Abstract
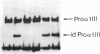
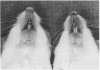
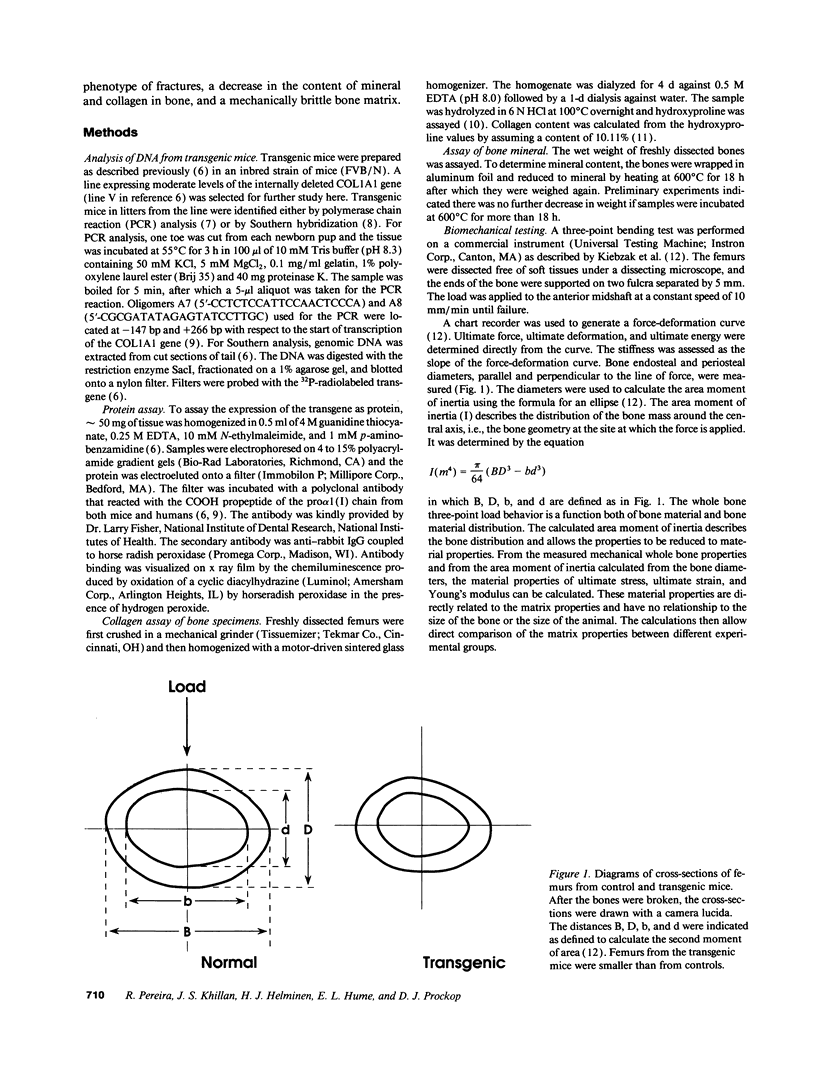
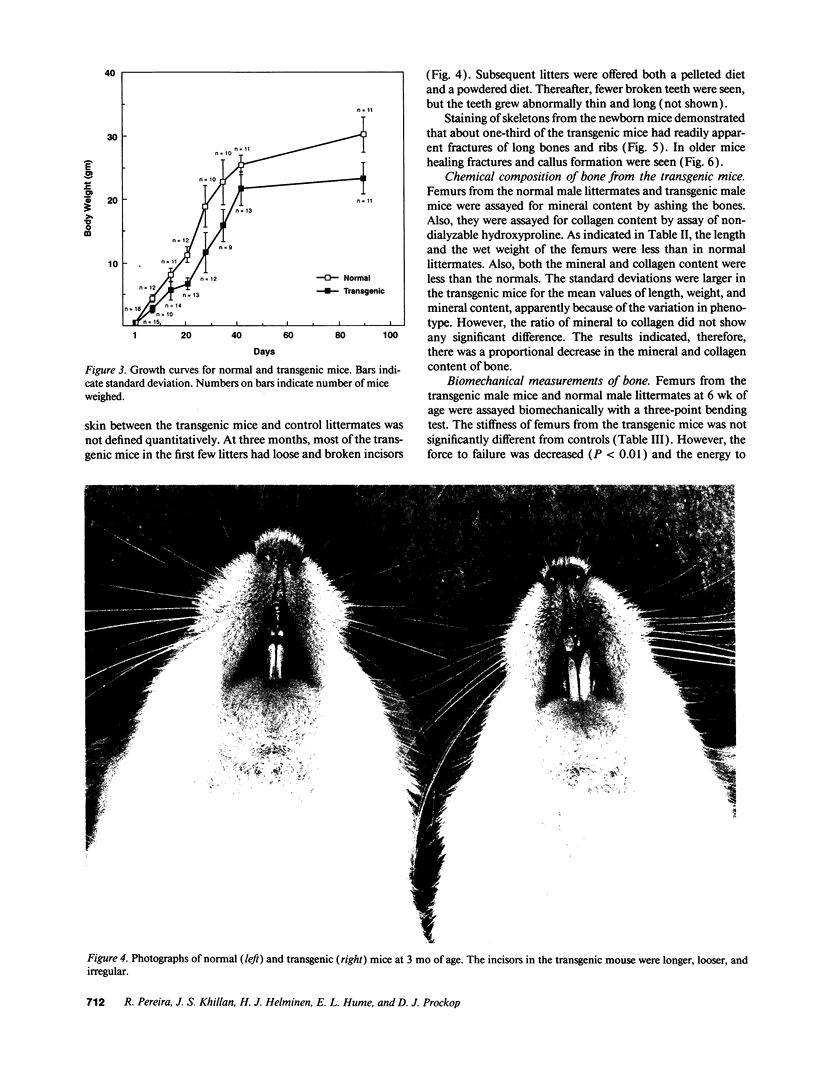
A line of transgenic mice was prepared that expressed moderate levels of an internally deleted human gene for the pro alpha 1(I) chain of type I procollagen. The gene construct was modeled after a sporadic in-frame deletion of the human gene that produced a lethal variant of osteogenesis imperfecta by causing biosynthesis of shortened pro alpha 1(I) chains. 89 transgenic mice from the line were examined. About 6% had a lethal phenotype with extensive fractures at birth, and 33% had fractures but were viable. The remaining 61% of the transgenic mice had no apparent fractures as assessed by x ray examination on the day of birth. Brother-sister matings produced eight litters in which approximately 40% of the mice had the lethal phenotype, an observation indicating that expression of the exogenous gene was more lethal in putative homozygous mice from the line. Examination of femurs from the transgenic mice indicated that the bones were significantly shorter in length and had a decrease in wet weight, mineral content, and collagen content. However, there was no statistically significant change in the mineral to collagen ratio. Biomechanical measurements on femurs from the mice at 6 wk indicated a decrease in force and energy to failure. There was also a decrease in strain to failure and an increase in Young's modulus of elasticity, observations indicating increased brittleness of bone matrix. The results suggested that the transgenic mice may be an appropriate model for testing potential therapies for osteogenesis imperfecta. They may also be a useful model for studying osteoporosis.
Full text
PDF

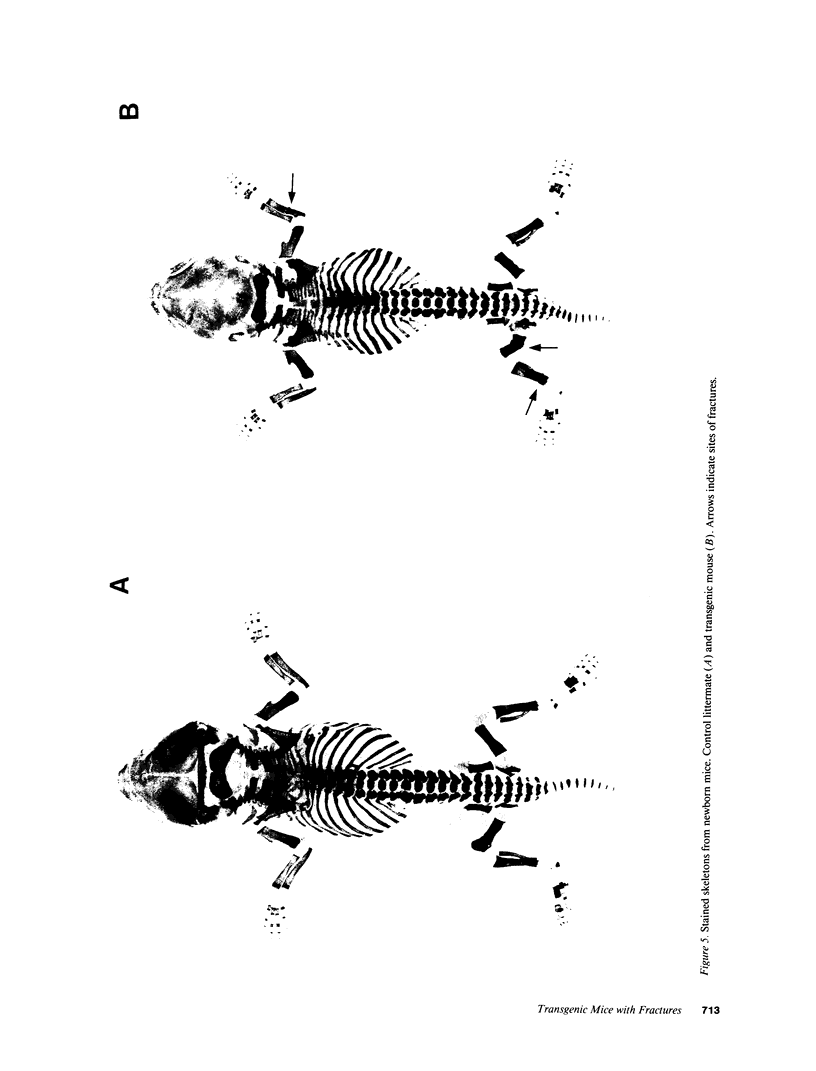
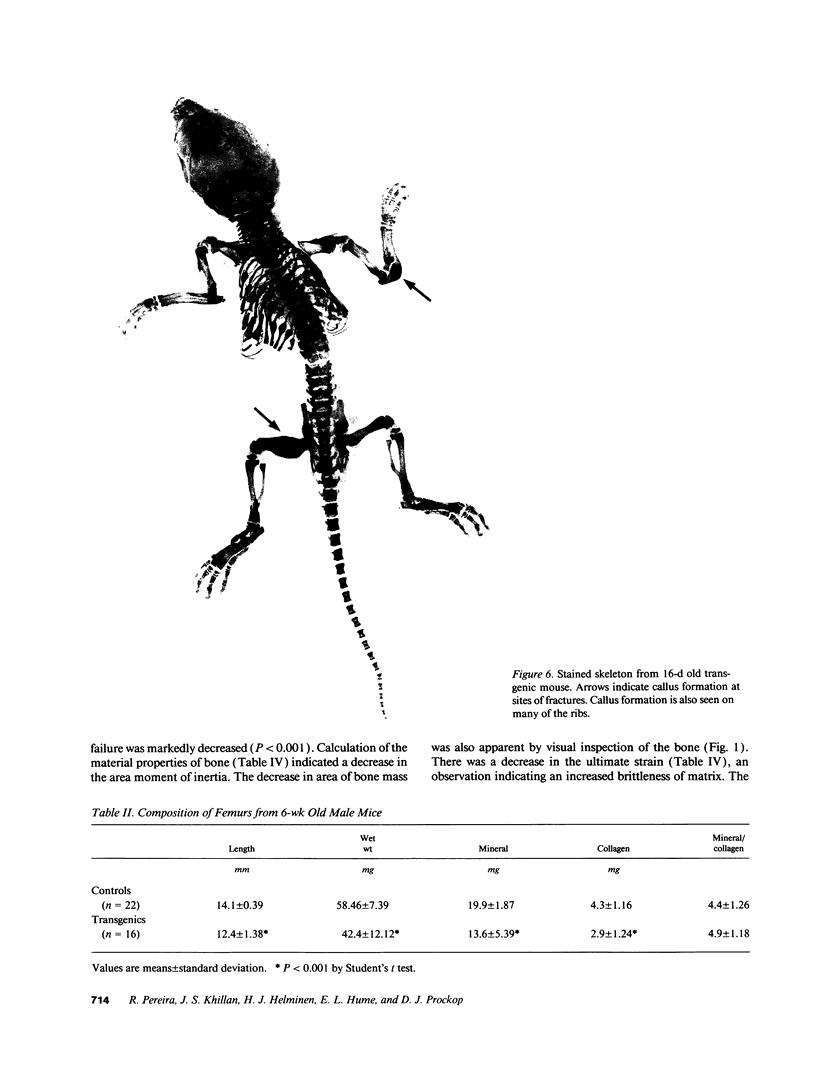
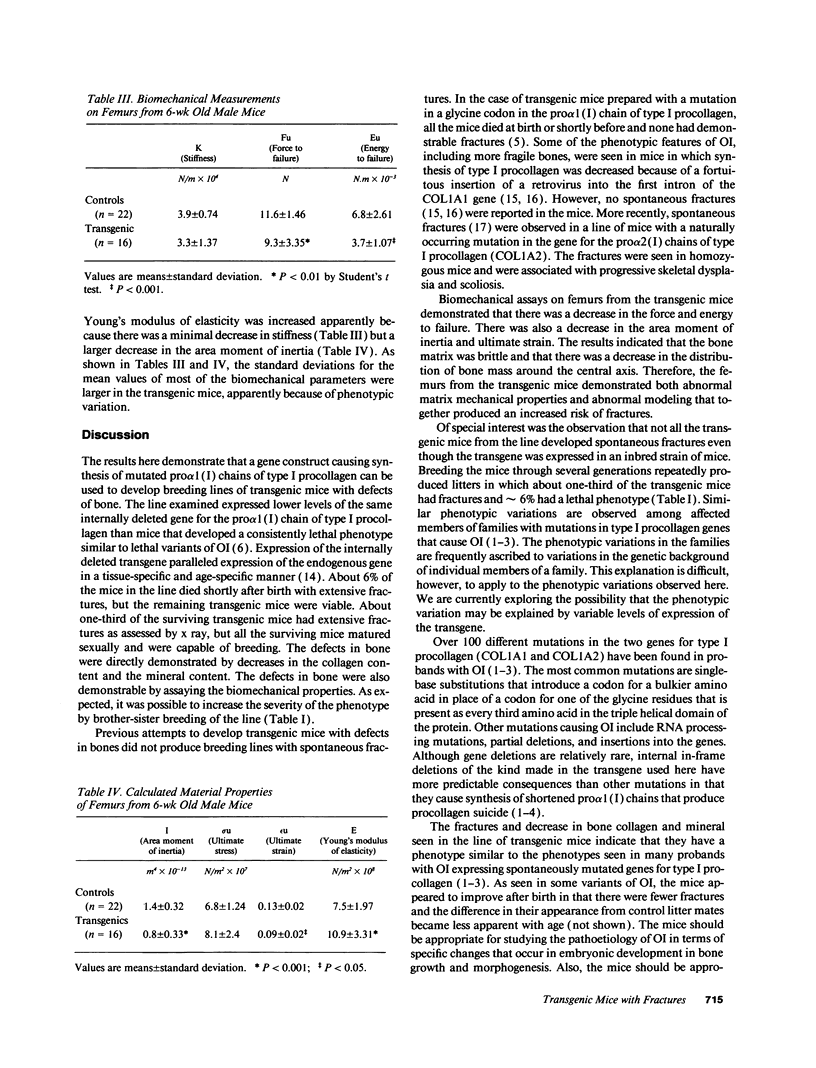

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonadio J., Saunders T. L., Tsai E., Goldstein S. A., Morris-Wiman J., Brinkley L., Dolan D. F., Altschuler R. A., Hawkins J. E., Jr, Bateman J. F. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7145–7149. doi: 10.1073/pnas.87.18.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Khillan J. S., Olsen A. S., Kontusaari S., Sokolov B., Prockop D. J. Transgenic mice that express a mini-gene version of the human gene for type I procollagen (COL1A1) develop a phenotype resembling a lethal form of osteogenesis imperfecta. J Biol Chem. 1991 Dec 5;266(34):23373–23379. [PubMed] [Google Scholar]
- Kiebzak G. M., Smith R., Gundberg C. C., Howe J. C., Sacktor B. Bone status of senescent male rats: chemical, morphometric, and mechanical analysis. J Bone Miner Res. 1988 Feb;3(1):37–45. doi: 10.1002/jbmr.5650030107. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Olsen A. S., Geddis A. E., Prockop D. J. High levels of expression of a minigene version of the human pro alpha 1 (I) collagen gene in stably transfected mouse fibroblasts. Effects of deleting putative regulatory sequences in the first intron. J Biol Chem. 1991 Jan 15;266(2):1117–1121. [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spotila L. D., Constantinou C. D., Sereda L., Ganguly A., Riggs B. L., Prockop D. J. Mutation in a gene for type I procollagen (COL1A2) in a woman with postmenopausal osteoporosis: evidence for phenotypic and genotypic overlap with mild osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5423–5427. doi: 10.1073/pnas.88.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Sykes B. Human genetics. Bone disease cracks genetics. Nature. 1990 Nov 1;348(6296):18–20. doi: 10.1038/348018a0. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]