Results
(a). Species richness and environment
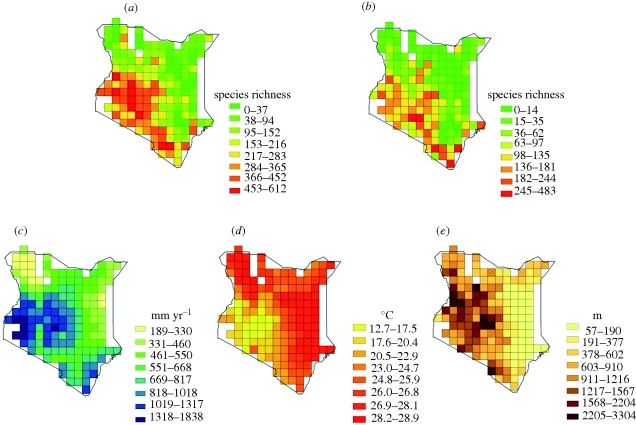
Although located at the equator, Kenya exhibits strong broad-scale geographical gradients in bird and woody plant species richness and in environmental variables (figure 1). Current patterns of bird species richness peak in the southwestern parts of the country where precipitation and topographic heterogeneity are highest. Woody plant species richness, in contrast, is highest in the southernmost part of the country and shows intermediate values in the southwestern part. Areas in the north and east of Kenya are characterized by high temperatures and low topographic heterogeneity and generally show low species numbers of both birds and woody plants (figure 1).
Figure 1.
Present-day geographical patterns of (a,b) species richness and (c–e) environment across Kenya at 0.5° resolution. (a) Observed species richness of birds, (b) observed species richness of woody plants, (c) mean annual precipitation (mm yr−1), (d) mean annual temperature (°C), and (e) altitudinal range measuring topographic heterogeneity (highest minus lowest elevation, m). Natural breaks classification is shown.
(b). Climate-change scenarios
Averaged across all climate models, PREC was predicted to increase between the periods 1960–1989 and 2069–2098 by a median value of +317 mm yr−1 across Kenya (table 1). However, projected future precipitation surfaces differed extremely between GCMs, including both decreases and massive increases in rainfall (table 1), suggesting very large uncertainties in forecasting regional rainfall patterns at tropical latitudes in Africa. Projections of future temperature surfaces across Kenya were more similar between different climate models and suggested median increases in TEMP of 1.2–3.2°C (table 1). Median TEMP across all climate-change projections was projected to increase on average by 2.4°C across Kenya between the periods 1960–1989 and 2069–2098 (table 1).
(c). Model selection and validation
Predicting bird species richness only from environmental variables had substantially lower success (Spearman's rank: Rs between 0.60 and 0.73) than when woody plant species richness was also used as a predictor (Rs between 0.82 and 0.85) (electronic supplementary material, table S1). Four regression models with bird species richness as a response variable showed relatively similar support given the data (highlighted in bold in electronic supplementary material, table S1). All four regression models had similar AIC values with ΔAIC ≤ 3 and the same explained variance (R2 = 0.73). Accounting for spatial autocorrelation, SLMs generally gave similar results to GLMs (electronic supplementary material, table S1). Additionally, our cross-validation of the predictive performance of both GLMs and SLMs within our dataset indicated that these four models had similar prediction success (Spearman's rank: Rs either 0.84 or 0.85). Predicting woody plant species richness within our dataset was most successful when PREC was included (electronic supplementary material, table S2). Similarly to the bird GLMs, three multiple regression models for woody plant richness showed relatively similar support given the data (electronic supplementary material, table S2). These GLMs had similar AIC values (ΔAIC ≤ 6), explained 44–47% of the variance in WOODRICH, and had similar high prediction success in our cross-validation (Spearman's rank: Rs between 0.68 and 0.71, electronic supplementary material, table S2). Again, SLMs for woody plant richness gave similar results to GLMs (electronic supplementary material, table S2). Because spatial autocorrelation had no notable effect, and because of the general consistency in coefficients across models (data not shown), we are confident that the GLMs provide a robust framework for prediction. We therefore chose the three plant GLMs and the four bird GLMs to derive model-averaged parameter estimates for predicting future plant and bird species richness.
(d). Forecasts of bird species richness
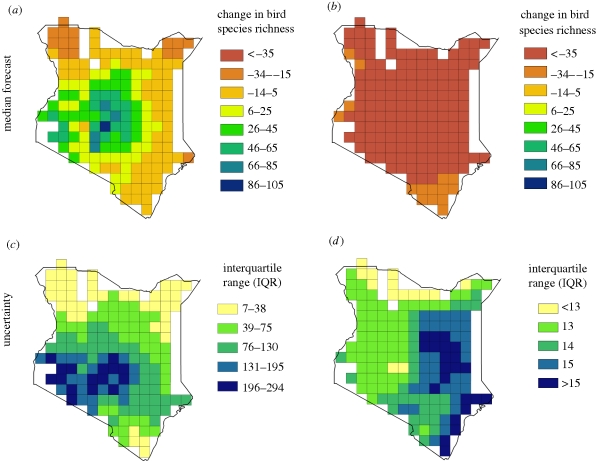
Model-averaged parameter estimates indicated that bird richness was most strongly determined by WOODRICH and TEMP, whereas WOODRICH was largely determined by PREC and TOPO (see standardized partial regression coefficients in table 2). Because of the great differences in precipitation predictions between the GCMs (table 1), our 15 predictions of potential future changes in bird species richness also differed widely between different climate-change scenarios, especially for Forecast 1 (table 3). This was mainly driven by the great variability in predicted PREC values that influenced future WOODRICH values. In Forecast 1, some climate models predicted median increases in bird species richness across Kenya, whereas other climate models predicted decreases (table 3). Predictions of Forecast 2 were more homogeneous between climate-change models and generally predicted losses in bird species richness (table 3). Median tendencies of predicted future patterns of bird species richness differed strongly between Forecasts 1 and 2 (figure 2a,b and table 3), with Forecast 1 having significantly more positive (and even reversed) predicted changes than Forecast 2 (t-test comparing predicted median changes of Forecast 1 with Forecast 2: t = 24.6, d.f. = 318, p < 0.001). Geographically, median predictions of Forecast 1 were more heterogeneous than those of Forecast 2 (figure 2a,b), suggesting an increase in bird species richness in the central parts of Kenya and decreases in some of the northern parts. Uncertainties (measured as IQRs) were significantly larger in Forecast 1 than Forecast 2 (t-test comparing log-transformed IQRs of Forecast 1 with 2: t = 24.3, d.f. = 318, p < 0.001), mainly because uncertainties in climate models (especially predictions of precipitation, table 1) played a more important role in Forecast 1 than in Forecast 2. Uncertainties of Forecast 1 were geographically structured, being largest in the central and southwestern parts of Kenya (figure 2c,d). Uncertainties of Forecast 2 were much less pronounced (compare IQRs values in figure 2c,d) and were slightly higher in the eastern parts of Kenya.
Table 2.
Parameter estimates for regression models used to predict future patterns of bird and woody plant species richness across Kenya. Parameter estimates (regression coefficients) were obtained from model averaging (see §2) using all regression models highlighted in bold in tables S1 and S2 in the electronic supplementary material. Standardized partial regression coefficients (ranging from 0 to 1) are given in brackets to indicate the relative importance of each predictor variable. Abbreviations of predictor variables: PREC, mean annual precipitation (mm); TEMP, mean annual temperature (°C); TOPO, topographic relief (highest minus lowest elevation, m); WOODRICH, woody plant species richness (square-root transformed in all analyses).
| variables | bird species richness | woody plant species richness (WOODRICH) |
|---|---|---|
| intercept | 425 | 0.831 |
| PREC | −0.0010 (−0.002) | 0.0070 (0.492) |
| TEMP | −1.513 (−0.385) | −0.0034 (−0.029) |
| TOPO | 0.0002 (0.001) | 0.0017 (0.250) |
| WOODRICH | 18.95 (0.568) | — |
Table 3.
Predicted changes in bird species richness under climate change. Values were derived from n = 160 grid cells across Kenya. Changes in bird species richness between the periods 1960–1989 and 2069–2098 were calculated for each individual grid cell as the difference between modelled current bird species richness and modelled future bird species richness. Bird species richness was either predicted from Forecast 1 (instantaneous change of woody plants) or Forecast 2 (no change of woody plants). Fifteen climate-change projections (codes as in table 1) were used for each type of forecast. Median forecasts and uncertainties (IQR, interquartile ranges) were first calculated for each grid cell across all 15 climate-change scenarios before the summary statistics (min, median, max) were calculated across the 160 cells.
| Forecast 1 (instantaneous change of woody plants) |
Forecast 2 (no change of woody plants) |
|||||
|---|---|---|---|---|---|---|
| climate scenario | min | median | max | min | median | max |
| GFDL-A2 | −87 | −59 | −21 | −45 | −43 | −40 |
| GFDL-A1B | −83 | −49 | −13 | −38 | −37 | −34 |
| GFDL-B1 | −55 | −30 | −6 | −24 | −24 | −23 |
| ECHAM-A2 | −31 | 145 | 399 | −51 | −49 | −48 |
| ECHAM-A1B | −45 | 13 | 166 | −49 | −47 | −46 |
| ECHAM-B1 | −86 | −37 | 69 | −34 | −32 | −31 |
| HadCM3-A2 | −29 | 299 | 1012 | −52 | −45 | −33 |
| HadCM3-A1B | −62 | 59 | 494 | −43 | −39 | −27 |
| HadCM3-B1 | −22 | 106 | 617 | −34 | −30 | −22 |
| MIROC-A2 | −29 | 17 | 115 | −54 | −45 | −36 |
| MIROC-A1B | −24 | 22 | 128 | −47 | −40 | −30 |
| MIROC-B1 | −19 | 14 | 77 | −33 | −27 | −23 |
| NCAR-A2 | −32 | −3 | 57 | −36 | −34 | −29 |
| NCAR-A1B | −23 | 1 | 81 | −30 | −28 | −26 |
| NCAR-B1 | −21 | −5 | 25 | −19 | −19 | −17 |
| median forecast | −28 | 7 | 98 | −39 | −36 | −33 |
| uncertainty (IQR) | 7 | 73 | 294 | 10 | 13 | 17 |
Figure 2.
(a,b) Projected future changes in bird species richness and (c,d) model uncertainties across Kenya. (a) Forecast 1 (instantaneous response of woody plant richness to climate change). (b) Forecast 2 (no change, i.e. a time lag, in the response of woody plant richness to climate change). (c) Uncertainty in Forecast 1. (d) Uncertainty in Forecast 2. Projected changes in bird species richness (a,b) are median values across all 15 climate-change scenarios for each grid cell (see tables 1 and 3). Uncertainties of forecasts (c,d) were measured as interquartile ranges (IQRs) from the same 15 predictions for each grid cell. Note that scales in IQR maps differ to make intra-model uncertainties better visible, but uncertainties are much larger in (c) Forecast 1 than in (d) Forecast 2.
4. Discussion
Our results clearly demonstrate that the response of woody plant species to climate change and regional uncertainties in climate models can dramatically influence forecasts of potential future changes in bird diversity. Models assuming a strong time lag in the response of woody plants to climate change (Forecast 2) forecasted significantly stronger decreases in bird species richness under climate change than models where woody plant species richness was allowed to show an instantaneous response to climatic change (Forecast 1). Thus, projected changes in bird species richness in Kenya strongly depend on woody plants and how they are included in forecast models. Moreover, strong variability in future precipitation patterns as predicted by GCMs at tropical latitudes can further introduce high levels of uncertainty in biodiversity forecasts. Overall, our study challenges the appropriateness of making forecasts of climate-change impacts on biodiversity without taking biotic interactions and uncertainties in regional climate models into account.
In our study, based on our previous findings (Kissling et al. 2008), we assumed that woody plant richness affects bird species richness (probably because it is an appropriate surrogate for bird habitat complexity) and showed that including lagged response times of woody plants has fundamental implications for assessing climate-change impacts on bird diversity. Time lags in the response of woody plants to climate change are not unrealistic, given the dispersal limitation, high age at maturity, and longevity of many woody plant species. Future changes in woody plant distributions are likely to be found somewhere in between the two extremes represented by Forecasts 1 and 2 (compare Midgley et al. 2006), but we know little about dispersal, establishment and seedling survival of woody plants in the tropics. For instance, long-distance dispersal events of woody plant species could reduce the predicted negative changes of Forecast 2, but such dispersal events are rare and highly stochastic, and their quantification remains challenging (Nathan 2006). On the other hand, it typically takes at least 50–100 years to establish a novel, mature forest tree and current rates of habitat loss, fragmentation and selective logging in tropical landscapes make tree recruitment often unlikely. We further note that our modelling approach is simplistic because we do not address the response of individual plant species, plant functional types, or other changes in plant community structure and composition. However, independent research on the potential climate change response of a California shrubland songbird also supports our results, showing that predicted future changes in the spatial distribution of a habitat specialist largely depends on the inclusion of structural vegetation changes (Preston et al. 2008). Climate change responses of both bird habitat generalists and specialists clearly deserve further study.
Besides habitat structure, future changes in the distribution of woody plants might also be important for birds from a trophic perspective. Especially in the tropics, many woody plants provide important food resources for bird consumers including fleshy-fruited trees and shrubs for frugivorous species (Shanahan et al. 2001; Kissling et al. 2007). This is similarly true for other vertebrate taxa such as mammals. Moreover, for many invertebrate species, such as phytophagous insects, tight interactions and specialized relationships with woody plants have been reported (Jaenike 1990; Novotny et al. 2006), suggesting that responses of such animal taxa to climate change will depend even more strongly on plants than those of vertebrates such as birds, for which we have better data. We therefore stress that the modelling and prediction of future changes in (woody) plant distributions will be fundamental to assessing the wider consequences of climate change for animal biodiversity, and see an urgent need for research into plant–animal interactions under climate change.
Current climate models (GCMs) have been demonstrated to reproduce observed features of recent climate and past climate changes and are believed to provide credible quantitative estimates of future climate change at continental and global spatial scales (Solomon et al. 2007). Regional scale climate models, however, remain in the exploratory phase and only in some regions has downscaling of climate-change simulations to the regional level been achieved (Christensen et al. 2007). Many climate models thus display substantial uncertainty in regional climate-change estimates, with uncertainties being much higher for precipitation than for temperature (Randall et al. 2007). Our downscaled climate data clearly demonstrate this for Kenya, but similar results might be obtained for other tropical regions because large uncertainties remain in the simulation of tropical winds, clouds and precipitation regimes (Randall et al. 2007). One way to deal with such uncertainty in outputs from climate models is to combine information from widely different projections and to extract the ‘signal’ that emerges from the noise associated with different model outputs (compare Araújo & New 2007). We employed such an approach by calculating average forecasts and geographical patterns of uncertainties across 15 climate models. This helped us to evaluate climate-related uncertainties in our biodiversity forecasts under climate change. Unfortunately, to date there is no clear guidance on how to select the most appropriate climate-change model for a given application or region (Beaumont et al. 2008), and quantifying variation in regional GCM projections thus remains crucial.
Many additional unknowns remain in forecasting future patterns of biodiversity (Davis et al. 1998; Pearson & Dawson 2003; Thomas et al. 2004; Araújo & Rahbek 2006). Current forecasting techniques often assume that species' distributions are at or near equilibrium with current climate (Pearson & Dawson 2003) and the potential effects of dispersal and recruitment limitation on current and future species distributions remain understudied (Davis et al. 1998; Nathan 2006; Svenning & Skov 2007). Even when the response of species richness to climate change can be adequately predicted, other attributes of ecological communities might not respond in a similar or predictable way (La Sorte et al. 2009). Further, we lack information about which species might be able to adapt to changing environmental conditions and how the emergence of novel climatic conditions (Williams et al. 2007) might promote the formation of novel species associations or changes in current biotic interactions. Land-use changes and interactions among drivers of biodiversity change (Sala et al. 2000; Jetz et al. 2007; Rüger et al. 2008) also need to be incorporated into global change analyses and could further reduce estimates of potential future species richness compared with purely climate-based models, especially in the tropics (Jetz et al. 2007). These are all areas where further research is urgently needed. However, none of this is likely to diminish (but may well enhance) the two central conclusions of this study: (i) that regional predictions of the response of biodiversity to climate change are strongly sensitive to current regional-scale uncertainties in climate models and (ii) that disregarding important biotic interactions and time lags of biotic partners is likely to bias such predictions.
In summary, our study highlights the urgent need for a new generation of forecasting techniques to assess climate-change impacts on biodiversity. We consider it particularly important to (i) address current and potential future patterns of biotic interactions at broad geographical scales (Araújo & Luoto 2007; Heikkinen et al. 2007; Kissling et al. 2007, 2008; Schweiger et al. 2008), (ii) identify, explore and quantify sources of uncertainty and expose the sensitivity of predictions to the assumptions underlying them (Thuiller 2004; Araújo et al. 2006; Pearson et al. 2006; Beaumont et al. 2008), and (iii) extend current modelling techniques and test novel forecasting approaches that allow better linking of species distribution data with biotic interactions and other processes, such as population dynamics, dispersal and adaptation (Midgley et al. 2006; Keith et al. 2008; Montoya et al. 2009; Scheiter & Higgins 2009). Especially at tropical latitudes, current regional climate-change projections are highly uncertain, high-resolution distribution data for many plant and animal species are lacking, and demographic processes and population dynamics of most species are unknown. We therefore urge researchers to obtain more data from the tropics, where the majority of global biodiversity is found, but where knowledge on global change impacts remains poor.
Acknowledgements
We thank James Brown, Frank La Sorte, Daniel Montoya, Matthias Schleuning and anonymous reviewers for comments on manuscript versions. We are also grateful to the modelling groups, the Programme for Climate Model Diagnosis and Intercomparison (PCMDI), and the WCRP's Working Group on Coupled Modelling (WGCM) for making the WCRP CMIP3 multi-model dataset available. Support for this dataset has been provided by the Office of Science, US Department of Energy. Our study was funded by the German Federal Ministry of Education and Research within the framework of BIOTA East Africa (01LC0405 and 01LC0625, subproject E11). W.D.K., K.B.-G. and R.F. designed the research, W.D.K. conducted analyses, H.K. contributed to model validation and U.H. compiled climate data. W.D.K. wrote the initial draft manuscript, and R.F., K.B.-G. and W.D.K. contributed to subsequent manuscript writing.
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Araújo M. B., Luoto M.2007The importance of biotic interactions for modeling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753 (doi:10.1111/j.1466-8238.2007.00359.x) [Google Scholar]
- Araújo M. B., New M.2007Ensemble forecasting of species distributions. Trends Ecol. Evol. 22, 42–47 (doi:10.1016/j.tree.2006.09.010) [DOI] [PubMed] [Google Scholar]
- Araújo M. B., Rahbek C.2006How does climate change affect biodiversity? Science 313, 1396–1397 (doi:10.1126/science.1131758) [DOI] [PubMed] [Google Scholar]
- Araújo M. B., Whittaker R. J., Ladle R. J., Erhard M.2006Reducing uncertainty in projections of extinction risk from climate change. Glob. Ecol. Biogeogr. 14, 529–538 (doi:10.1111/j.1466-822X.2005.00182.x) [Google Scholar]
- Beaumont L. J., Hughes L., Pitman A. J.2008Why is the choice of future climate scenarios for species distribution modelling important? Ecol. Lett. 11, 1135–1146 [DOI] [PubMed] [Google Scholar]
- Beentje H.1994Kenya trees, shrubs and lianas Nairobi, Kenya: National Museums of Kenya [Google Scholar]
- Bini L. M., et al. 2009Coefficient shifts in geographical ecology: an empirical evaluation of spatial and non-spatial regression. Ecography 32, 193–204 [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multimodel inference New York, NY: Springer [Google Scholar]
- Christensen J. H., et al. 2007Regional climate projections. In Climate Change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), pp. 847–940 Cambridge, UK: Cambridge University Press [Google Scholar]
- Cody M. L.1985Habitat selection in birds Orlando, FL: Academic Press [Google Scholar]
- Davis A. J., Jenkinson L. S., Lawton J. H., Shorrocks B., Wood S.1998Making mistakes when predicting shifts in species range in response to global warming. Nature 391, 783–786 (doi:10.1038/35842) [DOI] [PubMed] [Google Scholar]
- Field R., O'Brien E. M., Whittaker R. J.2005Global models for predicting woody plant species richness from climate: development and evaluation. Ecology 86, 2263–2277 (doi:10.1890/04-1910) [Google Scholar]
- Guisan A., Zimmermann N. E.2000Predictive habitat distribution models in ecology. Ecol. Model. 135, 147–186 (doi:10.1016/S0304-3800(00)00354-9) [Google Scholar]
- Hawkins B. A., et al. 2003Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (doi:10.1890/03-8006) [Google Scholar]
- Heikkinen R. K., Luoto M., Virkkala R., Pearson R. G., Körber H.2007Biotic interactions improve prediction of boreal bird distributions at macro-scales. Glob. Ecol. Biogeogr. 16, 754–763 (doi:10.1111/j.1466-8238.2007.00345.x) [Google Scholar]
- Huntley B., Collingham Y. C., Willis S. G., Green R. E.2008Potential impacts of climate change on European breeding birds. PLoS ONE 3, e1439 (doi:10.1371/journal.pone.0001439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson G. E.1959Homage to Santa Rosalia, or why are there so many kinds of animals? Am. Nat. 93, 145–159 (doi:10.1086/282070) [Google Scholar]
- Jaenike J.1990Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21, 243–273 (doi:10.1146/annurev.es.21.110190.001331) [Google Scholar]
- Jetz W., Rahbek C.2002Geographic range size and determinants of avian species richness. Science 297, 1548–1551 (doi:10.1126/science.1072779) [DOI] [PubMed] [Google Scholar]
- Jetz W., Wilcove D., Dobson A.2007Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 5, e157 (doi:10.1371/journal.pbio.0050157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D. A., Akcakaya H. R., Thuiller W., Midgley G. F., Pearson R. G., Phillips S. J., Regan H. M., Araújo M. B., Rebelo T. G.2008Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biol. Lett. 4, 560–563 (doi:10.1098/rsbl.2008.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling W. D., Carl G.2008Spatial autocorrelation and the selection of simultaneous autoregressive models. Global Ecol. Biogeogr. 17, 59–71 [Google Scholar]
- Kissling W. D., Rahbek C., Böhning-Gaese K.2007Food plant diversity as broad-scale determinant of avian frugivore richness. Proc. R. Soc. B 274, 799–808 (doi:10.1098/rspb.2006.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling W. D., Field R., Böhning-Gaese K.2008Spatial patterns of woody plant and bird diversity: functional relationships or environmental effects? Glob. Ecol. Biogeogr. 17, 327–339 (doi:10.1111/j.1466-8238.2007.00379.x) [Google Scholar]
- La Sorte F. A., Lee T. M., Wilman H., Jetz W.2009Disparities between observed and predicted impacts of climate change on winter bird assemblages. Proc. R. Soc. B 276, 3167–3174 (doi:10.1098/rspb.2009.0162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.-Y., Rotenberry J. T.2005Relationships between bird species and tree species assemblages in forested habitats of eastern North America. J. Biogeogr. 32, 1139–1150 (doi:10.1111/j.1365-2699.2005.01254.x) [Google Scholar]
- Legendre P.1993Spatial autocorrelation: trouble or new paradigm? Ecology 74, 1659–1673 (doi:10.2307/1939924) [Google Scholar]
- Lemoine N., Schaefer H.-C., Böhning-Gaese K.2007Species richness of migratory birds is influenced by global climate change. Glob. Ecol. Biogeogr. 16, 55–64 (doi:10.1111/j.1466-8238.2006.00252.x) [Google Scholar]
- Lewis A., Pomeroy D.1989A bird atlas of Kenya. Rotterdam, The Netherlands: Balkema [Google Scholar]
- Luoto M., Heikkinen R. K.2008Disregarding topographical heterogeneity biases species turnover assessments based on bioclimatic models. Glob. Change Biol. 14, 483–494 (doi:10.1111/j.1365-2486.2007.01527.x) [Google Scholar]
- MacArthur R. H., MacArthur J. W.1961On bird species diversity. Ecology 42, 594–598 (doi:10.2307/1932254) [Google Scholar]
- McCullagh P., Nelder J. A.1989Generalized linear models London, UK: Chapman and Hall [Google Scholar]
- Midgley G. F., Hughes G. O., Thuiller W., Rebelo A. G.2006Migration rate limitations on climate change-induced range shifts in Cape Proteaceae. Divers. Distrib. 12, 555–562 (doi:10.1111/j.1366-9516.2006.00273.x) [Google Scholar]
- Mitchell T. D., Jones P. D.2005An improved method of constructing a database of monthly climate observations and associated high-resolution grids. Int. J. Climatol. 25, 693–712 (doi:10.1002/joc.1181) [Google Scholar]
- Montoya D., Purves D. W., Urbieta I. R., Zavala M. A.2009Do species distribution models explain spatial structure within tree species ranges? Glob. Ecol. Biogeogr. 18, 662–673 (doi:10.1111/j.1466-8238.2009.00478.x) [Google Scholar]
- Nakicenovic N., Swart R.2000Special Report on Emission Scenarios of the Intergovernmental Panel on Climate Change New York, NY: Cambridge University Press [Google Scholar]
- Nathan R.2006Long-distance dispersal of plants. Science 313, 786–788 (doi:10.1126/science.1124975) [DOI] [PubMed] [Google Scholar]
- Novotny V., Drozd P., Miller S. E., Kulfan M., Janda M., Basset Y., Weiblen G. D.2006Why are there so many species of herbivorous insects in tropical rainforests? Science 313, 1115–1118 (doi:10.1126/science.1129237) [DOI] [PubMed] [Google Scholar]
- O'Brien E. M.1998Water–energy dynamics, climate, and prediction of woody plant species richness: an interim general model. J. Biogeogr. 25, 379–398 (doi:10.1046/j.1365-2699.1998.252166.x) [Google Scholar]
- O'Brien E. M., Whittaker R. J., Field R.1998Climate and woody plant diversity in southern Africa: relationships at species, genus and family levels. Ecography 21, 495–509 (doi:10.1111/j.1600-0587.1998.tb00441.x) [Google Scholar]
- O'Brien E. M., Field R., Whittaker R. J.2000Climatic gradients in woody plant (tree and shrub) diversity: water-energy dynamics, residual variation and topography. Oikos 89, 588–600 (doi:10.1034/j.1600-0706.2000.890319.x) [Google Scholar]
- Oesterle H., Gerstengarbe F. W., Werner P.2003Homogenisierung und Aktualisierung des Klimadatensatzes der Climate Research Unit der University of East Anglia, Norwich. Potsdam, Germany: Terra Nostra 6, Deutsche Klimatagung [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Pearson R. G., Dawson T. P.2003Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 (doi:10.1046/j.1466-822X.2003.00042.x) [Google Scholar]
- Pearson R., et al. 2006Model-based uncertainty in species range prediction. J. Biogeogr. 33, 1704–1711 (doi:10.1111/j.1365-2699.2006.01460.x) [Google Scholar]
- Preston K. L., Rotenberry J. T., Redak R. A., Allen M. F.2008Habitat shifts of endangered species under altered climate conditions: importance of biotic interactions. Glob. Change Biol. 14, 2501–2515 [Google Scholar]
- Qian H.2007Relationships between plant and animal species richness at a regional scale in China. Conserv. Biol. 21, 937–944 (doi:10.1111/j.1523-1739.2007.00692.x) [DOI] [PubMed] [Google Scholar]
- Raftery A. E.1995Bayesian model selection in social research (with Discussion). In Sociological methodology (ed. Marsden P. V.), pp. 111–196 Cambridge, MA: Blackwell [Google Scholar]
- Randall D. A., et al. 2007Climate models and their evaluation. In Climate Change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), pp. 589–662 Cambridge, UK: Cambridge University Press [Google Scholar]
- Rüger N., Williams-Linera G., Kissling W. D., Huth A.2008Long-term impacts of fuelwood extraction on a tropical montane cloud forest. Ecosystems 11, 868–881 (doi:10.1007/s10021-008-9166-8) [Google Scholar]
- Sala O. E., et al. 2000Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- Scheiter S., Higgins S. I.2009Impacts of climate change on the vegetation of Africa: an adaptive dynamic vegetation modelling approach. Glob. Change Biol. 15, 2224–2246 (doi:10.1111/j.1365-2486.2008.01838.x) [Google Scholar]
- Schweiger O., Settele J., Kudrna O., Klotz S., Kühn I.2008Climate change can cause spatial mismatch of trophically interacting species. Ecology 89, 3472–3479 (doi:10.1890/07-1748.1) [DOI] [PubMed] [Google Scholar]
- Shanahan M., So S., Compton S. G., Corlett R.2001Fig-eating by vertebrate frugivores: a global review. Biol. Rev. 76, 529–572 [DOI] [PubMed] [Google Scholar]
- Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L. (eds) 2007Climate Change 2007: the physical science basis. Cambridge, UK: Cambridge University Press; See http://ipcc-wg1.ucar.edu/wg1/wg1-report.html [Google Scholar]
- Svenning J.-C., Skov F.2007Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol. Lett. 10, 453–460 (doi:10.1111/j.1461-0248.2007.01038.x). [DOI] [PubMed] [Google Scholar]
- Thomas C. D., et al. 2004Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- Thuiller W.2004Patterns and uncertainties of species' range shifts under climate change. Glob. Change Biol. 10, 2020–2027 (doi:10.1111/j.1365-2486.2004.00859.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W., Lavorel S., Araújo M. B., Sykes M. T., Prentice I. C.2005Climate change threats to plant diversity in Europe. Proc. Natl Acad. Sci. USA 102, 8245–8250 (doi:10.1073/pnas.0409902102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J.-M., Hoegh-Guldberg O., Bairlein F.2002Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- Williams J. W., Jackson S. T., Kutzbach J. E.2007Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742 (doi:10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora R.2000Functional equivalence in plant-animal interactions: ecological and evolutionary consequences. Oikos 88, 442–447 (doi:10.1034/j.1600-0706.2000.880222.x) [Google Scholar]