Abstract
Climate change is expected to drive species extinct by reducing their survival, reproduction and habitat. Less well appreciated is the possibility that climate change could cause extinction by changing the ecological interactions between species. If ecologists, land managers and policy makers are to manage farmland biodiversity sustainably under global climate change, they need to understand the ways in which species interact with each other as this will affect the way they respond to climate change. Here, we consider the ability of nectar flower mixtures used in field margins to provide sufficient forage for bumble-bees under future climate change. We simulated the effect of global warming on the network of plant–pollinator interactions in two types of field margin: a four-species pollen and nectar mix and a six-species wildflower mix. While periods without flowering resources and periods with no food were rare, curtailment of the field season was very common for the bumble-bees in both mixtures. The effect of this, however, could be ameliorated by adding extra species at the start and end of the flowering season. The plant species that could be used to future-proof margins against global warming are discussed.
Keywords: climate change, field margin, bumble-bee
1. Introduction
Huge tracts of land are used for agricultural purposes worldwide. For example, 77 per cent of the land area in Great Britain is under agricultural production (defra 2002). Given that high levels of biodiversity are found on farmland (Hole et al. 2005), how agro-ecosystems respond to climate change has considerable implications for the maintenance and utilization of biodiversity. Global climate change is widely expected to drive species extinct by hampering reproductive success, reducing the amount and accessibility of suitable habitat, or eliminating organisms that are essential to a species' survival. Less well appreciated is the likelihood that climate change may directly disrupt or eliminate ecological interactions between species, even before extinctions occur (Visser et al. 1998; Memmott et al. 2007; Hegland et al. 2009).
Most research on the impact of climate change on species interactions relates to food chains, for example, to asynchronies between a bird species and one of its caterpillar prey species (Visser et al. 1998) or to frogs and their pathogens (Pounds et al. 2006). Recently though, there is some evidence of a shift in focus, for example Both et al. (2009) consider the effect of climate change on four trophic levels, one of which hosts four species, and Memmott et al. (2007) consider a network of interactions between 456 plant species and 1428 pollinator species (see also Van der Putten et al. 2010; Walther 2010). The vast majority of species, even those in relatively species-poor agro-ecosystems, are embedded within complex networks of interacting species (Gibson et al. 2006), in which disruptions of ecological interactions may have more far-reaching effects. For example, the removal of a handful of well-connected species could elicit an extinction cascade (Solé & Montoya 2001). To be able to make predictions about the likely impact of environmental stresses such as climate change, ecologists need to understand the architecture of ecological networks (McCann 2007) as this strongly influences their resilience to environmental stress (Dunne et al. 2002; Ives & Cardinale 2004). Moreover, by studying species interactions, ecologists are working in the currency of ecosystem services. Thus many services are the result of interactions, for example, pollination is the result of an interaction between a plant and a pollinator and pest control is the result of an interaction between a pest and its natural enemy. It is the conservative exploitation of these types of ecosystem services that provides the basis for a sustainable agriculture.
Most higher plant species, up to 90 per cent by some estimates (Nabhan & Buchmann 1997), rely on animals to pollinate their flowers in order to reproduce successfully. While vertebrates such as birds, bats and marsupials can act as pollinators, insects are the most important group of pollinators (Proctor et al. 1996). Pollinators are declining, along with insect-pollinated plants (Biesmeijer et al. 2006). In agro-ecosystems, bumble-bees (Bombus spp.) are known to be particularly badly affected (Carvell et al. 2006), although other groups are not as well studied and may be undergoing similar declines. Indeed, overall bee diversity, including that of solitary wild bee species, is known to have declined in large parts of Britain and the Netherlands (Biesmeijer et al. 2006). Only six species of bumble-bee remain common in the UK out of 22 extant species (Williams 2007), and similar reductions in abundance and geographical range have been documented for bumble-bee populations in other parts of Europe and in North America (Williams 1982; Goulson 2003).
Declines in the abundance and species richness of bumble-bees in Europe have been linked to agricultural intensification. Their need for a season-long supply of pollen and nectar (from February when the first queens emerge from hibernation, to September when the new queens need to build up reserves for hibernation) makes bumble-bees particularly susceptible to the effects of agricultural intensification. Changes in farming practice, such as the conversion of species-rich hay meadows to intensive grasslands, and the degradation of perennial vegetation in semi-natural habitats, such as field margins and hedgerows, are likely to have had negative effects on bumble-bees (Osborne & Corbet 1994; Carvell et al. 2006). In the UK, Environmental Stewardship Schemes offer monetary support for environmentally friendly farming and provide an opportunity to restore habitats of value to bumble-bees in intensively farmed areas. The schemes work by: (i) providing a series of management options that each earns a specific number of points and (ii) assigning each farm a target number of points based on its size and location (Natural England 2008). The farmer or wildlife advisor simply chooses sufficient options to reach the farm's points target. Once the management has been carried out, an annual subsidy is payable. Sowing ‘nectar flower mixtures’ in field margins has received relatively wide uptake in arable areas under Entry Level Stewardship (ELS) (Boatman et al. 2007) and can provide a good forage supply for bumble-bees (Carvell et al. 2007).
The nectar flower mixtures used in field margins and developed under today's climate regime could be vulnerable to the effects of global warming. In a study of 243 British plants, Fitter & Fitter (2002) reported that flowering phenology advances by 4.5 days per degree change in mean monthly temperature. Memmott et al. (2007) explored the potential disruption of this type of change on a plant–pollinator community. Using a large network describing the interactions between 429 plant species and 1419 pollinator species, they simulated the impact of phenological shifts in plant flowering and insect flight seasons on the pollinators' food supplies. Depending on model assumptions, phenological shifts reduced the floral resources available to 17–50% of all pollinator species by reducing availability of flowers during the pollinators' flight seasons. Reduced overlap between plants and pollinators also decreased the diet breadth of the pollinators. Overall, the predicted result of these disruptions was the extinction of pollinators, plants and their crucial interactions.
The insects feeding on flowers in field margins could be affected by temporal gaps in their resources if flower phenologies shift and no longer overlap seamlessly throughout their flight season. At worst, a species' flight season may not overlap with any of its flower resources. Or curtailment of foraging could occur if flowering resources are delayed at the start of the flight season or truncated at its end. If pollinator phenology responds to climate change in exactly the same way as floral phenology, there will be no problem for the pollinators; however, the cues responded to by different groups of species are likely to differ, leading to differential responses (Visser et al. 1998).
The aim of this paper is to explore how future climate change, caused by the doubling of atmospheric CO2 concentration forecasted for 2070–2100 (IPCC 2001), is likely to affect the ability of nectar flower mixtures used in field margins to provide sufficient forage for bumble-bees. This is an important and a pragmatic question to ask, as these high-quality restored habitats are our first line of defence against pollinator declines in agro-ecosystems. Moreover, it may be that only relatively small changes are needed in order to future-proof their benefits against likely changes in temperature. There are two objectives to our study: (i) to predict the likely asynchrony, caused by future climate change, between bumble-bees and their host plants in two types of field margin mixes: a four-species nectar flower mix and a six-species wildflower mix; and (ii) to make practical suggestions for the amelioration of the predicted impacts of global warming on field margins.
2. Material and methods
(a). Field sites and study species
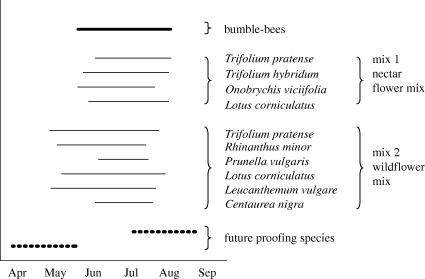
Field data on flowering phenology were collected over 3 years at six arable farm sites in central and eastern England for a study comparing five field margin management treatments with conventional cropping to the field edge in terms of their efficacy in providing forage supplies for bumble-bees (see Carvell et al. 2007 for details of the field site selection, site locations and farm management). On each site, six experimental plots of 50 m by 6 m were established in September 2001 along two cereal field margins within the same field. On each margin, these contiguous plots were managed according to one of six treatments. Here we use data from two of these treatments: the pollen and nectar seed mix and the wildflower seed mix. The former (here referred to as ‘nectar flower mix’ in accordance with their recent name change) consisted of four agricultural legume species and four fine grass species sown at 20 kg ha−1; the latter consisted of 21 native wildflower species and four fine grass species sown at 37 kg ha−1. Of the 21 wildflower species, six species established successfully, flowered at all six sites, and were visited by bumble-bees. Data from these six species were therefore included in our model, as they could be established widely under Environmental Stewardship options to create floristically enhanced field margins.
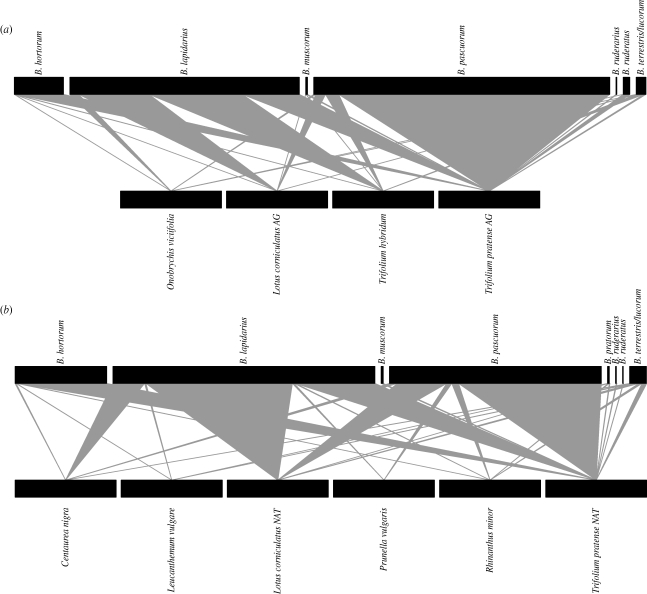
The flowering phenology of each plant species was recorded from early May to late August in 2002–2004 and averaged over the 3 years to provide a single representative flowering phenology (figure 1). Flowering days were based on the first and last day in each year on which a species was producing 1–25 flowers per plot on at least four of the six study sites. Foraging bumble-bees were recorded in each field margin on repeated bi-monthly transect walks in each of the 3 years (Carvell et al. 2007). Seven species of bumble-bee were recorded visiting flowers in the nectar flower mix, and eight species in the wildflower mix. Bombus terrestris/lucorum, Bombus lapidarius, Bombus pascuorum, Bombus hortorum, Bombus ruderarius, Bombus muscorum and Bombus ruderatus were found on both mixes; Bombus pratorum was found only on the wildflower mix. The two species Bombus terrestris and Bombus lucorum are lumped together as B. terrestris/lucorum as their workers are difficult to distinguish in the field. The bumble-bee visitation data for each plant species were summed across all sites and years and used to construct a visitation network for each of the two seed mixes (figure 2). Visitation networks can underestimate the number of flower species visited by pollinators (Bosch et al. 2009); however, they remain the network used by most pollination biologists as they are very straightforward to construct. Moreover, in a simple flower community such as that found in field margins and in an intensive sampling regime such as that used by Carvell et al. (2006), it is unlikely that interactions will be missed.
Figure 1.
The flowering phenology and identity of the species in the two margin mixes. The duration of the bumble-bee flight season under consideration is also shown, along with the two species added to the mixture to decrease periods of curtailment (labelled as future-proofing species). The two dotted lines represent the flowering phenologies of two hypothetical plant species used in our mitigation strategy.
Figure 2.
The plant–bumble-bee networks from field margins: (a) sown with nectar flower mix; (b) sown with wildflower mix. The squares at the bottom of each section represent the flowering plant species in the margins; the squares at the top represent bumble-bee species. All plant species are represented by similar-sized boxes, regardless of their abundance; bee species are represented by boxes proportionate in size to their abundance: bigger boxes represent higher numbers of visitations. The triangles or lines connecting the upper and lower boxes represent the frequency of visitations between each pair-wise combination of bee species and plant species.
A well-known limitation of field margin seed mixes is that they do not provide forage for the whole of the bees' flight season (February–September). We consider the effect of climate change on bee forage during the period when the margins do supply resources, the late spring and summer months (figure 1). Consequently, we define the bumble-bee flight season as the flowering season for the species in the nectar flower mix. This allows us to ask whether the six species in the wildflower seed mix provide increased protection against climate change, compared with the nectar flower mix.
(b). Objective 1: predicting the likely asynchrony, caused by future climate change, between bumble-bees and their host plants
We applied the approach used by Memmott et al. (2007) to simulate the effect of global warming on a large American plant–pollinator network to our visitation networks for bumble-bees in field margins. The methods for simulations can be divided into four main stages as follows.
(i). Predicting the impact of global warming on plant and insect phenology
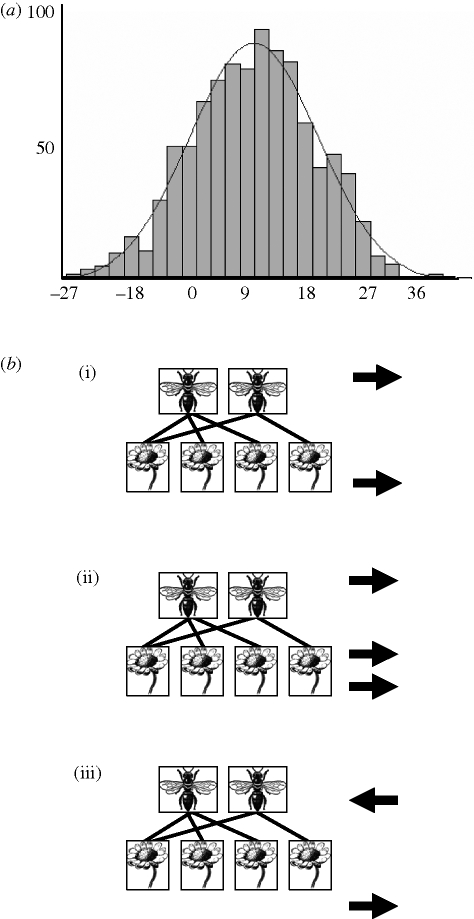
Fitter & Fitter (2002) reported that the mean flowering for 243 British flowering plants had advanced by an average of 4.5 days per degree change in mean monthly temperature over the last decade. In terms of pollinator phenology, butterflies appear to provide the only published estimates of shifts in onset of the flight season (Rusterholz & Erhardt 1998; Roy & Sparks 2000) and their response appears to be very similar to that of plants. We therefore used the same shifts in phenology for pollinators as for plants. While both plants and pollinators were subjected to the same average shifts in phenology, because their shifts were independently randomly picked from a distribution (see below and see figure 3), a given pair of interacting plant and pollinator species may show very different changes in phenology.
Figure 3.
(a) The distribution used to shift flowering and flight season phenology for the 9-day shift. The histogram shows the frequency of 1000 random numbers from a normal distribution with a mean of 9 and a standard deviation of 9. (b) Given that plant and pollinator shifts are picked independently from the distribution, the shifts of a given pair of interacting species can be different in both duration and direction. For example, (i) while most shifts are forward, (ii) shifts forward can differ in duration between plants and pollinators or (iii) be in different directions.
(ii). Predicting changes in temperature and advancement in plant and insect phenology
Climate models for the UK predict an increase in mean annual temperature of between 3.5 and 5°C under doubling of atmospheric CO2 (IPCC 2001). We therefore simulated changes of +2, +4 and +6°C to emulate best-case, worst-case and average scenarios. These increases, when multiplied by the estimated per degree mean phenological responses of 4.5 days per 1°C (Fitter & Fitter 2002), yield estimates of 9, 18 and 27 days for the predicted mean advance in British flowering phenology and insect flight seasons. The simple multiplicative approach used here assumes that future phenological shifts will scale linearly with the amount of climate warming, which may not be the case. In the absence of better information though, this seems a sensible approach (Memmott et al. 2007).
(iii). Simulation of climate change on the plant–pollinator network
We simulated the effect of future climate warming by advancing the onset of flowering of all plant species and of the flight activity periods of all pollinator species in our network by 9, 18 and 27 days. The durations of flowering and activity periods remained unchanged, as there is little evidence that warming alters the length of flowering seasons (Price & Waser 1998) and when it does, it tends to be early-flowering species that are affected (e.g. Dunne et al. 2009). Shifts for individual plant and pollinator species were drawn independently and at random from normal probability distributions with means of 9, 18 and 27 days and with standard deviations equal to the mean (figure 3). One thousand iterations were run for each of the three magnitudes of phenological shift.
(iv). Calculating the impact of global climate change on bumble-bee diet
For each iteration, we calculated the following parameters:
-
—
The proportion of the 1000 iterations in which at least one of the bumble-bee species no longer overlapped with any of its food plants (forthwith referred to as ‘no food’). Having no food as a flower-visiting insect means almost certain death for the individual pollinator, and in the case of social foragers, the entire colony.
-
—
The proportion of runs per simulation in which at least one of the bumble-bee species had a gap in its food resources (forthwith referred to as ‘gaps’).
-
—
The proportion of bee species with a shortened foraging season, i.e. forage either becomes available later in the season or it finishes earlier (forthwith referred to as ‘curtailment’). The timing of the curtailment (whether at the start or end of the flight season) and the length of curtailment were also calculated.
(c). Objective 2: can protection against global warming be built into field margins?
We suspect that the combination of species richness and flowering phenology will explain the outcome of our simulations (§3). Thus, in general, more plant species will provide greater protection against global warming, as high numbers of species reduce the probability of occurrence of gaps and periods with no food. Similarly, a longer flowering phenology means that bigger shifts are needed before gaps and curtailment occur. Using this information, we can explore possible solutions to the problem of curtailment through further simulations. We simulated two scenarios. In scenario 1, we added two hypothetical species to the wildflower mix, an early-flowering species and a late-flowering species and repeated the simulations. These ‘future-proofing’ species are predicted to reduce curtailment at both ends of the bee flight season. Extending the concept further in scenario 2, the phenology of the early flowering future proofer was increased (i.e. flowering started at the same time, but it flowered for longer).
3. Results
(a). Objective 1: predicting the likely asynchrony, caused by future climate change, between bumble-bees and their host plants
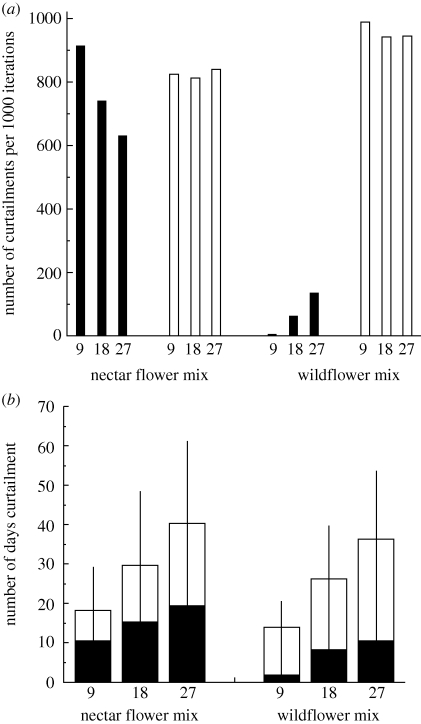
Very few of the bee species were left with no food in the simulations. In the runs simulating the effects of three levels of global warming for the pollen and nectar seed mix, the bees were without food for 0, 0.001 and 0.010 per cent of the 1000 iterations for the 9-, 18- and 27-day shifts, respectively. While very low, the number of days without food follow the expected pattern and increase as the degree of asynchrony increases. The wildflower mix provided greater protection from days with no food and only the extreme case of a 27-day shift resulted in any periods with no food.
Here, though there was little difference with the nectar flower mix (0.014 for the wildflower mix versus 0.010 for the nectar flower mix).
Gaps in food resources were rare, but again showed a logical pattern; there was an increase in gaps from 0 to 0.004 to 0.022 per cent of the 1000 runs as the number of days shifted increased from 9 to 18 to 27 for the nectar flower mix. The wildflower mix provided greater protection from gaps: they only occurred for the 27-day shift and then in only two of the 1000 runs.
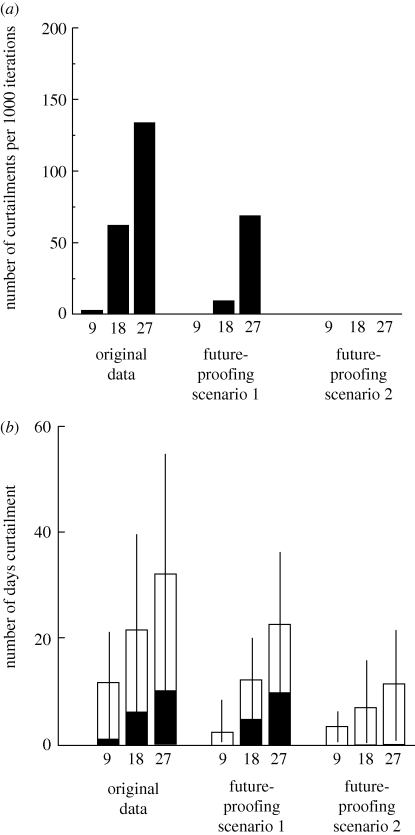
In contrast to the prevalence of no food and gaps in forage availability, curtailment was extremely common, occurring at either the start or the end of the flowering season in most of the iterations in the six sets of simulations (figure 4a). While there was curtailment in all six simulations, there were very few cases of curtailment at the start of the flight season in the wildflower mix. The total length of curtailment varied from ca two weeks to over a month; the shortest curtailment occurred in the 9-day shift in the wildflower mix and the longest in the 27-day shift for the nectar flower mix (figure 4b). While confidence intervals overlap, i.e. there is no significant difference in curtailment between the two mixes, a logical trend exists in that mean curtailment is longer for the nectar flower than for the wildflower mix.
Figure 4.
Curtailment in bumble-bee resource availability. (a) The number of times curtailment occurs in 1000 iterations of each simulation (9, 18 and 27 days), at the start and end of the flowering period for the two types of field margins; (b) curtailment duration for the two margin mixes at the start and the end of the flowering period. The error bars show the 95% confident intervals for the total curtailment duration. White bars, end curtailment; black bars, start curtailment.
(b). Objective 2: can protection against global warming be built into field margins?
In the scenario 1 simulations where two future-proofing plant species were added, the prevalence of curtailment at the start of the flight season was reduced; when the phenology of the early-flowering species was changed so that it flowered for longer (scenario 2), start curtailment did not occur at all (figure 5a). Length of end curtailment though, was not significantly different among the original mix and the two scenarios (figure 5b).
Figure 5.
The effect of adding extra plant species at the start and end of the original flowering season on number of days' curtailment for the wildflower mix. (a) The prevalence of start curtailment in 1000 iterations of the original wildflower mixture for scenario 1 and scenario 2; (b) the duration of the start and end curtailment in scenario 1 and scenario 2. White bars, end curtailment; black bars, start curtailment.
4. Discussion
The simulations suggest that for both types of field margin, gaps in resources and periods without food are unlikely to be a problem for bumble-bees under global climate change. Curtailment of food supply is much more likely to cause a problem, although it is likely that its impact can be reduced, at least in part, by strategic choice of flowering species in the margins.
(a). Limitations
In addition to the assumptions implicit in our models concerning likely changes in mean temperatures, there are three main limitations to our approach. First, we only considered the plants sown in the field margins, whereas in reality alternative forage was available for the bees both in the margins and elsewhere on the farm. For example, teasel (Dipsacus fullonum), spear thistle (Cirsium vulgare) and field scabious (Knautia arvensis) were present in margins, and alternative sources of forage were available in habitats such as hedges, woodlands and as weeds in crops. Moreover, crops themselves can be important sources of food for pollinators, for example peas, beans or oil seed rape. While the amount of alternative forage varied among the six study sites (C. Carvell 2004, personal observation), we may have overestimated the impact of curtailment on bees, because of these other sources of forage. Our aim here, however, was to quantify the effect of global warming on the efficacy of margin mixes to provide floral resources, not its impact on floral resources on the whole farm.
Second, we have assessed the value of the margins to a small proportion of the pollinators and flower visitors found in agro-ecosystems. In addition to bumble-bees, solitary bees, butterflies and moths, hoverflies and beetles all forage in field margins for pollen and/or nectar (Gibson et al. 2006). However, though bumble-bees are only a small proportion of the species visiting flowers, they carry much of the insect-borne pollen grains on their bodies (i.e. excluding pollen in the corbicula) when the entire visitor community is assayed for pollen. For example, in a heathland flower visitor community, the Hymenoptera, of which bumble-bees and honeybees were the dominant members, carried 97 per cent of the insect-borne pollen (Forup et al. 2008). Thus, if bumble-bees are protected by field margins, it is likely that a large part of the ecosystem service of pollination is also protected. While other species of flower visitors were not sampled in Carvell et al.'s (2007) study, they were observed visiting sown and unsown flowers in the margins (C. Carvell, personal observation). Though they are probably not as important to pollinators, these other plant species do provide insurance against the loss of the key pollinators, and should be considered more widely in field margin studies focusing on pollination.
Finally, our model only considers the effect of a change in average temperature, and this is only one of the many possible changes in climate predicted under global warming. However, rather than add complexity to what is in reality a very simple model, it would probably be more informative to first experimentally test our current predictions, perhaps by manipulating flowering phenology in the field.
(b). The simulations
Given the long flowering season deliberately chosen for the species included in both margin mixes, it is not a surprise that the shifts in phenology needed to induce a gap in resources happen only rarely; likewise, for all of the plant species to shift out of the bee flight season would be a very rare event indeed. The two margin mixes naturally have slightly different phenologies: the wildflower mix starts to flower considerably earlier and finishes slightly earlier than the nectar flower mix (figure 1). Consequently, there is end curtailment of resources in the wildflower mix in comparison to the pollen and nectar mix, even without global warming. This shortened season, along with the fact that there are only two late-flowering species in the wildflower mix in comparison to the three species in the nectar flower mix (figure 1), probably accounts for the larger curtailment seen in simulations at the end of the season in the wildflower mix (figure 4b). This result is somewhat counterintuitive; the expectation would be that the higher number of flowering species would provide greater protection against shifts in flowering phenology. However, it is not simply the number of flowering species that determines the network's response to global warming; flowering phenology and relative flower abundance (not considered here) also need to be incorporated into any calculations. Given that curtailment occurs in simulations with both margin mixes, it is difficult to class one as better than the other with respect to the protection they provide against global warming, as it is not known whether curtailment at the start or the end of a flight season is more detrimental to bumble-bee populations.
The results of the simulations are broadly in agreement with Memmott et al. (2007), in that curtailment of the foraging season is the most likely outcome of global warming. How much curtailment in resources matters to bumble-bees is not clear, as far less is known about their colony founding and hibernation requirements than, for example, their foraging ecology (Benton 2006; Lye et al. 2009). However, if curtailment has an effect, it is likely to be negative. At the start of the flowering period (May for the wildflower mix and June for the nectar flower mix), queen bumble-bees have established colonies and workers of most species are foraging. The quantity and quality of forage could affect the production of males and queens, which is the measure of the reproductive success of the colony (e.g. Muller & Schmidhempel 1992). At the end of the season, the new queens need to build up resources for hibernating, and a curtailment at this time could affect their likelihood of surviving hibernation.
Adding the future-proofing species had an ameliorative effect on curtailment at the start of the field season. Indeed, it was possible to eliminate start curtailment altogether by simulating an extension of the flowering season of these species. Practically, this extension could probably be engineered by adding another species whose flowering season starts when the original species is finished. Species of flowering plant that could fulfil the role of the future proofers are members of the deadnettle family, for example red or white deadnettle (Lamium purpureum or Lamium album), well used by bumble-bee queens in Lye et al. (2009) or members of the primula family such as cowslips (Primula veris) for the early-flowering species and members of the scabious family (K. arvensis or Succia pratensis) for the late-flowering species. These species have the right phenology, but whether or not they would establish themselves well in field margins is not known.
Species added to a seed mix do not always provide significant amounts of bee forage, as evidenced by the fact that the wildflower mix contained 21 species, but only six of these predictably established themselves and were used as forage plants across all sites (other species established and flowered but were not always visited by bumble-bees) (Carvell et al. 2007). Further trials are needed to determine which early- and late-flowering species will reliably flower under field margin conditions. Collaboration between entomologists, plant community ecologists and agronomists could aid the design of mixes that provide beneficial flowering field margins that are straightforward for farmers to manage.
Once field margins are established, they can be managed to maximize pollinator survival. For example, cutting can be varied in different margins on a farm or on different sections of the same margin, to extend flowering and provide heterogeneity in forage availability (Pywell et al. 2008). Current ELS policy (Natural England 2008) is that half the area of pollen and nectar margins on a farm should be cut in late June to stimulate late flowering, and the whole area should be cut in late September. Non-margin habitats on farms should also be optimized for bees, for example, by having flowering hedgerow plants, and farm woodlands with an ungrazed understorey. Nectar flower mixtures are not among the most widely adopted Environmental Stewardship options in England (Boatman et al. 2007), so their potential to have a countrywide beneficial effect on bumble-bee populations has yet to be realized. Simple solutions that can easily be implemented throughout the country are needed. The instigation of such solutions will require more effective communication and technology transfer between researchers, policy makers and land managers.
5. Conclusion
Considering species in agro-ecosystems as networks of interacting species allows new management tools to be used. Here, considering bumble-bees and the flowers they visit in margin mixes as pollination networks enabled the potential consequences of global warming to be modelled, along with some potential solutions to its negative effects. The idea that global warming affects interactions between species is not new; here though we have shown that warming can lead to a curtailment of the period during which interactions take place. Given that bumble-bees are regarded as key pollinators in both natural and managed systems, there may be knock-on effect in the provision of ecosystem services during these periods of curtailment unless remedial action is taken.
Acknowledgements
The bee and plant data used for our analyses were collected under the Buzz Project, funded by Defra, Syngenta and Unilever Plc.
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Benton T.2006Bumblebees. London, UK: Collins [Google Scholar]
- Biesmeijer J. C., et al. 2006Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 (doi:10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- Boatman N. D., Jones N. E., Garthwaite D., Pietravalle S.2007Option uptake in entry level scheme agreements in England. Aspects Appl. Biol. 81, 309–316 [Google Scholar]
- Bosch J., Gonzalez A. M. M., Rodrigo A., Navarro D.2009Plant–pollinator networks: adding the pollinator's perspective. Ecol. Lett. 12, 409–419 (doi:10.1111/j.1461-0248.2009.01296.x) [DOI] [PubMed] [Google Scholar]
- Both C., van Asch M., Bijlsma R. G., van den Burg A. B., Marcel M. E.2009Climate change and unequal phenological changes across four trophic levels: constraints or adaptations. J. Anim. Ecol. 78, 73–83 (doi:10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- Carvell C., Roy D. B., Smart S. M., Pywell R. F., Preston C. D., Goulson D.2006Declines in forage availability for bumblebees at a national scale. Biol. Conserv. 132, 481–489 (doi:10.1016/j.biocon.2006.05.008) [Google Scholar]
- Carvell C., Meek W. R., Pywell R. F., Goulson D., Nowakowski M.2007Comparing the efficacy of agri-environment schemes to enhance bumblebee abundance and diversity on arable field margins. J. Appl. Ecol. 44, 29–40 (doi:10.1111/j.1365-2664.2006.01249.x) [Google Scholar]
- defra. Action plan to develop organic farming in England. 2002. See http://www.defra.gov.uk/farm/organic/actionplan/indexhtm .
- Dunne J. A., Williams R. J., Martinez N. D.2002Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett. 5, 558–567 (doi:10.1046/j.1461-0248.2002.00354.x) [Google Scholar]
- Dunne J. A., Harte J., Taylor K. J.2009Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol. Monogr. 73, 69–86 (doi:10.1890/0012-9615(2003)073[0069:SMFPRT]2.0.CO;2) [Google Scholar]
- Fitter A. H., Fitter R. S. R.2002Rapid changes in flowering time in British plants. Science 296, 1689–1691 (doi:10.1126/science.1071617) [DOI] [PubMed] [Google Scholar]
- Forup M. L., Henson K. E., Craze P. G., Memmott J.2008The restoration of ecological interactions: plant–pollinator networks on ancient and restored heathlands. J. Appl. Ecol. 45, 742–752 (doi:10.1111/j.1365-2664.2007.01390.x) [Google Scholar]
- Gibson R. H., Nelson I. L., Hopkins G. W., Memmott J.2006Pollinator webs, plant communities and the conservation of rare plants: arable weeds as a case study. J. Appl. Ecol. 43, 246–257 (doi:10.1111/j.1365-2664.2006.01130.x) [Google Scholar]
- Goulson D.2003Bumblebees their behaviour and ecology , 2nd edn.Oxford, UK: Oxford University Press [Google Scholar]
- Hegland S. J., Nielsen A., Lazaro A., Bjerknes A. L., Totland O.2009How does climate warming affect plant–pollinator interactions? Ecol. Lett. 12, 184–195 (doi:10.1111/j.1461-0248.2008.01269.x) [DOI] [PubMed] [Google Scholar]
- Hole D. G., Perkins A. J., Wilson J. D., Alexander I. H., Grice F., Evans A. D.2005Does organic farming benefit biodiversity? Biol. Conserv. 122, 113–130 (doi:10.1016/j.biocon.2004.07.018) [Google Scholar]
- IPCC 2001Climate Change 2001: Third Assessment Report of the Intergovernmental Panel on Climate Change (WG 1 and 2). Cambridge, UK: Cambridge University Press [Google Scholar]
- Ives A. R., Cardinale B. J.2004Food-web interactions govern the resistance of communities after non-random extinctions. Nature 429, 174–177 (doi:10.1038/nature02515) [DOI] [PubMed] [Google Scholar]
- Lye G. C., Park K., Osborne J., Holland J., Goulson D.2009Assessing the value of Rural Stewardship schemes for providing foraging resources and nesting habitat for bumblebee queens (Hymenoptera: Apidae). Biol. Conserv. 142, 2023–2032 (doi:10.1016/j.biocon.2009.03.032) [Google Scholar]
- McCann K.2007Protecting biostructure. Nature 446, 29 (doi:10.1038/446029a) [DOI] [PubMed] [Google Scholar]
- Memmott J., Craze P. G., Waser N. M., Price M. V.2007Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 10, 710–717 (doi:10.1111/j.1461-0248.2007.01061.x) [DOI] [PubMed] [Google Scholar]
- Muller C. B., Schmidhempel P.1992Correlates of reproductive success among field colonies of Bombus lucorum—the importance of growth and parasites. Ecol. Entomol. 17, 343–353 (doi:10.1111/j.1365-2311.1992.tb01068.x) [Google Scholar]
- Nabhan G. P., Buchmann S. L.1997Services provided by pollinators. In Nature's services—societal dependence on natural ecosystems (ed. Daily G. E.), pp. 133–150 Washington, DC: Island Press [Google Scholar]
- Natural England 2008ELS handbook, 2nd edn. October 2008. See http://www.naturalengland.org.uk/Images/NE%20ES%20ELS_tcm6-6505.pdf [Google Scholar]
- Osborne J. L., Corbet S. A.1994Managing habitats for pollinators in farmland. Aspects Appl. Biol. 40, 207–215 [Google Scholar]
- Pounds J. A., et al. 2006Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167 (doi:10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- Price M. V., Waser N. M.1998Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79, 1261–1271 (doi:10.1890/0012-9658(1998)079[1261:EOEWOP]2.0.CO;2) [Google Scholar]
- Proctor M., Yeo P., Lack A.1996The natural history of pollination. London: Harper Collins Publishers [Google Scholar]
- Pywell R. F., Hulmes L., Meek W., Nowakowski M. Creation and management of pollen and nectar habitats on farmland: annual report 2007/8. 2008. See http://nora.nerc.ac.uk/6443/
- Roy D. B., Sparks T. H.2000Phenology of British butterflies and climate change. Glob. Change Biol. 6, 407–416 (doi:10.1046/j.1365-2486.2000.00322.x) [Google Scholar]
- Rusterholz H. P., Erhardt A.1998Effects of elevated CO2 on flowering phenology and nectar production of nectar plants important for butterflies of calcareous grasslands. Oecologia 113, 341–349 (doi:10.1007/s004420050385) [DOI] [PubMed] [Google Scholar]
- Solé R. V., Montoya M.2001Complexity and fragility in ecological networks. Proc. R. Soc. Lond. B 268, 2039–2045 (doi:10.1098/rspb.2001.1767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Putten W. H., Macel M., Visser M. E.2010Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Phil. Trans. R. Soc. B 365, 2025–2034 (doi:10.1098/rstb2010.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., van Noordwijk A. J., Tinbergen J. M., Lessells C. M.1998Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 (doi:10.1098/rspb.1998.0514) [Google Scholar]
- Walther G.-R.2010Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019–2024 (doi:10.1098/rstb.2010.0021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H.1982The distribution and decline of British bumble bees (Bombus Latr.). J. Apicult. Res. 21, 236–245 [Google Scholar]
- Williams P. Bombus: bumblebees of the world. 2007 See http://www.nhm.ac.uk/research-curation/research/projects/bombus/ [Google Scholar]