Abstract
Previsions of a warmer ocean as a consequence of climatic change point to a 2–6°C temperature rise during this century in surface oceanic waters. Heterotrophic bacteria occupy the central position of the marine microbial food web, and their metabolic activity and interactions with other compartments within the web are regulated by temperature. In particular, key ecosystem processes like bacterial production (BP), respiration (BR), growth efficiency and bacterial–grazer trophic interactions are likely to change in a warmer ocean. Different approaches can be used to predict these changes. Here we combine evidence of the effects of temperature on these processes and interactions coming from laboratory experiments, space-for-time substitutions, long-term data from microbial observatories and theoretical predictions. Some of the evidence we gathered shows opposite trends to warming depending on the spatio-temporal scale of observation, and the complexity of the system under study. In particular, we show that warming (i) increases BR, (ii) increases bacterial losses to their grazers, and thus bacterial–grazer biomass flux within the microbial food web, (iii) increases BP if enough resources are available (as labile organic matter derived from phytoplankton excretion or lysis), and (iv) increases bacterial losses to grazing at lower rates than BP, and hence decreasing the proportion of production removed by grazers. As a consequence, bacterial abundance would also increase and reinforce the already dominant role of microbes in the carbon cycle of a warmer ocean.
Keywords: temperature, bacteria, heterotrophic nanoflagellate, grazing, metabolic theory of ecology, activation energy
1. Climate change and the surface ocean: the microbial gap
Global change encompasses a multitude of environmental and ecological changes that have been observed during the last decades as a direct or indirect result of increasing human population, the burning of fossil fuels and consequent accumulation of greenhouse gases in the atmosphere. Changes in climate are the most prominent manifestation of global change. Climate change impacts the ocean through generalized temperature rise, shifts in wind and radiation regimes, changes in the hydrological cycle, alterations related to oceanic circulation and stratification, changes in the frequency of episodic extreme events (such as storms) and acidification. Here, we consider only temperature rise, since the science behind, for example, acidification effects is still at an early stage. We have also not considered subtle effects like changes in sea water viscosity on grazing rates, an important issue for micro-zooplankton. Unlike in terrestrial ecosystems, the direct link between climate change and temperature rise in the ocean is not straightforward, as changes in oceanic circulation or vertical mixing can actually decrease oceans' temperatures at a regional scale (e.g. Levitus et al. 2000).
The most immediate and direct effect of global change at a global ocean scale will be, however, the increment in the surface sea water temperature, down to tens of metres. Climatic models predict that water temperatures will increase by a few degrees during this century (Timmermann et al. 1999; Meehl et al. 2007). These predictions have been confirmed by long-term observations in European seas where sea surface temperature rates of increase have been around 0.01°C yr−1 since the 1860s (Wiltshire & Manly 2004; Vargas-Yañez et al. 2005; Mackensie & Schiedek 2007). It is likely that such changes will deeply affect different aspects of the structure and functioning of marine ecosystems (e.g. Edwards & Richardson 2004; Wiltshire et al. 2008; Montoya & Raffaelli 2010).
The vast majority of published works on the ecosystem effects of climate change (e.g. Stenseth et al. 2002; Walther et al. 2002; Parmesan & Yohe 2003) do not mention (or only very briefly allude to) the microbial world. An explanation for this gap could be that the lower trophic levels, such as the primary producers (phytoplankton) and decomposers (heterotrophic prokaryotes), are considered less sensitive to environmental change than their consumers or predators, since sensitivity to climate is believed to increase with trophic level (Voigt et al. 2003; Raffaelli 2004). However, minor effects at the base of the food web could be amplified through trophic chains, warranting interest in the effects of climate change on microbes. In addition, the microbial world is a ‘slippery field’, based on complex instrument-mediated observations, where the perception of changes may not be as obvious as in the perceived ‘real’ (macroscopic) world.
In this paper, we predict effects and review the empirical evidence of increasing temperatures on bacterial-associated ecosystem processes and trophic interactions within the microbial food web in the euphotic layer of the ocean, where susceptibility to warming is higher. We present and combine studies based on experimental manipulations, long-term observations, cross-comparisons between natural systems under different temperature regimes (also called space-for-time substitutions) and theoretical predictions derived from current ecological theory, in particular those resulting from the metabolic theory of ecology (MTE).
2. The marine microbial food web: why should we care about microbes?
The upper layer of the oceans constitutes a boundary between the atmosphere, where CO2 and other greenhouse-effect gases have accumulated during the previous decades, and the deep ocean, probably the largest carbon reservoir on Earth (Gruber et al. 2002, 2009; Sarmiento & Gruber 2002). It is in this euphotic (illuminated) zone that the solar energy supports an extraordinarily efficient photosynthesis, considering the scarcity of nutrients in the vast majority of the oceanic regions. Taking into account that two-thirds of the Earth surface is covered by oceans, it is not surprising that half of global primary production (PP) takes place in the sea. The uniqueness of the oceanic ecosystem is that these huge numbers, approximately 60 Gt of C per year, are actually processed by microbes (Field et al. 1998).
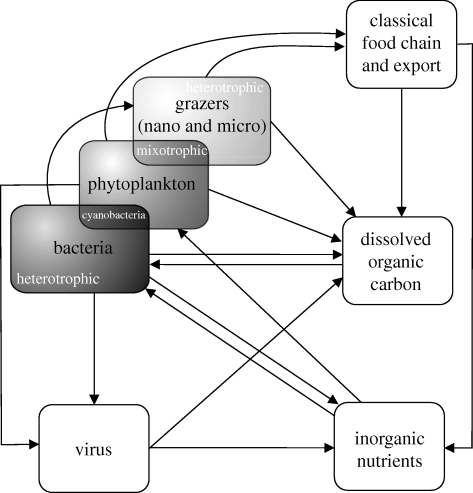
The microbial food web can be simplified in a small number of boxes (figure 1). This avoids the endless debate on the species definition of microbes and the entangled complexity generated by their huge diversity. These are functional boxes: the two largest boxes, in terms of biomass and, above all, carbon processing, are heterotrophic bacteria and phytoplankton. These two boxes are interconnected by a two-way flux: phytoplankton excretion or cell lysis is a source of organic matter for heterotrophic bacteria; mineralization of this organic matter by heterotrophic bacteria, in turn, provides nutrients for primary producers. The coexistence of phytoplankton and heterotrophic bacteria in high abundances in the sea implies a predominantly mutualistic relationship (Aota & Nakajima 2001), although algae can also produce antagonistic molecules that inhibit bacterial growth (e.g. Ribalet et al. 2008), and vice-versa (e.g. Mayali & Azam 2004).
Figure 1.
A simplified view of the major components of the marine microbial food web and their interactions (carbon fluxes) indicated by arrows.
Although bacterial production (BP) is highly variable in the oceans' euphotic layer, cell abundance is relatively constant (approx. 105 − 106 cells ml−1). On the contrary, phytoplankton abundance and productivity show large variations, both vertically (in the water column), and spatially (from one place to another). Large phytoplankton usually proliferate in regions with high inorganic nutrient content (e.g. upwelling zones, coastal areas, polar regions over the summer), but the vast majority of the total ocean surface corresponds to permanently stratified and extremely oligotrophic areas where small phytoplankton dominate autotrophic biomass (Falkowski et al. 1998; Behrenfeld et al. 2006).
Increasing temperatures affect both of these food web compartments. Paleontological and space-for-time analysis of phytoplankton cell size demonstrated that in a warmer ocean primary producers will tend to be smaller (e.g. Falkowski & Oliver 2007; Morán et al. 2010). For heterotrophic bacteria, the vast majority of studies have focused on metabolic rates, and all show an increase of bacterial specific growth rate, BP and bacterial respiration (BR) with temperature (e.g. White et al. 1991; López-Urrutia & Morán 2007; Vázquez-Domínguez et al. 2007).
The third box in our simplified food web corresponds to the grazers, comprising protists (nanoflagellates, ciliates, etc.) that are strictly heterotrophic or mixotrophic and incorporate carbon by ingesting small primary producers (e.g. cyanobacteria) and/or heterotrophic bacteria. Nano- and micro-grazers are, in turn, grazed by larger zooplankton, representing an energy input from the microbial food web into the classic food chain (phytoplankton–metazooplankton–fish). Things are much more complex than this simplistic representation: e.g. recent reports have shown that roughly half of the grazing impact on the bacterial community is due to small phytoplankton (Unrein et al. 2007; Zubkov & Tarran 2008), so that some individuals classically belonging to the phytoplankton box should also be included in the grazers box. Here too, it is well established that bacterial losses to protist grazing increase with temperature (as discussed below), although grazing rates depend also on prey and predator abundance (Peters 1994; Vaqué et al. 1994).
The fourth box, and by far the least well documented and understood, comprises marine viruses. We lack evidence on how temperature affects viral infection, and the environmental factors that trigger viral infection and lysogeny are still poorly understood. It is common to observe bacterial mortality owing to viruses at least as high as losses owing to grazing (Fuhrman 1999; Boras et al. 2009). Theoretically we would expect virus infection to be proportional to bacterial cell abundance, but further research is needed in this area (but see Weinbauer et al. 2009).
As a metaphor, one could compare the euphotic layer of the ocean to a cell membrane, constituting a highly complex and active boundary that keeps sharp gradients of different molecules between the cell interior and the outside world. In this sense, the upper ocean microbial food web (the autotrophs in particular) could be seen as a huge carbon-processing machine that would remove CO2 from the atmosphere and would push it downwards into the depths. However, part of the carbon fixed by autotrophy is actually respired in situ. Global ocean respiration estimates point to numbers at least as high as oceanic PP (del Giorgo & Duarte 2002; Karl et al. 2003; Riser & Johnson 2008; but see Williams et al. 2004). The majority (greater than 95%; del Giorgo & Duarte 2002) of respiration in the ocean is carried out by heterotrophic bacteria, with half of it (approx. 37 Gt of C per year) taking place in the euphotic layer (del Giorgio & Williams 2005). A fundamental feature that has changed our view of global biogeochemical processes is that oceanic microbes are the key players. Slight changes in the biomass stock or activity of any of the compartments of the microbial food web within the euphotic zone of the ocean should have major impacts on the global carbon cycle and could accelerate or compensate the processes associated with global change.
3. Interactions involving heterotrophic bacteria: temperature, resources and grazing
One of the biggest challenges in marine research is to forecast the effects of climate change on planktonic communities, especially when the effects of temperature on a particular box of the microbial food web depend on the feedback with other boxes that are also responding to environmental change. The final outcome, perceived as changes in metabolic rates (physiological response) or in species composition, can be a tradeoff of synergetic and antagonistic processes taking place within the food web. This is exemplified by the case of heterotrophic bacteria. Increasing temperature will increase respiration rates (e.g. Vázquez-Domínguez et al. 2007), but will not necessarily decrease bacterial growth efficiency (BGE, defined as BP/(BP + BR), where BR is bacterial respiration and BP is bacterial production) because the vast majority of the oceanic regions are oligotrophic and the limiting factor for bacterial growth is the availability of substrates, mostly organic matter derived from phytoplankton (López-Urrutia & Morán 2007).
With ocean warming, it is likely that the oligotrophic regions of the ocean will become more stratified, and nutrient segregation—sharp gradients of inorganic nutrients where exchanges between the nutrient-rich deep waters and the nutrient-poor upper layer are reduced as a result of enhanced stratification—will have a negative impact on net PP. Some studies have already reported strong evidence for ocean ‘oligotrophication’ as a direct effect of global warming (Falkowski & Wilson 1992; Karl et al. 2001; Behrenfeld et al. 2006; Falkowski & Oliver 2007; Polovina et al. 2008) and have forecasted shifts in the size structure of the autotrophic community (Morán et al. 2010). However, in terms of dissolved PP, these results can be misleading as temperature is known to stimulate phytoplankton exudation (Morán et al. 2006; but see Watanabe 1980; Verity 1981; Zlotnik & Dubinsky 1989) and consequently a temperature increase would increase substrate availability for heterotrophic bacteria. Thus, the lack of a consensual and unequivocal theoretical framework on how phytoplankton will respond to climate change (e.g. Falkowski & Oliver 2007; Falkowski & Oliver 2008; Peters 2008) strongly compromises our ability to make predictions about the effects of warming on heterotrophic bacteria and associated ecosystem processes (BP, BR, BGE) and on microzooplankton grazing rates. Approaches to this area correspond to three different spatio-temporal scales: the experimental approach (punctual and local scale), the use of ecological theory (time-independent and local–regional scale) and long-term observations (seasonal/inter-annual and regional–global scale).
(a). Experimental evidence
The design of temperature perturbation experiments is a key factor when evaluating the evidence for the effect of temperature increases on organism abundance and activity. Some experiments tend to highlight acclimatation issues and are designed to observe, under laboratory conditions, the changes produced by slight warming on very simplistic communities of micro-organisms growing during a relatively large numbers of generations. In laboratory microcosms, Petchey et al. (1999) studied how microbial food webs with different degrees of complexity responded to such a slow progressive warming. They found PP and decomposition rates increased directly through increased temperature-dependent physiological rates and indirectly through changes in trophic structure. The extinction of protist top predators and herbivores in warmed communities was evident, with a clear increase in the dominance of autotrophs and bacterivores. The communities used in these experiments were ‘custom-made’ microbial food webs strongly simplified compared with natural communities.
A different experimental approach is the use of temperature-controlled mesocosms in which the community is warmed over a short period, and then maintained at this temperature. In a mesocosm experiment of this kind carried out in the Baltic Sea, in which sea water was warmed by 2, 4 and 6°C, Hoppe et al. (2008) reported an acceleration of bacterial degradation of organic matter derived from a phytoplankton spring bloom and an increase in the average ratio between BP and PP with temperature. Community respiration increased in warmed conditions, with an increase in the contribution of the picoplankton (i.e. less than 3 µm fraction) to total respiration. The authors used these results to predict that warming during the winter/early spring in temperate climatic zones would favour bacterial degradation of organic matter by tightening the coupling between phytoplankton and bacteria. As highlighted by the authors, these predictions would only be valid if PP was not reduced by warming.
In another study with a similar experimental design (mesocosms warmed by 2, 4 and 6°C), Wohlers et al. (2009) also observed an acceleration of the respiratory consumption of organic carbon relative to autotrophic production with temperature, with a decrease in the biological drawdown of dissolved inorganic carbon of up to 31 per cent. In this experiment, warming shifted the partitioning between particulate and dissolved organic carbon towards an enhanced accumulation of dissolved compounds (higher phytoplankton exudation and less particle aggregation), and the loss of organic carbon through sinking was reduced at the highest temperatures. The main conclusions of this work were that changes in biogenic carbon flow by warming had the potential to reduce the transfer of primary produced organic matter to higher trophic levels, weakening the ocean's biological carbon pump and providing a positive feedback to rising of atmospheric CO2, in line with predictions derived from the model of Laws et al. (2000).
Using a slightly different approach, Vázquez-Domínguez et al. (2007) incubated samples in microcosms at different temperatures over short periods of time (24–48 h), and measured the different variables of interest throughout a seasonal cycle in a coastal Mediterranean site. In this study, the warmed (ca 2°C) samples had total bacterial carbon demand on average 20 per cent higher than unwarmed samples, without any effect on the partitioning of this demand into production or respiration (that is, BGE). In this case, the predictions suggested a positive feedback between warming of coastal waters and CO2 production.
In summary, evidence from experimental approaches suggests that warming will favour a more oligotrophic ocean, with a higher proportion of picophytoplankton among autotrophs and higher BR, thus making the microbial food web even more important. The studies reviewed suggest that the future warmed ocean will behave as it currently does in the parts of the ocean that experience the highest temperatures.
Perturbation experiments exploring the effect of temperature on bacterial losses to grazing are rare. In a temperate system, Marrasé et al. (1992) warmed samples to 20°C over a year cycle, and then measured bacterial losses to grazing. In the warmed samples, grazing on bacteria (GB) rates were higher with an increase proportional to the degree of warming. In Antarctic waters, Vaqué et al. (2009) performed a microcosm experiment with temperature manipulation (−1 to 5°C) and observed that a temperature increase affected grazing rates and BP differentially. In general, GB increased at maximal rates at temperatures lower than 2°C, while BP increased at higher rates at temperatures greater than 2°C, which suggests that BP and bacterial grazing would become uncoupled processes at higher temperatures. However, the implications for carbon fluxes of this apparent uncoupling must be considered carefully because acclimatation and taxa substitutions in microbial assemblages for optimized growth at different conditions need to be factored in (e.g. Karl et al. 2001; Morán et al. 2010).
Despite methodological considerations that make each experiment unique and the limitations for extrapolation to a global scale, most studies showed increases in microbial metabolism with temperature and an increase in the activity of the immediate trophic level, bacterial grazers. One approach to unify, validate and/or explain these common trends observed in various experiments is the use of ecological theory.
(b). The use of current ecological theory
Considerable evidence has accumulated in recent years on the merits of using theoretical approaches to explain patterns and predict changes in both disturbed and undisturbed marine systems. For instance, recent developments in the so-called MTE (sensu Brown et al. 2004) can provide important insights into the effects of impending warming on the structure and dynamics of marine ecosystems.
MTE takes individual metabolism as the fundamental process that regulates the flux of energy and matter through different levels of biological organization, from individuals to ecosystems. It assumes nutrients and energy are transported and optimized through a fractal-like distribution network (West et al. 1997), from which a set of metabolic processes can be predicted and quantified, and from which a number of analytical predictions emerge at the individual, population, community and ecosystem level. The fundamental variable in the MTE is individual metabolic rate—the power required to sustain an organism—represented by the general metabolic model (West et al. 1997; Gillooly et al. 2001; Brown et al. 2004):
| 2.1 |
where Bi is the basal metabolic rate (i.e. the rate of respiration for a heterotroph) of an individual i, b0 is a normalization constant independent of body size and temperature, e−E/kT is the Boltzmann factor that describes the temperature T dependence of metabolic rate, where k is Boltzmann's constant (8.62 × 10−5 eV K−1) and E is the activation energy of metabolism. Mi corresponds to the body mass of individual i, and α is the allometric scaling exponent (West et al. 1997; Brown et al. 2004). By summing the individual metabolic rates of all organisms within an ecosystem, it is possible to predict total ecosystem metabolic rates (Enquist et al. 2003; Allen et al. 2005; López-Urrutia et al. 2006).
This general metabolic model has been extended to describe and predict different community-level and ecosystem-level processes. It assumes these processes are dependent on two fundamental parameters: body mass (of the individual and their distribution within the community) and ambient temperature. Our interest here is on how some of the microbial processes change with temperature. This model offers a framework to forecast changes in ecosystem processes under different global warming scenarios.
In particular, we concentrate on BP and GB, which, as explained below, are two key processes within the microbial food web. Following MTE, BP can be expressed as
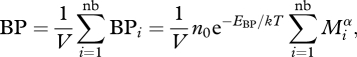 |
2.2 |
where nb is the number of bacteria in volume V, n0 is a normalization constant independent of body size Mi and temperature T, EBP is the activation energy governing the temperature dependence of BP and α is the allometric scaling exponent. The temperature dependence (i.e. EBP) is characterized by the kinetics of ATP synthesis in the respiratory complex of heterotrophic organisms, where the average activation energy governing the temperature dependence of respiratory reactions is ≈0.65 eV (Bernacchi et al. 2001; Allen et al. 2005; López-Urrutia et al. 2006).
Equation (2.2) can be linearized, resulting in
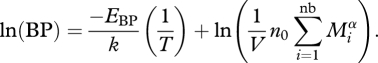 |
2.3 |
Because we wish to address the effects of warming alone on BP, we make the simplifying assumption that biomass abundance (i.e. 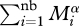 ) is constant, or at least invariant with temperature; that is, the product of number of bacteria and their mass does not change with temperature.
) is constant, or at least invariant with temperature; that is, the product of number of bacteria and their mass does not change with temperature.
Similarly, GB can be predicted using MTE. To satisfy its energetic requirements, an organism must consume food according to its metabolism, and here we assume that ingestion (grazing) rate GRj (the mass of food required per individual of species j) is proportional to its metabolic rate, and therefore
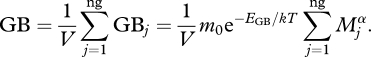 |
2.4 |
where ng is the number of grazers in volume V, m0 is a normalization constant independent of body size Mi and temperature T, EGB is the activation energy for the temperature dependence of grazing rate and α is the allometric scaling exponent. As grazing rate is a particular case of ingestion, we can assume the temperature dependency of grazing rate is equal to the temperature dependence of ingestion rate. Previous work (Emmerson & Raffaelli 2004; Montoya et al. 2005; Brose et al. 2006) has shown that ingestion rates scale as metabolic rates, and thus, the activation energy of grazing rate is equal to the activation energy of basal metabolic rates (≈0.75 eV) (Bernacchi et al. 2001; Allen et al. 2005; López-Urrutia et al. 2006), Equation (2.4) can be written as
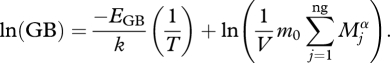 |
2.5 |
To summarize, following MTE, we would expect both BP and grazing rates on bacteria to increase with temperature with slopes of 0.65 and 0.75, respectively. With increasing temperatures, GB would be more tightly coupled to BP (a larger proportion of BP would be taken by grazers) because the slope would be higher. That said, this is the expectation assuming temperature only affects physiological changes in the organisms, all other things being equal.
We tested these theoretical predictions with two different datasets taken from the literature. First, we used data from perturbation experiments that measured BP and GB after warming water above its ambient temperature in the laboratory. Leucine incorporation (Kirchman et al. 1985) is the most commonly used method to estimate BP: water samples are incubated for 1–3 h under different temperature regimes in the presence of a saturating amount of 3H-leucine. GB can be estimated using different methods (reviewed by Vaqué et al. 1994), the most commonly used being microscopic enumeration of ingested fluorescent particles per individual protist (often restricted to a limited size/taxonomic class of grazers) and/or measuring the disappearance of fluorescent particles over time (which provides an estimate of total community losses to grazing). Here, we compiled GB data obtained by the disappearance of fluorescent particles method (Sherr et al. 1987; Vázquez-Domínguez et al. 1999) in order to include grazing by the whole community, in accordance with equations (2.4) and (2.5), where the effect of individual grazers is aggregated at the community level, and therefore community grazing is the predictive variable. We call these ‘perturbation experiments’ (in situ temperature is perturbed).
We also analysed experiments that measured both BP and GB under natural temperature conditions. In this case, the samples were taken from different oceans at different latitudes (electronic supplementary material), with a wide temperature range (−1.7 to 28.9°C). These are usually called space-for-time experiments or substitutions.
To test for the accuracy and precision of our theoretical predictions, we used empirical observations. We plotted ln(BP) versus 1/kT and ln(GB) versus 1/kT, and observed the slopes that correspond to their respective activation energies (table 1, figures 2 and 3). To assess the agreement between the theoretical prediction and empirical observations of activation energies, we performed a reiterative analysis of the confidence intervals of the regression slopes. This involves reducing the confidence level and therefore decreasing the amplitude of the confidence intervals for each regression until the theoretical prediction for the slope (i.e. activation energy) lies outside this interval. We call this confidence level the critical confidence level (critical CL) (table 1). A critical CL of 95 per cent means we are 95 per cent confident that the observed slope is in the range of the confidence interval and the theoretical prediction is within that interval too. This is a conservative approach, where we try to narrow the amplitude of the interval to see how robust the theoretical prediction is.
Table 1.
Summary of the equation parameters for the effect of the temperature (1/kT) on BP and on GB (µgC ml−1 h−1, prior to Ln transformation) in space-for-time measurements and perturbation experiments. Ln (PB) or Ln (GB) = a − b (1/kT); (n.s., not significant; c.i., 95% CI; CL, confidence level). References: (1), Vaqué et al. (1994) and references therein, Vaqué et al. (2002, 2001, 2008), Vázquez-Domínguez et al. (2005), Unrein et al. (2007), Boras et al. (2009); (2), J. M. Gasol (1998 unpublished data); (3), Pedrós-Alió et al. (2002); (4), Vaqué et al. (2009).
| a | b | [c.i.] | critical CL of the [c.i.] | p-value | n | r2 | references | ||
|---|---|---|---|---|---|---|---|---|---|
| space-for-time measurements | BP | 17.413 | 0.667 | [0.569–0.765] | <70% | F1,189 = 50.1, p < 0.0001 | 190 | 0.21 | (1) |
| GB | 9.770 | 0.456 | [0.329–0.584]a | 95%a | F1,127 = 50.0, p < 0.0001 | 128 | 0.28 | (1) | |
| perturbation experiments | BP | 7.204 | 0.385 | [0.116–0.653] | 95% | F1,11 = 10.2, p < 0.001 | 12 | 0.50 | (2) |
| 26.034 | 0.823 | [0.594–1.053] | <70% | F1,5 = 18.2, p < 0.01 | 6 | 0.82 | (2) | ||
| 1.762 | 0.256 | n.s. | 7 | (2) | |||||
| 7.904 | 0.387 | [0.060–0.714] | 99% | F1,5 = 29.6, p < 0.001 | 6 | 0.88 | (2) | ||
| 10.149 | 0.419 | n.s. | 6 | (2) | |||||
| 1.683 | 0.213 | n.s. | 8 | (2) | |||||
| 22.095 | 0.766 | [0.595–0.936] | <70% | F1,5 = 28.5, p < 0.001 | 6 | 0.88 | (3) | ||
| 43.281 | 1.274 | [0.175–2.724]a | 95%a | F1,4 = 7.8, p < 0.05 | 5 | 0.72 | (3) | ||
| 0.056 | 0.241 | n.s. | 6 | (3) | |||||
| 22.474 | 0.799 | [0.575–1.023] | <70% | F1,4 = 19.9, p < 0.01 | 5 | 0.87 | (3) | ||
| −10.969 | 0.008 | n.s. | 5 | (3) | |||||
| 9.804 | 0.483 | [0.289–0.676] | <70% | F1,5 = 8.8, p < 0.01 | 6 | 0.69 | (3) | ||
| 6.681 | 0.411 | n.s. | 6 | (3) | |||||
| average of significant | 19.828 | 0.702 | |||||||
| GB | −7.344 | 0.040 | n.s. | 5 | (4) | ||||
| 19.803 | 0.672 | [0.343–1.000] | <70% | F1,4 = 6.5, p < 0.05 | 5 | 0.68 | (4) | ||
| 28.403 | 0.898 | [0.600–1.196] | <70% | F1,4 = 14.2, p < 0.01 | 5 | 0.82 | (4) | ||
| 21.635 | 0.766 | n.s. | 5 | (4) | |||||
| −4.555 | 0.107 | n.s. | 5 | (4) | |||||
| 29.258 | 0.925 | n.s. | 5 | (4) | |||||
| 20.813 | 0.728 | [0.646–0.811] | <70% | F1,3 = 148.7, p < 0.001 | 4 | 0.99 | (4) | ||
| 7.444 | 0.424 | n.s. | 5 | (4) | |||||
| average of significant | 23.006 | 0.766 |
aTheoretical prediction slope values (0.65 for BP and 0.75 for GB) do not lie on the [c.i.] of the slopes for the field observations.
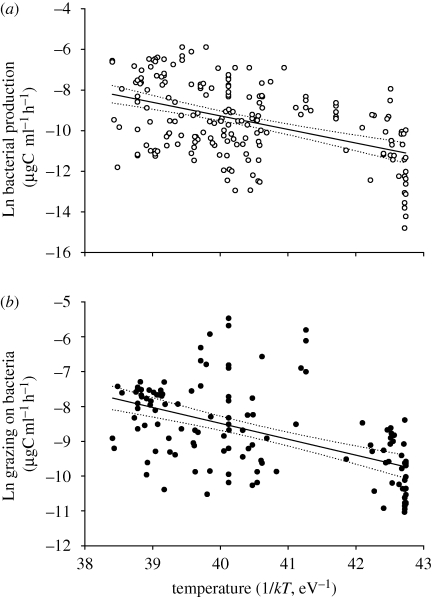
Figure 2.
(a) Arrhenius plot showing the effect of temperature (1/kT) on BP in natural systems (µgC ml−1 h−1, prior to Ln transformation). The black line represents the linear relationship between Ln (PB) and 1/kT (y = 17.413 – 0.667x, N = 190, r2 = 0.21, F1,189 = 50.1, p < 0.0001, the dashed line represents the 95% confidence interval of the slope); (b) Arrhenius plot showing the effect of temperature (1/kT) on total bacterial losses to grazing in natural systems (µgC ml−1 h−1, prior to Ln transformation).The black line represents the linear relationship between Ln (GB) and 1/kT (y = 9.770 – 0.456x, N = 128, r2 = 0.28, F1,127 = 50.0, p < 0.0001, the dashed line represents the 95% confidence interval of the slope).
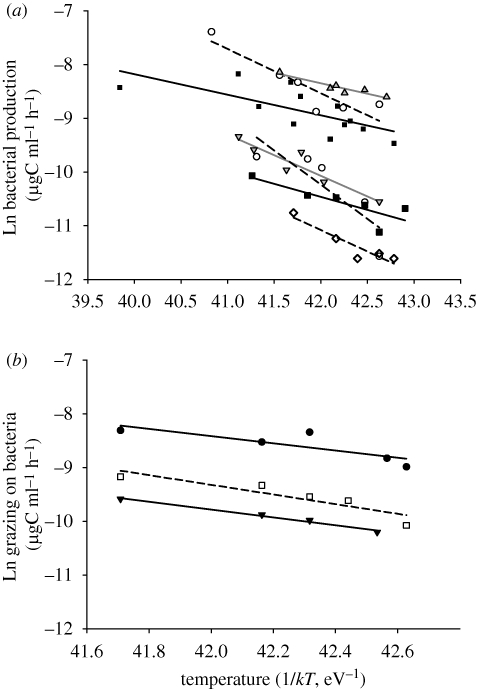
Figure 3.
Arrhenius plot showing the effect of the temperature (1/kT) on (a) BP and on (b) GB measured in perturbation experiments (µgC ml−1 h−1, prior to Ln transformation).
(i). Conclusions for perturbation experiments
For BP, seven out of 13 experiments showed a significant increase with T, with activation energies ranging from 0.38 to 1.27, and a mean value of 0.70 (table 1, figure 3), close to the theoretical prediction (0.65). For the individual regressions, the theoretical prediction lay within the confidence interval even at relatively narrow intervals (see table 1 for details).
For GB, only three out of eight experiments showed a significant increase with increasing temperatures. For those, slopes ranged from 0.67 to 0.90, with a mean of 0.77 (table 1, figure 3), close to the theoretical expectation of 0.75. Again, this theoretical prediction was within the confidence interval even at relatively narrow intervals (see table 1 for details).
Only around half of experiments showed a temperature dependency of either BP or GB. At present, we can offer no explanation for this, except for idiosyncratic ones (e.g. temperature control was not assured throughout the experiment). We certainly do not feel we should reject the hypothesis that metabolism is temperature dependent, given the fundamental nature of that relationship. But in those experiments where temperature was a determinant of process rate, observations fit theoretical predictions from MTE. In this manner, when temperature affects any of these two bacterial processes, its effects can be predicted and understood from relatively simple physiological mechanisms at the individual level. In those cases, theory and experimental manipulations suggest grazing rates on bacteria would increase faster with increasing temperatures than BP. This implies short-term environmental warming would change the bacterial–grazers biomass flux: there would be more total biomass flux, but also a larger fraction of the increased BP would be taken by grazers.
(ii). Conclusions for space-for-time measurements
In this case, both BP and GB significantly increase with increasing temperature, but with relatively low proportions of the variance explained by temperature (r2 values of 0.21 and 0.28, respectively, table 1). For BP, the observed activation energy was 0.67, very close to the theoretical prediction of 0.65, and within the confidence interval for different confidence levels (table 1). However, the observed activation energy for GB (0.46) was considerably smaller than the predicted MTE value (0.75), and the theoretical value was outwith the confidence interval.
If space-for-time substitutions are used to predict the effects of warming on bacteria and their associated grazers, we would predict BP to increase at a faster rate than bacterial grazing. Thus, bacterial–grazers biomass flux would increase overall, but the fraction of BP taken by grazers would be smaller in a warmer world. This is the contrary both to theoretical predictions and to the outcome of short-term experimental manipulations, where significant temperature effects were suggested.
In view of this, it is difficult to decide which approach is the best to forecast the effects of warming on bacterial-associated ecosystem processes: results derived from theory, short-term experimental manipulations and space-for-time substitutions can lead to different (and sometimes opposite) conclusions. In the present paper, temperature-induced changes in BP are coherent under the three approaches: when BP increases with increasing temperatures, it does so with a slope close to 0.65. For GB, theory and short-term manipulative experiments agree: when GB increases with temperature, it does so with a slope close to 0.75, larger than shown by space-for-time substitutions. Taken together, the three approaches imply that in a warmer ocean, BP and GB would increase and biomass flux from bacteria to grazers would also increase. With respect to the proportion of biomass production taken by grazers, the different approaches provide contrary insights. For those few experiments where temperature showed an effect, there was agreement that the proportion would increase, but in the case of space-for-time substitutions, the proportion would decrease. Two plausible mechanisms might help to explain these contradictions.
Both MTE and experimental results only contemplate the effects of temperature on organism physiology and its additive effects at the community level. Thus, they do not consider other factors that are affected by temperature in the natural systems used in space-for-time substitutions that can also affect grazing rates. First, we consider changes that may occur in other compartments within the food web. Predation rates on grazers are expected to increase with temperature in proportion to predator body mass (Emmerson & Raffaelli 2004; Brose et al. 2006). Under natural conditions, predators would decrease protist grazer abundances, and thus grazing rates on bacteria would not increase as fast as theory predicts and experiments that systematically exclude predators show.
Second, the proportion of the GB attributed to mixotrophic protists (capable of photosynthesis and particle grazing) may change along a temperature gradient (we have insufficient data to test this). These organisms use photosynthesis as a complementary source of energy, but MTE assumes that all organisms use the same energy source. Further, we assumed mixotrophy to be constant across systems (i.e. the same fraction of grazing and photosynthesis within the grazer guild) and changes in the proportion of grazing by mixotrophic protists throughout the temperature range covered could account for the lower than predicted observed activation energy. In other words, a higher proportion of mixotrophy within the grazer community would be an alternative way (other than bacterivory) for fulfilling the energy requirements associated with the high metabolic costs of living in a warmer environment. It has been shown that mixotrophic flagellates have lower specific ingestion rates than heterotrophic nanoflagellates (Unrein et al. 2007); but, owing to their high abundance (Jürgens & Massana 2008), their contribution to total GB can be extremely important. Zubkov & Tarran (2008) found that small algae carry out 40–95% of total GB in the euphotic layer of the temperate North Atlantic Ocean in summer and a similar range of 37–70% in the surface waters of the tropical Northeast Atlantic Ocean. However, both sets of measurements were performed in warm waters, so it is not possible to identify temperature effects.
(c). Long-term observations
In additions to changes within bacterial assemblages, a temperature increase is likely to change total bacterial abundance within a particular region. Theoretical predictions point towards a decrease in abundance with increasing temperatures (Brown et al. 2004). With some simplifying assumptions, it is even possible to quantify the precise changes in total abundance that could be expected. In particular, the MTE predicts the following relationship between abundance N and temperature T:
where NB is total bacterial abundance at the beginning of either the experiment or sampling collection, 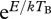 is the Boltzmann factor that describes the temperature dependence of abundance, where TB is the initial temperature, M corresponds to bacterial body mass and α is the allometric scaling exponent (West et al. 1997; Brown et al. 2004), and r0 is a normalization constant independent of body mass and temperature.
is the Boltzmann factor that describes the temperature dependence of abundance, where TB is the initial temperature, M corresponds to bacterial body mass and α is the allometric scaling exponent (West et al. 1997; Brown et al. 2004), and r0 is a normalization constant independent of body mass and temperature.
Similarly, the abundance at the end of the warming experiment or sampling collection, for the new temperature TE is given by:
For simplicity, we assume the same normalization constant for both cases (i.e. constant resource availability, sensu Brown et al. 2004), and the same body mass distribution within bacterial assemblages for both temperatures (MB = ME). Therefore, the ratio of change in total abundance between temperatures TB and TE is given by
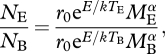 |
which, given the assumptions below, can be simplified and written as
and therefore, the ratio of decrease can be predicted by simply knowing TB and TE. A bacterial community containing 6 × 105 cells ml−1, typical of the summer situation in the NW Mediterranean (e.g. Alonso-Sáez et al. 2008), would decrease 18.6 per cent with a 2°C increase in temperature. A period of 10 years with the known increase in temperature of the NW Mediterranean sea of 0.032°C yr−1 (Salat & Pascual 2006) would represent a decrease of 3.2 per cent in bacterial abundance, a value probably too low to be detected with current microbial ecology techniques.
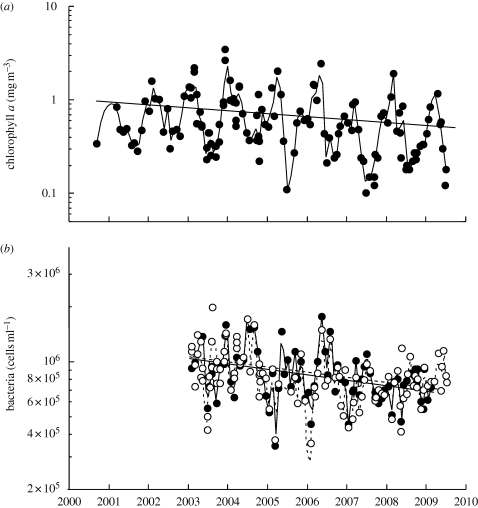
In the Blanes Bay Microbial Observatory, a coastal station in the NW Mediterranean, a trend of decreasing bacterial abundance has been detected in recent years (figure 4b), amounting to 51 000 cells ml−1 yr−1, 10 per cent per year. This decreasing trend is three times higher than the theoretical prediction, and might reflect the waste water depuration efforts undertaken in that part of the coast that have led to a decrease in nutrient inputs from the land, or perhaps to changes in land–ocean influx of organic materials if changes in rain patterns have occurred. Chlorophyll a also showed a significant decreasing trend (0.088 mg Chl a m−3 yr−1 on average, figure 4a) as did Secchi disc depth (water is becoming more transparent); silica and phosphate concentrations are also decreasing (data not shown).
Figure 4.
(a) Changes observed in the Microbial Observatory of Blanes Bay in the last decade in chlorophyll a and (b) in bacterial abundance determined with two different methodologies (DAPI counts on epifluorescence microscopy and flow cytometry). The relationships are: logChl a = 78.14–0.039 time (years), F1,115 = 7.77, p < 0.01 and logBact = 57.6–0.025 time (years), F1,62 = 5.5, p = 0.02 for DAPI counts and logBact = 53.3–0.023 time (years), F1,95 = 8.6, p < 0.01 for flow cytometry counts. Solid lines with filled circles, DAPI; dashed lines with open circles, flow cytometry.
Even if environmental signals owing to relatively small increases in temperature per year may be swamped by other environmental changes and thus remain difficult to detect unambiguously, the long-term observations in the Blanes Bay Microbial Observatory show a similar trend to the theoretical predictions. Long-term observations of marine ecosystems are rare, and time series of microbial abundance and diversity are even rarer (but see, e.g. Gerdts et al. 2004). Where they have been made in the past, they have allowed insights into changes occurring in the Pacific Ocean (e.g. Karl 1999), in the Arctic Ocean (Li et al. 2009) or in the body size of fish in European lakes (Daufresne et al. 2009). Long-term datasets have also been used to refute the hypothesis of a change in the North Sea spring bloom caused by warming (Wiltshire et al. 2008). As in the case of Blanes Bay mentioned above, several factors seem to contribute to the detected changes.
4. The role of each scientific approach
Experiments, which involve the generation of artificial variability by the manipulation of certain factors, allow the reduction of the complexity of a system so that hypothesis testing becomes more tractable (e.g. they provide opportunities to falsify hypotheses). However, their relevance to natural field situations is often debated and their utility probably lies more in identifying mechanisms and possible, rather than probable, outcomes. In particular, they are often conducted at scales not appropriate to the broader questions in which society has a stake and their highly controlled nature means that potential synergistic and antagonistic interactions between environmental forcing factors are often missed.
The unstated assumption behind the experiments that raise the temperature approximately 2°C in a water sample in order to predict what will occur in a 100 years time is that temperature has a physiological effect which is so pervasive that all micro-organisms will be equally affected. Indeed, with microbes growing at rates of ca 1 d−1, the expected 2°C that oceans will warm during this century represents ca 300 000 generations. In other words, each generation will experience, on average, a change of 0.000007°C, microbes will have plenty of time to adapt and it is likely that the sudden change of temperature in the experiment will affect them in ways fundamentally different from those likely to be experienced in reality.
Such problems have motivated many to explore other approaches to the problem. Ecological theory provides one such framework, and the one adopted here is based purely on physiological determinants of organism growth and grazing. However, and as has been illustrated above, temperature is not the only variable associated with climate change, and the effects of rising temperatures might even be contrary to the effects produced by other representations of global change, e.g. decreased input of land-derived nutrients as a consequence of decreased rainfall. Oligotrophic environments tend to be warmer, but with low nutrients, and a change in the nutrient inputs could be as important as temperature, both of which affect bacterial growth rates (White et al. 1991).
Long-term data series would be useful for exploring such complex interactions in the real world, but such observations are rare because of the short-term focus of funding bodies. Consequently, we now lack much of the information we could have obtained from such data series, in contrast to climatologists who have been able to use equivalent data.
5. Synopsis: knowns and unknowns concerning the effects of temperature on microbial food web interactions
In a warmer ocean, the fate of heterotrophic bacteria metabolism will strongly depend on the rates at which labile organic carbon derived from primary producers will become available to fuel BP. However, the prediction of metabolic rates in autotrophs and the partitioning of their carbon uptake into particulated (biomass) or dissolved (excretion and cell lysis) organic carbon remain uncertain. Characterizing the processes of production of dissolved organic molecules (DOM) by autotrophs and their lability (susceptibility to bacterial degradation) is undoubtedly one of the great challenges in the near future for marine research.
In addition, the way in which bacterial metabolism and community structure will be affected by a community shift in the phytoplankton is far from understood. It is possible to envision that a region of the ocean typically dominated by the cyanobacteria Synechococcus—typical picophytoplankton of the oligotrophic ocean in temperate regions—could shift to dominance by smaller cyanobacteria, Prochlorococcus—largely dominant in tropical regions (e.g. Zubkov et al. 2000; Vázquez-Domínguez et al. 2008). Intuitively, we would expect substantial differences in the quality and quantity of DOM produced in the first and the second case, as well as in the predators that benefit in each case. But the exact rate of change in the bacterial community and its metabolism in response to such a shift remains uncertain.
Nevertheless, we do have enough experimental evidence and a theoretical basis to predict that, in the upper layer of a stratified and oligotrophic ocean (corresponding to the largest proportion of the total ocean surface), temperature increases will probably result in an increase in BR of organic carbon and of bacterial losses to grazers, increasing the biomass flux between these two trophic levels within the microbial food web. If enough resources are available (as labile organic matter), BP would also increase, at rates probably higher than GB. In that case, bacterial abundance would also increase, and a higher proportion of inorganic nutrients would accumulate as bacterial biomass, thus strengthening the role of heterotrophy in the worlds' oceans. Planktonic communities would tend to decrease their average size (Falkowski & Oliver 2007; Daufresne et al. 2009; Morán et al. 2010), and a reinforcement of the already dominant role of microbes in the carbon cycle in a warmer ocean is highly probable.
Acknowledgements
H.S. benefited from grants from the Spanish MEC (SB2006-0060), and MCyI (Juan de la Cierva Fellowship JCI-2008-2727) and Portuguese FCT do Ministério da Ciência, Tecnologia e Ensino Superior (grant SFRH/BPD/34041/2006). J.M.M. is supported by the MCyI (Ramon y Cajal Fellowship RYC-2008-03664) and the Generalitat de Catalunya. Work of E.V.D. was supported by the FISIOCEAN project (PIF2008-30F0061). Contribution of D.V. was supported by U.E. ATP project (Contract no. 226248 to P.W.). Work of J.M.G. was supported by projects SUMMER (CTM2008-03309/MAR) and STORM (CTM2009-09352/MAR) from Spanish MCyI. We are thankful to Dave Raffaelli and to Xosé Anxelu G. Morán who helped improve the manuscript, and to Carles Pedrós-Alió and Fernando Unrein for facilitating the access to published data. We thank Ramon Massana for the DAPI counts in the Microbial Observatory of Blanes Bay dataset.
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Allen A. P., Gillooly J. F., Brown J. H.2005Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19, 202–213 (doi:10.1111/j.1365-2435.2005.00952.x) [Google Scholar]
- Alonso-Sáez L., et al. 2008Factors controlling the year-round variability in carbon flux through bacteria in a coastal marine system. Ecosystems 11, 397–409 (doi:10.1007/s10021-008-9129-0) [Google Scholar]
- Aota Y., Nakajima H.2001Mutualistic relationships between phytoplankton and bacteria caused by carbon excretion from phytoplankton. Ecol. Res. 16, 289–299 (doi:10.1046/j.1440-1703.2001.00396.x) [Google Scholar]
- Behrenfeld M. J., et al. 2006Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 (doi:10.1038/nature05317) [DOI] [PubMed] [Google Scholar]
- Bernacchi C. J., Singsaas E. L., Pimentel C., Portis A. R., Long S. P.2001Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 24, 253–259 (doi:10.1111/j.1365-3040.2001.00668.x) [Google Scholar]
- Boras J. A., Sala M. M., Vázquez-Domínguez E., Weinbauer M. G., Vaqué D.2009Annual changes of bacterial mortality due to viruses and protists in an oligotrophic coastal environment (NW Mediterranean). Environ. Microbiol. 11, 1181–1193 (doi:10.1111/j.1462-2920.2008.01849.x) [DOI] [PubMed] [Google Scholar]
- Brose U., Williams R. J., Martinez N. D.2006Allometric scaling enhances stability in complex food webs. Ecol. Lett. 9, 1228–1236 (doi:10.1111/j.1461-0248.2006.00978.x) [DOI] [PubMed] [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B.2004Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- Daufresne M., Lengfellner K., Sommer U.2009Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793 (doi:10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Giorgo P. A., Duarte C. M.2002Respiration in the open ocean. Nature 420, 379–384 (doi:10.1038/nature01165) [DOI] [PubMed] [Google Scholar]
- del Giorgio P. A., Williams P. J. l. B.2005The global significance of respiration in aquatic ecosystems: from single cells to the biosphere. In Respiration in aquatic ecosystems (eds del Giorgio P. A., Williams P.), pp. 267–316 New York, NY: Academic Press [Google Scholar]
- Edwards M., Richardson A. J.2004Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (doi:10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- Emmerson M. C., Raffaelli D.2004Predator–prey body size, interaction strength and the stability of a real food web. J. Anim. Ecol. 73, 399–409 (doi:10.1111/j.0021-8790.2004.00818.x) [Google Scholar]
- Enquist B. J., Economo E. P., Huxman T. E., Allen A. P., Ignace D. D., Gillooly J. F.2003Scaling metabolism from organisms to ecosystems. Nature 423, 639–642 (doi:10.1038/nature01671) [DOI] [PubMed] [Google Scholar]
- Falkowski P. G., Oliver M. J.2007Mix and match: how climate selects phytoplankton. Nature Rev. Microbiol. 5, 813–819 (doi:10.1038/nrmicro1751) [DOI] [PubMed] [Google Scholar]
- Falkowski P. G., Oliver M. J.2008Diatoms in a future ocean-stirring it up: reply from Falkowski and Oliver. Nature Rev. Microbiol. 6, 407–407 [DOI] [PubMed] [Google Scholar]
- Falkowski P. G., Wilson C.1992Phytoplankton productivity in the North Pacific Ocean since 1900 and implications for absorption of anthropogenic CO2. Nature 358, 741–743 (doi:10.1038/358741a0) [Google Scholar]
- Falkowski P. G., Barber R. T., Smetacek V.1998Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–206 (doi:10.1126/science.281.5374.200) [DOI] [PubMed] [Google Scholar]
- Field C. B., Behrenfeld M. J., Randerson J. T., Falkowski P.1998Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240 (doi:10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A.1999Marine viruses and their biogeochemical and ecological effects. Nature 399, 541–548 (doi:10.1038/21119) [DOI] [PubMed] [Google Scholar]
- Gerdts G., Wichels A., Döpke H., Klings K.-W., Gunkel W., Schütt C.200440-year long-term study of microbial parameters near Helgoland (German Bight, North Sea): historical view and future perspectives. Helgoland Mar. Res. 58, 230–242 (doi:10.1007/s10152-004-0189-z) [Google Scholar]
- Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L.2001Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- Gruber N., Keeling C. D., Bates N. R.2002Interannual variability in the North Atlantic Ocean carbon sink. Science 298, 2374–2378 (doi:10.1126/science.1077077) [DOI] [PubMed] [Google Scholar]
- Gruber N., et al. 2009Oceanic sources, sinks, and transport of atmospheric CO2. Glob. Biogeochem. Cycles 23, GB1005 (doi:10.1029/2008GB003349) [Google Scholar]
- Hoppe H. G., Breithaupt P., Walther K., Koppe R., Bleck S., Sommer U., Jürgens K.2008Climate warming in winter affects the coupling between phytoplankton and bacteria during the spring bloom: a mesocosm study. Aquat. Microb. Ecol. 51, 105–115 (doi:10.3354/ame01198) [Google Scholar]
- Jürgens K., Massana R.2008Protistan grazing on marine bacterioplankton. In Microbial ecology of the oceans (ed. Kirchman D. L.), 2nd edn, pp. 383–441 [Google Scholar]
- Karl D. M.1999A sea of change: biogeochemical variability in the North Pacific Subtropical Gyre. Ecosystems 2, 181–214 (doi:10.1007/s100219900068) [Google Scholar]
- Karl D. M., Bidigare R. R., Letelier R. M.2001Long-term changes in plankton community structure and productivity in the North Pacific Subtropical Gyre: the domain shift hypothesis. Deep-Sea Res. Pt II 48, 1449–1470 [Google Scholar]
- Karl D. M., Laws E. A., Morris P., Williams P. J. l., Emerson S.2003Global carbon cycle (communication arising): metabolic balance of the open sea. Nature 426, 32–32 (doi:10.1038/426032a) [DOI] [PubMed] [Google Scholar]
- Kirchman D., K'nees E., Hodson R.1985Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl. Environ. Microb. 49, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws E. A., Falkowski P. G., Smith W. O., Jr, Ducklow H., McCarthy J. J.2000Temperature effects on export production in the open ocean. Glob. Biogeochem. Cycles 14, 1231–1246 (doi:10.1029/1999GB001229) [Google Scholar]
- Levitus S., Antonov J. I., Boyer T. P., Stephens C.2000Warming of the world ocean. Science 287, 2225–2229 (doi:10.1126/science.287.5461.2225) [Google Scholar]
- Li W. K. W., McLaughlin F. A., Lovejoy C., Carmack E. C.2009Smallest algae thrive as the Arctic Ocean freshens. Science 326, 539–539 (doi:10.1126/science.1179798) [DOI] [PubMed] [Google Scholar]
- López-Urrutia A., Morán X. A. G.2007Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 88, 817–822 (doi:10.1890/06-1641) [DOI] [PubMed] [Google Scholar]
- López-Urrutia Á., San Martin E., Harris R. P., Irigoien X.2006Scaling the metabolic balance of the oceans. Proc. Natl Acad. Sci. USA 103, 8739–8744 (doi:10.1073/pnas.0601137103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackensie B. R., Schiedek D.2007Daily ocean monitoring since the 1860s shows record warming of northern European seas. Glob. Change Biol. 13, 1335–1347 [Google Scholar]
- Marrasé C., Lim E. L., Caron D. A.1992Seasonal and daily changes in bacterivory in a coastal plankton community. Mar. Ecol. Prog. Ser. 82, 281–289 (doi:10.3354/meps082281) [Google Scholar]
- Mayali X., Azam F.2004Algicidal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 51, 139–144 (doi:10.1111/j.1550-7408.2004.tb00538.x) [DOI] [PubMed] [Google Scholar]
- Meehl G. A., et al. 2007The physical science basis. In Climate change 2007 IPCC report, pp. 748–845 Cambridge, UK: Cambridge University Press [Google Scholar]
- Montoya J. M., Raffaelli D.2010Climate change, biotic interactions and ecosystem services. Phil. Trans. R. Soc. B 365, 2013–2018 (doi:10.1098/rstb.2010.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J. M., Emmerson M. C., Solé R. V., Woodward G.2005Perturbation and indirect effects in complex food webs. In Dynamic food webs (eds Ruiter P. C. D., Wolters V., Moore J. C.), pp. 369–380 New York, NY: Academic Press [Google Scholar]
- Morán X. A. G., Sebastián M., Pedrós-Alió C., Estrada M.2006Response of southern ocean phytoplankton and bacterioplankton production to short-term experimental warming. Limnol. Oceanogr. 51, 1791–1800 [Google Scholar]
- Morán X. A. G., López-Urrutia A., Calvo-Díaz L., Li W. K. W.2010Increasing importance of small phytoplankton in a warmer ocean. Glob. Change Biol. 16, 1137–1144 (doi:10.1111/j.1365-2486.2009.01960.x) [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Pedrós-Alió C., Vaqué D., Guixa-Boixereu N., Gasol J. M.2002Prokaryotic plankton biomass and heterotrophic production in western Antarctic waters during the 1995–1996 Austral summer. Deep-Sea Res. Pt II 49, 805–825 [Google Scholar]
- Petchey O. L., McPhearson P. T., Casey T. M., Morin P. J.1999Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72 (doi:10.1038/47023) [Google Scholar]
- Peters F.1994Prediction of planktonic protistan grazing rates. Limnol. Oceanogr. 39, 195–206 [Google Scholar]
- Peters F.2008Diatoms in a future ocean–stirring it up. Nature Rev. Microbiol. 6, 407–407 [DOI] [PubMed] [Google Scholar]
- Polovina J. J., Howell E. A., Abecassis M.2008Ocean's least productive waters are expanding. Geophys. Res. Lett. 35, L03618 doi:10.1029/2007GL031745 [Google Scholar]
- Raffaelli D. G.2004How extinction patterns affect ecosystems. Science 306, 1141–1142 (doi:10.1126/science.1106365) [DOI] [PubMed] [Google Scholar]
- Ribalet F., Intertaglia L., Lebaron P., Casotti R.2008Differential effect of three polyunsaturated aldehydes on marine bacterial isolates. Aquat. Toxicol. 86, 249–255 (doi:10.1016/j.aquatox.2007.11.005) [DOI] [PubMed] [Google Scholar]
- Riser S. C., Johnson K. S.2008Net production of oxygen in the subtropical ocean. Nature 451, 323–325 (doi:10.1038/nature06441) [DOI] [PubMed] [Google Scholar]
- Salat J., Pascual J.2006Principales tendencias climatológicas en el Mediterráneo noroccidental, a partir de más de 30 años de observaciones oceanográfcas en la costa catalana. In Clima, sociedad y medio ambiente, vol. 5 (eds Prats J. M. C., Sánchez M. A. S., Serrano S. M. V., Lanjeri S., Arrillaga N. d. L., González-Hidalgo J. C.), pp. 284–290 Zaragoza, Spain: Publicaciones de la Asociación Española de Climatología (AEC) serie A [Google Scholar]
- Sarmiento J. L., Gruber N.2002Sinks for anthropogenic carbon. Phys. Today 55, 30–36 (doi:10.1063/1.1510279) [Google Scholar]
- Sherr B., Sherr E., Fallon R.1987Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl. Environ. Microb. 53, 958–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N. C., Mysterud A., Ottersen G., Hurrell J. W., Chan K.-S., Lima M.2002Ecological effects of climate fluctuations. Science 297, 1292–1296 (doi:10.1126/science.1071281) [DOI] [PubMed] [Google Scholar]
- Timmermann A., Oberhuber J., Bacher A., Esch M., Latif M., Roeckner E.1999Increased El Nino frequency in a climate model forced by future greenhouse warming. Nature 398, 694–697 [Google Scholar]
- Unrein F., Massana R., Alonso-Sáez L., Gasol J. M.2007Significant year-round effect of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnol. Oceanogr. 52, 456–469 [Google Scholar]
- Vaqué D., Gasol J. M., Marrasé C.1994Grazing rates on bacteria—the significance of methodology and ecological factors. Mar. Ecol. Prog. Ser. 109, 263–274 (doi:10.3354/meps109263) [Google Scholar]
- Vaqué D., Casamayor E. O., Gasol J. M.2001Dynamics of whole community bacterial production and grazing losses in seawater incubations as related to the changes in the proportions of bacteria with different DNA content. Aquat. Microb. Ecol. 25, 163–177 (doi:10.3354/ame025163) [Google Scholar]
- Vaqué D., Calderón-Paz J. I., Guixa-Boixereu N., Pedrós-Alió C.2002Spatial distribution of microbial biomass and activity (bacterivory and bacterial production) in the northern Weddell Sea during the austral summer (January 1994). Aquat. Microb. Ecol. 29, 107–121 (doi:10.3354/ame029107) [Google Scholar]
- Vaqué D., Guadayol O., Peters F., Felipe J., Ángel-Ripoll L., Terrado R., Lovejoy C., Pedrós-Alió C.2008Seasonal changes in planktonic bacterivory rates under the ice-covered coastal Arctic Ocean. Limnol. Oceanogr. 53, 2427–2438 [Google Scholar]
- Vaqué D., Guadayol O., Peters F., Felipe J., Malits A., Pedrós-Alió C.2009Differential response of grazing and bacterial heterotrophic production to experimental warming in Antarctic waters. Aquat. Microb. Ecol. 54, 101–112 (doi:10.3354/ame01259) [Google Scholar]
- Vargas-Yañez M., Salat J., Fernandez de Puelles M. L., Lopez-Jurado J. L., Pascual J., Ramirez T., Cortés D., Franco I.2005Trends and time varibility in the Northern continental shelf of the western Mediterranean. J. Geophys. Res. 110, 1–18 [Google Scholar]
- Vázquez-Domínguez E., Peters F., Gasol J. M., Vaqué D.1999Measuring the grazing losses of picoplankton: methodological improvements in the use of fluorescently labeled tracers combined with flow cytometry. Aquat. Microb. Ecol. 20, 119–128 (doi:10.3354/ame020119) [Google Scholar]
- Vázquez-Domínguez E., Gasol J. M., Agustí S., Duarte C. M., Vaqué D.2005Growth and grazing losses of prokaryotes in the central Atlantic Ocean. J. Plankton Res. 27, 1055–1066 (doi:10.1093/plankt/fbi074) [Google Scholar]
- Vázquez-Domínguez E., Vaqué D., Gasol A. M.2007Ocean warming enhances respiration and carbon demand of coastal microbial plankton. Glob. Change Biol. 13, 1327–1334 (doi:10.1111/j.1365-2486.2007.01377.x) [Google Scholar]
- Vázquez-Domínguez E., Duarte C. M., Agustí S., Jürgens K., Vaqué D., Gasol J. M.2008Microbial plankton abundance and heterotrophic activity across the Central Atlantic Ocean. Prog. Oceanogr. 79, 83–94 [Google Scholar]
- Verity P. G.1981Effects of temperature, irradiance, and daylength on the marine diatom Leptocylindrus danicus Cleve. I. Photosynthesis and cellular composition. J. Exp. Mar. Biol. Ecol. 55, 79–91 (doi:10.1016/0022-0981(81)90094-0) [Google Scholar]
- Voigt W., et al. 2003Trophic levels are differentially sensitive to climate. Ecology 84, 2444–2453 (doi:10.1890/02-0266) [Google Scholar]
- Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J.-M., Hoegh-Guldberg O., Bairlein F.2002Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- Watanabe Y.1980A Study of the excretion and extracellular products of natural phytoplankton in Lake Nakanuma, Japan. Int. Rev. Ges. Hydrobiol. 65, 809–834 (doi:10.1002/iroh.19800650606) [Google Scholar]
- Weinbauer M. G., Arrieta J. M., Griebler C., Herndl G. J.2009Enhanced viral production and infection of bacterioplankton during an iron-induced phytoplankton bloom in the Southern Ocean. Limnol. Oceanogr. 54, 774–784 [Google Scholar]
- West G. B., Brown J. H., Enquist B. J.1997A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 (doi:10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]
- White P. A., Kalff J., Rasmussen J. B., Gasol J. M.1991The effect of temperature and algal biomass on bacterial production and specific growth-rate in fresh-water and marine habitats. Microb. Ecol. 21, 99–118 (doi:10.1007/BF02539147) [DOI] [PubMed] [Google Scholar]
- Williams P. J. l. B., Morris P. J., Karl D. M.2004Net community production and metabolic balance at the oligotrophic ocean site, station ALOHA. Deep-Sea Res. Pt I 51, 1563–1578 [Google Scholar]
- Wiltshire K. H., Manly B. F. J.2004The warming trend at Helgoland Roads, North Sea: phytoplankton response. Helgoland Mar. Res. 58, 269–273 (doi:10.1007/s10152-004-0196-0) [Google Scholar]
- Wiltshire K. H., Malzahn A. M., Wirtz K., Greve W., Janisch S., Mangelsdorf P., Manly B. F. J., Boersma M.2008Resilience of North Sea phytoplankton spring bloom dynamics: an analysis of long-term data at Helgoland Roads. Limnol. Oceanogr. 53, 1294–1302 [Google Scholar]
- Wohlers J., Engel A., Zöllner E., Breithaupt P., Jürgens K., Hoppe H.-G., Sommer U., Riebesell U.2009Changes in biogenic carbon flow in response to sea surface warming. Proc. Natl Acad. Sci. USA 106, 7067–7072 (doi:10.1073/pnas.0812743106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Dubinsky Z.1989The effect of light and temperature on DOC excretion by phytoplankton. Limnol. Oceanogr. 34, 831–839 [Google Scholar]
- Zubkov M., Sleigh M., Burkill P., Leakey R.2000Picoplankton community structure on the Atlantic Meridional Transect: a comparison between seasons. Prog. Oceanogr. 45, 369–386 (doi:10.1016/S0079-6611(00)00008-2) [Google Scholar]
- Zubkov M. V., Tarran G. A.2008High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature 455, 224–226 (doi:10.1038/nature07236) [DOI] [PubMed] [Google Scholar]