Abstract
Climate change will exacerbate the degree of abiotic stress experienced by semi-arid ecosystems. While abiotic stress profoundly affects biotic interactions, their potential role as modulators of ecosystem responses to climate change is largely unknown. Using plants and biological soil crusts, we tested the relative importance of facilitative–competitive interactions and other community attributes (cover, species richness and species evenness) as drivers of ecosystem functioning along stress gradients in semi-arid Mediterranean ecosystems. Biotic interactions shifted from facilitation to competition along stress gradients driven by water availability and temperature. These changes were, however, dependent on the spatial scale and the community considered. We found little evidence to suggest that biotic interactions are a major direct influence upon indicators of ecosystem functioning (soil respiration, organic carbon, water-holding capacity, compaction and the activity of enzymes related to the carbon, nitrogen and phosphorus cycles) along stress gradients. However, attributes such as cover and species richness showed a direct effect on ecosystem functioning. Our results do not agree with predictions emphasizing that the importance of plant–plant interactions will be increased under climate change in dry environments, and indicate that reductions in the cover of plant and biological soil crust communities will negatively impact ecosystems under future climatic conditions.
Keywords: facilitation, competition, climate change, Mediterranean, biotic interactions
1. Introduction
The study of biotic interactions among plants, understood as ‘the effect of one individual plant on another individual of either the same or a different species’ (Brooker 2006), has been a core research theme since the early days of ecology (Oosting 1948; Keddy 2001; Callaway 2007). These plant–plant interactions are ubiquitous in most terrestrial ecosystems, and interact with habitat suitability and dispersal to determine the structure of plant populations and communities (Callaway et al. 2005; Valiente-Banuet & Verdú 2007; Chu et al. 2008; but see Mitchell et al. 2009). Because the important roles they play in determining the array of functional traits within plant communities, which interact with the environment to affect processes such as nutrient-cycling, positive (facilitative) and negative (competitive) interactions have been frequently invoked as major controls of ecosystem functioning (Hooper et al. 2005; Michalet et al. 2006; Yachi & Loreau 2007). However, relatively few studies have empirically examined how these interactions affect the functioning of whole ecosystems (Mulder et al. 2001; Kikvidze et al. 2005). Therefore, the importance (sensu Welden & Slauson 1986) of facilitation and competition for maintaining ecosystem functioning is largely unknown (Callaway 2007).
Assessing the direct effects of biotic interactions on ecosystem functioning using the large body of literature available is not an easy task because the vast majority of studies have simplified the complexity of natural communities by evaluating the interaction between a single or a few pairs of species. Such studies constituted over 81 per cent of the ca 400 studies reviewed in recent syntheses on facilitation in plant communities (Flores & Jurado 2003; Brooker et al. 2008). In response, the study of facilitative–competitive interactions among all the members of a given community is now gaining increased attention (e.g. Cavieres et al. 2006; Dullinger et al. 2007; Valiente-Banuet & Verdú 2007; Maestre et al. 2008), and there is a clear need to devote more research efforts to explore such interactions at this level if we aim to substantially advance our understanding of their role as drivers of ecosystem functioning (Brooker et al. 2008; Maestre et al. 2009a). However, conducting field experiments to assess the outcome of biotic interactions at the community level is logistically difficult, if not impossible, in most ecosystems. Although the attribution of patterns to processes cannot be made without uncertainty using solely observational approaches, they are being increasingly used and recommended for this aim (Brooker et al. 2008; Maestre et al. 2009a). In this direction, recent experimental studies provide evidence that spatial aggregation promotes coexistence in plant communities (Stoll & Prati 2001; Monzeglio & Stoll 2005). Other studies have shown that processes such as competition and facilitation may be inferred through the observation of segregation and aggregation patterns, respectively (Purves & Law 2002; Tirado & Pugnaire 2005), and that changes in the net outcome of interactions promoted by abiotic stress may be tracked by parallel shifts in the fine-scale spatial arrangement and aggregation of plant communities (Kikvidze et al. 2005).
Semi-arid ecosystems, which cover 41 per cent of Earth's land surface and support over 38 per cent of the total global population of 6.5 billion (Reynolds et al. 2007), are among the most sensitive ecosystems to climate change (Körner 2000). In the semi-arid areas of the Mediterranean basin, predicted modifications in climate—a sharp decrease in water availability, an increase of temperature by up to 7°C in summer by the end of the twenty-first century and a higher overall climate variability (de Castro et al. 2005; Rowell & Jones 2006)—are going to substantially exacerbate the degree of abiotic stress these communities experience (Schröter et al. 2005). Substantial research efforts have been devoted over the last decade to predict how the interplay of facilitative and competitive interactions varies along abiotic stress gradients driven by precipitation and temperature (e.g. Callaway et al. 2002; Maestre & Cortina 2004; Holzapfel et al. 2006). This body of research has shown that modifications in the degree of abiotic stress have major impacts on plant–plant interactions, although the specific effects of such modifications on the magnitude and direction of these interactions are still being debated (Maestre et al. 2005a, 2006, 2009a; Lortie & Callaway 2006; Smit et al. 2009).
Because co-occurring species differ in their tolerance to abiotic stress, which in turn affects the outcome of plant–plant interactions (Liancourt et al. 2005; Wang et al. 2008), the study of one or a few particular pairs of species—followed by most facilitation–competition research carried out to date (Keddy 2001; Callaway 2007)—may not be sufficient to accurately predict how biotic interactions within a given community will change along abiotic stress gradients. Despite its importance, very few empirical studies have evaluated how the outcome of facilitative–competitive interactions at the community level vary along wide-ranging abiotic stress gradients (i.e. involving more than two levels; Kikvidze et al. 2005; Dullinger et al. 2007; Maestre et al. 2009b), and how joint changes in these interactions and in climate affect ecosystem functioning (Kikvidze et al. 2005). Therefore, and despite it having been hypothesized that climate change may also exert indirect effects on ecosystem functioning by influencing competitive and facilitative interactions (see Brooker 2006; Tylianakis et al. 2008 for reviews), it is difficult to know whether these interactions can control ecosystem responses to climate change, particularly when compared with community attributes with important functional roles, such as diversity (Reiss et al. 2009).
In this article we focus on the following question: do biotic interactions and other community attributes modulate ecosystem functioning along climatic gradients in semi-arid Mediterranean ecosystems? We hypothesize that both biotic interactions and attributes such as cover and diversity (species richness and evenness) will drive variations in ecosystem functioning along abiotic stress gradients, and thus have the potential to drive ecosystem responses to climate change. To test this hypothesis, we used multiple biotic communities (vascular plants and biological soil crusts (BSCs) formed by mosses and lichens) and experimental approaches (natural and manipulated climatic gradients at different spatial scales). We also sought to include a comprehensive set of ecosystem processes and variables related to ecosystem functioning, something critical when assessing the functional role of biotic communities (Reiss et al. 2009). Our combined use of multiple experimental approaches, biotic communities and spatial scales to test the role of stress and biotic interactions on ecosystem functioning has not, to our knowledge, been attempted before. Such an integrated approach can provide broader insights on the functional role of biotic interactions and other attributes of biotic communities, and on their respective potential as drivers of ecosystem responses to climate change.
2. Material and methods
To achieve our objective, four independent studies have been carried out in various semi-arid environments of Spain. Three of these studies (hereafter named as Studies 1, 2 and 3) use BSCs as a model system. The last study (Study 4) targets vascular perennial vegetation. In Studies 1, 2 and 4, variations in abiotic stress were induced by natural changes in climatic and/or topographic conditions, while in Study 3 such variations were promoted through experimental manipulations. We decided to focus on BSC in most of the studies because they are a key biotic component of semi-arid environments (Belnap & Lange 2003), a good model system to explore the question posed here (Bowker et al. 2010) and are clearly under-represented in the facilitation–competition literature (e.g. Callaway 2007). The results presented from Studies 1 and 4 are new re-analyses of previously published data (Maestre et al. 2008, 2009b; Maestre & Escudero 2009).
(a). Measuring ecosystem functioning
In each study we measured between one and seven of the following soil variables: respiration (Studies 3 and 4), organic carbon (Study 4), water-holding capacity (Study 4), soil compaction (Study 4) and the activity of three enzymes related to the carbon (β-glucosidase; Studies 1, 2 and 4), nitrogen (urease; Studies 1 and 4) and phosphorus (phosphatase; Studies 1, 2 and 4) cycles. These variables either measure ‘true’ ecosystem functions (sensu Reiss et al. 2009; e.g. respiration) or are key determinants of processes such as infiltration (compaction and water-holding capacity; Castellano & Valone 2007) and nutrient-cycling (organic carbon and soil enzymes; Wallenstein & Weintraub 2008), which are critical determinants of the functioning of semi-arid ecosystems (Whitford 2002).
In Studies 1, 2 and 4, these variables were measured in the laboratory using air-dried samples as described in the electronic supplementary material, appendix A. In Study 3, soil respiration was measured 14 times during the study period in all plots using a LI-COR 8100 Automated Soil Respirometer (LI-COR, Lincoln, NE, USA) as described in the electronic supplementary material, appendix A.
(b). Study 1: variations in biotic attributes, interactions and ecosystem functioning in BSC communities along a small-scale natural environmental gradient
This study was conducted in gypsum outcrops located next to Belmonte del Tajo, in Central Spain (40°7′3″ N, 3°18′30″ W, 686 m.a.s.l.). The climate is Mediterranean semi-arid, with a mean annual temperature and rainfall of 14°C and 452 mm, respectively. The studied gypsum outcrops are surrounded by a well-preserved forest of Quercus ilex L. and Pinus halepensis Miller, but perennial plant cover within them remains below 20 per cent (electronic supplementary material, figure S1).
A total of 63 plots (50 × 50 cm), spread over a homogeneous area of 1.3 ha, were placed non-randomly on bare ground areas with well-developed BSC-forming lichen communities. This non-random placement of plots is commonly followed with BSCs because of the small size and high within-site spatial variability of the organisms constituting them (Maestre et al. 2005b; Bowker et al. 2006; Martínez et al. 2006). However, a minimum separation distance of 0.7 m between sampling units was established to minimize the risk of sampling non-independent areas owing to the spatial structure of BSC. Much of the spatial variation in the cover of BSC organisms in semi-arid Mediterranean areas occurs at spatial scales smaller than the 50 × 50 cm quadrats used (Maestre 2003), and with this separation distance we sought to remove potential sources of non-independence between sampling quadrats. With this survey, we aimed to capture the greatest possible contrast in lichen and moss community composition and structure, avoiding changes in the proportion of suitable habitat among the plots that could confound the interpretation of the co-occurrence patterns observed (Dullinger et al. 2007).
Species richness was estimated as the number of lichen species present in each plot. For the estimation of cover, species evenness and biotic interactions (described below), each plot was divided into 100 sampling quadrats of 5 × 5 cm, and the cover of every lichen species was estimated. The average of the cover of all lichens in the 100 quadrats was used as our estimate of total plot cover. Species evenness was calculated using the Pielou's J index (Pielou 1975). Abiotic stress promoted by changes in resource availability was indirectly measured in every plot using two variables: slope angle and soil surface roughness (for details see the electronic supplementary material, appendix A). Slope angle is related to the radiation dose and heat load, and is an important abiotic factor controlling the distribution of soil lichens (Hauck et al. 2007). Soil surface roughness is related to the runoff and infiltration dynamics, and thus to water availability. An independent calibration proved that soil surface roughness measured with this index was negatively related to soil moisture at the 0–2 cm depth after spring rainfalls at the study site (electronic supplementary material, figure S2).
(c). Study 2: variations in biotic attributes, interactions and ecosystem functioning in BSC communities along a regional natural environmental gradient
This study was conducted at eight sites located along a 350 km gradient from central to south-eastern Spain. Among the sites, average annual precipitation and temperatures ranged from 334 to 497 mm, and from 13 to 15°C, respectively. Vegetation was in most cases open grasslands dominated by Stipa tenacissima L. (electronic supplementary material, figure S3), whereas three were open woodlands dominated by P. halepensis. Five sites were located on soils derived from limestone, while three sites were located on gypsum-rich soils. All selected sites exhibited continuous or, more commonly, patchy biological crusts in interspaces between plants, with sharp differences in community structure between the limestone-derived and gypsiferous soils. On gypsum soils, BSC were dominated by lichens such as Diploschistes diacapsis (Ach.) Lumbsch, Squamarina lentigera (Weber) Poelt, Fulgensia subbracteata (Nyl.) Poelt and Psora decipiens (Hedw.) Hoffm. On calcareous soils they were dominated by the mosses Pleurochaete squarrosa (Brid.) Lindb., Didymodon sp. and by the lichen Cladonia convoluta (Lam.) Anders.
Within each site, 7–10 line intercept transects (150 cm in length) were sampled for the assessment of community properties. Transects were non-randomly placed in multi-specific BSC patches that were dense enough to calculate biotic interactions. Transect placement was in all cases greater than 30 cm from the nearest perennial shrub or tussock grass. A minimum separation distance of 1 m between transects was established to minimize the risk of sampling non-independent areas. In a few cases, small interspace size made it difficult to place 150 cm transects, thus two parallel transects of 75 cm, and spaced 30 cm apart were sampled. Species richness was estimated as the total number of moss and lichen species found in each transect. Cover was estimated as the proportion of the total length of the transect covered by these organisms. Species evenness was calculated using the Pielou's J index. For the estimation of biotic interactions (detailed below), we divided each transect into 30 segments of 5 cm and for each, we recorded the presence of every bryophyte and lichen species intercepted along the segment. Climatic attributes (annual radiation, temperature and rainfall) were collected for each site using the available climatic interpolations for the Iberian Peninsula (Ninyerola et al. 2005).
(d). Study 3: simulated climate change impacts on ecosystem functioning in BSC communities
This study is part of an ongoing research programme, started in July 2008, devoted to evaluate climate change impacts on BSC-dominated ecosystems. It is being conducted in gypsum outcrops located in the vicinity of Aranjuez, in the centre of the Iberian Peninsula (40°02′ N–3° 37′ W; 590 m.a.s.l.). The climate is Mediterranean semi-arid, with an average annual rainfall and temperature of 456 mm and 13.8°C, respectively. Perennial plant cover is below 40 per cent, and is dominated by S. tenacissima; isolated individuals of the evergreen shrub Retama sphaerocarpa (L.) Boiss. are also present. The open areas between perennial plants are colonized by a well-developed BSC, dominated by lichens such as D. diacapsis, Squamarina lentigera, F. subbracteata and Psora decipiens.
We set-up a factorial experiment at this site with three treatments: BSC (poorly developed BSC communities with cover less than 5% versus well-developed BSC communities with cover greater than 50%); temperature increase (control versus increased temperature); and rainfall reduction (control versus a 20% reduction in annual rainfall). The working plots (1 m2 size) were randomly selected among suitable bare ground areas, and the eight combinations of treatments were randomly assigned to the plots. Ten replicates per combination of treatments were established, resulting in a total of 80 plots. As the rainfall reduction treatment was installed in November 2008, we report here preliminary results of the temperature increase treatment (July 2008–September 2009). In this experiment, we aim to achieve an annual increase in air temperature of 2–4°C, according to model predictions for Central Spain by the late twenty-first century (de Castro et al. 2005). For doing this, we used open top chambers (OTC) as described in the electronic supplementary material, appendix A (see also in the electronic supplementary material, figures S4 and S5).
Within each plot, we installed a PVC collar (20 cm diameter, 8 cm height) for soil CO2 measurements. The cover, species richness and evenness (Pielou's J index) of the BSC community (bryophytes and lichens) were estimated within each collar at the beginning of the experiment using the point-sampling method (1 × 1 cm grid; 120 sampling points per collar). For the estimation of biotic interactions (detailed below), we divided each collar into 19 circles with a diameter of 4 cm, spaced by about 2 cm which was not sampled, and for each we recorded the presence of every bryophyte or lichen species.
(e). Study 4: variations in biotic attributes, interactions and ecosystem functioning in S. tenacissima grasslands along a regional natural environmental gradient
This study was conducted in 29 S. tenacissima steppes located along the same central to southeastern Spain gradient described for Study 2. Most sites (22) were located on soils derived from limestone, while seven sites were located on gypsum-rich soils. All sites were placed on south-facing gentle slopes. Vegetation was in all cases open grasslands dominated by S. tenacissima (electronic supplementary material, figure S2), and contained shrub species like Quercus coccifera L. and Rosmarinus officinalis L. in calcareous soils, and Lepidium subulatum L. and Gypsophila struthium L. in gypsum soils. Perennial plant cover ranged between 15 and 68 per cent (see Maestre & Escudero (2009) for further information on the study sites).
In each site, we established a 30 × 30 m plot for assessing the attributes of the perennial plant community. In the upper left corner of each plot, we located one 30 m long transect downslope. Three parallel transects of the same length, each 8 m apart across the slope, were added. In each transect, we collected a continuous record of all perennial vegetation patches intercepting the transect for estimating total cover. In each transect, we also placed 20 consecutive quadrats (1.5 × 1.5 m size), and the cover of every perennial species was visually recorded. These data were used to assess biotic interactions (described below) and species evenness (Pielou's J index). The number of perennial species per 900 m2 plot was used as an estimate of total species richness. Climatic attributes (annual radiation, temperature and rainfall) were also estimated for each site using the available climatic interpolations for the Iberian Peninsula (Ninyerola et al. 2005).
(f). Assessment of biotic interactions through co-occurrence patterns
To estimate the outcome of biotic interactions at the community level, we carried out null model analyses of co-occurrence patterns (Gotelli 2000). This approach has often been employed to evaluate the importance of competitive interactions as a force structuring biotic communities (see Gotelli & Graves 1996 for a review), and in recent years it is being used to explore both competitive and facilitative interactions in vascular plant and lichen communities (Dullinger et al. 2007; Maestre et al. 2008; Rooney 2008). We acknowledge that species co-occurrence can be affected by processes such as limited dispersal, habitat selection and clonal growth (Gotelli & Graves 1996; Abrahamson et al. 2005). However, we believe that these aspects can only marginally affect co-occurrence in the studied communities because of the characteristics of the surveys employed (which minimized the sampling of non-suitable habitat), the dispersal characteristics of the species studied (which make them quite unlikely to be dispersal-limited in the studied sites) and the prevalence of clonal species such as S. tenacissima in all the grasslands evaluated.
For each plot/transect, the data were organized as a presence–absence matrix, where each row and column represents a different species and quadrat/transect segment, respectively. We estimated co-occurrence in each of the sampled plots/transects (matrices) with the C-score index. It is calculated for each pair of species as (Ri−S)(Rj−S), where Ri and Rj are the matrix row totals for species i and j, and S is the number of squares in which both species occur; this score is then averaged over all possible pairs of species in the matrix (Gotelli 2000). If a community is structured by competitive or facilitative interactions, the C-score should be significantly larger or smaller than expected by chance, respectively. We selected the C-score among different available indices because it is robust to the presence of noise in the data and has good statistical properties (see Gotelli 2000 for a review).
The indices obtained from each matrix were compared with those derived from 10 000 randomly assembled matrices (null matrices), generated using a ‘fixed rows-equiprobable columns’ null model (see Gotelli 2000 for details). This null model has low type I error, good power to detect non-randomness, is particularly suitable for standardized samples collected in homogeneous habitats, and is appropriate for detecting patterns caused by species interactions (Gotelli 2000; Gotelli & Entsminger 2003).
Because the values of the C-score are dependent on the number of species and co-occurrences within each plot, we obtained a standardized effect size (SES) as (Iobs − Isim)/Ssim, where Iobs is the observed value of the C-score, and Isim and Ssim are the mean and standard deviation, respectively, of this index obtained from the 10 000 null communities (Gotelli & Entsminger 2006). Values of SES higher and lower than 0, respectively, indicate prevailing spatial segregation and aggregation among the species within a community. Null model analyses were conducted with EcoSim 7.22 (Gotelli & Entsminger 2006).
(g). Statistical analyses
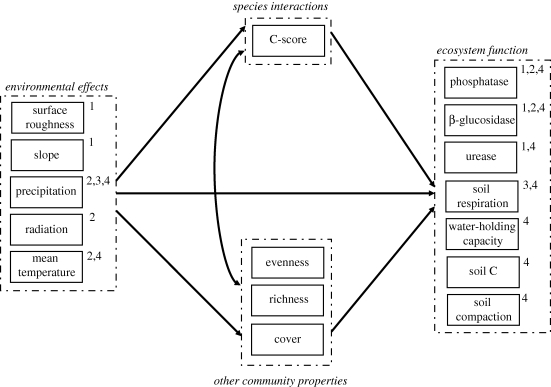
In Studies 1 and 2, we evaluated the relationships between biotic interactions, other attributes of biotic communities (species richness and cover), and the different surrogates of ecosystem functioning using structural equation modelling (SEM; Grace 2006). Generally, an investigator will propose an a priori model that features variables and hypothesized relationships among variables in a path diagram (figure 1). The second step is to estimate path coefficients using the maximum likelihood estimation technique. These are obtained for each pathway of the model by optimally adjusting the observed variance–covariance matrix to the path diagram. Standardized path coefficients range from 0 to 1, are analogous to regression weights or partial correlation coefficients, and describe the effect size of relationships in the model. At this time, researchers typically test the overall goodness-of-fit of the model against the dataset, poor goodness-of-fit indicating that a model is not a plausible causal scenario, which could have resulted in the covariance matrix of the dataset (Grace 2006). Our a priori model was saturated, meaning that there was a direct uni- or bi-directional relationship between every possible pairing of variables, because all were plausible hypotheses. We fitted the saturated model to estimate the path coefficients, and worked backward removing weak pathways which did not strongly impact the model fit (generally path with coefficients <0.05). The exception was that we retained paths from the C-score to ecosystem function variables in all models because these were a primary focus of the study. In this way we simplified to more parsimonious models which could be subjected to goodness-of-fit criteria, and various models may differ somewhat in their final structure. We used the traditional χ2 goodness-of-fit test, but since it is prone to some type I error, the RMSEA index and the Bollen-Stine bootstrap test were also considered as alternative indices of model fit (Grace 2006). These generally agree closely, and yield the probability of the implied covariance structure of the model fitting that of the data. Thus, and unlike many statistical tests, low probability values are not desired.
Figure 1.
Generalized a priori conceptual model depicting pathways by which climate, and therefore climate change, and species interactions may influence ecosystem function, along with important covariates. Dashed boxes depict conceptual variables which may be represented by multiple measurements. Solid boxes depict measurements available in at least one of three datasets. Single-headed arrows signify a hypothesized causal influence of one variable upon another. Double-headed arrows signify a correlation in which no direction is specified, possibly owing to shared causal influences; though not shown for simplicity, measured variables within dashed boxes are expected to intercorrelate. Superscript numbers denote which of the four studies each variable was used in.
In our SEM models we employ composite variables, which allow an additive combination of the effects of multiple conceptually related variables upon a response variable (Grace 2006). Composite variables are primarily a graphical and numerical interpretation tool, and do not change the underlying model. In both datasets, we used a composite entitled ‘community properties’ that represents the summed effects of total cover, species richness and species evenness. Likewise, we used composites (‘environmental effects’) to pool the effects of variables related to climate or microclimate: annual precipitation, mean annual temperature and mean annual radiation in the dataset from Study 2, and slope angle and surface roughness in that from Study 1. However, and to determine which individual variables were explicitly active within each composite, we also examined the underlying observed variable models with the composite variables removed. SEM analyses were performed using AMOS (SPSS Inc., Chicago, IL, USA).
In the case of the dataset gathered from Study 4, there was insufficient sample size for carrying out SEM analyses (n = 29), and instead we used path analysis (Shipley 2001) based upon partial Mantel statistics (Smouse et al. 1986) to construct a model with similar structure. The interpretation is similar, except that a ‘variable’ is actually a distance matrix based upon multiple conceptually related variables using squared Euclidean distance. The environment matrix contained annual precipitation, mean annual temperature and mean annual radiation. The community properties matrix contained total cover, species richness and species evenness. The ecosystem function matrix contained soil compaction, soil C, respiration, water-holding capacity and enzyme (phosphatase, β-glucosidase and urease) activities. The C-score matrix was simply the C-score data expressed in distance matrix format. Our approach was similar to that of Leduc et al. (1992), except that we did not remove paths based upon probability values, and emphasize the path coefficient (equivalent to the partial Mantel statistic) as our measure of effect size. Partial and bivariate Mantel statistics were obtained in R 2.6.2 (www.r-project.org), using the Ecodist package (Goslee & Urban 2007). r2 of endogenous variables was calculated using the formula in McCune & Grace (2002). As a post hoc test to determine the individual effects of particular community attributes on ecosystem functions, we repeated the analyses three times, substituting the community attributes matrix with matrices representing cover, richness and evenness alone. We did the same with the environment matrix to see the effect of each abiotic factor measured separately on the C-score.
Soil respiration data from Study 3 were analysed separately for plots with low and high BSC cover. Data from the former were analysed using repeated-measures analysis of variance (ANOVA), with OTC as a fixed factor. Data from the high cover plots were analysed using repeated-measures analysis of covariance (ANCOVA), with OTC as a fixed factor and biotic interactions, cover, species evenness and species richness as covariates. Separate analyses were carried out for each of these covariates. Prior to these analyses, respiration data were log-transformed to achieve the homogeneity of variances in their distribution. ANOVA analyses were performed using SPSS for Windows 17.0 (SPSS Inc.).
3. Results
(a). Variations in biotic attributes, interactions and ecosystem functioning in BSC communities
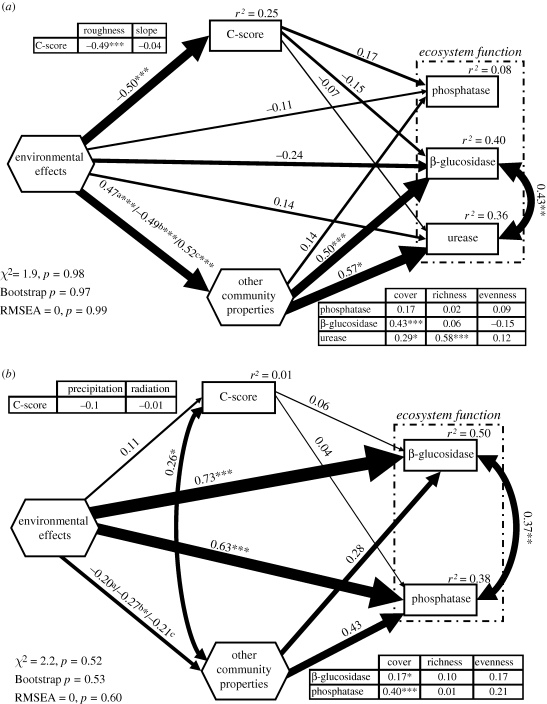
In Study 1 (figure 2a), our SEM model was able to explain greater than one-third of the variance in both β-glucosidase and urease (r2 > 0.35 in both cases), respectively, but had little explanatory power for phosphatase (r2 = 0.08). This model was satisfactorily fitted to our data, as indicated by the different goodness-of-fit statistics (figure 2a). Most of the variance was accounted for by community properties other than species interactions, particularly cover, whereas direct effects of environmental variables and species interactions were relatively small (absolute value of r ≤ 0.26). The abiotic variables measured exerted important indirect effects on the soil enzymes via their direct influence upon species interactions and other community properties (absolute value of r ≥ 0.47).
Figure 2.
Final SEM models for the datasets obtained from Studies (a) 1 and (b) 2. Numbers adjacent to arrows are path coefficients, analogous to regression weights and indicative of the effect size of the relationship. Width of arrows is proportional to the strength of path coefficients. As in other linear models, r2 signifies proportion of variance explained and appears above every response variable in the model. Goodness-of-fit statistics for each model are shown in the lower left corner. There are some differences between the a priori model and the final model structures owing to removal of paths with coefficients close to zero; these modifications make only trivial changes in the models. Inset tables reflect the individual path coefficients from variables that compose the composite variables, and C-score or function variables. a, b and c denote path coefficients from environmental effects composite to species richness, total cover and species evenness, respectively. Significance levels are as follows: *p < 0.05, **p < 0.01 and ***p < 0.001.
In Study 2 (figure 2b), our SEM was able to explain 50 and 38 per cent of the variance in β-glucosidase and phosphatase, respectively. Our data satisfactorily fitted this model, as shown by the different goodness-of-fit statistics (figure 2b). In contrast to experiment 1, the largest contribution to the variance explained was the direct effect regional climatic variables (β-glucosidase r = 0.73, phosphatase r = 0.63). Of secondary importance were the effects of community properties such as total cover, which in the case of phosphatase were moderately strong (r = 0.40). These effects were in turn moderately affected by the environmental gradients (absolute value of r between 0.20 and 0.30). Both the effects of species interactions and evenness upon the soil enzymes evaluated and the effects of environmental gradients upon species interactions were weak. However, species interactions indirectly affected ecosystem functioning via their moderate effects on community properties.
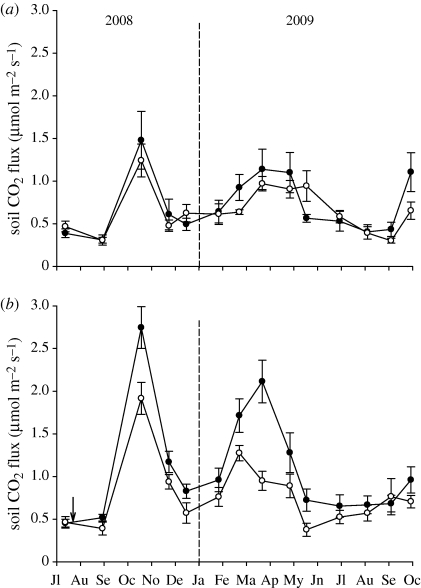
In Study 3, plots with well-developed BSCs showed higher soil respiration than plots without BSCs (figure 3). The OTCs promoted an average increase in temperature of 2.6°C compared with the control treatment over the study period (electronic supplementary material, figure S5). This temperature augment promoted a slight, but not significant, increase in soil CO2 flux in the plots without BSCs (figure 3a; repeated-measures ANOVA; FOTC = 0.05, d.f. = 1,18, p = 0.818). However, such an increase was clearly significant in plots with well-developed BSC cover, regardless of the covariate considered (figure 3b; repeated-measures ANCOVA; FOTC > 8.7, d.f. = 1,18, p < 0.010 in all cases). The magnitude of these differences was not affected by biotic interactions, species richness or evenness (F1,18 < 1.82, p > 0.195 in all cases), but the effect of total BSC cover was marginally significant (F1,18 = 3.91, p = 0.063). Although this covariable did not affect the overall OTC effect, the amount of CO2 respired over the study period per unit of BSC cover was higher in the elevated temperature plots (0.017 ± 0.0014 versus 0.011 ± 0.0009 (unitless), means ± s.e., n = 10 and 11, respectively; Mann–Whitney test, Z = −2.817, p = 0.004).
Figure 3.
Soil CO2 flux in plots with low (a) or high (b) biological soil crust cover subjected to ambient (control, open circles) or elevated (OTC, filled circles) temperature in the Aranjuez study site, Central Spain. The arrow indicates the date when the elevated temperature treatment was installed. The first datapoint (July 2008) is not included in the statistical analyses. Data represent means ± s.e. (n = 10).
(b). Variations in biotic attributes, interactions and ecosystem functioning in S. tenacissima grasslands
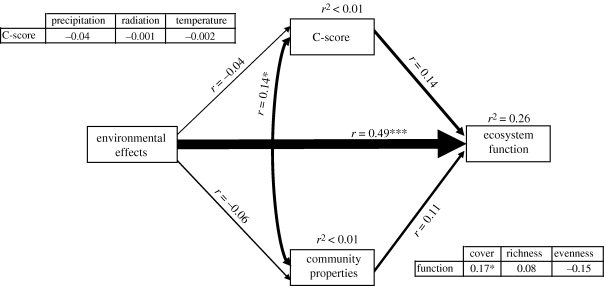
In Study 4, the overall variation explained by the model was relatively low (r2 = 0.26; figure 4). Variations in both the C-score and the attributes of biotic communities were poorly related to changes in abiotic conditions (r2 < 0.01 in both cases). The greatest direct effect upon the ecosystem function matrix was the environmental matrix (r = 0.49). The effects of both the C-score and the community properties matrix on the ecosystem function matrix were non-significant. However, when the effects of the different biotic attributes were considered in isolation from each other, the cover was significantly related to ecosystem functioning (r = 0.17).
Figure 4.
Final partial Mantel path analysis for the dataset gathered in Study 4. Boxes represent matrices of conceptually related variables. Numbers adjacent to arrows are partial Mantel coefficients, analogous to regression weights among matrices rather than univariate variables, and indicative of the effect size of the relationship. Width of arrows is proportional to path coefficients. Inset tables reflect the results from separate post hoc tests, wherein univariate matrices of the various community attributes and abiotic variables substituted the ‘community properties’ and ‘environmental effects’ matrices, respectively. The changes in the rest of the paths observed during these tests are negligible, and are not shown for clarity. Significance levels are as follows: *p < 0.05, **p < 0.01 and ***p < 0.001.
4. Discussion
Overall, we found little evidence to suggest that biotic interactions, as measured by the standardized effect of the C-scores, are a major direct influence upon indicators of ecosystem function along abiotic stress gradients driven by changes in water availability and temperature. This result was consistent in all the studies conducted, regardless of the spatial scale considered, the experimental approach followed and the organisms being targeted. Despite the lack of direct effects of species interactions, in most cases over one-quarter of the variation was explained in most ecosystem function indicators by direct effects of environmental gradients or other community properties. Semi-arid ecosystems are primarily abiotically driven (Whitford 2002), and the ecosystems studied are not an exception. However, attributes such as cover, and to a lesser degree, species richness directly and significantly influenced many of the functional surrogates measured. Therefore, our results provide evidence for a strong biotic control on ecosystem functioning in Mediterranean semi-arid ecosystems dominated by both plants and BSCs.
(a). Effects of biotic interactions and community attributes on ecosystem functioning
Although currently under revision (Maestre et al. 2009a; Smit et al. 2009), conceptual models widely employed when studying the outcome of plant–plant interactions along abiotic stress gradients (e.g. the ‘stress-gradient hypothesis’ of Bertness & Callaway (1994)) have promoted the idea that facilitative interactions should be more frequent, and thus more important for ecosystem structure and functioning, under high abiotic stress conditions (see Callaway 2007 for a review). Indeed, it has been suggested that the importance of facilitation in dry Mediterranean environments should increase with the ongoing climatic change (Brooker 2006). On the other hand, and across latitudinal gradients, there is a large body of literature on trophic interactions suggesting that biotic interactions are more important at low latitudes (see Schemske et al. 2009 for a recent review). Co-occurrence patterns, our surrogate for biotic interactions, were found to vary substantially with abiotic stress only at small spatial scales (figure 1b). Regardless of these variation patterns, our results clearly indicate that the relative importance of the outcome of facilitative–competitive interactions at the community level as a driver of variations in ecosystem functioning along abiotic stress gradients is lower than that of other attributes of biotic communities. In other words, the importance of such outcomes (sensu Welden & Slauson 1986) for the functioning of the studied ecosystems is not as high as one would expect, given the frequency and the functional roles commonly attributed to biotic interactions in arid and semi-arid environments (Whitford 2002; Callaway 2007). Do our results indicate that biotic interactions are not important for ecosystem functioning? While this may be the first impression drawn from our analyses, we cannot extrapolate beyond our data and the ecosystems studied, and more empirical examples are clearly needed to evaluate the generality of our results. It is worth noting that finding ample evidence of competition and facilitation does not imply that such processes are necessarily playing a predominant role in ecosystem processes (Brooker et al. 2008). While the intensity of these interactions can be very high, their impact relative to other processes (i.e. their importance) to determine ecosystem structure and functioning may vary from high to low (see Brooker et al. 2005; Lamb & Cahill 2008; Mitchell et al. 2009 for recent examples).
Our findings resemble those from previous studies evaluating the relative importance of spatial pattern, which is commonly determined by biotic interactions (e.g. Eccles et al. 1999), and cover/richness as drivers of ecosystem functioning (Maestre et al. 2005b; Maestre & Escudero 2009). These studies, conducted in the same ecosystems studied here, found that the magnitude of the relationship between spatial pattern and the surrogates of ecosystem functioning was always lower than that between cover/species richness and the same surrogates. The patterns found also agree with many studies conducted in a wide variety of environments, including Mediterranean shrublands and semi-arid steppes, which have found strong and positive relationships between biotic attributes, such as cover and species richness on different surrogates of ecosystem functioning (e.g. Troumbis & Memtsas 2000; Martínez-Mena et al. 2002; Bastida et al. 2008; Montès et al. 2008).
Direct effects of biotic interactions on ecosystem functioning might be related to an increase in the efficiency of the whole community for using resources and recycling nutrients, the so-called ‘complementarity effect’—that implies a reduction in the competitive effects of some species on others (Callaway 2007)—or to direct or indirect facilitative effects leading to an increase in community productivity and ecosystem functioning (Knops et al. 1999; Mulder et al. 2001). In semi-arid environments, it is well known that facilitative–competitive interactions among plant species are important determinants of the heterogeneous spatial distribution of vegetation commonly found in these areas and of the formation of resource islands under the canopy of isolated shrub and grass species (Aguiar & Sala 1999). Why are we not observing significant effects of biotic interactions upon the surrogates of ecosystem functioning measured, particularly when compared with attributes such as total cover? While the mechanisms highlighted above can be important drivers of changes in vascular plant productivity, the surrogate of ecosystem functioning used in virtually all the relevant studies (Callaway 2007), they may not be so important when using soil variables acting as surrogates of processes related to nutrient-cycling. Direct effects of biotic interactions on these variables are likely to work primarily at the scale of plant patches (Cortina & Maestre 2005), and may not be translated to the inter-patch areas, which are the dominant land cover in arid and semi-arid ecosystems. However, soil attributes in these areas may be largely affected by plant patches (Maestre et al. 2009c), as these can modify the microclimate in their surroundings through shading, and can provide carbon, water and nutrients through processes such as hydraulic lift, lateral root growth and litter inputs (Breshears et al. 1998; Caldwell et al. 1998; Boeken & Orenstein 2001). The influence of plant patches on the functioning of the inter-patch areas is going to be increasingly important with changes in cover, irrespective of whether such changes are driven by facilitation or not.
Regarding BSC communities, positive interactions among soil lichens can occur through mechanisms such as increased nutrient availability close to N-fixing species like Collema spp. (Belnap 2002), and increased water availability in the surroundings of those species capturing dew (Kidron et al. 2002). On the other hand, negative interactions can arise through mechanisms such as allelopathy (Souza-Egipsy et al. 2002), genuine competitive displacement (Armstrong & Welch 2007) and reduced moisture availability by those species sealing the soil surface (Cantón et al. 2004). While the outcome of these processes will have functional consequences at the scale of the interacting organisms, the multiplicity of competitive and facilitative interactions happening within whole communities, and their opposite effects on processes such as nutrient-cycling (e.g. N fixation versus reduced soil moisture) can dilute the functional importance of biotic interactions at this scale. Our results provide, to our knowledge, the first empirical test of the relative importance of biotic interactions, cover and richness as drivers of ecosystem functioning in BSC-dominated ecosystems. They also agree with recent studies and syntheses conducted in BSC-dominated areas describing an important control of total cover and richness on soil nutrient-cycling (Maestre et al. 2005b; Bowker et al. 2010).
(b). Indirect and direct effects of changes in abiotic stress on ecosystem functioning
While the direct effects of increased temperature and modifications in rainfall amount and pattern are causing the most evident impacts of climate change on ecosystem functioning (e.g. Dormann & Woodin 2002; Fay et al. 2003; Emmett et al. 2004), indirect effects mediated through impacts upon biota can also play an important role in the way ecosystem function responds to such changes (Canadell et al. 2007). Recent studies conducted with model grassland communities have shown that biotic attributes such as biodiversity interact in complex ways with global change drivers such as elevated CO2 and increases in nutrient availability to determine ecosystem productivity and community nutrient use (e.g. Reich et al. 2004; Maestre & Reynolds 2006). Despite the recognized importance of these interactions and indirect effects, no studies like these have been conducted with biotic communities other than vascular plants.
Although widely employed to explore the impacts of climate change on organisms and ecosystem processes, results from observational approaches such as those employed here must be interpreted with caution because response to climate change might involve adaptation/plasticity that is neglected when working on natural environmental gradients, as populations are possibly already adapted to the local conditions (Giménez-Benavides et al. 2007; but see Gimeno et al. 2008). These limitations can be overcome by using experimental approaches such as those employed in Study 3. The preliminary results obtained from this study provide evidence of strong short-term effects of climate change on soil respiration, a response that was controlled by the total cover of BSCs (i.e. areas of low BSC cover did not show any response to the warming treatment), but not by species richness, evenness or biotic interactions. If warming increases soil respiration, particularly in areas of high BSC cover, BSC-forming organisms such as lichens and mosses may be at risk of C deficits, particularly when air temperatures are high and moisture is limited (Wilske et al. 2008). This situation may be exacerbated by other ongoing climatic alterations, such as increases in UV radiation, which has been shown to negatively affect the physiological functioning of BSC-forming organisms (Belnap et al. 2008). Our results indicate that BSCs will probably experience added stress under the forecasted future climatic conditions, which may in turn have negative feedbacks on many ecosystem functions modulated by these organisms (see Belnap & Lange 2003 for a review). The increase in soil respiration observed in the OTC treatment also agree with results from warming experiments conducted in shrublands and grasslands (Emmett et al. 2004; Zhou et al. 2006; but see Luo et al. (2001) and Lellei-Kovács et al. (2008)). Our measurement period is not long enough to meaningfully extrapolate the observed soil respiration responses, and to discuss the mechanisms driving them, but we would like to highlight the role played by BSC cover as a driver of these responses to experimental warming. These experimental results further add to the observations obtained from Studies 1, 2 and 4 to emphasize the role of total cover as another potentially key biotic attribute modulating the responses of semi-arid ecosystems to the ongoing environmental change.
5. Concluding remarks
Important research efforts are being devoted to incorporating the multiplicity of factors affecting ecosystem functioning when evaluating its responses to environmental change, when using both experimental (e.g. Reich et al. 2004; Maestre & Reynolds 2006) and modelling (e.g. Savage et al. 2007; Shen et al. 2008) approaches. At the same time, the study of the facilitation–competition continuum along environmental gradients has been a major topic of study during the last two decades (Callaway 2007; Brooker et al. 2008). However, most of this research has targeted single or a few pairs of species, have not directly evaluated the potential roles of climate, biotic interactions and other community attributes as drivers of ecosystem functioning and have been conducted using environmental gradients involving just two levels. Our results contribute to filling this gap, and indicate that attributes of biotic communities such as total cover, but not the outcome of biotic interactions, exert important controls on ecosystem functioning along environmental gradients in semi-arid ecosystems dominated by BSCs and vascular plants. They also indicate that factors negatively affecting biotic attributes such as cover, and to a lesser degree richness, will probably have a major negative impact on ecosystem functioning under future climatic conditions, exacerbating the impacts of climate on this functioning. Although challenging (Freckleton et al. 2009), future facilitation–competition research should explicitly consider the importance of biotic interactions, as this is crucial for a full understanding of the role of these interactions as drivers of ecosystem functioning and of its responses to climate change.
Acknowledgements
We thank José M. Montoya for inviting us to write this contribution for the special issue, Marta Carpio, Aran Luzuriaga, Patricia Valiente, Becky Mou and Ana Sánchez for their help during field and laboratory work, and Nicholas Gotelli, José M. Montoya and two anonymous reviewers for their thorough and constructive comments on a previous version of this manuscript. F.T.M. is supported by the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement no. 242658. M.A.B. was supported by a ‘Juan de la Cierva’ contract from the Spanish Ministerio de Ciencia e Innovación (MICINN), co-funded by the European Social Fund. C.E.M. and A.P.C. were supported by Studentships from the British Ecological Society (231/1975) and the Fundación BBVA (BIOCON06/105 grant), respectively. S.S. and P.G.P. were supported by fellowships from Fundación Biodiversidad-CINTRA (EXPERTAL grant). This research was supported by grants from the British Ecological Society (Early Career Project Grant 231/607 and Studentship 231/1975), Fundación BBVA (BIOCON06/105), Comunidad de Madrid (S-0505/AMB/0335), Universidad Rey Juan Carlos (URJC-RNT-063-2) and MICINN (CGL2008-00986-E/BOS).
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Abrahamson W. G., Ball Dobley K., Houseknecht H. R., Pecone C. A.2005Ecological divergence among five co-occurring species of old-field goldenrods. Plant Ecol. 177, 43–56 (doi:10.1007/s11258-005-2069-2) [Google Scholar]
- Aguiar M. R., Sala O. E.1999Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends Ecol. Evol. 14, 273–277 (doi:10.1016/S0169-5347(99)01612-2) [DOI] [PubMed] [Google Scholar]
- Armstrong R. A., Welch A. R.2007Competition in lichen communities. Symbiosis 43, 1–12 [Google Scholar]
- Bastida F., Barbera G. G., Garcia C., Hernandez T.2008Influence of orientation, vegetation and season on soil microbial and biochemical characteristics under semiarid conditions. Appl. Soil Ecol. 38, 62–70 (doi:10.1016/j.apsoil.2007.09.002) [Google Scholar]
- Belnap J.2002Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol. Fert. Soils 35, 128–135 (doi:10.1007/s00374-002-0452-x) [Google Scholar]
- Belnap J., Lange O. L. (eds) 2003Biological soil crusts: structure, function, and management Berlin, Germany: Springer [Google Scholar]
- Belnap J., Phillips S. L., Flints S., Money J., Caldwell M.2008Global change and biological soil crusts: effects of ultraviolet augmentation under altered precipitation regimes and nitrogen additions. Global Change Biol. 14, 670–686 (doi:10.1111/j.1365-2486.2007.01509.x) [Google Scholar]
- Bertness M., Callaway R. M.1994Positive interactions in communities. Trends Ecol. Evol. 9, 191–193 (doi:10.1016/0169-5347(94)90088-4) [DOI] [PubMed] [Google Scholar]
- Boeken B., Orenstein D.2001The effect of plant litter on ecosystem properties in a Mediterranean semi-arid shrubland. J. Veg. Sci. 12, 825–832 (doi:10.2307/3236870) [Google Scholar]
- Bowker M. A., Belnap J., Davidson D. W., Goldstein H.2006Correlates of biological soil crust abundance across a continuum of spatial scales: support for a hierarchical conceptual model. J. Appl. Ecol. 43, 152–163 (doi:10.1111/j.1365-2664.2006.01122.x) [Google Scholar]
- Bowker M. A., Maestre F. T., Escolar C.2010Biological crusts as a model system for examining the biodiversity–ecosystem function relationship in soils. Soil Biol. Biochem. 42, 405–417 (doi:10.1016/j.soilbio.2009.10.025) [Google Scholar]
- Breshears D. D., Nyhan J. W., Heil C. E., Wilcox B. P.1998Effects of woody plants on microclimate in a semiarid woodland: soil temperature and evaporation in canopy and intercanopy patches. Int. J. Plant Sci. 159, 1010–1017 (doi:10.1086/314083) [Google Scholar]
- Brooker R. W.2006Plant–plant interactions and environmental change. New Phytol. 171, 271–289 (doi:10.1111/j.1469-8137.2006.01752.x) [DOI] [PubMed] [Google Scholar]
- Brooker R., Kikvidze Z., Pugnaire F. I., Callaway R. M., Choler P., Lortie C. J., Michalet R.2005The importance of importance. Oikos 109, 63–70 (doi:10.1111/j.0030-1299.2005.13557.x) [Google Scholar]
- Brooker R. W., et al. 2008Facilitation in plant communities: the past, the present and the future. J. Ecol. 96, 18–34 (doi:10.1111/j.1365-2745.2007.01295.x) [Google Scholar]
- Caldwell M. M., Dawson T. E., Richards J. H.1998Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113, 151–161 (doi:10.1007/s004420050363) [DOI] [PubMed] [Google Scholar]
- Callaway R. M.2007Positive interactions and interdependence in plant communities Dordrecht, The Netherlands: Springer [Google Scholar]
- Callaway R. M., et al. 2002Positive interactions among alpine plants increase with stress. Nature 417, 844–848 (doi:10.1038/nature00812) [DOI] [PubMed] [Google Scholar]
- Callaway R. M., Kikodze D., Chiboshvili M., Khetsuriani L.2005Unpalatable plants protect neighbors from grazing and increase plant community diversity. Ecology 86, 1856–1862 (doi:10.1890/04-0784) [Google Scholar]
- Canadell J., Pataki D., Pitelka L. F. (eds)2007Terrestrial ecosystems in a changing world Berlin, Germany: Springer [Google Scholar]
- Cantón Y., Solé-Benet A., Domingo F.2004Temporal and spatial patterns of soil moisture in semiarid badlands of SE Spain. J. Hydrol. 285, 199–214 (doi:10.1016/j.jhydrol.2003.08.018) [Google Scholar]
- Castellano M. J., Valone T. J.2007Livestock, soil compaction and water infiltration rate: evaluating a potential desertification recovery mechanism. J. Arid Environ. 71, 97–108 (doi:10.1016/j.jaridenv.2007.03.009) [Google Scholar]
- Cavieres L., Badano E. I., Sierra-Almeida A., Gómez-González S., Molina-Montenegro M. A.2006Positive interactions between alpine plant species and the nurse cushion Laretia acaulis do not increase with elevation in the Andes of central Chile. New Phytol. 169, 59–69 (doi:10.1111/j.1469-8137.2005.01573.x) [DOI] [PubMed] [Google Scholar]
- Chu C. J., Maestre F. T., Xiao S., Weiner J., Wang Y. S., Duan Z. H., Wang G.2008Balance between facilitation and resource competition determines biomass–density relationships in plant populations. Ecol. Lett. 11, 1189–1197 (doi:10.1111/j.1461-0248.2008.01228.x) [DOI] [PubMed] [Google Scholar]
- Cortina J., Maestre F. T.2005Plant effects on soils in drylands: implications on community dynamics and ecosystem restoration. In Tree species effects on soils: implications for global change (eds Binkley D., Menyailo O.), pp. 85–118 Berlin, Germany: Springer [Google Scholar]
- de Castro M., Martín-Vide J., Alonso S.2005El clima de España: pasado, presente y escenarios de clima para el siglo XXI. In Evaluación Preliminar General de los Impactos en España por Efecto del Cambio Climático (ed. Moreno J. M.), pp. 1–64 Madrid, Spain: Ministerio de Medio Ambiente [Google Scholar]
- Dormann C. F., Woodin S. J.2002Climate change in the Arctic: using plant functional types in a meta-analysis of field experiments. Funct. Ecol. 16, 4–17 (doi:10.1046/j.0269-8463.2001.00596.x) [Google Scholar]
- Dullinger S., et al. 2007Weak and variable relationships between environmental severity and small-scale co-occurrence in alpine plant communities. J. Ecol. 95, 1284–1295 (doi:10.1111/j.1365-2745.2007.01288.x) [Google Scholar]
- Eccles N. S., Esler K. J., Cowling R. M.1999Spatial pattern analysis in Namaqualand desert plant communities: evidence for general positive interactions. Plant Ecol. 142, 71–85 (doi:10.1023/A:1009857824912) [Google Scholar]
- Emmett B., Beier C., Estiarte M., Tietema A., Kristensen H. L., Williams D., Peñuelas J., Schmidt I., Sowerby A.2004The response of soil processes to climate change: results from manipulation studies of shrublands across an environmental gradient. Ecosystems 7, 625–637 (doi:10.1007/s10021-004-0220-x) [Google Scholar]
- Fay P. A., Carlisle J. D., Knapp A. K., Blair J. M., Collins S. L.2003Productivity responses to altered rainfall patterns in a C4-dominated grassland. Oecologia 137, 245–251 (doi:10.1007/s00442-003-1331-3) [DOI] [PubMed] [Google Scholar]
- Flores J., Jurado E.2003Are nurse-protégé interactions more common among plants from arid environments? J. Veg. Sci. 14, 911–916 (doi:10.1658/1100-9233(2003)014[0911:ANIMCA]2.0.CO;2) [Google Scholar]
- Freckleton R. P., Watkinson A. R., Rees M.2009Measuring the importance of competition in plant communities. J. Ecol. 97, 379–384 (doi:10.1111/j.1365-2745.2009.01497.x) [Google Scholar]
- Giménez-Benavides L., Escudero A., Iriondo J. M.2007Local adaptation enhances seedling recruitment along an altitudinal gradient in a high mountain Mediterranean plant. Ann. Bot. 99, 723–734 (doi:10.1093/aob/mcm007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno T., Pías B., Lemos Fihlo J. P., Valladares F.2008Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol. 29, 87–98 (doi:10.1093/treephys/tpn007) [DOI] [PubMed] [Google Scholar]
- Goslee S. C., Urban D. L.2007The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Soft. 22, 1–19 [Google Scholar]
- Gotelli N. J.2000Null model analysis of species co-occurrence patterns. Ecology 81, 2606–2621 (doi:10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2) [Google Scholar]
- Gotelli N. J., Entsminger G. L.2003Swap algorithms in null model analysis. Ecology 84, 532–535 (doi:10.1890/0012-9658(2003)084[0532:SAINMA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Gotelli N. J., Entsminger G. L.2006. EcoSim: null models software for ecology, version 7. Acquired Intelligence Inc. and Kesey-Bear; See http://garyentsminger.com/ ecosim.htm [Google Scholar]
- Gotelli N. J., Graves G. R.1996Null models in ecology Washington, DC: Smithsonian Institution Press [Google Scholar]
- Grace J. B.2006Structural equation modeling and natural systems Cambridge, UK: Cambridge University Press [Google Scholar]
- Hauck M., Dulamsuren C., Muhlenberg M.2007Lichen diversity on steppe slopes in the northern Mongolian mountain taiga and its dependence on microclimate. Flora 202, 530–546 (doi:10.1016/j.flora.2006.11.003) [Google Scholar]
- Holzapfel C., Tielbörger K., Parag H. A., Kigel J., Sternberg M.2006Annual plant–shrub interactions along an aridity gradient. Basic Appl. Ecol. 7, 268–279 (doi:10.1016/j.baae.2005.08.003) [Google Scholar]
- Hooper D. U., et al. 2005Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 (doi:10.1890/04-0922) [Google Scholar]
- Keddy P. A.2001Competition, 2nd ed.Dordrecht, The Netherlands: Kluwer [Google Scholar]
- Kidron G. J., Herrnstadt I., Barzilay E.2002The role of dew as a moisture source for sand microbiotic crusts in the Negev Desert, Israel. J. Arid Environ. 52, 517–533 (doi:10.1006/jare.2002.1014) [Google Scholar]
- Kikvidze Z., Pugnaire F. I., Brooker R. W., Choler P., Lortie C. J., Michalet R., Callaway R. M.2005Linking patterns and processes in alpine plant communities: a global study. Ecology 86, 1395–1400 (doi:10.1890/04-1926) [Google Scholar]
- Knops J. M. H., et al. 1999Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances, and diversity. Ecol. Lett. 2, 286–293 (doi:10.1046/j.1461-0248.1999.00083.x) [DOI] [PubMed] [Google Scholar]
- Körner C. H.2000Biosphere responses to CO2-enrichment. Ecol. Appl. 10, 1590–1619 (doi:10.1890/1051-0761(2000)010[1590:BRTCE]2.0.CO;2) [Google Scholar]
- Lamb E. G., Cahill J. F., Jr2008When competition does not matter: grassland diversity and community composition. Am. Nat. 171, 777–787 (doi:10.1086/587528) [DOI] [PubMed] [Google Scholar]
- Leduc A., Drapeau P., Bergeron Y., Legendre P.1992Study of spatial components of forest cover using partial Mantel tests and path analysis. J. Veg. Sci. 3, 69–78 (doi:10.2307/3236000) [Google Scholar]
- Lellei-Kovács E., Kovács-Láng E., Kalapos T., Botta-Dukát Z., Barabás S., Beier C.2008Experimental warming does not enhance soil respiration in a semiarid temperate forest-steppe ecosystem. Commun. Ecol. 9, 29–37 (doi:10.1556/ComEc.9.2008.1.4) [Google Scholar]
- Liancourt P., Callaway R. M., Michalet R.2005Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology 86, 1611–1618 (doi:10.1890/04-1398) [Google Scholar]
- Lortie C. J., Callaway R. M.2006Re-analysis of meta-analysis: support for the stress-gradient hypothesis. J. Ecol. 94, 7–16 (doi:10.1111/j.1365-2745.2005.01066.x) [Google Scholar]
- Luo Y., Wan S., Hui D., Wallace L. L.2001Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–624 (doi:10.1038/35098065) [DOI] [PubMed] [Google Scholar]
- Maestre F. T.2003Small-scale spatial patterns of two soil lichens in semi-arid Mediterranean steppe. Lichenologist 35, 71–81 (doi:10.1006/lich.2002.0425) [Google Scholar]
- Maestre F. T., Cortina J.2004Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. Proc. R. Soc. Lond. B 271, S331–S333 (doi:10.1098/rsbl.2004.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre F. T., Escudero A.2009Is the patch-size distribution of vegetation a suitable indicator of desertification processes? Ecology 90, 1729–1735 (doi:10.1890/08-2096.1) [DOI] [PubMed] [Google Scholar]
- Maestre F. T., Reynolds J. F.2006Spatial heterogeneity in soil nutrient supply modulates nutrient and biomass responses to multiple global change drivers in model grassland communities. Global Change Biol. 12, 2431–2441 (doi:10.1111/j.1365-2486.2006.01262.x) [Google Scholar]
- Maestre F. T., Valladares F., Reynolds J. F.2005aIs the change of plant–plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J. Ecol. 93, 748–757 (doi:10.1111/j.1365-2745.2005.01017.x) [Google Scholar]
- Maestre F. T., Escudero A., Martínez I., Guerrero C., Rubio A.2005bDoes spatial pattern matter to ecosystem functioning? Insights from biological soil crusts. Funct. Ecol. 19, 566–573 (doi:10.1111/j.1365-2435.2005.01000.x) [Google Scholar]
- Maestre F. T., Valladares F., Reynolds J. F.2006The stress-gradient hypothesis does not fit all relationships between plant–plant interactions and abiotic stress: further insights from arid environments. J. Ecol. 94, 17–22 (doi:10.1111/j.1365-2745.2005.01089.x) [Google Scholar]
- Maestre F. T., Escolar C., Martínez I., Escudero A.2008Are soil lichen communities structured by biotic interactions? A null model analysis. J. Veg. Sci. 19, 261–266 (doi:10.3170/2007-8-18366) [Google Scholar]
- Maestre F. T., Callaway R. M., Valladares F., Lortie C.2009aRefining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 97, 199–205 (doi:10.1111/j.1365-2745.2008.01476.x) [Google Scholar]
- Maestre F. T., Martínez I., Escolar C., Escudero A.2009bOn the relationship between abiotic stress and co-occurrence patterns: an assessment at the community level using soil lichen communities and multiple stress gradients. Oikos 118, 1015–1022 (doi:10.1111/j.1600-0706.2009.17362.x) [Google Scholar]
- Maestre F. T., et al. 2009cShrub encroachment can reverse desertification in Mediterranean semiarid grasslands. Ecol. Lett. 12, 930–941 (doi:10.1111/j.1461-0248.2009.01352.x) [DOI] [PubMed] [Google Scholar]
- Martínez-Mena M., Rogel A. J., Castillo V., Albaladejo J.2002Organic carbon and nitrogen losses influenced by vegetation removal in a semiarid mediterranean soil. Biogeochemistry 61, 309–321 (doi:10.1023/A:1020257208048) [Google Scholar]
- Martínez I., Escudero A., Maestre F. T., de la Cruz A., Guerrero C., Rubio A.2006Small-scale patterns of abundance of mosses and lichens forming biological soil crusts in two semi-arid gypsum environments. Aust. J. Bot. 54, 339–348 (doi:10.1071/BT05078) [Google Scholar]
- McCune B., Grace J. B.2002Analysis of ecological communties Gleneden Beach, USA: MJM Software Design [Google Scholar]
- Michalet R., Brooker R. W., Cavieres L. A., Kikvidze Z., Lortie C. J., Pugnaire F. I., Valiente-Banuet A., Callaway R. M.2006Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 9, 767–773 (doi:10.1111/j.1461-0248.2006.00935.x) [DOI] [PubMed] [Google Scholar]
- Mitchell M., Cahill J. F., Hik D. S.2009The unimportance of plant interactions in a subarctic-alpine plant community. Ecology 90, 2360–2367 (doi:10.1890/08-0924.1) [DOI] [PubMed] [Google Scholar]
- Montès N., Maestre F. T., Ballini C., Baldy V., Gauquelin T., Planquette M., Greff S., Dupouyet S., Perret J. B.2008On the relative importance of the effects of selection and complementarity as drivers of diversity–productivity relationships in Mediterranean shrublands. Oikos 117, 1345–1350 (doi:10.1111/j.2008.0030-1299.16910.x) [Google Scholar]
- Monzeglio U., Stoll P.2005Spatial patterns and species performances in experimental plant communities. Oecologia 145, 619–628 (doi:10.1007/s00442-005-0168-3) [DOI] [PubMed] [Google Scholar]
- Mulder C. P. H., Uliassi D. D., Doak D. F.2001Physical stress and diversity–productivity relationships: the role of positive interactions. Proc. Natl Acad. Sci. USA 98, 6704–6708 (doi:10.1073/pnas.111055298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninyerola M., Pons X., Roure J. M.2005Atlas climático digital de la Península Ibérica. Metodología y aplicaciones enbioclimatología y geobotánica Barcelona, Spain: Universidad Autónoma de Barcelona; See http://www.opengis.uab.es/wms/iberia/mms/index.htm [Google Scholar]
- Oosting H. J.1948The study of plant communities: an introduction to plant ecology San Francisco, CA: W. H. Freeman [Google Scholar]
- Pielou E. C.1975Ecological diversity New York, NY: Wiley [Google Scholar]
- Purves D. W., Law R.2002Fine-scale spatial structure in a grassland community: quantifying the plant's-eye view. J. Ecol. 90, 121–129 (doi:10.1046/j.0022-0477.2001.00652.x) [Google Scholar]
- Reich P. B., Tilman D., Naeem S., Ellsworth D. S., Knops J., Craine J., Wedin D., Trost J.2004Species and functional group diversity independently influence biomass accumulation and its response to CO2 and N. Proc. Natl Acad. Sci. USA 101, 10 101–10 106 (doi:10.1073/pnas.0306602101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss J., Bridle J. R., Montoya J. M., Woodward G.2009Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol 24, 505–514 (doi:10.1016/j.tree.2009.03.018) [DOI] [PubMed] [Google Scholar]
- Reynolds J. F., et al. 2007Global desertification: building a science for dryland development. Science 316, 847–851 (doi:10.1126/science.1131634) [DOI] [PubMed] [Google Scholar]
- Rooney T. P.2008Comparison of co-occurrence structure of temperate forest herb-layer communities in 1949 and 2000. Acta Oecol. 34, 354–360 (doi:10.1016/j.actao.2008.06.011) [Google Scholar]
- Rowell D. P., Jones R. G.2006Causes and uncertainty of future summer drying over Europe. Clim. Dyn. 27, 281–299 (doi:10.1007/s00382-006-0125-9) [Google Scholar]
- Savage V. M., Webb C. T., Norberg J.2007A general multi-trait-based framework for studying the effects of biodiversity on ecosystem functioning. J. Theor. Biol. 247, 213–229 (doi:10.1016/j.jtbi.2007.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemske D. W., Mittelbach G. G., Cornell H. V., Sobel J. M., Roy K.2009Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269 (doi:10.1146/annurev.ecolsys.39.110707.173430) [Google Scholar]
- Schröter D., et al. 2005Ecosystem service supply and vulnerability to global change in Europe. Science 310, 1333–1337 (doi:10.1126/science.1115233) [DOI] [PubMed] [Google Scholar]
- Shen W., Jenerette G. D., Hui D., Phillips R. P., Ren H.2008Effects of changing precipitation regimes on dryland soil respiration and C pool dynamics at rainfall event, seasonal and interannual scales. J. Geophys. Res. 113, G03024 (doi:10.1029/2008JG000685) [Google Scholar]
- Shipley B.2001Cause and correlation in biology: a users guide to path analysis, structural equations and causal inference Cambridge, UK: Cambridge University Press [Google Scholar]
- Smit C., Rietkerk M., Wassen M. J.2009Inclusion of biotic stress (consumer pressure) alters predictions from the stress gradient hypothesis. J. Ecol. 97, 1215–1219 (doi:10.1111/j.1365-2745.2009.01555.x) [Google Scholar]
- Smouse P. E., Long J. C., Sokal R. R.1986Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst. Zool. 35, 627–632 (doi:10.2307/2413122) [Google Scholar]
- Souza-Egipsy V., Wierzchos J., García-Ramos J. V., Ascaso C.2002Chemical and ultrastructural features of the lichen-volcanic/sedimentary rock interface in a semiarid region (Almería, Spain). Lichenologist 34, 155–167 (doi:10.1006/lich.2001.0371) [Google Scholar]
- Stoll P., Prati D.2001Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 82, 319–327 (doi:10.1890/0012-9658(2001)082[0319:IAACII]2.0.CO;2) [Google Scholar]
- Tirado R., Pugnaire F. I.2005Community structure and positive interactions in constraining environments. Oikos 111, 437–444 (doi:10.1111/j.1600-0706.2005.14094.x) [Google Scholar]
- Troumbis A. Y., Memtsas D.2000Observational evidence that diversity may increase productivity in Mediterranean shrublands. Oecologia 125, 101–108 (doi:10.1007/PL00008880) [DOI] [PubMed] [Google Scholar]
- Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A.2008Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- Valiente-Banuet A., Verdú M.2007Facilitation can increase the phylogenetic diversity of plant communities. Ecol. Lett. 10, 1029–1036 (doi:10.1111/j.1461-0248.2007.01100.x) [DOI] [PubMed] [Google Scholar]
- Wallenstein M. D., Weintraub M. N.2008Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem. 40, 2098–2106 (doi:10.1016/j.soilbio.2008.01.024) [Google Scholar]
- Wang Y. S., Chu C. J., Maestre F. T., Wang G.2008On the relevance of facilitation in alpine meadow communities: an experimental assessment with multiple species differing in their ecological optimum. Acta Oecol. 33, 108–113 (doi:10.1016/j.actao.2007.10.002) [Google Scholar]
- Welden C. W., Slauson W. L.1986The intensity of competition versus its importance: an overlooked distinction and some implications. Q. Rev. Biol. 61, 23–44 (doi:10.1086/414724) [DOI] [PubMed] [Google Scholar]
- Whitford W. G.2002Ecology of desert systems London, UK: Academic Press [Google Scholar]
- Wilske B., Burgheimer J., Karnieli A., Zaady E., Andreae M. O., Yakir D., Kesselmeier J.2008The CO2 exchange of biological soil crusts in a semiarid grass-shrubland at the northern transition zone of the Negev desert, Israel. Biogeosciences 5, 1411–1423 [Google Scholar]
- Yachi S., Loreau M.2007Does complementary resource use enhance ecosystem functioning? A model of light competition in plant communities. Ecol. Lett. 10, 54–62 (doi:10.1111/j.1461-0248.2006.00994.x) [DOI] [PubMed] [Google Scholar]
- Zhou X., Sherry R. A., An Y., Wallace L. L., Luo Y.2006Main and interactive effects of warming, clipping, and doubled precipitation on soil CO2 efflux in a grassland ecosystem. Global Biogeochem. Cycles 20, GB1003 (doi:10.1029/2005GB002526) [Google Scholar]