Abstract
The carbon cycle modulates climate change, via the regulation of atmospheric CO2, and it represents one of the most important services provided by ecosystems. However, considerable uncertainties remain concerning potential feedback between the biota and the climate. In particular, it is unclear how global warming will affect the metabolic balance between the photosynthetic fixation and respiratory release of CO2 at the ecosystem scale. Here, we present a combination of experimental field data from freshwater mesocosms, and theoretical predictions derived from the metabolic theory of ecology to investigate whether warming will alter the capacity of ecosystems to absorb CO2. Our manipulative experiment simulated the temperature increases predicted for the end of the century and revealed that ecosystem respiration increased at a faster rate than primary production, reducing carbon sequestration by 13 per cent. These results confirmed our theoretical predictions based on the differential activation energies of these two processes. Using only the activation energies for whole ecosystem photosynthesis and respiration we provide a theoretical prediction that accurately quantified the precise magnitude of the reduction in carbon sequestration observed experimentally. We suggest the combination of whole-ecosystem manipulative experiments and ecological theory is one of the most promising and fruitful research areas to predict the impacts of climate change on key ecosystem services.
Keywords: global warming, carbon sequestration, carbon cycle, metabolic theory, gross primary production, ecosystem respiration
1. Introduction
The biosphere is in the midst of a pronounced warming trend. Global surface temperature has risen by approximately 0.74°C in the past century and is projected to increase by a further 3–5°C over the next 100 years (Houghton 2001; IPCC 2007). Evidence for the ecological impacts of global warming on individual taxa is now unequivocal as represented by range expansions and poleward migrations (Walther et al. 2002; Parmesan & Yohe 2003; Rosenzweig 2008) but the potential responses of whole ecosystems are uncertain (Walther et al. 2002). This may be at least partially due to the perceived difficulties of dealing with such seemingly complex systems (Walther et al. 2002; Montoya et al. 2006; Memmott et al. 2007).
Changes to the carbon cycle are regarded as one of the greatest impacts on ecosystem service supply associated with climate change (Schroter et al. 2005). These changes include direct effects—e.g. on productivity, CO2 sequestration, resource quality—but also indirect effects—e.g. on precipitation patterns, water availability, crop production.
Of special interest are those changes in the biogeochemical cycling of carbon that could potentially alter the ‘metabolic balance’ of ecosystems. This balance is defined as the rate of carbon fixation by photosynthesis relative to remineralization by respiration, and it determines whether an ecosystem acts as a source or a sink for atmospheric CO2 (Woodwell et al. 1998; del Giorgio & Duarte 2002; Woodward 2007).
Some recent evidence has highlighted the potential for feedback between warming and ecosystem CO2 sequestration (Cox et al. 2000; Canadell et al. 2007; Piao et al. 2008). For instance, in terrestrial ecosystems there is a strong positive feedback between temperature and CO2 emission due to elevated rates of soil respiration (Lloyd & Taylor 1994; Cox et al. 2000; Knorr et al. 2005; Davidson & Janssens 2006; Arnone et al. 2008), and it has also been suggested that as the oceans warm their ability to sequester CO2 from the atmosphere may weaken (del Giorgio & Duarte 2002; López-Urrutia et al. 2006).
Recently, several attempts have been made with coupled climate–carbon models to incorporate some of the key biotic components of the carbon cycle (Cox et al. 2000; Friedlingstein et al. 2006). However, there is little agreement as to exactly how this should be done in a systematic and predictive manner. In relation to this point the two fundamental questions that we address here, are:
-
—
How will the metabolic balance of ecosystems respond to warming?
-
—
Can we predict the precise magnitude of such changes for any probable warming scenario?
To answer these questions we combine a whole-system experiment with predictive ecological theory, to enable us to simulate experimentally future warming scenarios and their probable consequences on ecosystem processes. In particular, the experimental component permits direct comparisons to be made between contemporary ecosystems with their ‘future’ warmed counterparts, and also gives us the opportunity to explore the underlying drivers behind the observed responses. Furthermore, by using materially closed systems (i.e. the only inputs of carbon are through gaseous exchange with the atmosphere) we are able to avoid the confounding effects of changes in the movements of allochthonous carbon into and out of the system and focus on the mechanisms affecting changes in the balance of autochthonous carbon.
Here, we first present and test the metabolic theory of ecology (MTE) (sensu Brown et al. 2004) by attempting to establish the temperature dependence of the fundamental components of the carbon cycle (net primary production (NPP), gross primary production (GPP) and ecosystem respiration (ER), respectively) and their dependence on individual metabolism. We then use the theoretical platform of the MTE to explore whether warming will alter carbon sequestration rates in ecosystems. Finally, through extension of the MTE, we attempt to predict quantitative changes in the metabolic balance of ecosystems in response to a probable warming scenario predicted for the end of the next century (IPCC 2007). We tested our predictions at the ecosystem scale using a whole system manipulative experiment in aquatic mesocosms that mimicked this degree of warming.
Lentic freshwater ecosystems are tractable as mesocosms because the unit of the ecosystem is easily delimited and replicable. Importantly, these systems enable the assembly of functioning ecosystems, which although simplifications of their natural counterparts, allow us to understand the mechanisms behind the ecosystem level changes that may occur as a result of warming. Furthermore, freshwater ecosystems (e.g. wetlands) are known to be fundamental components of the global carbon cycle with respect to carbon sequestration (Whiting & Chanton 2001). Therefore, understanding how carbon sequestration rates behave in response to warming in these systems is critical.
2. Theoretical framework
Metabolism is a fundamental process that regulates the flux of energy and matter through multiple levels of biological organization, from individuals to ecosystems (West et al. 1997; Brown et al. 2004). According to the MTE, individual metabolic rate (i.e. the power required to sustain an organism), can be explained by the general metabolic model (West et al. 1997; Gillooly et al. 2001; Brown et al. 2004)
| 2.1 |
where Bi is the basal metabolic rate of an individual i, b0 is a normalization constant independent of body size and temperature, e−E/kT is the Boltzmann factor that describes the temperature, T, dependence of metabolic rate, where k is Boltzmann's constant (8.62×10−5 eV K−1) and E is the activation energy of metabolism. Mi corresponds to the body mass of an individual i, and α is the allometric scaling exponent (West et al. 1997; Brown et al. 2004). By summing the individual metabolic rates of all the organisms within an ecosystem it is possible to predict total ecosystem metabolic rates (Enquist et al. 2003; Allen et al. 2005; López-Urrutia et al. 2006). This general metabolic model has been extended to describe three ecosystem processes that underpin the carbon cycle: NPP, GPP and ER (Enquist et al. 2003; Allen et al. 2005; López-Urrutia et al. 2006).
The rate of GPP for a whole ecosystem can be estimated from the sum of the individual photosynthetic rates of all of its autotrophic organisms (Allen et al. 2005; López-Urrutia et al. 2006):
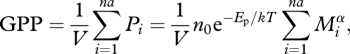 |
2.2 |
where na is the number of autotrophic organisms in volume V, n0 is a normalization constant independent of body size Mi and temperature T, Ep is the effective activation energy governing the temperature dependence of photosynthetic reactions reported in the literature (approx. 0.32 eV; Allen et al. 2005; López-Urrutia et al. 2006), and α is the allometric scaling exponent. The parameter Ep, which is the ‘effective’ activation energy of photosynthesis, approximates the hyperbolic temperature dependence of photosynthesis with an exponential function over the temperature range (0–30°C) to permit direct comparison with the exponential temperature dependence of respiration. (Allen et al. 2005; López-Urrutia et al. 2006). The photosynthesis–temperature response is typically hyperbolic, declining at high temperatures due to deactivation of the component reactions (Bernacchi et al. 2001; Medlyn et al. 2002). However, photosynthetic temperature optima are generally correlated with the environmental temperature range experienced by plants and deactivation is uncommon within the annual environmental temperature range experienced by plants in their natural environment (Larcher 1995). We, therefore, approximate the hyperbolic photosynthesis–temperature relationship with Ep, following Allen et al. (2005) using a well-established model of leaf photosynthesis (Farquhar et al. 1980) and reasonable assumptions (internal CO2 concentrations are about 70% of ambient, co-limitation of photosynthesis by Rubisco, similar kinetic properties for Rubisco across species) that are frequently used in carbon cycling models. It is important to note here, that the derivation of Ep is based on the expected concentrations of CO2 at the sites of photosynthesis in terrestrial plants (Allen et al. 2005). Therefore, potential differences between aquatic and terrestrial photosynthesis, for instance, changes in the concentration gradient of CO2 at the site of photosynthesis, due to Henry's law or slight differences in Rubisco kinetics between aquatic and terrestrial plants, may result in a divergence from the expected Ep of approximately 0.32 eV in aquatic ecosystems, a point that has been previously neglected in tests of MTE in aquatic systems (López-Urrutia et al. 2006).
The GPP of an ecosystem is the gross absorption of CO2. Therefore, GPP also accounts for the photosynthate that is respired by autotrophs. Because autotrophic respiration is ultimately limited by, and tightly coupled to, photosynthate production within individual autotrophs (i.e. by substrate availability; Dewar et al. 1999; Atkin & Tjoelker 2003) the temperature dependence of autotrophic respiration should be constrained by the photosynthetic activation energy over temporal scales as short as days to weeks. This process is called type-I respiratory acclimation, and has been observed empirically (Atkin & Tjoelker 2003) and experimentally (Dewar et al. 1999). In our model for GPP we therefore assume that autotrophic respiration (AR) has an activation energy equivalent to Ep over the comparatively long temporal scale of our experiment (Allen et al. 2005).
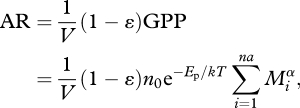 |
2.3 |
where (1 − ɛ) is the fraction of photosynthate that is respired by autotrophs.
The NPP of an ecosystem is defined as its GPP minus the carbon respired by autotrophs, AR (i.e. the net fixation of CO2 into plant biomass; Allen et al. 2005; Woodward 2007):
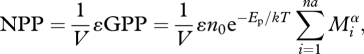 |
2.4 |
where ɛ is the fraction of photosynthate allocated to the net primary production of producer biomass.
In a similar way, the rate of ecosystem respiration (ER) can be estimated from the individual respiratory rates of all of its autotrophic (AR) and heterotrophic (HR) organisms (Enquist et al. 2003; Allen et al. 2005; López-Urrutia et al. 2006):
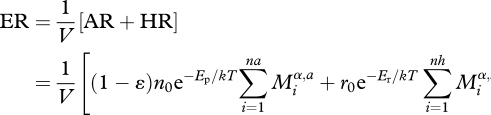 |
2.5 |
where na is the total number of autotrophic organisms and nh is the number of heterotrophic organisms in a volume V, r0 is a normalization constant which is independent of Mi and T. We assume the scaling exponent α is the same for autotrophs, a and heterotrophs, h, (West et al. 1997; Gillooly et al. 2001; Brown et al. 2004). The average activation energy governing the temperature dependence of respiratory reactions, Er is approximately 0.65 eV (Gillooly et al. 2001; Enquist et al. 2003).
In equation (2.5) because ER is the sum of both HR and AR it does not have a simple exponential temperature dependence governed by a single activation energy, unlike NPP or GPP. At steady state, in a closed system, ER is limited for substrate and must equal GPP over the course of a year. Therefore, under conditions of substrate limitation the activation energy for heterotrophic metabolism, Er, should approach the activation energy for photosynthetic reactions, Ep, resulting in equivalent temperature dependences between GPP and ER over the relevant temporal scale (Allen et al. 2005). However, when an ecosystem deviates from steady state (i.e. ER < GPP or ER > GPP), ER is not constrained by GPP. During non-steady-state dynamics, providing there is sufficient stored carbon, heterotrophic respiration may exceed NPP (i.e. the potential contemporary carbon substrate) over temporal scales dependent on the turnover time of the carbon stores. Under such conditions heterotrophic metabolism can proceed at maximum capacity. Therefore, during non-steady-state dynamics, because Er > Ep, ER should have a temperature dependence approaching that of heterotrophic metabolism, Er, and therefore greater than the activation energy for GPP.
Equations (2.2), (2.4) and (2.5) yield general expressions for the temperature dependence of NPP, GPP and ER and highlight the importance of the activation energies for individual metabolism in controlling the temperature response of whole ecosystem metabolic rates. Importantly, the theory outlined above differs from previous work modelling the temperature dependence of the carbon cycle based on individual metabolism (Allen et al. 2005). Here, we do not make the assumption of steady state. Rather, because we are simulating the consequences of global warming on ecosystem metabolism (i.e. a perturbation) we attempt to understand what happens to the metabolic balance of ecosystems during the transitory phase between steady states. As such, GPP and ER have the potential to go out of balance. In a scenario where ER > GPP, ER may be fuelled by baseline respiration (i.e. respiration uncoupled from contemporary primary production) which is dependent on the carbon stored within the system (Trumbore 2000; del Giorgio & Williams 2005). On the other hand, when ER < GPP, ER is not substrate limited. In either case ER is not constrained by GPP and can exhibit non-steady-state dynamics in response to warming.
The theory above provides a platform from which a mechanistic understanding of the potential consequences of global warming on the metabolic balance of ecosystems can be drawn and leads to a number of important predictions, which we tested experimentally. First, the temperature dependence of NPP is governed by the effective activation energy that characterizes photosynthetic reactions, and the relationship between ln(NPP) and 1/kT should approximate a slope of Ep ≈ 0.32 eV. Second, the temperature dependence of GPP is constrained by the activation energy for photosynthetic reactions because of the acclimation of AR and the slope of the relationship between ln(GPP) and 1/kT should be indistinguishable from that of NPP. Third, assuming non-steady-state dynamics, the temperature dependence of ER should be greater than that of GPP and the slope of the relationship between ln(ER) and 1/kT should approach the activation energy of heterotrophic metabolism, Er ≈ 0.65 eV. Finally, and most importantly, because of the differential temperature dependences of the two processes, ecosystem respiration should increase more rapidly than primary production as ecosystems warm.
With an understanding of the mechanisms controlling the temperature dependence of NPP, GPP and ER it is possible to predict how the metabolic balance (ER/GPP)—which is the ability of an ecosystem to sequester carbon—will respond to warming. We define RH : U as the ratio of the metabolic balance (ERH/GPPH) in the heated, i.e. future, systems to the ratio of the metabolic balance (ERU/GPPU) in the unheated, i.e. present, ecosystems, which is given by
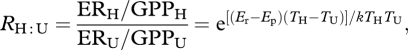 |
2.6 |
where Er and Ep are the activation energies for ecosystem respiration and photosynthesis, respectively, and TH and TU are the temperatures of heated and unheated ecosystems (see the electronic supplementary material, S1 for a full derivation of the theory). Equation (2.6) suggests that the response of the metabolic balance of an ecosystem to warming can be predicted and quantified from the knowledge of two parameters: the difference between the activation energies of respiration and photosynthesis (Er − Ep) and the temperature increase affecting the system (TH − TU).
3. Material and methods
(a). Experimental set-up
We tested these predictions by comparing ecosystem metabolism rates in freshwater mesocosms designed specifically for ecosystem scale manipulations (figure 1). The field-based study was carried out between December 2005 and April 2008 at the Freshwater Biological Association River Laboratory (2°10′ W, 50°13′ N), East Stoke, Dorset, UK. We used 20 artificial ponds, each holding 1 m3 of water: this scale of mesocosm reproduces the key elements of community structure (e.g. diversity, trophic complexity) and functioning (e.g. nutrient cycling) of shallow lake ecosystems (Jones et al. 2002; McKee et al. 2003; Ventura et al. 2008). Half were warmed 3–5°C (mean 4°C) above ambient temperature (see the electronic supplementary material, figures S3 and S4, and table S6), in accordance with global warming projections for the next 100 years for temperate areas in the Northern Hemisphere (IPCC 2007). Experimental warming was achieved by an electronic heating element connected to a thermocouple which monitored the temperature in a given heated and unheated treatment pair of mesocosms. Treatments were arranged in a randomized block design (five blocks of four mesocosms) such that each block contained two replicates of each treatment. The mesocosms were seeded in December 2005 with organic substrates and a suite of organisms, representing an interconnected pelagic and benthic community that contained representative species from primary producers (phytoplankton, macrophytes) to top predators (Roach, Rutilus rutilus), and a suite of intermediate invertebrate consumers (Zooplankton, including Daphnia and Bosmina, and benthic macroinvertebrates, including Mollusca, Malacostraca, Trichoptera, Ephemeroptera and Odonata; see the electronic supplementary material, S7 for a full species list) to mimic the organismal composition, trophic complexity and physical structure of shallow lake ecosystems (Jones et al. 2002; McKee et al. 2003; Ventura et al. 2008). The biota was left to establish for 10 months prior to experimental warming, which commenced on 11 September 2006, thereby allowing time for further natural colonization before the onset of the study on 11 April 2007. NPP, GPP and ER were measured every two months for one year.
Figure 1.
(a) Aerial view of the global warming mesocosm experiment in April 2008. The experimental plot consisted of 20 mesocosms: 10 heated and 10 unheated. (b) Close-up of mesocosm 1 (heated) in April 2008, highlighting the presence of diverse floral and faunal assemblages.
(b). Calculation of metabolic parameters
Whole ecosystem metabolic fluxes (NPP, GPP and ER) were measured over a 24 h diel cycle for each replicate of each treatment on alternate months over the course of one year (April 2007 to April 2008) using the dissolved oxygen (DO) change technique (Marzolf et al. 1994; Mulholland et al. 2001; see the electronic supplementary material, S2 for additional details). This technique assumes that changes in DO concentration over a diel cycle represent the metabolic activity (photosynthetic and respiratory) of an aquatic ecosystem.
The record of continuous DO measurements was used to calculate the NPP, GPP and ER for each pond on each sampling occasion. The DO change (ΔDO) for each 15 min time interval was calculated as the difference in O2 concentration between t1 and t2 (i.e. t2 − t1; see the electronic supplementary material, figure S5). The daylight and night-time analysis periods were delimited as follows: the total analysis period was defined from the minimum O2 concentration on the 1st night and extended for 24 h to include the minimum O2 concentration on the 2nd night. Photosynthetic dawn was identified as the minimum O2 concentration after which all subsequent values were greater than it. Photosynthetic dusk was defined as the maximum O2 concentration after which all subsequent values were lower (see the electronic supplementary material, figure S5; Bales & Nardi 2007). Each O2 change value was then assigned to a day- or night-time category. Subsequently the metabolic parameters were calculated by numerical integration. NPP was calculated as
| 3.1 |
GPP was calculated as
| 3.2 |
where Rday is the day-time respiration. Since it is impossible to directly measure Rday, it was estimated, in keeping with the literature, by extrapolating the mean night-time respiration value across the hours of daylight (Marzolf et al. 1994; Mulholland et al. 2001; Bales & Nardi 2007). ER was calculated as
| 3.3 |
The metabolic balance of each replicate of each treatment was then determined as the ratio of ER : GPP. In the rare event of significant instrument drift or failure, the entire replicate was removed from the final analysis (nine measurements were removed from a total of 140; n = 131).
(c). Statistical analyses
Statistical analyses of the temperature dependence of ln(NPP), ln(GPP) and ln(ER) (treating temperature (1/kT) as a continuous variable) using analysis of co-variance (ANCOVA) were computed in R statistical software (R Developmental Core Team 2006). To account for temporal pseudoreplication in the statistical model we included pond identity nested within sampling occasion to account for temporal random effects. Comparison of NPP, GPP, ER and the ratio of ER/GPP among treatments (treating temperature as a categorical factor) was conducted with restricted maximum likelihood methods (PROC MIXED) in SAS, using a blocked, factorial design with repeated measures. This procedure is comparable to repeated measures ANOVA, in that temporal pseudo-replication is accounted for, but has a covariance structure that enables measurements to be included where replicates are not present on all occasions, a prerequisite of other repeated measures tests (Wolfinger & Chang 1998).
4. Results
(a). The temperature dependence of NPP, GPP and ER
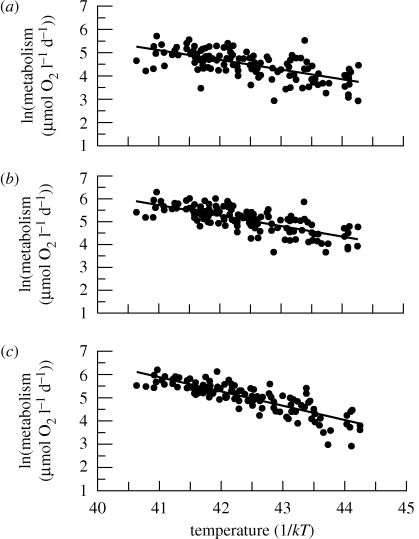
NPP, GPP and ER all increased with temperature (figure 2a–c and table 1). There were no significant differences in the slopes or intercepts of the temperature dependences of NPP, GPP or ER between heated and unheated mesocosms (table 1). Furthermore, we observed no significant interactions between temperature, treatment, pond identity or sampling occasion for NPP, GPP or ER, suggesting that temporal random effects did not influence our results and their temperature dependencies were equivalent across months and between sampling occasions (table 1). This facilitated the use of a single linear model to characterize each of the empirically determined temperature dependences of NPP, GPP and ER (figure 2a–c). Empirical measures of photosynthetic and respiratory activation energies were close to our theoretical expectations and those values reported in the literature. For NPP, Ep was 0.41 eV (95% CI 0.32–0.5 eV, n = 131) which is slightly steeper than the predicted value (Ep ≈ 0.32 eV; Allen et al. 2005; figure 1a). This small overestimate may be ascribed to the fact that NPP measures based on O2 production are inevitably influenced to some extent by heterotrophic metabolism and may therefore more accurately be described as ‘net ecosystem production’ (Bales & Nardi 2007). Because it is currently impossible to completely disentangle autotrophic and heterotrophic processes in a systematic way at the ecosystem level (Baldocchi et al. 2001; see the electronic supplementary material, S2), heterotrophic metabolism could not be isolated from our measurements of O2 production. Nevertheless, the effective activation energy of NPP reported in the literature (Ep ≈ 0.32 eV; Allen et al. 2005) falls within the 95 per cent confidence limits of our empirically determined activation energy for NPP. For GPP, Ep was 0.45 eV (95% CI 0.38–0.53 eV, n = 131; figure 2b), which was statistically indistinguishable from the activation energy from NPP (table 1) though slightly steeper than predicted from the activation energy of photosynthesis. This discrepancy is likely to again be attributable to the inability to isolate autotrophic and heterotrophic processes when measuring whole system metabolism with measurements of O2 change. Additionally, deviations between our predictions and experimental results may arise from assuming that the activation energies of aquatic and terrestrial photosynthesis are equivalent in the derivation of equations (2.2) and (2.4) after Allen et al. (2005). Nevertheless, these results provide substantial evidence for our assumption that the temperature dependence of GPP is governed by the activation energy for photosynthesis due to the type-I acclimation of AR to photosynthate production over periods of months to years (e.g. Dewar et al. 1999; Atkin & Tjoelker 2003).
Figure 2.
Temperature dependence of (a) net primary production, NPP, (b) gross primary production, GPP and (c) whole ecosystem respiration, ER. The slope of the temperature response equates to the activation energy of the respective process rate. Each data point corresponds to either the NPP, GPP or ER of a single mesocosm on each of the seven sampling occasions. The slope of the temperature dependence of ER was more sensitive to increases in temperature than NPP and GPP (see main text). (a) y = −0.41x +22.1; r2 = 0.4; (b) y = −0.45x +24.4; r2 = 0.5; (c) y = −0.62x +31.2; r2 = 0.7.
Table 1.
Results from analysis of co-variance (ANCOVA). The first ANCOVA tests for relationships between ecosystem level metabolic rates (NPP, GPP or ER) and sampling occasions and temperature, parallelism between treatments and differences between intercepts. Metabolic rates are used as dependent variables, temperature (1/kT) as the covariate, and treatment (heated or control) as the independent variable. The second ANCOVA tests for differences in the slope of the temperature dependence between metabolic rates (i.e. ER × NPP and ER × GPP). Here metabolic rate is used as the dependent variable, temperature (1/kT) as the covariate and metabolic rate ID (e.g. NPP or ER) as the independent variable.
| relationship | d.f. | f-ratio | p-value |
|---|---|---|---|
| ln(NPP) versus 1/kT | 1,123 | 85.9 | <0.0001 |
| ln(GPP) versus 1/kT | 1,123 | 146.6 | <0.0001 |
| ln(ER) versus 1/kT | 1,123 | 294.85 | <0.0001 |
| difference in slope of ln(NPP) versus 1/kT between treatments | 1,123 | 0.51 | 0.47 |
| difference in intercept of ln(NPP) versus 1/kT between treatments | 1,123 | 2.05 | 0.15 |
| difference in slope of ln(GPP) versus 1/kT between treatments | 1,123 | 0.23 | 0.82 |
| difference in intercept of ln(GPP) versus 1/kT between treatments | 1,123 | 2.55 | 0.11 |
| difference in slope of ln(ER) versus 1/kT between treatments | 1,123 | 0.56 | 0.46 |
| difference in intercept of ln(ER) versus 1/kT between treatments | 1,123 | 2.27 | 0.13 |
| difference in slope between ln(GPP) × ln(NPP) versus 1/kT | 1,254 | 0.45 | 0.5 |
| difference in slope between ln(ER) × ln(NPP) versus 1/kT | 1,254 | 12.88 | <0.001 |
| difference in slope between ln(ER) × ln(GPP) versus 1/kT | 1,254 | 3.2 | 0.0015 |
For ER, the activation energy was 0.62 eV (95% CI 0.55 to 0.69 eV, n = 131), and approached the activation energy expected for heterotrophic metabolism (Er ≈ 0.65 eV; Gillooly et al. 2001; Enquist et al. 2003; Allen et al. 2005; figure 2c). Importantly, the activation energy for ER was greater than that of GPP, validating our assumption that ER and heterotrophic metabolism were not limited by GPP (i.e. the mesocosms exhibit non-steady-state dynamics). Further, the empirically determined temperature dependence of NPP and GPP differed from ER (table 1) and, as predicted, ER was more sensitive to temperature increases than NPP and GPP, further substantiating the non-steady-state dynamics exhibited by the experiment.
The conformity between our empirical data and theoretical predictions provides strong support for the suggestion that the rate of ecosystem metabolism is ultimately constrained by the activation energies of photosynthesis and respiration at the individual level (Enquist et al. 2003; Brown et al. 2004; Allen et al. 2005). Further, because ER responds more rapidly to rising temperatures than NPP and GPP, warming could alter the metabolic balance (i.e. the balance between GPP and ER) and carbon sequestration rates within ecosystems.
(b). Whole-system metabolic balance: quantitative predictions
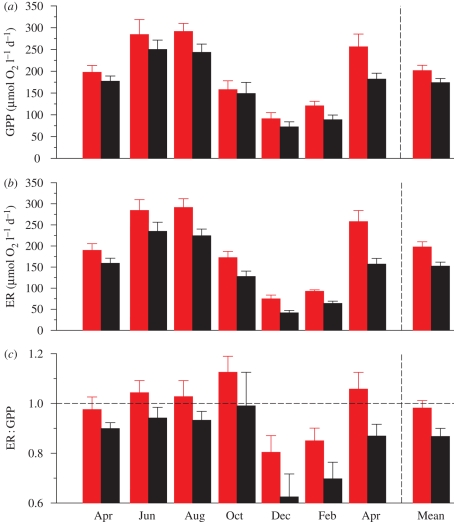
Our experimental manipulation showed that GPP and ER were consistently elevated (both within and across seasons) in the warmed mesocosms (figure 3a,b). Correspondingly, mean annual GPP (F1,113 = 9.58, p = 0.0025; figure 3a) and mean annual ER (F1,113 = 33.37, p < 0.0001; figure 3b) were significantly higher in the warmed mesocosms, but the magnitude of their responses to warming differed markedly. In agreement with our qualitative theoretical predictions, ER increased at a faster rate under experimental warming than did GPP. As such, experimental warming increased ER considerably more than GPP which showed smaller differences between warmed and control mesocosms (figure 3a,b).
Figure 3.
Differences in ecosystem metabolism (±s.e.) between heated (red bars) and unheated (black bars) experimental treatments. Both (a) gross primary production, GPP, and (b) ecosystem respiration, ER, were consistently elevated in warmed treatments. The magnitude of the increase in ER between warmed and unheated systems was markedly greater than the increase in GPP, reflecting its stronger temperature dependence. Correspondingly, there was a highly significant treatment effect on the (c) ER : GPP ratio, such that the metabolic balance of the warmed mesocosms shifted towards heterotrophy, both seasonally and over the whole year (represented by mean annual values). The dotted line represents the metabolic balance (ER = GPP). Warmed ecosystems were net sources of CO2 to the atmosphere in June, August, October and April (i.e. ER : GPP > 1).
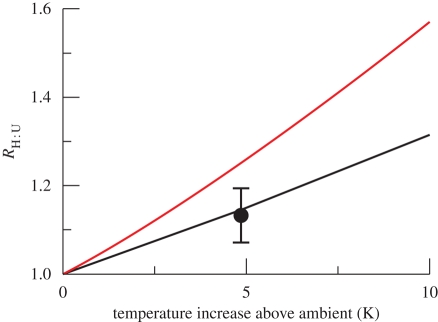
Given the differential responses of GPP and ER to warming, which were governed by their activation energies at the individual level, we sought to predict how the metabolic balance of our mesocosms would respond to warming (i.e. equation (2.6) RH : U). In figure 4, we show how the metabolic balance of a given ecosystem should change quantitatively with increasing temperatures. For a constant reference value of TU (e.g. present-day temperatures), carbon sequestration is reduced (RH : U increases) as TH increases. The magnitude of the increase (i.e. the slope) is governed by the difference in the activation energies of respiration and photosynthesis (Er − Ep).
Figure 4.
Quantitative changes in the ratio of the metabolic balance between warmed and ambient ecosystems (RH : U in equation (2.6) as temperature, TH, increases. The black line corresponds to the prediction for the experimentally observed activation energies for respiration, Er, and photosynthesis, Ep (0.62 and 0.42 eV, respectively). The red line is the prediction for the mean value reported in the literature, based on Er of 0.65 eV and Ep of 0.32 eV. The dot corresponds to the mean annual value (and 95% CIs) measured empirically in our mesocosms, which is undistinguishable from our theoretical prediction.
We tested this general prediction with data from our experiment. Here, Er and Ep were our empirically observed values of 0.62 and 0.45 eV, respectively. We used TH and TU as the mean annual absolute temperatures in the heated and unheated mesocosms (290.9 and 286.1 K, respectively). After substituting the empirical values into equation (2.6) we would expect the ratio RH : U to be 1.12, i.e. carbon sequestration will be reduced by 12 per cent in the warming scenario. Our empirically measured RH : U (mean annual ratio) was 1.13 (95% CI 1.07–1.19), and was statistically indistinguishable from our theoretical prediction (figure 4). Accordingly, the metabolic balance (ER : GPP ratio) of the warmed mesocosms was significantly elevated over the course of the year (F1,113 = 12.71, p < 0.005, figure 3c). In fact, in four months during the study (June, August and October 2007 and April 2008) the ER : GPP ratio was greater than 1, suggesting that the warmed systems became net sources of CO2 to the atmosphere over the growing season.
5. Discussion
Our results suggest that the temperature dependences of whole ecosystem respiration and primary production are fundamentally different, as suggested by their activation energies at the individual level. This finding provides a simple mechanistic platform with strong predictive power for understanding how global warming may alter carbon sequestration rates within ecosystems. Because the activation energy for ecosystem respiration is higher than that of primary production, ecosystem respiration increased proportionately more than production under the experimentally induced global warming scenarios predicted for the end of the century. The shift in the metabolic balance of the warmed ecosystems in our experiment suggests that a larger fraction of the carbon fixed by photosynthesis was remineralized and released as CO2, thus compromising the capacity of these systems to sequester carbon as they warm.
In our experiment both warmed and control mesocosms were net sinks for CO2. However, the carbon sequestration capacity of the warmed systems relative to the control systems was severely compromised. In both warmed and control mesocosms the carbon balance deviated from steady state because ER/GPP was less than 1 averaged over the year, validating the assumptions of our theoretical models. Importantly, in the control mesocosms, at ambient temperature, ER/GPP averaged over the year was considerably lower than 1, indicating that these systems were strong sinks for CO2. In the warmed mesocosms ER/GPP was less than 1 when averaged over the year; however, during the summer and autumn months these systems were net CO2 sources (i.e. ER/GPP > 1) indicating that a portion of heterotrophic metabolism was fuelled by stored organic carbon. Because the mesocosms were not at steady state (i.e. ER/GPP < 1 or ER/GPP > 1), ER was not substrate limited by contemporary NPP. As such, heterotrophic metabolism increased in response to warming, and was unconstrained by the weaker temperature dependence of GPP. This corroborates our assumption that the activation energy for ER closely reflected the activation energy for heterotrophic metabolism in response to warming. In our mesocosm experiment the temperature response of ER was not constrained by GPP and warming increased the fraction of absorbed carbon (GPP) that was respired (ER), thereby reducing carbon sequestration.
In general, caution must be exercised when extrapolating from mesocosm experiments to natural ecosystems. Having a general theoretical framework that is supported by experimental observations may assist this extrapolation. In particular, the effects of temperature on the metabolic balance observed in our whole-ecosystem manipulations should be treated somewhat cautiously when extrapolating to other systems where other limiting resources (e.g. light, nutrients, organic carbon) might alter the temperature response of primary production and, to a lesser extent, ecosystem respiration (Woodwell et al. 1998). For instance, in both marine (López-Urrutia & Moran 2007) and terrestrial (Woodwell et al. 1998) systems it has been suggested that resource limitation may override the effects of temperature on primary production at the ecosystem level. Nevertheless, if at higher temperatures resource limitation were to curtail the temperature response of primary production to a greater extent than respiration, as seen in oceanic carbon cycling (López-Urrutia & Moran 2007), we might expect the shift in the metabolic balance to be further amplified over temporal scales relevant to the turnover times of stored organic carbon pools. This is because the large stores of organic carbon in these systems will be available to fuel ER even if contemporary primary production is reduced.
Acclimation is of fundamental importance to any discussion of the potential effects of warming on the metabolic balance of ecosystems (Dewar et al. 1999; Melillo et al. 2002; Atkin & Tjoelker 2003; Allen et al. 2005). It has often been suggested that over temporal scales relevant to the study of the effects of global warming ER must balance GPP (i.e. the ecosystems reach steady state; Gifford 2003; Allen et al. 2005). The acclimation of ER to GPP arises from the assumption that oxidative metabolism is ultimately limited by carbon from GPP (Gifford 2003; Allen et al. 2005). If this is correct, the consequences of warming revealed in our study may be viewed as transient non-steady-state effects which, in natural ecosystems, would eventually reach metabolic equilibrium. However, the consequences of warming for the carbon balance of natural ecosystems depend fundamentally on the turnover times of the organic carbon pools. For instance, studies of soil organic carbon (SOC) pools suggest that the majority of contemporary respiration is driven by organic matter fixed more than 2 years but less than 30 years ago (Trumbore 2000). Furthermore, the effects of warming on soil respiration are most pronounced on the non-labile SOC pools that have large turnover times (decades to centuries), which increases the potential for strong long-term positive feedback to warming (Knorr et al. 2005). Given the considerable reserves of ‘stored’ organic carbon in natural ecosystems (Trumbore 2000; del Giorgio & Williams 2005), particularly in soils and aquatic sediments, any increase in baseline respiration (i.e. respiration uncoupled from contemporary primary production) relative to primary production driven by the differential activation energies of heterotrophic and autotrophic processes could shift the carbon balance of many ecosystems from being net sinks for atmospheric CO2 to becoming net sources.
Importantly, and as we have shown in our experiments, ecosystems are likely to exhibit non-steady-state dynamics with respect to carbon sequestration in response to warming. Over geological time-scales these ‘transient’ dynamics must reach steady state because ultimately ER requires fixed carbon as a substrate. However, understanding the effects of global warming on the carbon sequestration of ecosystems is crucial over much shorter temporal scales, and those which are relevant to the manifestations of positive feedback which may hasten global warming (i.e. decades). In this context, the use of manipulative experiments to inform short-term consequences of warming can be very useful (Benton et al. 2007).
6. Conclusion
The biotic regulation of atmospheric CO2 constitutes one of the most important ‘ecosystem services’ of value to humans (Schroter et al. 2005). It is surprising then, that there is still no general consensus as to how the metabolic balance of ecosystems will respond to projected global warming (del Giorgio & Duarte 2002; Knorr et al. 2005; López-Urrutia et al. 2006; López-Urrutia & Moran 2007). In addressing these problems we have used a combination of ecological theory, tested explicitly in experimental ecosystems. Our approach revealed a fundamental mechanism, ultimately driven by the metabolic rates of individuals, which dictated the effects of temperature on the metabolic balance of ecosystems. Furthermore, our results demonstrate that predicting how the metabolic balance of ecosystems may respond to environmental warming may not require a bespoke model plagued with detail and numerous parameters specific to the system under study. A significant portion of the biological complexity of an ecosystem (Montoya et al. 2006; e.g. community composition, trophic architecture) can be reduced to two fundamental parameters: the activation energies for the metabolic processes and temperature. However, given the inherent complexity and diversity of biotic and abiotic factors influencing the dynamics of carbon cycling in natural ecosystems, caution should be exercised in extrapolating our findings in mesocosms to natural systems. The generality of the quantitative predictions developed here to other systems may be achieved after verification in other natural ecosystem types (e.g. terrestrial and marine). Nevertheless, our models and their experimental verification provide an important baseline and foundation for understanding the mechanisms dictating the effects of temperature on the metabolic balance of ecosystems, and for predicting future change.
Acknowledgements
We thank Brian Godfrey, Dan Perkins, and the Freshwater Biological Association for their help with the experiment. Andrew P. Allen and Ricard Solé discussed ideas and provided comments on early drafts. G. Yvon-Durocher was supported by a Natural Environment Research Council studentship (NER/S/A2006/14 029). J. Montoya was funded by the NERC Fellowship Scheme (NE/C002 105/1), a Ramon y Cajal Fellowship (RYC-2008-03 664) and Generalitat de Catalunya.
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Allen A. P., Gillooly J. F., Brown J. H.2005Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19, 202–213 (doi:10.1111/j.1365-2435.2005.00952.x) [Google Scholar]
- Arnone J. A., et al. 2008Prolonged suppression of ecosystem carbon dioxide uptake after an anomalously warm year. Nature 455, 383–386 (doi:10.1038/nature07296) [DOI] [PubMed] [Google Scholar]
- Atkin O. K., Tjoelker M. G.2003Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 8, 343–351 (doi:10.1016/S1360-1385(03)00136-5) [DOI] [PubMed] [Google Scholar]
- Baldocchi D., et al. 2001FLUXNET: a new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull. Am. Meteorol. Soc. 82, 2415–2434 (doi:10.1175/1520-0477(2001)082<2415:FANTTS>2.3.CO;2) [Google Scholar]
- Bales J. D., Nardi M. R. (eds.) 2007Automated routines for calculating whole stream metabolism. Theoretical background and users guide: US Geological Survey techniques and methods 4-C2 US Geological Survey; See http://pubs.water.usgs.gov/tm4c2/ [Google Scholar]
- Benton T. G., Solan M., Travis J. M. J., Sait S. M.2007Microcosm experiments can inform global ecological problems. Trends Ecol. Evol. 22, 516–521 (doi:10.1016/j.tree.2007.08.003) [DOI] [PubMed] [Google Scholar]
- Bernacchi C. J., Singsaas E. L., Pimentel C., Portis A. R., Long S. P.2001Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 24, 253–259 (doi:10.1111/j.1365-3040.2001.00668.x) [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B.2004Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- Canadell J. G., et al. 2007Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl Acad. Sci. USA 104, 18 866–18 870 (doi:10.1073/pnas.0702737104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. M., Betts R. A., Jones C. D., Spall S. A., Totterdell I. J.2000Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408, 750 (doi:10.1038/35047138) [DOI] [PubMed] [Google Scholar]
- Davidson E. A., Janssens I. A.2006Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173 (doi:10.1038/nature04514) [DOI] [PubMed] [Google Scholar]
- del Giorgio P. A., Duarte C. M.2002Respiration in the open ocean. Nature 420, 379–384 (doi:10.1038/nature01165) [DOI] [PubMed] [Google Scholar]
- del Giorgio P. A., Williams P. J. l.2005The global significance of respiration in aquatic ecosystems: from single cells to the biosphere. In Respiration in aquatic ecosystems (eds del Giorgio P. A., leB. Williams P. J.), pp. 267–303 Oxford, UK: Oxford University Press [Google Scholar]
- Dewar R. C., Medlyn B. E., McMurtrie R. E.1999Acclimation of the respiration photosynthesis ratio to temperature: insights from a model. Global Change Biol. 5, 615–622 (doi:10.1046/j.1365-2486.1999.00253.x) [Google Scholar]
- Enquist B. J., et al. 2003Scaling metabolism from organisms to ecosystems. Nature 423, 639–642 (doi:10.1038/nature01671) [DOI] [PubMed] [Google Scholar]
- Farquhar G. D., von Caemmerer S., Berry J. A.1980A biochemical model of photosynthetic CO2 assimilation in leaves C3 species. Planta 149, 78–90 (doi:10.1007/BF00386231) [DOI] [PubMed] [Google Scholar]
- Friedlingstein P., et al. 2006Climate-carbon cycle feedback analysis: results from the (CMIP)-M-4 model intercomparison. J. Clim. 19, 3337–3353 (doi:10.1175/JCLI3800.1) [Google Scholar]
- Gifford R. M.2003Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct. Plant Biol. 30, 171–186 (doi:10.1071/FP02083) [DOI] [PubMed] [Google Scholar]
- Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L.2001Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- Houghton J.2001The science of global warming. Interdisciplinary Sci. Rev. 26, 247–257 (doi:10.1179/030801801679485) [Google Scholar]
- IPCC 2007Climate change 2007: the physical sciences basis. In Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. (eds Parry M. L., Canziani O. F., Palutikof J. P., van der Linden P. J., Hanson C. E.), pp. 7–22 Cambridge, UK: Cambridge University Press [Google Scholar]
- Jones J. I., Young J. O., Eaton J. W., Moss B.2002The influence of nutrient loading, dissolved inorganic carbon and higher trophic levels on the interaction between submerged plants and periphyton. J. Ecol. 90, 12–24 (doi:10.1046/j.0022-0477.2001.00620.x) [Google Scholar]
- Knorr W., Prentice I. C., House J. I., Holland E. A.2005Long-term sensitivity of soil carbon turnover to warming. Nature 433, 298–301 (doi:10.1038/nature03226) [DOI] [PubMed] [Google Scholar]
- Larcher W.1995Physiological plant ecology, 3rd edn.Berlin, Germany: Springer [Google Scholar]
- Lloyd J., Taylor J. A.1994On the temperature dependence of soil respiration. Funct. Ecol. 8, 315–323 (doi:10.2307/2389824) [Google Scholar]
- López-Urrutia A., Moran X. A. G.2007Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 88, 817–822 (doi:10.1890/06-1641) [DOI] [PubMed] [Google Scholar]
- López-Urrutia A., San Martin E., Harris R. P., Irigoien X.2006Scaling the metabolic balance of the oceans. Proc. Natl Acad. Sci. USA 103, 8739–8744 (doi:10.1073/pnas.0601137103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolf E. R., Mulholland P. J., Steinman A. D.1994Improvements to the diurnal upstream-downstream dissolved-oxygen change techniques for determining whole stream metabolism in small streams. Can. J. Fish. Aquat. Sci. 51, 1591–1599 (doi:10.1139/f94-158) [Google Scholar]
- McKee D., et al. 2003Response of freshwater microcosm communities to nutrients, fish, and elevated temperature during winter and summer. Limnol. Oceanogr. 48, 707–722 [Google Scholar]
- Medlyn B. E., et al. 2002Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 25, 1167–1179 (doi:10.1046/j.1365-3040.2002.00891.x) [Google Scholar]
- Melillo J. M., et al. 2002Soil warming and carbon-cycle feedbacks to the climate system. Science 298, 2173–2176 (doi:10.1126/science.1074153) [DOI] [PubMed] [Google Scholar]
- Memmott J., Craze P. G., Waser N. M., Price M. V.2007Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 10, 710–717 (doi:10.1111/j.1461-0248.2007.01061.x) [DOI] [PubMed] [Google Scholar]
- Montoya J. M., Pimm S. L., Sole R. V.2006Ecological networks and their fragility. Nature 442, 259–264 (doi:10.1038/nature04927) [DOI] [PubMed] [Google Scholar]
- Mulholland P. J., et al. 2001Inter-biome comparison of factors controlling stream metabolism. Freshwater Biol. 46, 1503–1517 (doi:10.1046/j.1365-2427.2001.00773.x) [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Piao S. L., et al. 2008Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451, 49–52 (doi:10.1038/nature06444) [DOI] [PubMed] [Google Scholar]
- R Developmental Core Team 2006R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Rosenzweig C. D.2008Attributing physical and biological impacts to anthropogenic climate change. Nature 453, 353–357 (doi:10.1038/nature06937) [DOI] [PubMed] [Google Scholar]
- Schroter D., et al. 2005Ecosystem service supply and vulnerability to global change in Europe. Science 310, 1333–1337 (doi:10.1126/science.1115233) [DOI] [PubMed] [Google Scholar]
- Trumbore S.2000Age of soil organic matter and soil respiration: radiocarbon constraints on belowground C dynamics. Ecol. Appl. 10, 399–411 (doi:10.1890/1051-0761(2000)010[0399:AOSOMA]2.0.CO;2) [Google Scholar]
- Ventura M., Liboriussen L., Lauridsen T., Sondergaard M., Jeppesen E.2008Effects of increased temperature and nutrient enrichment on the stoichiometry of primary producers and consumers in temperate shallow lakes. Freshwater Biol. 53, 1434–1452 (doi:10.1111/j.1365-2427.2008.01975.x) [Google Scholar]
- Walther G. R., et al. 2002Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- West G. B., Brown J. H., Enquist B. J.1997A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 (doi:10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]
- Whiting G. J., Chanton J. P.2001Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration. Tellus Ser. B Chem. Phys. Meteorol. 53, 521–528 [Google Scholar]
- Wolfinger R. D., Chang M.1998Comparing the SAS GLM and MIXED procedures for repeated measures. Cary, NC: SAS Institute [Google Scholar]
- Woodward F. I.2007Global primary production. Curr. Biol. 17, 269–273 [DOI] [PubMed] [Google Scholar]
- Woodwell G. M., et al. 1998Biotic feedbacks in the warming of the earth. Clim. Change 40, 495–518 (doi:10.1023/A:1005345429236) [Google Scholar]