Abstract
Current predictions on species responses to climate change strongly rely on projecting altered environmental conditions on species distributions. However, it is increasingly acknowledged that climate change also influences species interactions. We review and synthesize literature information on biotic interactions and use it to argue that the abundance of species and the direction of selection during climate change vary depending on how their trophic interactions become disrupted. Plant abundance can be controlled by aboveground and belowground multitrophic level interactions with herbivores, pathogens, symbionts and their enemies. We discuss how these interactions may alter during climate change and the resulting species range shifts. We suggest conceptual analogies between species responses to climate warming and exotic species introduced in new ranges. There are also important differences: the herbivores, pathogens and mutualistic symbionts of range-expanding species and their enemies may co-migrate, and the continuous gene flow under climate warming can make adaptation in the expansion zone of range expanders different from that of cross-continental exotic species. We conclude that under climate change, results of altered species interactions may vary, ranging from species becoming rare to disproportionately abundant. Taking these possibilities into account will provide a new perspective on predicting species distribution under climate change.
Keywords: climate warming, climate envelope, aboveground–belowground interactions, predictive modelling, range shift, biological invasion
1. Introduction
A major challenge for ecology is to predict the possible consequences of climate change and, based on this, propose adaptation and mitigation measures in order to sustain human societies. One of the key factors that limit predictions is that climate change may not only affect species performances, but also species interactions (Tylianakis et al. 2008). Species interact in complex food webs with different trophic levels, and species within or between the different trophic levels do not necessarily react to climate change in a similar way (Van der Putten et al. 2004; Schweiger et al. 2008). Therefore, when predictions on species distributions and species abundances would be limited to effects of altered temperature and habitat quality, as is often the case, consequences of altered species interactions will be largely missed. Here, we review climate change effects on community interactions and on species range shifts from a multitrophic interactions perspective. Our aim is to deepen insights into species interactions, identify where there are short-comings in current knowledge in relation to climate change, identify new knowledge and propose how that knowledge can be used to enhance the ecological relevance of predictive modelling.
Effects of climate change are expressed at various levels. The most proximate effects of climate change are in the phenology of species. For example, spring temperature strongly determines germination and bud burst of plants, as well as foraging and reproductive activities of animals. As enhanced spring temperature does not necessarily influence the phenology of interacting species in the same way, it can decouple interactions between predators and preys (Visser & Both 2005). Moreover, air temperature responds more immediately than soil temperature, which can also cause time lags in responses between aboveground and belowground subsystems (Gehrig-Fasel et al. 2008). As such effects vary from year to year, they have strong effects on annual fluctuations in species physiology, fitness and abundance. However, when changes in temperature proceed in one direction, they may affect species interactions more permanently, which could make selection pressures change in one direction as well. Currently, in temperate and arctic regions, the progressive warming causes the life cycles of consumers and resources to drift apart in directions that lead to more frequent mismatches in life histories (Post & Forchhammer 2008). When the rate of evolutionary changes is not sufficient in order to enable adaptation to the changes in phenology of the species at other trophic levels (Visser 2008), species may reach the end of the limits of their capacities to persist.
Climate change can alter the setting of range limits, leading to range expansion, or range contraction. Many such range shifts have been reported over the past decades and this process is supposed to continue (Walther et al. 2002; Parmesan & Yohe 2003; McLachlan et al. 2005). The current process of climate warming takes place at unprecedented rates when compared with historical records (Jones & Mann 2004). Also, range shifts are known to differ between species, or when hosts and enemies both shift ranges, they may not necessarily interact in the new range as they did in the original range (Menendez et al. 2008). Therefore, range shifts may result in decoupling of trophic interactions by a number of different causes, including differences in dispersal rate and the inability of predators and preys to interact in the new range. Both these and other factors may influence selection pressures and evolution of species.
Current climate envelope model predictions suppose severe loss of biodiversity owing to climate warming (Thomas et al. 2004; Thuiller et al. 2005), because species disperse slower than the climate changes, and because habitat specialists are limited in their capacity to keep up with climate warming by range-shifting. These climate envelope model predictions are strongly based on abiotic prerequisites of species occurrences. Indeed, when a climate envelope migrates faster than a species, the latter undoubtedly will have difficulties in keeping up with their preferred abiotic conditions. Moreover, when climate envelopes move towards regions where essential specific habitat conditions are absent, species may be unable to keep up with shifting climate conditions by mere range-shifting (Warren et al. 2001). However, there are also many open questions. For example, how much is known about what other factors may limit species ranges (Holt & Barfield 2009)? Can current observations be extrapolated to future conditions and how important may long-distance dispersal be in the response of species to climate change? These questions have given rise to further work, mainly by theoretical modelling. A number of these climate models also incorporate biotic interactions (Moore et al. 2007) in order to get a more accurate assessment of predictions on future species distributions and abundances (Brooker et al. 2007; Gillson et al. 2008; Preston et al. 2008; Pathikonda et al. 2009). However, the biotic interactions in most models are still limited to those within the same trophic level (to include competitive and facilitative interactions), whereas interactions between species of higher or lower trophic position are quite under-represented.
In our review, we discuss possible effects of climate change from a multitrophic interactions perspective and how differential responses of interacting species to climate warming may influence our predictions on distributions and abundances. Our main focus is on plants, and we will consider interactions between plants, invertebrates, micro-organisms and vertebrates. We will use and combine conceptual developments in the field of multitrophic interactions, biological invasions and evolutionary ecology in order to discuss possible outcomes of species and community responses to climate change as a result of species-specific patterns in range-shifting. We discuss possible outcomes of disrupted multitrophic interactions. Such disruptions may occur both in the new and original ranges, and we discuss possible consequences for the distribution and abundance of species, as well as for evolutionary processes. Such pleas have been made by several colleagues using a variety of arguments (Davis et al. 1998; Pearson & Dawson 2003; Schmitz et al. 2003; Berg et al. 2010). Our contribution to this discussion is to enhance our conceptual understanding of how altered biotic interactions may operate in the native and new ranges, assuming that species may either gain or suffer from disrupted interactions with higher trophic level organisms.
2. Biotic interactions modify plant species abundance
Species occurrence and abundance aboveground as well as belowground are controlled by a variety of factors that are hierarchically structured (Lavelle et al. 1993; Whittaker et al. 2001). At a continental scale, climate is strongly determining which species can occur where (Field et al. 2009), and this also applies to exotic species when invading a new range (Milbau et al. 2009). At smaller spatial scales, however, climate variation does not explain species richness anymore and one of the explanations is that at such relatively small spatial scales, other factors explain species richness (Field et al. 2009). These other factors will include biotic interactions between plants and their aboveground and belowground herbivores, pathogens, symbiotic mutualists and decomposer organisms, although there may be contrasting views about their contributions relative to each other and to abiotic factors, for example the availability of nutrients and moisture.
Aboveground–belowground biotic interactions influence plant community diversity and evenness: root herbivores preferring dominant plants enhance diversity in the plant community, as they reduce interspecific competition of the dominant over the rare plants (De Deyn et al. 2003). Aboveground and belowground biotic interactions can have additive or synergistic effects on each other. For example, aboveground herbivores may enhance root exudation patterns, which stimulate soil decomposer organism activities and plant resource availability. That can result in enhanced primary productivity (Bardgett et al. 1998; Mikola et al. 2001).
Aboveground and belowground biotic interactions vary with seasonal and successional time (Brown & Gange 1992; Schädler et al. 2004; Bardgett et al. 2005), as well as with latitudinal position (De Deyn & Van der Putten 2005). As fast growing plants are less well-defended by secondary chemistry than slow-growing plants (De Jong 1995), early succession plants in general are supposed to be less well directly defended to herbivores than late successional species (Price 1984). Early successional plants may avoid herbivores in other ways, such as by fast growth or effective dispersal. For example, the North American tree species Prunus serotina (black cherry) accumulates soil pathogens that kill seedlings during their establishment phase (Packer & Clay 2000). Since its cherries are preferred by birds that subsequently disperse the seeds, these seeds may land on sites away from the influence of the parent trees and their associated soil pathogens. At those so-called enemy-free sites the seeds will be better able to establish new trees unhampered by soil pathogens, rather than close to the parent trees. A potential disadvantage is that dispersal also disconnects offspring from the symbiotic mutualists, such as arbuscular and ectomycorrhizal fungi that are accumulated around the parent plants. These and other unmentioned biotic interactions all may influence plant performance and plant abundance.
3. Responses to climate warming by changes in phenology
Climate warming alters the existing coupling between photoperiod and temperature (Fuhrer 2003). Many species, including birds (Visser 2008), insects (Bale et al. 2002) and plants (Marchand et al. 2004) use a combination of photoperiod and temperature for timing their yearly life cycle processes. Species interactions have evolved under fluctuating conditions of ambient temperature, whereas effects of photoperiod are much more fixed to latitudinal position. Effects of warming may decouple trophic interactions when one trophic level responds to temperature and the other to day length. For example, temperature rise in the Arctic initiates plant growth earlier, whereas calving of caribou is day length-related. This is currently causing a trophic mismatch, because now the caribous produce calves too long after the food has become available (Post & Forchhammer 2008).
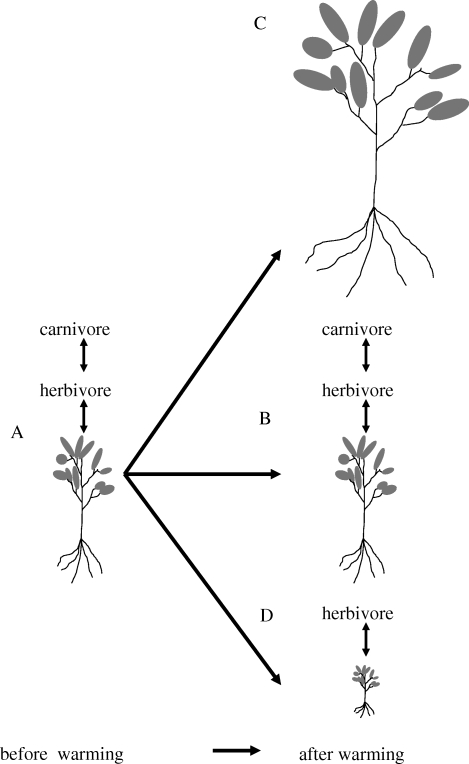
Climate warming may also decouple plant and insect life histories. For example, winter warming resulted in the earlier development of St John's wort (Hypericum perforatum), which led to reduced damage from gall forming and sucking insects in spring (Fox et al. 1999). In that case, warming reduced plant exposure to herbivorous insects in turn leading to increased plant abundance. The outcomes of plant–enemy interactions also depend on the responses of carnivores. In an Ecotron experiment with the peach potato aphid (Myzus persicae) and the parasitoid Aphidius matricariae, elevated temperature decreased plant biomass, and increased plant nitrogen concentration and aphid abundance, whereas the parasitoid showed only a trend of increase (Bezemer et al. 1998). In that case, climate warming decoupled trophic interactions at positions between primary consumers (herbivores) and their predators. The trophic position where the decoupling occurs will determine the sort of outbreak: decoupling between plants and herbivores will result in enhanced plant dominance, whereas decoupling between herbivores and carnivores will enhance herbivore outbreaks, consequently reducing plant dominance (figure 1).
Figure 1.
Different outcomes of plant performance depending on how climate warming influences interactions between plants, their herbivores (read as: and also pathogens) and their natural enemies. Plant performance is indicated by plant size. Average-sized plants represent plants that are normally controlled by aboveground and belowground herbivores (and pathogens) and that have intermediate abundance. Large-sized plants represent plants that are less controlled by herbivores and that can become dominant in plant communities owing to an indirect advantage in competition over other plant species that are controlled by herbivores and pathogens. Small-sized plants are heavily controlled by aboveground and belowground herbivores and are interstitial, or rare in plant communities. Symbiotic mutualists, for example arbuscular mycorrhizal fungi, are not indicated, but their effects can be easily replaced with the herbivores, taking into account that their effects will be generally positive, so that loss of symbionts will result in smaller plants. (A) Plant performance before warming is controlled by herbivores, which in turn are controlled by their natural enemies. (B) When plants, herbivores and their enemies respond to warming in exactly the same way (i.e. no changes in phenology or no different capacities for range-shifting), plant size will not be affected. (C) When herbivores (and their enemies) cannot keep up with the responses of the plants, plant size will increase in the post-warming era in the original range, or in the new range where climate warming enables the plants to become established. (D) When only herbivores, but not their enemies can keep up with the plants, the plants will be overexploited and may become subordinate or rare in both the warmed original range, or in the new range into which they have shifted. That release from enemies can promote local plant abundance that has been demonstrated for invasive exotic plants (e.g. Reinhart et al. 2003; Callaway et al. 2004).
Soil warming may enhance decomposition and nutrient cycling, provided that moisture is available for these processes (Chapin et al. 1995). Experimental warming in Arctic conditions resulted in higher bacterial and fungal biomass, as well as higher numbers of bacterivorous and fungivorous nematodes (Ruess et al. 1999). These responses will be mostly due to enhanced plant productivity. Soil warming may affect mycorrhizal effectiveness positively (Rillig et al. 2002), whereas it does not necessarily change mycorrhizal community composition (Fujimura et al. 2008). In general, it is assumed that plant–mycorrhizal fungi interactions benefit from climate warming, especially in Arctic regions (Kytoviita & Ruotsalainen 2007). However, these responses may depend on how warming affects plant species identities (Heinemeyer & Fitter 2004). Therefore, responses of the mycorrhizal community to climate warming (and drought) are probably influenced mostly through changes in plant community composition (Staddon et al. 2003) and less through direct effects of warming on the soil organisms (Fujimura et al. 2008). These effects of warming on nutrient-cycling and mycorrhizal associations may influence plant nutritional status, which can influence aboveground plant–insect–enemy interactions, depending on the feeding mode and diet breadth of the insects, as well as on the identity of the mycorrhizal fungal species involved (Koricheva et al. 2009). This also applies to endophytic fungi, which can show responses that are in contrast with those of mycorrhizal fungi (Hartley & Gange 2009).
There is a considerable amount of evidence that climate warming will enhance plant pathogen incidence, although this requires specific weather conditions, moisture especially being important for rapid pathogen outbreaks. However, opposite to the amount of knowledge on crop plants, relatively little is known about how wild plants will experience a change in exposure to pathogens. Most of that work has been done on aboveground plant pathogens, whereas relatively little is known on consequences of climate warming for soil pathogens and their effects on plants. In one study, the soil-borne pathogen Pythium cinnamomi that causes oak decline increased its activity under climate warming (Brasier 1996).
Possibly, soil pathogen and root herbivore communities will be influenced strongly when plant community composition is altered (Viketoft et al. 2009), which would be in analogy with how mycorrhizal fungi are influenced. Altered shoot to root ratios might influence plant sensitivity to root herbivory and soil pathogen sensitivity. Clearly, these effects need to be explored in further depth in order to determine their influences on community organization and ecosystem functioning, but climate change obviously will affect the current interactions of plants with their root pathogens and herbivores, although the outcomes are still difficult to predict. Other studies have examined how warming can affect soil community composition and system functioning. Warming pulses can be affecting soil community composition and ecosystem functions more than gradual increases in temperature, which complicates predictive modelling even further (Barrett et al. 2008).
4. Responses to climate warming by range expansion
The literature on range limits includes many exceptions and few generalities (Gaston 2009). Besides hard boundaries and temperature gradients, species ranges can also be limited by biotic conditions (Moore et al. 2007). For example, specialized parasites or pollinators cannot spread beyond the range of their hosts, or pollinator-dependent plants cannot spread beyond their pollinators (Klein et al. 2008). Nevertheless, the current range shifts towards higher latitudes as well as altitudes make it quite plausible that climate warming enables range expansion of species from warm climate regions into previously colder regions (Walther et al. 2002; Parmesan & Yohe 2003). In The Netherlands, a database survey revealed that most of the immigrant plants are from warm climate regions (Tamis et al. 2005). Of course, there is a much larger pool size of species from warm climate regions, but when for example land use change is the main cause of range shifts, species should shift in any direction where land use is changing. This is not happening as massively as from warm to previously cold ranges.
Species vary largely in their ability to actively shift ranges. In general, birds may shift faster than mammals, insects, plants or soil organisms (Berg et al. 2010). However, dispersal distance can also vary largely within species groups. For example, plants with heavy seeds can drop their seeds just next to the mother plants, whereas seeds of wind-dispersed plants can move kilometres away (Soons & Bullock 2008). Maximum dispersal potential may not be reached when dispersal is limited by physical barriers, when habitats are fragmented or when species require special conditions in the recipient habitats (Soons et al. 2005). Dispersal limitations may be overcome by vector organisms, such as waterfowl that disperse marsh plants across long distances during spring migration (Charalambidou & Santamaria 2005). Therefore, average dispersal distances can be helpful to some extent in predicting how species interactions may change as a result of climate change, but because of the large variation among species or environmental conditions, the variety of possible outcomes still will be substantial.
Any analysis based on dispersal capacities will be complicated further by long-distance dispersal (Hastings et al. 2005). Often, dispersal data used in modelling studies are based on short-dispersal distance information, which would be an underestimation of the real dispersal possibilities. Therefore, deterministic models use estimated fractions of the total population that achieve long-distance dispersal (Clark et al. 2003). This will still result in uncertainties in the model estimates. Moreover, other factors influence the outcomes of modelling, such as the conditions of the dispersal corridors or habitat suitability; in one analysis wide corridors promoted short-distance dispersal of plants, whereas suitable habitats in the receptor range promoted the success of long-distance dispersal (van Dorp et al. 1997). Actual colonization patterns, for example of Lactuca serriola in The Netherlands over the past 50 years, clearly show that long-distance dispersal results in populations far away from the expansion zone, which then colonize the surrounding area by short-distance dispersal (Hooftman et al. 2006). Such isolated populations may not be traced easily by specialist enemies or by the enemies' enemies (Kruess & Tscharntke 1994). This insight in dispersal possibilities and limitations might also apply to belowground species and species interactions, although that information is far less available.
As range-shift capacities also depend on the habitat requirements of the species involved, current climate warming-induced range-shifts will differ from those in the pre-industrial era. Current habitat fragmentation, farming practices and urbanization will reduce the capacity of many species to respond to climate change by range-shifting. For example, several studies in the UK have shown that habitat-generalist and habitat-specialist butterflies respond differently to climate warming. Habitat-generalists are more limited by climate warming, whereas habitat-specialists are limited most by the availability of suitable host plants (Warren et al. 2001; Menendez et al. 2007). As a result of limiting suitable habitats, or host plants, habitat-specialists are more susceptible to extinction owing to climate warming than habitat-generalists (Warren et al. 2001). Pollinator networks, on the other hand are probably robust against rapid current climate warming (Hegland et al. 2009). Recent advances in responses of pollinator networks to climate change are discussed elsewhere in this special issue (Memmott 2010). For soil decomposer communities, habitat fragmentation does not seem to play a major role (Rantalainen et al. 2008). Moreover, soil decomposer communities are supposed to be quite general in their decomposition activities and most soil organisms present in the rhizosphere originate from the soil surrounding the plant roots, rather than from further distance (de Ridder-Duine et al. 2005).
When predators and preys both disperse, they do not necessarily interact with each other in the new range. The brown argus butterfly, Aricia agestis, expanded its range as a response to warming. In the new range, the larvae suffered less mortality from parasitization than in long established populations, although in the new range, the parasitoids of Aricia were present on the common blue butterfly, Polyommatus icarus. Therefore, trophic interactions may not necessarily become reconnected, even when both prey and predator move to the same new range (Menendez et al. 2008).
5. Conceptual analogies and differences between climate warming and biological invasions
There are a number of analogies between introduced exotic plants and plants that are subjected to climate change. Plants may either perform better or worse when disconnected from higher trophic level interactions, or when exposed to fewer specialist herbivores (table 1). The various outcomes in figure 1 depending on where trophic control is disrupted may be owing to the decoupling of trophic interactions in existing communities, as well as to altered trophic interactions following range-shifts; both processes can be covered by the conceptual representations in figure 1. For example, when winter warming releases St John's wort from insect damage (Fox et al. 1999), the plants can become more abundant, when the insects were a key control factor and when this role is not taken over by another factor. In another climate change-related example, range-shifts may disconnect plants from their natural enemies in the soil (van Grunsven et al. 2010). This has an analogy with the release of exotic plants from natural soil-borne enemies when introduced to a new continent (Reinhart et al. 2003; Callaway et al. 2004). Range expansion could also lead to the loss of symbiotic arbuscular mycorrhizal fungi or pollinating insects, but these effects have been supposed to be less negative, because of their relatively generalist nature (Richardson et al. 2000). If plants do not become released from their enemies but they lose the enemies of the herbivores, plants may be less abundant under warming (figure 1).
Table 1.
Overview of similarities and differences between successful intracontinental range-expanding plants and intercontinental invasive plants based on literature information and our own estimations based on common knowledge.
| type of range shift |
|||
|---|---|---|---|
| process | altitudinal shifts | intracontinental range expansion | intercontinental invasion |
| enemy release | short-term, mainly from soil-borne enemiesa | mid-long-term, probably stronger from soil-borne enemies than from aboveground enemiesb | long term, both belowground and aboveground enemiesc |
| dispersal barriersa | no | moderate | high |
| range limitationd | probably temperature | probably temperature and photoperiod | probably temperature and photoperiod |
| availability of symbiotic mutualistse | pollinators will be available; mycorrhizal fungi and nitrogen fixing microbes close by or present | pollinators may, or may not be available; mycorrhizal fungi and nitrogen fixing microbes probably available | pollinators will be available; mycorrhizal fungi and nitrogen fixing microbes will be available |
| continuity of gene flow from original populationsa | yes | yes | no |
| hybridization with local speciesa | yes | yes | yes |
| hybridization with other populations from same speciesa | yes | yes | depends on number of introductions and geographical spread of source populations |
aOur own estimations based on common knowledge.
The question is whether effective range-expanders have different properties than related residents. In one study, range-expanding species from warm climate regions were better defended against polyphagous shoot feeders than related plant species that were native in the invaded region (Engelkes et al. 2008). This supports the view that the high insect diversity in tropical and Mediterranean ecosystems has contributed to the selection of a high diversity and high levels of secondary plant compounds (Coley & Barone 1996), although that issue needs further study in the case of range-expanding plants. In eastern USA, on salt marsh plants at lower latitudes, damage due to leaf chewers was 2–10 times lower than in higher latitude salt marshes (Pennings et al. 2009). In palatability tests, herbivores mostly preferred higher latitude plants over lower latitude plants (Pennings et al. 2001). These different selection pressures on lower latitude plants could also have an effect on decomposition. However, a worldwide survey showed that there is larger variation within latitudes due to plant trait variation for decomposability than there is a clear latitudinal pattern in litter decomposition (Cornwell et al. 2008). For decomposition of low-latitude plants in high-latitude environments, local temperature and moisture conditions might be more important in controlling the rate of litter decomposition (Wall et al. 2008).
When low-latitude plants expand their range towards former colder regions, this will go along with a number of changes. First, successful range-expanding plants may have less negative feedback interactions with the soil community from the new range than related species that are native in that range, which results in an enemy release effect (Van Grunsven et al. 2007; Engelkes et al. 2008). Second, the shoots of those range-expanding plants are of less quality to polyphagous generalist insects than the shoots of related native plants (Engelkes et al. 2008). Third, interactions between range-expanding plants and mycorrhizal fungi and other symbionts have not yet been tested, but given the reported specificity in at least some host–symbiont interactions (Klironomos 2003), plant–mutualism interactions might be less effective in the new range when compared with the original range. Fourth, the different traits of lower latitude plants could influence decomposition of the leaf material, but effects may depend predominantly on the traits of the range-shifting plant species and not on their origin (Cornwell et al. 2008). Fifth, the range-expanders may hybridize with related local plant species as has also been shown for cross-continental exotic plants (Ellstrand & Schierenbeck 2000). These hybrids may be superior and become invasive, as has been shown for invasive lizards, which produce hybrids in the new range that have superior invasive capacity (Kolbe et al. 2004).
Intracontinental range-expanding plants may also differ from intercontinental exotic plants in a number of respects (table 1). For example, the limitations of natural enemies of intracontinental range-expanders to co-migrate are mainly due to different dispersal rates and not to dispersal barriers, such as oceans or mountain ridges. Ultimately, these natural enemies, as well as their predators, might show up in the expansion range of their host as well, albeit there is uncertainty whether the original interactions will become re-established (Menendez et al. 2008). Whether or not these interactions will become re-established and at what trophic level the re-establishment may fail there is uncertainty will influence the pattern of abundance of range-expanding plants in their new habitat (figure 1). Moreover, plants that expand their range within a continent will be more exposed to an ongoing gene flow from their source areas than plants that are introduced artificially to new continents. This will influence the rate of adaptation to the new environmental conditions. In order to further elucidate these patterns and processes, experimental studies are needed to determine the factors that control species abundance in their current habitat, and to assess how these species may, or may not, be controlled when either the climate conditions in that habitat change, or when species shift range to higher latitudes or altitudes.
6. Discussion
Including knowledge on multitrophic level interactions in current model predictions on climate warming effects will be a major challenge for ecology (Tylianakis et al. 2008). Until this refinement of the models is accomplished, predictions will not account for the effects of loss or gain of natural enemies, symbionts and their enemies in the new range. Improved estimates require closer interaction of predictive modelling and empirical validation of the input parameters. Enhanced insights into the factors that ultimately determine the chances of species to keep up with climate warming will enrich ecology with a better understanding of factors that determine the abundance and distribution of species, which are two core aims of this field of research. Moreover, that information will enhance the adequateness of policy-making and the necessary mitigation and adaptation management practices.
Interestingly, marine researchers have already made considerable progress in including trophic relationships into their analyses of climate warming effects on community composition and ecosystem processes. They are able to combine information on the ecology of predator–prey interactions, foodweb relationships and databases on numbers and size distribution of fish and other species (Mouritsen et al. 2005). In the marine world, there is also awareness that marine invaders may shift range owing to climate warming and that this has important consequences on trophic interactions and material flows (Occhipinti-Ambrogi 2007). Such information is largely lacking in terrestrial systems and, probably, network theory could contribute to predictions to be tested in empirical studies. Also, consequences of range shifts for ecosystem processes and nutrient-cycling have not yet been explored, although those consequences may be more related to the traits of species than to their origin (Cornwell et al. 2008). Ultimately, combinations of habitat suitability, life-history strategy, enemy release and genetic processes in the native and new ranges will determine whether or not species can keep up with climate warming and how they will perform under the new conditions. Some studies in terrestrial systems have already shown successfully that including biotic interactions across trophic levels improves the outcomes of climate models, even at macro-ecological scales (Araujo & Luoto 2007). These approaches need further development in order to reduce the uncertainties in the current predictions.
An important question is what role selection and adaptation will play in enabling species to respond to climate change and to altered abiotic and biotic environments. In a transplant experiment on a continental scale, two plant species with a wide-spread occurrence showed differences in adaptation to local climate, whereas there was no adaptation to local soil conditions (Macel et al. 2007). This suggests that genetic responses to climate change can be species-specific. Another way of plants to deal with different environments is phenotypic plasticity, where generalist genotypes can cope with various environmental conditions. The moving of the invasive weed Verbascum thapsus to higher elevations in the Sierra Nevada was probably due to ready-for-purpose genotypes rather than to rapid evolution (Parker et al. 2003). Along the same line, the sea beet (Beta vulgaris ssp. maritima) shows considerable genetic variability for photoperiod, which may enable them to easily keep up with climate warming when moving to higher latitudes (Van Dijk & Hautekeete 2007). However, it is unknown to what extent species that shift range are subject to rapid selection and to what extent continuous gene flow from the original range may prevent adaptation in the expansion range.
We propose that more accurate predictions on the responses of plant species to climate change will benefit from acknowledging that plants are in between aboveground and belowground multitrophic interactions; mind that the plant–herbivore–carnivore interactions depicted in figure 1 can be active aboveground, as well as belowground. It has already been acknowledged that climate warming can enable invasive species to expand their range into previously colder biomes (Walther et al. 2009). Here, we argue that changes in local trophic interactions, as well as changes in trophic interactions during natural climate warming-induced range expansion can cause unexpected changes in the abundance of individual species. This will affect biodiversity. In principle, the outcomes of those interactions may result in rare species going extinct when their herbivores and pathogens are released from their enemies, to becoming invasive when successfully released from herbivores and pathogens above- and/or below-ground.
7. Conclusions
— An increasing number of examples show that interactions between plants and aboveground and belowground higher trophic level organisms can become, at least temporarily, disconnected during climate warming. Understanding that process from an ecological-evolutionary perspective is key to explain why some plants may and others may not become rare or abundant in their native or new range due to climate change.
— As biotic interactions across trophic levels are an important driver of selection processes, disruption of connections between preys and their predators, or between plants and their herbivores, pathogens or symbionts may enhance or counteract the ability of species to become adapted to their new climate, or to their new range. On the other hand, gene flow from the original range may limit adaptation in the new range.
— Recent studies have pointed at the need of including biotic interactions in climate envelope models. Some of them only consider biotic interactions within the same trophic level, whereas considering interactions with herbivores, pathogens, carnivores and symbionts could change the outcomes of climate change considerably.
— Enhancing the predictions requires more intensive interactions between modelling and empirical studies, including information from the current conditions and the future conditions, as well as from the native and the expansion range.
— In the short term, the reward for doing so may seem low, as it will turn out that many empirical studies are needed to quantify and generalize the outcomes of multitrophic level interactions now and in the future. However, coupling these empirical and modelling studies may further enhance our knowledge on ecological interactions and factors that control the abundance and distribution of species.
Acknowledgements
This manuscript was developed as a result of a session at the British Ecological Society meeting at Imperial College, London in September 2008. W.H.Vd.P. and M.E.V. acknowledge their NWO-ALW VICI grants. This is publication 4738 of the Netherlands Institute of Ecology (NIOO-KNAW).
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Araujo M. B., Luoto M.2007The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753 (doi:10.1111/j.1466-8238.2007.00359.x) [Google Scholar]
- Bale J. S., et al. 2002Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1–16 (doi:10.1046/j.1365-2486.2002.00451.x) [Google Scholar]
- Bardgett R. D., Wardle D. A., Yeates G. W.1998Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol. Biochem. 30, 1867–1878 (doi:10.1016/S0038-0717(98)00069-8) [Google Scholar]
- Bardgett R. D., Bowman W. D., Kaufmann R., Schmidt S. K.2005A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 20, 634–641 (doi:10.1016/j.tree.2005.08.005) [DOI] [PubMed] [Google Scholar]
- Barrett J. E., Virginia R. A., Wall D. H., Doran P. T., Fountain A. G., Welch K. A., Lyons W. B.2008Persistent effects of a discrete warming event on a polar desert ecosystem. Glob. Change Biol. 14, 2249–2261 (doi:10.1111/j.1365-2486.2008.01641.x) [Google Scholar]
- Berg M. P., Kiers E. T., Driessen G., Van der Heijden M. G. A., Kooi W. B. W., Kuenen F., Liefting M., Verhoef H. A., Ellers J.2010Adapt or disperse: understanding species persistence in a changing world. Glob. Change Biol. 16, 587–598 (doi:10.1111/j.1365-2486.2009.02014.x) [Google Scholar]
- Bezemer T. M., Jones T. H., Knight K. J.1998Long-term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and its parasitoid Aphidius matricariae. Oecologia 116, 128–135 (doi:10.1007/s004420050571) [DOI] [PubMed] [Google Scholar]
- Brasier C. M.1996Phytophthora cinnamomi and oak decline in southern Europe. Ann. Sci. Forest. 53, 347–358 (doi:10.1051/forest:19960217) [Google Scholar]
- Brooker R. W., Travis J. M. J., Clark E. J., Dytham C.2007Modelling species' range shifts in a changing climate: the impacts of biotic interactions, dispersal distance and the rate of climate change. J. Theoret. Biol. 245, 59–65 (doi:10.1016/j.jtbi.2006.09.033) [DOI] [PubMed] [Google Scholar]
- Brown V. K., Gange A. C.1992Secondary plant succession: how is it modified by insect herbivory? Vegetatio 101, 3–13 (doi:10.1007/BF00031910) [Google Scholar]
- Callaway R. M., Thelen G. C., Rodriguez A., Holben W. E.2004Soil biota and exotic plant invasion. Nature 427, 731–733 (doi:10.1038/nature02322) [DOI] [PubMed] [Google Scholar]
- Chapin F. S., Shaver G. R., Giblin A. E., Nadelhoffer K. J., Laundre J. A.1995Responses of Arctic Tundra to experimental and observed changes in climate. Ecology 76, 694–711 [Google Scholar]
- Charalambidou I., Santamaria L.2005Field evidence for the potential of waterbirds as dispersers of aquatic organisms. Wetlands 25, 252–258 (doi:10.1672/2) [Google Scholar]
- Clark J. S., Lewis M., McLachlan J. S., Hille Ris Lambers J.2003Estimating population spread: what can we forecast and how well? Ecology 84, 1979–1988 (doi:10.1890/01-0618) [Google Scholar]
- Coley P. D., Barone J. A.1996Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 27, 305–335 (doi:10.1146/annurev.ecolsys.27.1.305) [Google Scholar]
- Cornwell W. K., et al. 2008Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071 (doi:10.1111/j.1461-0248.2008.01219.x) [DOI] [PubMed] [Google Scholar]
- Davis A. J., Jenkinson L. S., Lawton J. H., Shorrocks B., Wood S.1998Making mistakes when predicting shifts in species range in response to global warming. Nature 391, 783–786 (doi:10.1038/35842) [DOI] [PubMed] [Google Scholar]
- De Deyn G. B., Van der Putten W. H.2005Linking aboveground and belowground diversity. Trends Ecol. Evol. 20, 625–633 (doi:10.1016/j.tree.2005.08.009) [DOI] [PubMed] [Google Scholar]
- De Deyn G. B., Raaijmakers C. E., Zoomer H. R., Berg M. P., de Ruiter P. C., Verhoef H. A., Bezemer T. M., Van der Putten W. H.2003Soil invertebrate fauna enhances grassland succession and diversity. Nature 422, 711–713 (doi:10.1038/nature01548) [DOI] [PubMed] [Google Scholar]
- De Jong T. J.1995Why fast-growing plants do not bother about defence. Oikos 74, 545–548 (doi:10.2307/3546002) [Google Scholar]
- de Ridder-Duine A. S., Kowalchuk G. A., Gunnewiek P., Smant W., Van Veen J. A., De Boer W.2005Rhizosphere bacterial community composition in natural stands of Carex arenaria (sand sedge) is determined by bulk soil community composition. Soil Biol. Biochem. 37, 349–357 [Google Scholar]
- Ellstrand N. C., Schierenbeck K. A.2000Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl Acad. Sci. USA 97, 7043–7050 (doi:10.1073/pnas.97.13.7043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkes T., Morriën E., Verhoeven K. J. F., Bezemer T. M., Biere A., Harvey J. A., McIntyre L. M., Tamis W. L. M., Van der Putten W. H.2008Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456, 946–948 (doi:10.1038/nature07474) [DOI] [PubMed] [Google Scholar]
- Field R., et al. 2009Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147 (doi:10.1111/j.1365-2699.2008.01963.x) [Google Scholar]
- Fox L. R., Ribeiro S. P., Brown V. K., Masters G. J., Clarke I. P.1999Direct and indirect effects of climate change on St John's wort, Hypericum perforatum L. (Hypericaceae). Oecologia 120, 113–122 (doi:10.1007/s004420050839) [DOI] [PubMed] [Google Scholar]
- Fuhrer J.2003Agroecosystern responses to combinations of elevated CO2, ozone, and global climate change. Agric. Ecosyst. Environ. 97, 1–20 (doi:10.1016/S0167-8809(03)00125-7) [Google Scholar]
- Fujimura K. E., Egger K. N., Henry G. H.2008The effect of experimental warming on the root-associated fungal community of Salix arctica. ISME J. 2, 105–114 (doi:10.1038/ismej.2007.89) [DOI] [PubMed] [Google Scholar]
- Gaston K. J.2009Geographic range limits: achieving synthesis. Proc. R. Soc. B 276, 1395–1406 (doi:10.1098/rspb.2008.1480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig-Fasel J., Guisan A., Zimmermann N. E.2008Evaluating thermal treeline indicators based on air and soil temperature using an air-to-soil temperature transfer model. Ecol. Mod. 213, 345–355 [Google Scholar]
- Gillson L., Ekblom A., Willis K. J., Froyd C.2008Holocene palaeo-invasions: the link between pattern, process and scale in invasion ecology? Landsc. Ecol. 23, 757–769 (doi:10.1007/s10980-008-9243-6) [Google Scholar]
- Hartley S. E., Gange A. C.2009Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu. Rev. Entomol. 54, 323–342 (doi:10.1146/annurev.ento.54.110807.090614) [DOI] [PubMed] [Google Scholar]
- Hastings A., et al. 2005The spatial spread of invasions: new developments in theory and evidence. Ecol. Lett. 8, 91–101 (doi:10.1111/j.1461-0248.2004.00687.x) [Google Scholar]
- Hegland S. J., Nielsen A., Lazaro A., Bjerknes A. L., Totland O.2009How does climate warming affect plant–pollinator interactions? Ecol. Lett. 12, 184–195 (doi:10.1111/j.1461-0248.2008.01269.x) [DOI] [PubMed] [Google Scholar]
- Heinemeyer A., Fitter A. H.2004Impact of temperature on the arbuscular mycorrhizal (AM) symbiosis: growth responses of the host plant and its AM fungal partner. J. Exp. Bot. 55, 525–534 (doi:10.1093/jxb/erh049) [DOI] [PubMed] [Google Scholar]
- Holt R. D., Barfield M.2009Trophic interactions and range limits: the diverse roles of predation. Proc. R. Soc. B 276, 1435–1442 (doi:10.1098/rspb.2008.1536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooftman D. A. P., Oostermeijer J. G. B., Den Nijs J. C. M.2006Invasive behaviour of Lactuca serriola (Asteraceae) in the Netherlands: spatial distribution and ecological amplitude. Basic Appl. Ecol. 7, 507–519 (doi:10.1016/j.baae.2005.12.006) [Google Scholar]
- Jones P. D., Mann M. E.2004Climate over past millennia. Rev. Geophys. 42, RG2002 (doi:10.1029/2003RG000143) [Google Scholar]
- Klein D. R., Bruun H. H., Lundgren R., Philipp M.2008Climate change influences on species interrelationships and distributions in high-Arctic Greenland. Adv. Ecol. Res. 40, 81–100 (doi:10.1016/S0065-2504(07)00004-9) [Google Scholar]
- Klironomos J. N.2003Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84, 2292–2301 (doi:10.1890/02-0413) [Google Scholar]
- Kolbe J. J., Glor R. E., Schettino L. R. G., Lara A. C., Larson A., Losos J. B.2004Genetic variation increases during biological invasion by a Cuban lizard. Nature 431, 177–181 (doi:10.1038/nature02807) [DOI] [PubMed] [Google Scholar]
- Koricheva J., Gange A. C., Jones T.2009Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90, 2088–2097 (doi:10.1890/08-1555.1) [DOI] [PubMed] [Google Scholar]
- Kruess A., Tscharntke T.1994Habitat fragmentation, species loss, and biological control. Science 264, 1581–1584 (doi:10.1126/science.264.5165.1581) [DOI] [PubMed] [Google Scholar]
- Kytoviita M. M., Ruotsalainen A. D.2007Mycorrhizal benefit in two low arctic herbs increases with increasing temperature. Am. J. Bot. 94, 1309–1315 (doi:10.3732/ajb.94.8.1309) [DOI] [PubMed] [Google Scholar]
- Lavelle P., Blanchart E., Martin A., Martin S., Spain A., Toutain F., Barois I., Schaefer R.1993A hierarchical model for decomposition in terrestrial ecosystems: application to soils of the humid tropics. Biotropica 25, 130–150 (doi:10.2307/2389178) [Google Scholar]
- Macel M., et al. 2007Climate vs. soil factors in local adaptation of two common plant species. Ecology 88, 424–433 (doi:10.1890/0012-9658(2007)88[424:CVSFIL]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Marchand F. L., Nijs I., Heuer M., Mertens S., Kockelbergh F., Pontailler J. Y., Impens I., Beyens L.2004Climate warming postpones senescence in high Arctic tundra. Arctic Antarctic Alp. Res. 36, 390–394 (doi:10.1657/1523-0430(2004)036[0390:CWPSIH]2.0.CO;2) [Google Scholar]
- McLachlan J. S., Clark J. S., Manos P. S.2005Molecular indicators of tree migration capacity under rapid climate change. Ecology 86, 2088–2098 (doi:10.1890/04-1036) [Google Scholar]
- Memmott J., Carvell C., Pywell R. F., Craze P. G.2010The potential impact of global warming on the efficacy of field margins sown for the conservation of bumble-bees. Phil. Trans. R. Soc. B 365, 2071–2079 (doi:10.1098/rstb.2010.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez R., Gonzalez-Megias A., Collingham Y., Fox R., Roy D. B., Ohlemuller R., Thomas C. D.2007Direct and indirect effects of climate and habitat factors on butterfly diversity. Ecology 88, 605–611 (doi:10.1890/06-0539) [DOI] [PubMed] [Google Scholar]
- Menendez R., Gonzalez-Megias A., Lewis O. T., Shaw M. R., Thomas C. D.2008Escape from natural enemies during climate-driven range expansion: a case study. Ecol. Entomol. 33, 413–421 (doi:10.1111/j.1365-2311.2008.00985.x) [Google Scholar]
- Mikola J., Yeates G. W., Barker G. M., Wardle D. A., Bonner K. I.2001Effects of defoliation intensity on soil food-web properties in an experimental grassland community. Oikos 92, 333–343 (doi:10.1034/j.1600-0706.2001.920216.x) [Google Scholar]
- Milbau A., Stout J. C., Graae B. J., Nijs I.2009A hierarchical framework for integrating invasibility experiments incorporating different factors and spatial scales. Biol. Inv. 11, 941–950 (doi:10.1007/s10530-008-9306-2) [Google Scholar]
- Moore P., Hawkins S. J., Thompson R. C.2007Role of biological habitat amelioration in altering the relative responses of congeneric species to climate change. Mar. Ecol.-Prog. Ser. 334, 11–19 (doi:10.3354/meps334011) [Google Scholar]
- Mouritsen K. N., Tompkins D. M., Poulin R.2005Climate warming may cause a parasite-induced collapse in coastal amphipod populations. Oecologia 146, 476–483 (doi:10.1007/s00442-005-0223-0) [DOI] [PubMed] [Google Scholar]
- Occhipinti-Ambrogi A.2007Global change and marine communities: alien species and climate change. Mar. Pollut. Bull. 55, 342–352 [DOI] [PubMed] [Google Scholar]
- Packer A., Clay K.2000Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404, 278–281 (doi:10.1038/35005072) [DOI] [PubMed] [Google Scholar]
- Parker I. M., Rodriguez J., Loik M. E.2003An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conserv. Biol. 17, 59–72 (doi:10.1046/j.1523-1739.2003.02019.x) [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Pathikonda S., Ackleh A. S., Hasenstein K. H., Mopper S.2009Invasion, disturbance, and competition: modeling the fate of coastal plant populations. Conserv. Biol. 23, 164–173 (doi:10.1111/j.1523-1739.2008.01073.x) [DOI] [PubMed] [Google Scholar]
- Pearson R. G., Dawson T. P.2003Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 (doi:10.1046/j.1466-822X.2003.00042.x) [Google Scholar]
- Pennings S. C., Siska E. L., Bertness M. D.2001Latitudinal differences in plant palatability in Atlantic coast salt marshes. Ecology 82, 1344–1359 (doi:10.1890/0012-9658(2001)082[1344:LDIPPI]2.0.CO;2) [Google Scholar]
- Pennings S. C., Ho C. K., Salgado C. S., Wieski K., Dave N., Kunza A. E., Wason E. L.2009Latitudinal variation in herbivore pressure in Atlantic Coast salt marshes. Ecology 90, 183–195 (doi:10.1890/08-0222.1) [DOI] [PubMed] [Google Scholar]
- Post E., Forchhammer M. C.2008Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375 (doi:10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K., Rotenberry J. T., Redak R. A., Allen M. F.2008Habitat shifts of endangered species under altered climate conditions: importance of biotic interactions. Glob. Change Biol. 14, 2501–2515 [Google Scholar]
- Price P. W.1984The concept of the ecosystem. In Ecological entomology (eds Huffaker C. B., Robb R. L.), pp. 19–52 New York: John Wiley & Sons [Google Scholar]
- Rantalainen M. L., Haimi J., Fritze H., Pennanen T., Setälä H.2008Soil decomposer community as a model system in studying the effects of habitat fragmentation and habitat corridors. Soil Biol. Biochem. 40, 853–863 (doi:10.1016/j.soilbio.2007.11.008) [Google Scholar]
- Reinhart K. O., Packer A., Van der Putten W. H., Clay K.2003Plant-soil biota interactions and spatial distribution of black cherry in its native and invasive ranges. Ecol. Lett. 6, 1046–1050 (doi:10.1046/j.1461-0248.2003.00539.x) [Google Scholar]
- Richardson D. M., Allsopp N., D'Antonio C. M., Milton S. J., Rejmanek M.2000Plant invasions: the role of mutualisms. Biol. Rev. 75, 65–93 (doi:10.1017/S0006323199005435) [DOI] [PubMed] [Google Scholar]
- Rillig M. C., Wright S. F., Shaw M. R., Field C. B.2002Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 97, 52–58 (doi:10.1034/j.1600-0706.2002.970105.x) [Google Scholar]
- Ruess L., Michelsen A., Schmidt I. K., Jonasson S.1999Simulated climate change affecting microorganisms, nematode density and biodiversity in subarctic soils. Plant Soil 212, 63–73 [Google Scholar]
- Schädler M., Jung G., Brändl R., Auge H.2004Secondary succession is influenced by belowground insect herbivory on a productive site. Oecologia 138, 242–252 (doi:10.1007/s00442-003-1425-y) [DOI] [PubMed] [Google Scholar]
- Schmitz O. J., Post E., Burns C. E., Johnston K. M.2003Ecosystem responses to global climate change: moving beyond color mapping. Bioscience 53, 1199–1205 (doi:10.1641/0006-3568(2003)053[1199:ERTGCC]2.0.CO;2) [Google Scholar]
- Schweiger O., Settele J., Kudrna O., Klötz S., Kuhn I.2008Climate change can cause spatial mismatch of trophically interacting species. Ecology 89, 3472–3479 (doi:10.1890/07-1748.1) [DOI] [PubMed] [Google Scholar]
- Soons M. B., Bullock J. M.2008Non-random seed abscission, long-distance wind dispersal and plant migration rates. J. Ecol. 96, 581–590 (doi:10.1111/j.1365-2745.2008.01370.x) [Google Scholar]
- Soons M. B., Messelink J. H., Jongejans E., Heil G. W.2005Habitat fragmentation reduces grassland connectivity for both short-distance and long-distance wind-dispersed forbs. J. Ecol. 93, 1214–1225 (doi:10.1111/j.1365-2745.2005.01064.x) [Google Scholar]
- Staddon P. L., Thompson K., Jakobsen I., Grime J. P., Askew A. P., Fitter A. H.2003Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob. Change Biol. 9, 186–194 (doi:10.1046/j.1365-2486.2003.00593.x) [Google Scholar]
- Tamis W. L. M., Van't Zelfde M., Van der Meijden R., De Haes H. A. U.2005Changes in vascular plant biodiversity in the Netherlands in the 20th century explained by their climatic and other environmental characteristics. Clim. Change 72, 37–56 (doi:10.1007/s10584-005-5287-7) [Google Scholar]
- Thomas C. D., et al. 2004Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- Thuiller W., Lavorel S., Araujo M. B., Sykes M. T., Prentice I. C.2005Climate change threats to plant diversity in Europe. Proc. Natl Acad. Sci. USA 102, 8245–8250 (doi:10.1073/pnas.0409902102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A.2008Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- Van der Putten W. H., de Ruiter P. C., Bezemer T. M., Harvey J. A., Wassen M., Wolters V.2004Trophic interactions in a changing world. Basic Appl. Ecol. 5, 487–494 [Google Scholar]
- Van Dijk H., Hautekeete N.2007Long day plants and the response to global warming: rapid evolutionary change in day length sensitivity is possible in wild beet. J. Evol. Biol. 20, 349–357 (doi:10.1111/j.1420-9101.2006.01192.x) [DOI] [PubMed] [Google Scholar]
- Van Dorp D., Schippers P., vanGroenendael J. M.1997Migration rates of grassland plants along corridors in fragmented landscapes assessed with a cellular automation model. Landsc. Ecol. 12, 39–50 [Google Scholar]
- Van Grunsven R. H. A., Van der Putten W. H., Bezemer T. M., Tamis W. L. M., Berendse F., Veenendaal E. M.2007Reduced plant-soil feedback of plant species expanding their range as compared to natives. J. Ecol. 95, 1050–1057 (doi:10.1111/j.1365-2745.2007.01282.x) [Google Scholar]
- Van Grunsven R. H. A., Van der Putten W. H., Bezemer T. M., Berendse F., Veenendaal E. M.2010Plant–soil interactions in the expansion and native range of a poleward shifting plant species. Glob. Change Biol. 16, 380–385 (doi:10.1111/j.1365-2486.2009.01996.x) [Google Scholar]
- Viketoft M., Bengtsson J., Sohlenius B., Berg M. P., Petchey O., Palmborg C., Huss-Danell K.2009Long-term effects of plant diversity and composition on soil nematode communities in model grasslands. Ecology 90, 90–99 (doi:10.1890/08-0382.1) [DOI] [PubMed] [Google Scholar]
- Visser M. E.2008Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Both C.2005Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. H., et al. 2008Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Change Biol. 14, 2661–2677 [Google Scholar]
- Walther G. R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J. M., Hoegh-Guldberg O., Bairlein F.2002Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- Walther G. R., et al. 2009Alien species in a warmer world: risks and opportunities. Trends Ecol. Evol. 24, 686–693 (doi:10.1016/j.tree.2009.06.008) [DOI] [PubMed] [Google Scholar]
- Warren M. S., et al. 2001Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414, 65–69 (doi:10.1038/35102054) [DOI] [PubMed] [Google Scholar]
- Whittaker R. J., Willis K. J., Field R.2001Scale and species richness: towards a general, hierarchical theory of species diversity. J. Biogeogr. 28, 453–470 (doi:10.1046/j.1365-2699.2001.00563.x) [Google Scholar]