Abstract
Fresh waters are particularly vulnerable to climate change because (i) many species within these fragmented habitats have limited abilities to disperse as the environment changes; (ii) water temperature and availability are climate-dependent; and (iii) many systems are already exposed to numerous anthropogenic stressors. Most climate change studies to date have focused on individuals or species populations, rather than the higher levels of organization (i.e. communities, food webs, ecosystems). We propose that an understanding of the connections between these different levels, which are all ultimately based on individuals, can help to develop a more coherent theoretical framework based on metabolic scaling, foraging theory and ecological stoichiometry, to predict the ecological consequences of climate change. For instance, individual basal metabolic rate scales with body size (which also constrains food web structure and dynamics) and temperature (which determines many ecosystem processes and key aspects of foraging behaviour). In addition, increasing atmospheric CO2 is predicted to alter molar CNP ratios of detrital inputs, which could lead to profound shifts in the stoichiometry of elemental fluxes between consumers and resources at the base of the food web. The different components of climate change (e.g. temperature, hydrology and atmospheric composition) not only affect multiple levels of biological organization, but they may also interact with the many other stressors to which fresh waters are exposed, and future research needs to address these potentially important synergies.
Keywords: biodiversity–ecosystem functioning, ecological stoichiometry, food webs, foraging theory, global warming, metabolic scaling
1. Introduction: climate change and levels of biological organization
Climatic variations occur naturally over seasonal to millennial time scales, yet the unprecedented rates of warming observed in recent decades threaten to undermine the functioning of natural ecosystems, especially when combined with the myriad additional anthropogenic stresses to which many fresh waters are subjected (Malmqvist et al. 2008). Climate change itself represents a complex amalgam of stressors, including alterations in temperature (Webb et al. 2008), elevated atmospheric CO2 (IPCC 2007), and increased frequency and intensity of droughts and extreme flow events (Barnett et al. 2005; Milly et al. 2006). There are also other more subtle associated effects, including invasions and extinctions of species as cold stenotherms are squeezed polewards and onto higher ground by the expansion of eurythermic species (e.g. Krajick 2004). Synergies with other stressors could amplify the effects of climate change: for instance, summer droughts will not only lead to elevated temperatures and habitat fragmentation, but they may also exacerbate the impacts of eutrophication and toxins by increasing pollutant concentrations. Our focus in this review, however, is primarily on the more direct impacts of warming per se and, to a lesser extent, rising CO2 levels, and how these might scale across multiple levels of biological organization, from individuals to ecosystems.
Fresh waters are particularly vulnerable to climate change because they are relatively isolated and physically fragmented within a largely terrestrial landscape, and they are also already heavily exploited by humans for the provision of ‘goods and services’ (e.g. drinking water and food; Woodward 2009). Furthermore, freshwater biodiversity is disproportionately at risk on a global scale, because while fresh waters cover only 0.8 per cent of the Earth's surface, they are home to an estimated 6 per cent of all species (Dudgeon et al. 2006). From an applied perspective, climate change has the potential to undermine many existing freshwater biomonitoring schemes, which are based largely on responses to organic pollution with little consideration of the increasing influence of climatic effects (Woodward et al. 2009). Thus, the ways in which we currently assess ‘ecological status’ could become increasingly obsolete over time, as the baseline drifts away from earlier (and cooler) reference conditions.
Climate change is arguably the greatest emerging threat to global biodiversity and the functioning of local ecosystems (IPCC 2007). Although the effects will be felt worldwide, they will not be evenly distributed, with systems at higher latitudes and altitudes experiencing some of the fastest rates of warming on the planet (Hassan et al. 2005). Fresh waters in these areas can be considered as ‘sentinel systems’, in the sense that they could provide early warning of wider scale change, and by studying them we are likely to gain better insight into climate change impacts on the more complex (i.e. species rich) systems we find in warmer climatic zones (Woodward et al. 2009; Layer et al. 2010; Perkins et al. in press). Despite the multitude of studies examining potential climate change effects in fresh waters, however, most have focused on individuals or species populations (e.g. McKee & Atkinson 2000) rather than the higher levels of organization (i.e. communities, food webs, ecosystems) (but see Kishi et al. 2005; Burgmer et al. 2007; Buisson et al. 2008; Friberg et al. 2009; Woodward et al. 2009; Yvon-Durocher et al. 2010). This is especially true for fresh waters, and this is a cause for considerable concern because climate change will not only affect all organizational levels (e.g. ecosystem metabolism is the sum of all individuals' metabolic rates, which are themselves a function of temperature) but the dominance of ectotherms in fresh waters means the effects of temperature change will be particularly pervasive. Further, it is not necessarily possible to predict ecosystem responses by simply extrapolating from the lower levels of organization, because of the potential for emergent properties to be manifested in these more complex systems (Petchey et al. 2004; Woodward 2009; Walther 2010).
In this review we address the need to understand biological responses at the higher levels of organization, suggest ways by which we might link these changes to energetic constraints imposed on individuals, and outline the potential for integrating these approaches to develop the more coherent theoretical framework that is needed to predict future impacts. Since energy is arguably the ultimate currency in ecology, and individual metabolism is driven by a combination of body size and temperature (e.g. Brown et al. 2004), changes in thermal regimes are likely to have profound effects that will ramify through the entire ecosystem. Similarly, temperature has strong impacts on the foraging behaviour of individuals (e.g. increased encounter rates, decreased handling times), which will determine the strength of interactions. Furthermore, it has been suggested that rising temperatures might shift the size spectrum in local communities towards greater dominance by smaller organisms as large individuals are lost disproportionately (e.g. Petchey et al. 1999), and this has important implication for freshwater food webs, which appear to be far more strongly size-structured than their terrestrial counterparts (Woodward et al. 2005; Ings et al. 2009). These fundamental energetic constraints on metabolism, behaviour, and the architecture and dynamics of trophic networks underpin many of the key points addressed in this review.
2. Scales of study and levels of biological organization
One of the key challenges facing freshwater ecologists is to develop a suite of tools for detecting the impacts of climate change in complex natural systems that can be applied across multiple spatio-temporal scales and levels of organization. We will also need to integrate long-term, empirical survey data with models and manipulative experiments if we are to develop a truly mechanistic, and hence predictive, understanding of responses to future change.
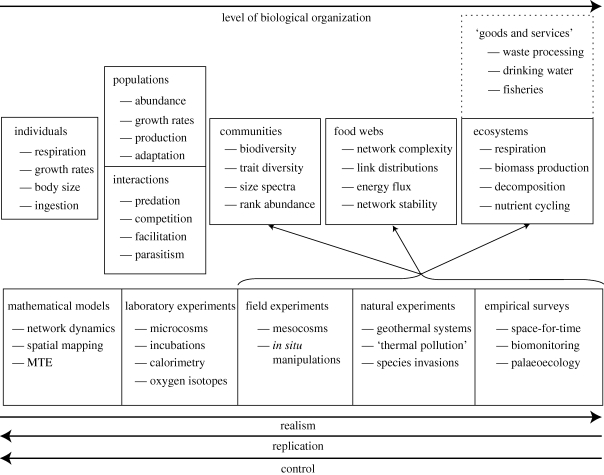
To date, most freshwater climate change research has focused on responses of individuals (e.g. growth and metabolism under warming; Sweeney & Schanck 1977) or focal species populations (e.g. polewards or altitudinal range shifts; Babaluk et al. 2000). Many data are also correlational and therefore potentially vulnerable to other confounding factors: ‘space-for-time’ surveys, for instance, often conflate temperature differences with biogeographical or latitudinal gradients. Conversely, microcosm experiments, although they can be controlled and replicated with a high degree of precision, are unable to capture the full range of complexity of natural systems. Clearly, no single approach is perfect and we must make full use of the armoury of techniques at our disposal to address the problem (figure 1).
Figure 1.
Schematic of studies into climate change impacts in fresh waters arranged along gradients of control, replication and realism, highlighting the links between different levels of organization investigated and the approaches used.
Some of the earliest biodiversity–ecosystem functioning (B-EF) experiments to investigate the effects of warming were carried out in freshwater systems, albeit under laboratory conditions. Petchey and co-workers (Petchey et al. 1999; Petchey 2000) pioneered much of this field, by carrying out a series of experiments using protist assemblages in microcosms to study trajectories of community change and food web responses to experimental warming. Top predators and herbivores were disproportionately affected, assemblages became dominated by autotrophs and bacterivores, and ecosystem functioning was altered beyond the scale expected solely from predictions based on temperature-dependent physiological rates, owing to changes in the relative abundance of functional groups (Petchey et al. 1999; Petchey 2000). Similarly, Dang et al. (2009) have shown that warming alters the composition of fungal assemblages, thereby influencing rates of organic matter decomposition. Work on unionid mussels in laboratory experiments has revealed that resource assimilation and key ecosystem properties (e.g. nutrient cycling, benthic–pelagic coupling) carried out by these organisms are also strongly temperature dependent (Spooner & Vaughn 2008).
More recently, experiments have been carried out on a more ambitious scale, both in terms of the physical size of the arenas, as larger mesocosm studies have gained increasing prominence, but also in the ecological complexity of the systems being studied. Examples include those that formed part of the EU sixth Framework EUROLIMPACS programme involving long-term warming studies carried out in pond mesocosms (e.g. Meerhoff et al. 2007). Some of these experimental manipulations are still running several years after their inception: 24 ponds in Silkeborg (Denmark) have been warmed continuously since 2003 (Liboriussen et al. 2005), another set of 20 ambient/warmed ponds at the FBA River Lab in Dorset (UK) have been running since 2006 (Yvon-Durocher et al. 2010) and 48 similar-sized ponds have also been subjected to a comparable level of warming in Ness Gardens at the University of Liverpool (UK) since 1998, albeit intermittently (e.g. McKee et al. 2003). The former have been used to assess shifts in community structure and ecosystem properties, both of which responded to elevated temperatures, as the ponds tended towards more turbid, plankton-dominated systems at higher temperatures (Meerhoff et al. 2007), and the FBA set-up has revealed that warming pushes the systems towards increased heterotrophy and reduced CO2 sequestration (Yvon-Durocher et al. 2010).
A few field experiments have been carried out in situ in natural systems, rather than in self-contained experimental arenas. Hogg et al. (1995), for instance, raised the water temperature of a stream by 2.0–3.5°C over 2 years, which suppressed total invertebrate abundance but stimulated growth rates. Barlocher et al. (2008) heated the hyporheic zone of streams by up to 4.3°C, a change which accelerated leaf litter decomposition rates, and in turn led to lower abundance and diversity of aquatic hyphomycetes owing to reduced substrate availability. In addition to these more recent field-based manipulations of temperature, it is also worth remembering that a large body of data was collected on the effects of thermal pollution in fresh waters during the 1970s and 1980s (e.g. Langford 1990), before global warming per se appeared on the scientific agenda, and these early studies could provide potentially useful new insights if revisited from a climate change perspective.
The associated effects of drought and habitat fragmentation have been less well-studied than those of temperature in the context of climate change, but both are likely to be critical stressors in many fresh waters. A recent study subjected a suite of replicated experimental stream channels to different intensities of drought designed to mimic the likely effects of patch scale dewatering resulting from the reduced flows and increased abstraction predicted for the coming decades (Harris et al. 2007). The channel flows were diverted from an adjacent stream, to ensure a common source of water and potential colonists, and each one was divided into three sections that were then assigned to the experimental treatments (unmanipulated controls, three-monthly dewatering and monthly dewatering). In the absence of disturbance, epilithic space was dominated by the green encrusting alga, Gongrosira incrustans. However, droughts consistently reduced the dominance of the green alga, and crust abundance decreased, opening up space for a diversity of mat-forming diatoms (Ledger et al. 2008). The invertebrate community structure also shifted in the most stressed treatments relative to the controls, with a decrease in large, rare species higher in the food web.
The next step along the gradient of ecological realism from experiments to natural systems (figure 1) involves the use of ‘natural experiments’, and geothermally heated systems are useful here, provided other potentially confounding variables (e.g. sulphur contamination) can be avoided. Such systems have proved invaluable for investigating aspects of ecosystem thermodynamics in the past (Brock & Brock 1966). More recently, Friberg et al. (2009) explored changes in both community structure and ecosystem functioning (detrital processing and algal production) in a set of 10 Icelandic geothermal streams. The streams spanned an approximately 20°C thermal gradient, with no other discernable differences in water chemistry; i.e. temperature was not confounded with other variables. All 10 streams were constituents of the same catchment and linked to the mainstem; thus the local assemblages were derived from the same regional species pool. Friberg et al. (2009) found that both primary production and decomposition rates rose dramatically with temperature, and this was also associated with clear shifts in community structure. In the warmer streams, the herbivore assemblage was dominated by large-bodied and very efficient grazers, in the form of the snail Radix peregra, whereas in the colder streams this niche was occupied by small chironomid midge larvae. This taxonomic change, represented by a functional shift in the community, in turn resulted in stronger top-down control of algal production in the warmer streams. More recently, Woodward et al. (in press) characterized community structure in 15 of these streams and found that food chain length increased with a temperature change from approximately 5 to 25°C, with fish (brown trout, Salmo trutta L.) replacing invertebrates as the top predators in the warmer streams. In addition, mean individual body mass of the trout also increased with temperature, mirroring the increases in the trophic height of the food web. Such natural experiments offer additional potential if they can be combined with field-based manipulations, such as species transplantations or nutrient addition experiments (e.g. Friberg et al. 2009).
The arena of large-scale empirical surveys is arguably where the most realistic manifestations of ecosystem responses to climate change can be observed, because such studies are able to quantify ambient conditions in natural systems (figure 1). They do suffer, however, from the drawbacks of little or no replication, potential confounding variables and an inability to demonstrate causal relationships unless some form of experimental data is also available. One common approach involves making inferences based on space-for-time substitutions, whereby contemporary systems are sampled along a proxy thermal gradient. Such approaches may be undertaken along latitudinal or longitudinal (e.g. upstream–downstream) gradients or at a series of independent spatial locations within a defined area. The principal limitation is that there are often also underlying confounding gradients in physicochemistry, biogeography or disturbance that can make it difficult to justify extrapolating from such data (e.g. Castella et al. 2001; Johnson & Miyanishi 2008). Nevertheless, if used carefully, such approaches can provide answers to important parts of the puzzle that cannot be addressed by experiments, which inevitably fail to capture the full complexity of natural systems or to allow for local adaptation (but see Acuna et al. 2008).
One well-documented use of a space-for-time chronosequence approach involves studies carried out in Glacier Bay, southeast Alaska (e.g. Milner et al. 2000, 2007, 2008), where new streams have been formed following glacier retreat. Water temperature was an important determinant of colonization for many macroinvertebrates in the youngest streams, where remnant ice influences were most evident. Coupled with these data, long-term monitoring (1977–present) of one stream (Wolf Point Creek) has further illustrated clear temperature-dependent thresholds at which these stream ecosystems change fundamentally. Taxon richness over the study period increased with water temperature, but while many species clearly benefited from warmer conditions others were lost. Milner et al. (2008) hypothesized that stream colonization could initially be deterministic (i.e. predominantly temperature influenced) up to approximately 10°C. However, several other streams in Glacier Bay with present day thermal regimes similar to Wolf Point Creek now have different macroinvertebrate assemblages, suggesting that beyond this threshold temperature community development becomes more stochastic, owing to combinations of life-history attributes, dispersal constraints and other environmental variables. Predicting the subsequent effects of further warming could therefore become unreliable with empirical chronosequence surveys if ecosystems' trajectories diverge (Johnson & Miyanishi 2008).
True long-term interannual gradients may be measured in a few instances, where time series of biomonitoring data (e.g. the UK Environmental Change Network; Lane 1997 or the US Long Term Ecological Research Network; Kratz et al. 2003; Layer et al. 2010) or palaeoecological data from sediment cores are available. Unfortunately, the former type of data is extremely rare, with few temporal series spanning more than a couple of decades (e.g. Jeppesen et al. 2003; Milner et al. 2008; Layer et al. 2010). This makes it difficult to pick up more subtle trends, or to remove non-directional climatic effects (Jeppesen et al. 2003), such as responses to the North Atlantic Oscillation (e.g. Bradley & Ormerod 2001; Durance & Ormerod 2007, 2009).
There is growing evidence that climatic change can induce phenological mismatches within food chains, whereby trophic interactions between consumers and resources become weakened or broken. Winder & Schindler (2004) examined the effects of warming in Lake Washington, northwest USA, dating back to the early 1960s and found clear disruption of trophic links between phytoplankton and zooplankton. Spring thermal stratification now occurs some 21 days earlier than in the 1960s and the associated phytoplankton bloom has shifted accordingly, but these changes have not been observed for Daphnia, which are no longer sufficiently synchronized with their algal resources to maximize their exploitation of peak food availability.
Palaeoecological data enable us to peer back further through time and certain groups (e.g. diatoms) may be sufficiently well-preserved in the fossil record to permit the reconstruction of assemblages that existed in past centuries or even millennia (Lotter & Birks 2003). Indeed, such approaches have revealed that long-term warming is a key driver of changes in Arctic lake communities (Douglas et al. 1994; Sorvari et al. 2002; Michelutti et al. 2003; Smol et al. 2005). A meta-analysis of 55 sediment cores, collected from lakes in the circumpolar Arctic, has provided compelling evidence of widespread shifts in algal assemblages since 1850 (Smol et al. 2005). Owing to the remoteness of many sites from human settlements, changes have been attributed to increasing temperatures, longer growing seasons and associated changes in lake physico-chemical properties. Similar patterns have been observed in Alpine lakes, but ascribing these to temperature alone is problematic owing to confounding influences of atmospheric pollution (e.g. Koinig et al. 2002). Evidence from Arctic cores also indicates compositional changes among herbivorous invertebrates, and Smol et al. (2005) showed that these shifts were not due to new colonization events because the taxa that have undergone recent population expansions have long been present, albeit at low abundance. Rather, it appears that these lakes have seen major regime shifts owing to warming (Brooks & Birks 2004; Smol et al. 2005), leading to more diverse and productive communities and more complex food webs.
There are limitations to palaeoecological approaches, not least the fact that taxa are preserved differentially and many important groups (e.g. oligochaete worms) are often missing from the fossil record, making it logistically challenging to reconstruct complete food webs (Rawcliffe et al. 2010). Increasingly, multiproxy techniques are being used to reduce the potential errors associated with focusing on one group of organisms, with more recent studies attempting to integrate diatom, macrophyte and invertebrate data to gain more of a community-level perspective (Battarbee 2000). As with space-for-time surveys there are potentially confounding variables, but in this case these are related to the temporal gradient: many fresh waters have altered significantly since the agricultural and industrial revolutions, owing to increasing anthropogenic influences (Durance & Ormerod 2009). As a result it is often extremely difficult to find extant contemporary systems to compare with those reconstructed from ancient fossil remains (Dunne et al. 2008). Finally, although this method may be viable in many lakes and wetlands, it is largely useless in most running waters where the sediment is continuously mixed or flushed downstream.
3. Non-random species change in fresh waters and the fragmentation of food webs in time and space
Climate change is clearly altering the composition, diversity and functioning of many freshwater ecosystems, but it is unlikely that species will be lost or gained in a random or a haphazard manner (e.g. figures 2 and 3). The trajectories of community change will be highly non-random, with certain taxa, especially those higher in the food web, typically being more vulnerable to local extinction (Ings et al. 2009; Woodward 2009). Essentially, all organisms face the same options as the climate changes: adapt, migrate or perish. However, an organism's success in implementing the first two strategies will depend largely on its life history and dispersal traits in relation to habitat fragmentation and the rate at which its environment changes. For example, it has been suggested that very small organisms (e.g. protists) form panmictic populations, such that almost any given species can be found almost anywhere on the planet, if local conditions are suitable (Finlay & Esteban 2007). Thus, identical protist taxa can be found in Antarctica, America and Europe, and a single intensively sampled small pond, Priest Pot, in England is home to a large proportion of the global species pool of these microscopic organisms (Finlay 2002; Finlay & Esteban 2007). By extension, this indicates that as the climate warms, many so-called ‘cryptic taxa’ that are usually hidden within what is effectively a global seed bank could manifest themselves by emerging from their resting phases as environmental conditions better approximate to their optima (e.g. Esteban & Finlay 2003; Smol et al. 2005), and start interacting with other members of the food web.
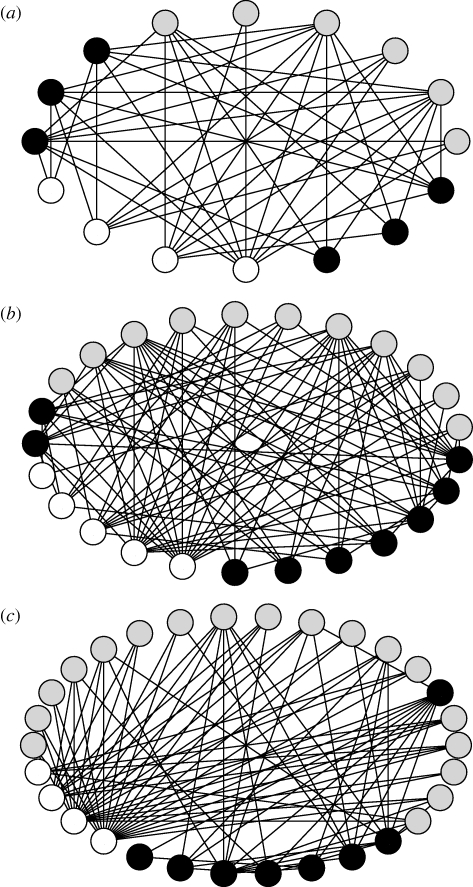
Figure 2.
Stream benthic food webs along a thermal gradient in the Estaragne Basin, French Pyrénées. Open circles denote basal resources; grey denotes primary consumers; black denotes predators. (a) 2370 m altitude, maximum water temperature (Tmax) = 4.5°C, no. species (S) = 16, secondary production (2P) = 4.9 g m−2 y−1. (b) 2150 m altitude, Tmax = 8.5°C, S = 25, 2P=6.55 g m−2 y−1. (c) 1850 m altitude, Tmax = 13°C, S = 30, 2P = 7.6 g m−2 y−1. Figures redrawn from Lavandier & Décamps (1983).
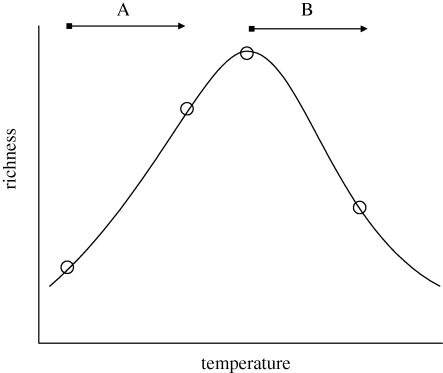
Figure 3.
Hypothetical unimodal relationship between local species richness and temperature. In relatively equitable environments, where most species are close to their thermal optima, we might expect richness to decline with warming (trajectory B). However, this scenario is unlikely to be ubiquitous: in systems close to the physiological limits of existence for most organisms (e.g. in polar regions: trajectory A), increases in temperature may lead to rises in local richness following invasion by less cold-tolerant species. This scenario, however, implies a loss of global diversity as specialist cold stenotherms are replaced by the more diverse eurytherms.
This does not apply, however, to taxa higher in the food web, whose regional species pools are more restricted in their distribution by the dispersal ability of the constituent members. Insects, which dominate many freshwater food webs, have the advantage of an aerial adult phase, which enables them to mitigate dispersal constraints, at least to some extent (e.g. Masters et al. 2007). A simple analysis relating the latitude of various streams in North America and their annual degree days showed that a 4°C increase in stream temperatures would displace the regression line by approximately 680 km northwards, indicating the distance required for the biota to maintain populations in fresh waters at their current thermal regime (Sweeney et al. 1992). Surprisingly little is known about the ability of aquatic organisms to colonize new areas, but it appears that only a few gravid females may be required to populate a water body (Hildrew et al. 2004; Petersen et al. 2004; Wilcock et al. 2007) and even large, physical barriers such as mountain ranges and narrow coastal straits between islands may be insufficient to prevent this from occurring (Milner et al. 2000, 2008). For smaller invertebrates (e.g. meiofaunal copepods), habitat availability might be a stronger determinant of establishment success because dispersal ability may be high owing to movement of these organisms by birds and mammals acting as vectors (Robertson & Milner 2006).
Other taxa are even more limited in their ability to move between systems: fish for example remain in their aqueous medium for their entire lives, and because relatively few species in either group have the physiological capacity to move between fresh and saline water many populations are effectively ‘landlocked’. These taxa must therefore either adapt to changing environmental conditions or perish, and many cold stenotherms such as Arctic Charr (Salvelinus alpines) appear to be in a particularly precarious position as global temperatures rise (Rouse et al. 1997). Fish migration within river systems is also often severely restricted by habitat fragmentation resulting from man-made obstacles (e.g. Zwick 1992), and this could be exacerbated further if localized droughts are induced by climate change. These differential abilities of species to persist within changing ecosystems suggest that many local food webs are likely to undergo radical restructuring in the face of climate change (Babaluk et al. 2000). This could lead to spatial disconnections between consumers and resources, in addition to any phenological separation arising owing to altered life histories (McDonald et al. 1996).
Latitudinal differences in the effects of climate change are also important in this context (IPCC 2007), as restructuring of aquatic assemblages is generally predicted to be observed first at northern high latitudes where the magnitude of warming and disruption to the hydrological cycle are predicted to be greatest. Potentially, some species populations, especially those of cold-adapted stenotherms, are likely to be extirpated from some areas as thermal tolerances are exceeded (Wrona et al. 2006). Hence, many high-latitude species are currently close to their physiological limits and are likely to experience dramatic range contractions, as space for tracking suitable thermal conditions diminishes. However, an interesting paradox arises here: although systems at high latitudes face greater rates of change, they typically experience greater natural annual climate variability. Thus, ectotherms at high latitudes (at least from terrestrial systems; Deutsch et al. 2008) appear to show broader thermal ranges than organisms in the tropics and therefore might be better able to withstand extreme thermal events over shorter time scales.
The effects of warming are expected to be particularly marked in cold high altitude/latitude systems (Barnett et al. 2005; Wrona et al. 2006; Brown et al. 2009; Milner et al. 2009) owing to the strong linkages between climate, cryospheric processes (e.g. snow and ice-melt) and community structure (Hannah et al. 2007). In the current phase of climate warming, many glaciers worldwide are shrinking, and loss of snow and ice is expected to alter the dynamics of river basin runoff leading to warmer water temperatures and greater community abundance and diversity (Brown et al. 2007). Studies along spatial gradients of decreasing snowmelt influence (thus increasing water temperature) in alpine rivers illustrate the potential for future climate change to lead to larger food webs with broader body size spectra, increased primary and secondary production, and wider diets among predatory invertebrates (Lavandier & Décamps 1983; figure 2). The retreat of glaciers will also create new habitats for some freshwater species (Milner et al. 2008), but many others will be unable to adapt. For example, Brown et al. (2007) outline how some endemic cold stenotherms in Pyrenean glacier-fed rivers are likely to become extinct as the small remnant ice masses on which they depend disappear. Hari et al. (2006) have also documented declines in alpine brown trout populations as suitable habitats shrink. The food web and ecosystem-scale ramifications of these species losses have yet to be examined in detail.
4. Linking across spatio-temporal scales and levels of organization: from individual metabolism to whole-ecosystem responses
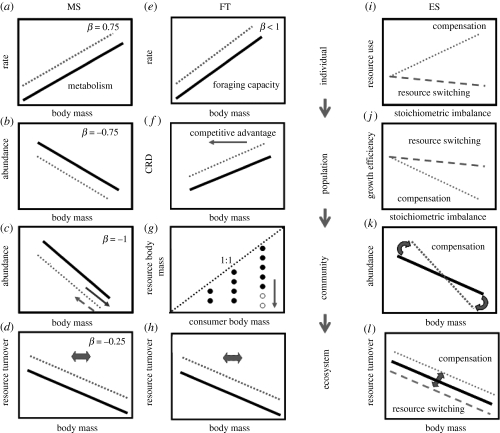
Figure 1 highlights the impacts of climate change upon different levels of organization, the methods commonly used to investigate biological responses in fresh waters and connections between levels. Figure 4 illustrates some of the potential impacts of warming in line with predictions and arguments based on three main theoretical approaches: metabolic allometric scaling (MS), ecological stoichiometry (ES) and foraging theory (FT). This highlights commonalities and a few intriguing discrepancies between the three approaches, which we address below.
Figure 4.
Potential effects of elevated temperature and altered nutrient quality of resources on metabolic (a,b,c,d), foraging (e,f,g,h) and stoichiometric constraints (i,j,k,l) over multiple levels of biological organization (individual, population, community and ecosystem). The relationships with temperature relate primarily to the ectotherms that dominate freshwater communities. All axes are log–log scales. (a) Per capita BMR increases with body mass (indicated by the solid line) with the scaling exponent, β = ¾ (Peters 1983). Metabolic demands will rise with warming (Brown et al. 2004; Yvon-Durocher et al. 2010; dashed line). (b) Mass-dependence of population abundance (solid line), where a common exponent of β =−¾ is predicted (Brown et al. 2004). As individual metabolic rates rise, warming is likely to result in a decrease in total biomass, given steady-state conditions (Brown et al. 2004). (c) Body-mass–abundance relationships across trophic levels (solid line), where a scaling exponent β =−1 is predicted (Brown et al. 2004; Woodward et al. 2005). In addition to changes in total biomass (dashed line), warming should induce transient changes in body size spectra as large, rare species high in the food web are lost first (e.g. Petchey et al. 1999; dashed arrow). In contrast, in very cold systems under physiological stress, warming could stimulate productivity, allowing food chains to lengthen and the size spectrum to broaden (Lavandier & Décamps 1983; Friberg et al. 2009; Milner et al. 2009; solid arrow). (d) Relationship between rates of resource turnover and average consumer body mass (solid line). Resources flux faster through assemblages of small individuals: the exponent of this relationship follows that of mass-specific metabolic rates, β = −¼ (Brown et al. 2004). Thus, warming will elevate process rates and changes in community size structure will also have consequences at the ecosystem level (bi-directional arrow). (e) Per capita foraging capacity (solid line) increases with body mass with an exponent β < 1 (Persson et al. 2000; Bystrom & Andersson 2005). Warming will elevate individual foraging rates (dashed line) (Woodward & Hildrew 2002). (f) Critical resource density (CRD), whereby energy intake balances metabolic demands, increases with increasing body size (solid line). Within a population, this could give smaller individuals a competitive advantage (Persson 1985) as warming should increase the CRD required by consumers (dashed line) and may further favour small individuals in the population (solid arrow). (g) Predation matrix describing a food web with resources (rows) and consumers (columns) (e.g. Beckerman et al. 2006), ordered in body size bins. Circles denote realized feeding links, and those below the 1 : 1 line indicate consumers feeding on resources smaller than themselves (Petchey et al. 2008). Here, the largest consumer displays adaptive feeding on successively smaller resources (open circles) as a result of size-dependent foraging and higher metabolic offset with warming (solid arrow). (h) Mass-specific resource use decreases with body mass (solid line) and is likely to be displaced by warming (dashed line) in a comparable manner to the relationship predicted by MS (d). Similarly, associated changes in community size structure have consequences at the ecosystem level (bi-directional arrow). (i) Per capita rates of exploitation of a given resource may (a) increase with increasing consumer–resource stoichiometric imbalances (e.g. C : N or C : P ratios) if individuals feed in a compensatory manner (dotted line), or (b) are temporally impaired if consumers switch to alternative resources (dashed line) (e.g. Sterner & Elser 2002). (j) Increased inefficiencies resulting from consumer–resource stoichiometric imbalances should slow population biomass growth rates (e.g. Tuchman et al. 2002; dotted line). These effects might be mitigated to some extent if stoichiometric demands can be met by switching to alternative resources (e.g. herbivory versus detritivory, after Ledger & Hildrew 2001; dashed line). (k) Body mass–abundance relationships (solid line) comparable to those predicted by MS (c). The scaling exponent could increase if assimilation efficiency across trophic levels is reduced via lower nutritional quality of resources and suppressed biomass production (e.g. Tuchman et al. 2002; dashed line). (l) Ecosystem rates of resource turnover increase (at least transiently) with increasing stoichiometric imbalances due to the compensatory feeding performances of individuals (dotted line), or decrease due to resource switching and/or reduced biomass production (dashed line).
One way in which we might derive a new more integrated theoretical framework is to consider what constrains individual organisms, since the higher levels are all ultimately composed of numerous interacting individuals. A promising place to start, and one that is particularly pertinent to climate change, is to consider the relationship between individual metabolism and body size (Brown et al. 2004; Ings et al. 2009). This allometric scaling association is very tight, with a central tendency of slopes of ¾ over many orders of magnitude of body size, from protists to large vertebrates (Peters 1983; Brown et al. 2004). Since energy is a key currency in ecology, this strong temperature dependence of individual metabolism has important implications that ramify to the higher, more complex levels of organization (e.g. whole-ecosystem metabolism; Woodward et al. 2005). It has been suggested that the reason for the prevalence of approximate quarter-power law scaling of so many ecological processes with body size is directly related to the metabolic constraints imposed by fractal resource supply networks with similar sized termini (e.g. capillaries in animals) irrespective of an organism's size (Brown et al. 2004). While the existence of this underlying mechanism is still hotly debated (e.g. Clarke 2006), it provides a useful heuristic framework, and the ubiquity of allometric scaling relations and the influence of temperature on metabolism cannot be denied. As such, MS offers a potentially powerful means of linking individuals to populations, communities and ultimately to food webs and ecosystems (e.g. Petchey et al. 2008; Berlow et al. 2009).
Essentially, because individual basal metabolic rate (BMR) is set by body size and temperature, respiratory costs will rise as BMR increases, and this will be most pronounced among larger organisms at higher temperatures (Brown et al. 2004; figure 4a). Body size per se also determines both food web structure (e.g. hierarchies of feeding niches; diet width) and dynamics (e.g. interaction strength), and temperature determines many key ecosystem process rates directly. Metabolic scaling theories have sought to couple both well-established body size allometries (e.g. Peters 1983) and temperature scaling based upon fundamental physical principles (e.g. Brown et al. 2004), enabling predictions to be made over multiple levels of organization. For instance, individual body-mass versus metabolism relations mean that mass-abundance scaling is predicted to follow a −¾ power law within trophic levels (figure 4b). Steeper scaling is predicted in multitrophic communities, however, because energy is lost between trophic levels (figure 4c). Rates of resource turnover in ecosystems are predicted to scale with body mass to the −¼ power (figure 4d), the same as for mass specific metabolic rate. Thus, an assemblage comprising of smaller individuals should process metabolic substrates faster than an assemblage of equivalent total biomass comprising solely large individuals (figure 4e).
Non-random species losses will be evident in many local communities, with large, rare species high in the food web being among the first to be lost as temperatures rise (e.g. Petchey et al. 1999). As smaller, faster growing organisms become increasingly dominant, this could alter food web dynamics profoundly (e.g. Strecker et al. 2004). However, it is important to consider that food web response will be observed across a spectrum of thermal regimes; freshwater systems that are currently at the colder end of the spectrum and under physiological stress (e.g. those at high altitude/latitude fed by snow and ice) are likely to be invaded by more and larger organisms as temperatures rise (Milner et al. 2009). As a more productive resource base forms and the richness of these communities increases, these changes should theoretically result in increased food chain lengths, broader size spectra and fewer generalist feeders (e.g. Friberg et al. 2009). Within the food web, life-history traits of populations will change owing to metabolic constraints (Winder & Schindler 2004), leading to potential phenological disconnections between consumers and resources in food chains (i.e. the match–mismatch hypothesis; Durant et al. 2007). These changes will be particularly pronounced in highly seasonal environments where growth and reproduction are concentrated in distinct periods of the annual cycle (e.g. cold environments during ice-free periods; Smol et al. 2005).
Foraging theory (FT) has been proposed as a means for predicting resource-partitioning among species, or among size classes within species. Recent advances using this approach have been made by acknowledging that maps of foraging decisions being made by individual consumers lie embedded within a food web (Beckerman et al. 2006; Woodward & Warren 2007; Petchey et al. 2008). Like MS, individual body size is central to FT, but in addition to energy requirements, the ability to find, capture and process food as well as the ability to avoid predators is used to predict interactions between consumers and resources (Persson et al. 1998; Beckerman et al. 2006; Petchey et al. 2008). The ability to gain energy can be separated into two size-dependent components: the search efficiency (encounter rate, attack rate) and the capacity to process food (handling; Lundberg & Persson 1993; Persson et al. 1998): together these make up the foraging capacity of an individual (Bystrom & Andersson 2005; Bystrom et al. 2006). The results of recent studies have revealed how complex food webs can arise from simple rules related to FT (Beckerman et al. 2006; Petchey et al. 2008), but the effects of temperature are yet to be fully incorporated in these new models, despite its strong influence on foraging rates (e.g. Persson 1986; Woodward & Hildrew 2002). Prey selection patterns, however, cannot necessarily be extrapolated to other temperatures since different components of the predation sequence may scale differently with temperature (Persson 1986), and this represents an important gap in our current knowledge. This is especially pertinent in the context of climate warming because fresh waters are composed predominately of ectotherms, whose foraging and metabolic rates will be determined largely by ambient temperatures.
The strong size dependencies in foraging capacity and metabolic requirements could potentially constrain an organism's competitive ability (Persson 1985). For instance, the body mass scaling of foraging capacity increases with an exponent of less than unity (Persson et al. 2000; Bystrom & Andersson 2005; figure 4e), so the critical resource density (CRD) at which energy intake balances metabolic demands increases with body size (Persson et al. 2000; Bystrom & Andersson 2005). When differently sized individuals of the same species compete for resources, the smaller individuals may therefore have an advantage (Persson 1985; figure 4f) if they can grow and sustain themselves at a lower resource level than larger conspecifics (Lundberg & Persson 1993; Persson et al. 1998). Mesocosm and in situ experiments have shown that the metabolic demands of Arctic Char increase with temperature at a faster rate than their foraging capacity (Bystrom et al. 2006). If warming increases CRD disproportionately for larger individuals, adaptive foraging by exploiting a larger size range of prey might be able to mitigate these energetic requirements (figure 4g). Temperature-dependent shifts in competitive interactions have also been shown in experimental studies on zooplankton (Lynch 1978) and fish (Persson 1986), reflecting size-related differences in temperature optima for growth.
A third body of theory, ecological stoichiometry (ES) has also sought to explain trophic interactions, but unlike MS and FT this is based upon principles of mass balance for multiple chemical elements (Sterner & Elser 2002). Here, in contrast to energy being the focal biological currency, the role of molar ratios of essential chemical elements (primarily C, N and P) is seen as key to mediating consumer–resource interactions. This framework could therefore provide novel insights into aspects of climate change related to increases in atmospheric CO2 concentrations, which are predicted to double by the end of this century (IPCC 2007). Elevated CO2 levels have been associated with an increase in stoichiometric imbalances in carbon : nutrient ratios of primary producers (Norby et al. 2001). Terrestrial leaf litter which is a critical basal resource in many fresh waters, is therefore likely to become considerably more recalcitrant in the future, as its relative carbon content rises (e.g Tuchman et al. 2002). If C : N and C : P ratios rise among detrital resources, consumers will need to feed faster to extract the same amount of nutriment (Sterner & Elser 2002) or switch to an alternative resource, such as algae (figure 4i).
Algal grazing by facultative herbivore–detritivores could increase interspecific competition with specialist herbivores and this might ultimately lead to competitive exclusion of certain taxa: analogous scenarios can be found in response to pH, whereby generalist stoneflies are squeezed out of local assemblages by specialist herbivores (mayflies and snails) that are more efficient exploiters of algal resources at higher pH (Woodward 2009). If diet switching is not feasible and feeding rates cannot be elevated sufficiently by increased activity, then metabolic costs of nutrient acquisition will rise, potentially leading to a slowing of biomass growth rates of individuals (figure 4j) and, by extension, of secondary production within the food web as a whole (Tuchman et al. 2002). In turn, stochiometric mismatches between consumers and resources could lead to disconnections and restructuring of trophic interactions, not only altering the architecture of aquatic food webs but also the rate of flux of nutrients to the higher trophic levels (figure 4k). This could also lead to increases in the relative importance of autochthonous algal-based pathways in the food web owing to greater carbon availability, potentially weakening the stabilizing effect of ‘slow’ detrital-based pathways (Rooney et al. 2006).
5. Unifying concepts within a Biodiversity-Ecosystem Functioning context
A plethora of B-EF studies over the past two decades have revealed clear positive relationships between biodiversity and key ecosystem processes (see Giller et al. 2004; Woodward 2009 and references therein). However, the unprecedented rates of climate change (IPCC 2007) and global biodiversity loss threaten to disrupt such relationships and hence the supply of valuable ecosystem ‘goods and services’ (Woodward 2009). While such research has provided invaluable insights into the form of B-EF relationships, and bridges pure and applied science by linking ecological theory to conservation objectives (Schwartz et al. 2000), few studies have examined how these relationships might shift in response to climate change.
Since B-EF experiments aim to assess how biodiversity (genetic, taxonomic and functional diversity) mediates ecosystem processes, it is essential to be able to quantify the relevant traits of the organisms that are driving the process in question (Reiss et al. 2009). Body size is an obvious and easily measured functional ‘trait’ that determines an individual's (and ultimately a population's) contribution to many critical process rates (e.g. nutrient cycling, biomass production) via the allometric relations and foraging constraints described above. For instance, an individual's use of resources is largely dictated by its metabolic needs (figure 4a) and this can be scaled up to the wider consumer assemblage (figure 4d). Despite this, most freshwater B-EF research has either controlled for biomass a priori or effectively ignored the effects of body mass altogether.
A few examples from experiments employing proxy assays of ‘ecosystem metabolism’ (such as leaf litter decomposition) have sought to correct for the metabolic capacity of consumer assemblages via allometric scaling (e.g. McKie et al. 2008), thereby partitioning this from any potential species richness or identity effects per se. However, in such cases an average body size per species is applied, thus losing much of the individual-based information required to scale up to the assemblage level: whether total assemblage biomass is made up of small or large individuals is important here because differences in mass-specific metabolic rates (figure 4a,d) need to be scaled with temperature to assess the metabolic capacity of the system as a whole. Such an approach would provide an explicit link between individual metabolism and high-level effects of biodiversity and species identity, which could then be separated via variance partitioning techniques (Reiss et al. 2009). Quantifying individual body size allows a range of null models to be created, against which the importance of different components of biodiversity (i.e. species traits, identity, richness and interactions) on ecosystem processes can be quantified (e.g. figure 4d).
Metabolic scaling provides a theoretical framework that links individuals to ecosystem level processes; but its integration into B-EF research has yet to really emerge (but see McKie et al. 2008), in contrast to the more extensive embedding of such approaches within food web research (e.g. Emmerson et al. 2005; Berlow et al. 2009). Freshwater systems are well-suited to such an approach because individual body size can be quantified relatively easily for many taxa (Woodward 2009). Other recent theoretical approaches have attempted to advance the field even further, by integrating MS and ES to better predict the cycling of nutrients across organizational levels (Allen & Gillooly 2009). Essentially, these new ideas combine the two fundamental biological currencies of energy and matter. Again, while ES has become increasingly integral to a range of food web and ecosystem functioning studies (Elser & Hessen 2005; Hladyz et al. 2009), it remains largely absent from freshwater B-EF research per se (Woodward 2009). A novel application in this context could include an additional covariate relating to the elemental ratios (i.e. C : N and C : P) of consumers and resources being incorporated explicitly into the design and analysis of B-EF experiments. Quantified measures of consumer—resource C : nutrient imbalances (or mismatches) could be used to gain insight into the nutrient requirements of consumer assemblages on different resource types (e.g. Hladyz et al. 2009). Potentially, such measures could then be combined with the metabolic capacity of assemblages (derived from body size and temperature scaling) to account for energetic and nutrient demands from which the strength of any residual variation explained by biodiversity effects can be assessed. Such approaches could provide a powerful integrative framework in which to predict the potential synergistic effects of climate-induced shifts in basal resource quality, from both a metabolic and stoichiometric perspective. Foraging theory is also relevant within this context: for instance, to predict shifts in adaptive feeding via switching behaviour of consumers in response to changing resource quality and quantity.
Finally, if we are to extrapolate B-EF relations across the large biogeographical scales over which climate change operates, we will also need to consider the role of intraspecific variation as a driver of ecosystem functioning (Schmid et al. 2002). For instance, species-level responses to temperature changes may be modulated at the individual level, via adaptation within populations to local environmental conditions (McKie et al. 2004), but this level of intraspecific genetic biodiversity has yet to be considered in B-EF climate change experiments. This is particularly pertinent in cold regions, which are often characterized by relatively low intraspecific genetic diversity, at least among the macrofauna (e.g. Costello et al. 2003) and, by implication, reduced ability to adapt to the rapid warming that is predicted in the coming decades.
6. Additional drivers and synergies
To date, vanishingly few studies have considered the potential influence of synergies between components of climate change (e.g. warming, and atmospheric and hydrological changes) and with other biotic and abiotic stressors (e.g. eutrophication, acidification, overexploitation of fisheries) in aquatic ecosystems (but see Moss et al. 2003). For instance, beyond compositional shifts within local assemblages, climate change will also offer opportunities for new species to invade (Rahel & Olden 2008; Milner et al. 2009) and this could cause disruption to ecosystems and ‘goods and services’ they supply. In temperate regions, as the vectors of previously tropical diseases move polewards, they will carry with them the vast financial costs of medical care for the recipient human populations (McMichael et al. 2006; Rahel & Olden 2008).
Non-additive responses to multiple stressors could either amplify or mitigate the effects of climate change (Giller et al. 2004; Feuchtmayr et al. 2009). For example, warmer temperatures could increase the summer, yet decrease the winter, likelihood of anoxia (Rahel & Olden 2008), whereas nutrient inputs derived from run-off will be higher under wetter conditions (Hessen et al. 1997), thus making interactions between climate change and eutrophication difficult to gauge without direct experimentation. The use of whole-system mesocosm experiments, such as those in Denmark (e.g. Liboriussen et al. 2005) and England (e.g. Moss et al. 2003; Yvon-Durocher et al. 2010), seem particularly well-suited to addressing these questions at an appropriate scale and organizational level, and some have already revealed important interactions between the effects of nutrient enrichment and warming on ecosystem functioning and community structure (e.g. Feuchtmayr et al. 2009).
7. Summary
If we are to predict future impacts of climate change in fresh waters, we need to achieve a better mechanistic understanding via the application of models, experiments and survey data across multiple scales and levels of organization. Developing a more unified theoretical framework is a challenging task and the integration of metabolic, stoichiometric and foraging theories that can be scaled from individuals to more complex biological entities, including entire ecosystems, offers a potentially promising way forward (Ings et al. 2009; Reiss et al. 2009).
Acknowledgements
We would like to thank the Natural Environment Research Council (grant reference: NE/D013305/1) and NERC Centre for Population Biology for financial support awarded to G.W. and the British Ecological Society for supporting the thematic topic at their Annual Scientific Meeting in 2008, which initiated the production of this manuscript. L.B.'s contribution was partly supported by NERC grant NE/E003729/1.
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Acuna V., Wolf A., Uehlinger U., Tockner K.2008Temperature dependence of stream benthic respiration in an Alpine river network under global warming. Freshw. Biol. 53, 2076–2088 (doi:10.1111/j.1365-2427.2008.02028.x) [Google Scholar]
- Allen A. P., Gillooly J. F.2009Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 12, 369–384 (doi:10.1111/j.1461-0248.2009.01302.x) [DOI] [PubMed] [Google Scholar]
- Babaluk J. A., Reist J. A., Johnson J. D., Johnson L.2000First records of sockeye (Oncorhynchus nerka) and pink salmon (O. gorbuscha) from Banks Island and other records of Pacific salmon in Northwest Territories, Canada. Arctic 53, 161–162 [Google Scholar]
- Barlocher F., Seena S., Wilson K. P., Williams D. D.2008Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshw. Biol 53, 368–379 [Google Scholar]
- Barnett T. P., Adam J. C., Lettenmaier D. P.2005Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438, 303–309 (doi:10.1038/nature04141) [DOI] [PubMed] [Google Scholar]
- Battarbee R. W.2000Palaeolimnological approaches to climate change, with special regard to the biological record. Quat. Sci. Rev. 19, 107–124 (doi:10.1016/S0277-3791(99)00057-8) [Google Scholar]
- Beckerman A. P., Petchey O. L., Warren P. H.2006Foraging biology predicts food web complexity. Proc. Natl Acad. Sci. USA 103, 13 745–13 749 (doi:10.1073/pnas.0603039103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlow E. A., Dunne J. A., Martinez N. D., Starke P. B., Williams R. J., Brose U.2009Simple prediction of interaction strengths in complex food webs. Proc. Natl Acad. Sci. USA 106, 187–191 (doi:10.1073/pnas.0806823106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. C., Ormerod S. J.2001Community persistence among stream invertebrates tracks the North Atlantic Oscillation. J. Anim. Ecol. 70, 987–996 (doi:10.1046/j.0021-8790.2001.00551.x) [Google Scholar]
- Brock D. T., Brock M. L.1966Temperature optima for algal development in Yellowstone and Iceland hot springs. Nature 209, 733–734 (doi:10.1038/209733a0) [Google Scholar]
- Brooks S. J., Birks H. J. B.2004The dynamics of Chironomidae (Insecta: Diptera) assemblages in response to environmental change during the past 700 years on Svalbard. J. Paleolimnol. 31, 483–498 (doi:10.1023/B:JOPL.0000022547.98465.d3) [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B.2004Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- Brown L. E., Hannah D. M., Milner A. M.2007Vulnerability of alpine stream biodiversity to shrinking glaciers and snowpacks. Global Change Biol. 13, 958–966 (doi:10.1111/j.1365-2486.2007.01341.x) [Google Scholar]
- Brown L. E., Céréghino R., Compin A.2009Endemic aquatic invertebrates from southern France: diversity, distribution and conservation implications. Biol. Conserv. 142, 2613–2619 (doi:10.1016/j.biocon.2009.06.009) [Google Scholar]
- Buisson L., Thuiller W., Lek S., Limp P., Grenouillet G.2008Climate change hastens the turnover of stream fish assemblages. Global Change Biol. 14, 2232–2248 (doi:10.1111/j.1365-2486.2008.01657.x) [Google Scholar]
- Burgmer T., Hillebrand H., Pfenninger M.2007Effects of climate-driven temperature changes on the diversity of freshwater macroinvertebrates. Oecologia 151, 93–100 (doi:10.1007/s00442-006-0542-9) [DOI] [PubMed] [Google Scholar]
- Bystrom P., Andersson J.2005Size-dependent foraging capacities and intercohort competition in an ontogenetic omnivore (Arctic char). Oikos 110, 523–536 (doi:10.1111/j.0030-1299.2005.13543.x) [Google Scholar]
- Bystrom P., Andersson J., Kiessling A., Eriksson L. O.2006Size and temperature dependent foraging capacities and metabolism: consequences for winter starvation mortality in fish. Oikos 115, 43–52 (doi:10.1111/j.2006.0030-1299.15014.x) [Google Scholar]
- Castella E., et al. 2001Macrobenthic invertebrate richness and composition along a latitudinal gradient of European glacier-fed streams. Freshw. Biol. 46, 1811–1831 (doi:10.1046/j.1365-2427.2001.00860.x) [Google Scholar]
- Clarke A.2006Temperature and the metabolic theory of ecology. Funct. Ecol. 20, 405–412 (doi:10.1111/j.1365-2435.2006.01109.x) [Google Scholar]
- Costello A. B., Down T. E., Pollard S. M., Pacas C. J., Taylor E. B.2003The influence of history and contemporary stream hydrology on the evolution of genetic diversity. Evolution 57, 328–344 [DOI] [PubMed] [Google Scholar]
- Dang C. K., Schindler M., Chauvet E., Gessner M. O.2009Temperature oscillation coupled with fungal community shifts can modulate warming effects on litter decomposition. Ecology 90, 122–131 (doi:10.1890/07-1974.1) [DOI] [PubMed] [Google Scholar]
- Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., Martin P. R.2008Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. S. V., Smol J. P., Blake W.1994Marked post-18th century environmental change in high-Arctic ecosystems. Science 266, 416–419 (doi:10.1126/science.266.5184.416) [DOI] [PubMed] [Google Scholar]
- Dudgeon D., et al. 2006Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182 (doi:10.1017/S1464793105006950) [DOI] [PubMed] [Google Scholar]
- Dunne J. A., Williams R. J., Martinez N. D., Wood R. A., Erwin D. E.2008Compilation and network analyses of Cambrian food webs. PLoS Biol. 6, 693–708 (doi:10.1371/journal.pbio.0060102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durance I., Ormerod S. J.2007Effects of climatic variation on upland stream invertebrates over a 25 year period. Global Change Biol. 13, 942–957 (doi:10.1111/j.1365-2486.2007.01340.x) [Google Scholar]
- Durance I., Ormerod S.2009Trends in water quality and discharge confound long-term warming effects on river macroinvertebrates. Freshw. Biol. 54, 388–405 (doi:10.1111/j.1365-2427.2008.02112.x) [Google Scholar]
- Durant J. M., Hjermann D. O., Ottersen G., Stenseth N. C.2007Climate and the match or mismatch between predator requirements and resource availability. Climate Res. 33, 271–283 (doi:10.3354/cr033271) [Google Scholar]
- Elser J. J., Hessen D. O.2005Biosimplicity via stochiometry; the evolution of food web structure and processes. In Aquatic food webs: an ecosystem approach (eds Belgrano A., Scharler U. M., Dunne J., Ulanowicz R. E.), pp. 7–18 Oxford, UK: Oxford University Press [Google Scholar]
- Emmerson M. E., Montoya J. M., Woodward G.2005Allometric scaling and body-size constraints in complex food webs. In Dynamic food webs: multispecies assemblages, ecosystem development, and environmental change (eds de Ruiter P. C., Wolters V., Moore J. C.). San Diego, CA: Academic Press [Google Scholar]
- Esteban G. F., Finlay B. J.2003Cryptic freshwater ciliates in a hypersaline lagoon. Protist 154, 411–418 (doi:10.1078/143446103322454149) [DOI] [PubMed] [Google Scholar]
- Feuchtmayr H., Moran R., Hatton K., Connor L., Heyes T., Moss B., Harvey I., Atkinson D.2009Global warming and eutrophication: effects on water chemistry and autotrophic communities in experimental hypertrophic shallow lake mesocosms. J. Appl. Ecol. 46, 713–723 (doi:10.1111/j.1365-2664.2009.01644.x) [Google Scholar]
- Finlay B. J.2002Global dispersal of free-living microbial eukaryote species. Science 296, 1061–1063 (doi:10.1126/science.1070710) [DOI] [PubMed] [Google Scholar]
- Finlay B. J., Esteban G. F.2007Body size and biogeography. In Body size: the structure and function of aquatic ecosystems (eds Hildrew A., Raffaelli D., Edmonds-Brown R.), pp. 167–185 Cambridge, UK: Cambridge University Press [Google Scholar]
- Friberg N., Christensen J. B., Olafsson J. S., Gislason G. M., Larsen S. E., Lauridsen T. L.2009Relationship between structure and function in streams contrasting in temperature: possible impacts of climate change on running water ecosystems. Freshw. Biol. 54, 2051–2206 (doi:10.1111/j.1365-2427.2009.02234.x) [Google Scholar]
- Giller P. S., et al. 2004Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos 104, 423–436 [Google Scholar]
- Hannah D. M., Brown L. E., Milner A. M., Gurnell A. M., McGregor G. R., Petts G. E., Smith B. P. G., Snook D. L.2007Integrating climate-hydrology-ecology for alpine river systems. Aquat. Conserv. Mar. Freshw. Ecosyst. 17, 636–656 (doi:10.1002/aqc.800) [Google Scholar]
- Hari R. E., Livingstone D. M., Siber R., Burkhardt-Holm P., Guttinger H.2006Consequences of climate change for water temperature and brown trout populations in Alpine rivers and streams. Global Change Biol. 12, 10–26 (doi:10.1111/j.1365-2486.2005.001051.x) [Google Scholar]
- Harris R. M. L., Milner A. M. M., Armitage P. D., Ledger M. E.2007Replicability of physicochemistry and macroinvertebrate assemblages in stream mesocosms: implications for experimental research. Freshw. Biol. 52, 2434–2443 (doi:10.1111/j.1365-2427.2007.01839.x) [Google Scholar]
- Hassan R., Scholes R., Ash N.2005Millenium Ecosystem Assessment. Ecosystems and human well-being: current state and trends, vol. 1 Washington, DC: Island Press [Google Scholar]
- Hessen D. O., Hindar A., Holtan G.1997The significance of nitrogen runoff for eutrophication of freshwater and marine recipients. Ambio 26, 312–320 [Google Scholar]
- Hildrew A. G., Woodward G., Winterbottom J. H., Orton S.2004Strong density dependence in a predatory insect: large scale experiments in a stream. J. Anim. Ecol. 73, 448–458 (doi:10.1111/j.0021-8790.2004.00819.x) [Google Scholar]
- Hladyz S., Gessner M. O., Giller P. S., Pozo J., Woodward G.2009Resource quality and stoichiometric constraints in a stream food web. Freshw. Biol. 54, 957–970 (doi:10.1111/j.1365-2427.2008.02138.x) [Google Scholar]
- Hogg I. D., Williams D. D., Eadie J. M., Butt S. A.1995The consequences of global warming for stream invertebrates: a field simulation. J. Thermal Biol. 20, 199–206 (doi:10.1016/0306-4565(94)00057-P) [Google Scholar]
- Ings T. C., et al. 2009Ecological networks: beyond food webs. J. Anim. Ecol. 78, 253–269 (doi:10.1111/j.1365-2656.2008.01460.x) [DOI] [PubMed] [Google Scholar]
- IPCC 2007The physical sciences basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Parry M., Canziani O., Palutkof J., Van der Linden P., Hanson C.). Cambridge, UK: Cambridge University Press [Google Scholar]
- Jeppesen E., Søndergaard M., Jensen J. P.2003Climatic warming and regime shifts in lake food webs: some comments. Limnol. Oceanogr. 48, 1346–1349 [Google Scholar]
- Johnson E. A., Miyanishi K.2008Testing the assumptions of chronosequences in succession. Ecol. Lett. 11, 419–431 (doi:10.1111/j.1461-0248.2008.01173.x) [DOI] [PubMed] [Google Scholar]
- Kishi D., Murakami M., Nakano S., Maekawa K.2005Water temperature determines strength of top-down control in a stream food web. Freshw. Biol. 50, 1315–1322 (doi:10.1111/j.1365-2427.2005.01404.x) [Google Scholar]
- Kratz T. K., Deegan L. A., Harmon M. E., Lauenroth W. K.2003Ecological variability in space and time: insights gained from the US LTER program. BioScience 53, 57–67 (doi:10.1641/0006-3568(2003)053[0057:EVISAT]2.0.CO;2) [Google Scholar]
- Koinig K. A., et al. 2002Environmental changes in an alpine lake (Gossenköllesee, Austria) over the last two centuries—the influence of air temperature on biological parameters. J. Paleolimnol. 28, 147–160 [Google Scholar]
- Krajick K.2004All downhill from here? Science 303, 1601–1602 (doi:10.1126/science.303.5664.1600) [DOI] [PubMed] [Google Scholar]
- Lane A. M. J.1997The UK environmental change network database: an integrated information resource for long-term monitoring and research. J. Environ. Manage. 51, 87–105 (doi:10.1016/S0301-4797(97)80003-5) [Google Scholar]
- Langford T. E.1990Ecological effects of thermal discharges Berlin, Germany: Springer [Google Scholar]
- Lavandier P., Décamps H.1983Un torrent d'altitude dans les Pyrénées: l'Estaragne. In Ecosystèmes limniques (eds Lamotte M., Bourlière F.), pp. 81–111 Paris, France: Masson [Google Scholar]
- Layer K., Hildrew A. G., Monteith D., Woodward G.In press Long-term variation in the littoral food web of an acidified mountain lake. Global Change Biol. (doi:10.1111/j.1365-2486.2010.02195.x) [Google Scholar]
- Ledger M. E., Hildrew A. G.2001Recolonization by the benthos of an acid stream following a drought. Arch. Hydrobiol. 152, 1–17 [Google Scholar]
- Ledger M. E., Harris R. M. L., Armitage P. D., Milner A. M.2008Disturbance frequency influences patch dynamics in stream benthic algal communities. Oecologia 155, 809–819 (doi:10.1007/s00442-007-0950-5) [DOI] [PubMed] [Google Scholar]
- Liboriussen L., et al. 2005Global warming: design of a flow-through shallow lake mesocosm climate experiment. Limnol. Oceanogr. Methods 3, 1–9 [Google Scholar]
- Lotter A. F., Birks H. J. B.2003Holocene sediments of Sägistalsee, a small lake at the present-day tree-line in the Swiss Alps. J. Palaeolimnol. 30, 253–260 (doi:10.1023/A:1026041030967) [Google Scholar]
- Lundberg S., Persson L.1993Optimal body-size and resource density. J. Theoret. Biol. 164, 163–180 (doi:10.1006/jtbi.1993.1146) [Google Scholar]
- Lynch M.1978Complex interactions between natural coexploiters Daphnia and Ceriodaphnia. Ecology 59, 552–564 (doi:10.2307/1936585) [Google Scholar]
- Malmqvist B., Rundle S. D., Covich A. P., Hildrew A. G., Robinson C. T., Townsend C. R.2008Prospects for streams and rivers: an ecological perspective. In Aquatic systems: trends and global perspectives (ed. Polunin N.), pp. 19–29 Cambridge, UK: Cambridge University Press [Google Scholar]
- Masters Z., Petersen I., Hildrew A. G., Ormerod S. J.2007Insect dispersal does not limit the biological recovery of streams from acidification. Aquat. Conserv. Mar. Freshw. Ecosyst. 17, 375–383 (doi:10.1002/aqc.794) [Google Scholar]
- McDonald M., Hershey A. E., Miller M. C.1996Global warming impacts on lake trout in Arctic lakes. Limnol. Oceanogr. 41, 1102–1108 [Google Scholar]
- McKee D., Atkinson D.2000The influence of climate change scenarios on populations of the mayfly Cloeon dipterum. Hydrobiologia 441, 55–62 (doi:10.1023/A:1017595223819) [Google Scholar]
- McKee D., et al. 2003Response of freshwater microcosm communities to nutrients, fish, and elevated temperature during winter and summer. Limnol. Oceanogr. 48, 707–722 [Google Scholar]
- McKie B. G., Cranston P. S., Pearson R. G.2004Gondwanan mesotherms and cosmopolitan eurytherms: effects of temperature on the development and survival of Australian Chironomidae (Diptera) from tropical and temperate populations. Mar. Freshw. Res. 55, 759–768 (doi:10.1071/MF04023) [Google Scholar]
- McKie B. G., Woodward G., Hladyz S., Nistorescu M., Preda E., Popescu C., Giller P. S., Malmqvist B.2008Ecosystem functioning in stream assemblages from different regions: contrasting responses to variation in detritivore richness, evenness and density. J. Anim. Ecol. 77, 495–504 (doi:10.1111/j.1365-2656.2008.01357.x) [DOI] [PubMed] [Google Scholar]
- McMichael A., Woodruff R., Hales S.2006Climate change and human health: present and future risks. Lancet 367, 859–869 (doi:10.1016/S0140-6736(06)68079-3) [DOI] [PubMed] [Google Scholar]
- Meerhoff M., Clemente J. M., De Mello F. T., Iglesias C., Pedersen A. R., Jeppesen E.2007Can warm climate-related structure of littoral predator assemblies weaken the clear water state in shallow lakes? Global Change Biol. 13, 1888–1897 (doi:10.1111/j.1365-2486.2007.01408.x) [Google Scholar]
- Michelutti N., Douglas M. S. V., Smol K.2003Diatom response to recent climatic change in a high arctic lake (Char Lake, Cornwallis Island, Nunavut). Global Planet. Change 38, 257–271 (doi:10.1016/S0921-8181(02)00260-6) [Google Scholar]
- Milly P. C. D., Dunne K. A., Vecchia A. V.2006Global pattern of trends in streamflow and water availability in a changing climate. Nature 438, 347–350 (doi:10.1038/nature04312) [DOI] [PubMed] [Google Scholar]
- Milner A. M., Knudsen E. E., Soiseth C., Robertson A. L., Schell D., Phillips I. T., Magnusson K.2000Colonization and development of stream communities across a 200-year gradient in Glacier Bay National Park, Alaska. Can. J. Fish Aquat. Sci. 57, 2319–2335 (doi:10.1139/cjfas-57-11-2319) [Google Scholar]
- Milner A. M., Fastie C. L., Chapin S., III, Engstrom D. R., Sharman L. C.2007Interactions and linkages among ecosystems during landscape evolution. BioScience 57, 237–247 (doi:10.1641/B570307) [Google Scholar]
- Milner A. M., Robertson A. E., Monaghan K., Veal A. J., Flory E. A.2008Colonization and development of a stream community over 28 years; Wolf Point Creek in Glacier Bay, Alaska. Frontiers Ecol. Environ. 6, 413–419 (doi:10.1890/060149) [Google Scholar]
- Milner A. M., Brown L. E., Hannah D. M.2009Hydroecological effects of shrinking glaciers. Hydrol. Processes 23, 62–77 (doi:10.1002/hyp.7197) [Google Scholar]
- Moss B., et al. 2003How important is climate? Effects of warming, nutrient addition and fish on phytoplankton in shallow lake microcosms. J. Appl. Ecol. 40, 782–792 (doi:10.1046/j.1365-2664.2003.00839.x) [Google Scholar]
- Norby R. J., Cotrufo M. F., Ineson P., O'Neill E. G., Canadell J. G.2001Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127, 153–165 (doi:10.1007/s004420000615) [DOI] [PubMed] [Google Scholar]
- Perkins D. M., Reiss J., Yvon-Durocher G., Woodward G.In press Global change and food webs in running waters. Hydrobiologia (doi:10.1007/s10750-009-0080-7) [Google Scholar]
- Persson L.1985Asymmetrical competition—are larger animals competitively superior? Am. Nat. 126, 261–266 (doi:10.1086/284413) [Google Scholar]
- Persson L.1986Temperature-induced shift in foraging ability in 2 fish species, roach (Rutilus rutilus) and perch (Perca fluviatilis): implications for coexistence between poikliotherms. J. Anim. Ecol. 55, 829–839 [Google Scholar]
- Persson L., Leonardsson K., de Roos A. M., Gyllenberg M., Christensen B.1998Ontogenetic scaling of foraging rates and the dynamics of a size-structured consumer-resource model. Theoret. Popul. Biol. 54, 270–293 (doi:10.1006/tpbi.1998.1380) [DOI] [PubMed] [Google Scholar]
- Persson L., Bystrom P., Wahlstrom E., Nijlunsing A., Rosema S.2000Resource limitation during early ontogeny: constraints induced by growth capacity in larval and juvenile fish. Oecologia 122, 459–469 (doi:10.1007/s004420050967) [DOI] [PubMed] [Google Scholar]
- Petchey O. L.2000Prey diversity, prey composition, and predator population stability in experimental microcosms. J. Anim. Ecol. 69, 874–882 (doi:10.1046/j.1365-2656.2000.00446.x) [DOI] [PubMed] [Google Scholar]
- Petchey O. L., McPhearson P. T., Casey T. M., Morin P. J.1999Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72 (doi:10.1038/47023) [Google Scholar]
- Petchey O. L., Downing A. L., Mittelbach G. G., Persson L., Steiner C. F., Warren P. H., Woodward G.2004Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 104, 467–478 (doi:10.1111/j.0030-1299.2004.13257.x) [Google Scholar]
- Petchey O. L., Beckerman A. P., Riede J. O., Warren P. H.2008Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA 105, 4191–4196 (doi:10.1073/pnas.0710672105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. H.1983The ecological implications of body size (eds Beck E., Birks H. J. B., Connor E. F.), pp. 24–44 Cambridge, UK: Cambridge University Press [Google Scholar]
- Petersen I., Masters Z., Hildrew A. G., Ormerod S. J.2004Dispersal of adult aquatic insects in catchments of differing land use. J. Appl. Ecol. 41, 934–950 (doi:10.1111/j.0021-8901.2004.00942.x) [Google Scholar]
- Rahel F. J., Olden J. D.2008Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. 22, 521–533 (doi:10.1111/j.1523-1739.2008.00950.x) [DOI] [PubMed] [Google Scholar]
- Rawcliffe R., Sayer C. D., Woodward G., Grey J., Davidson T. A., Jones J. I.2010Back to the future: using palaeolimnology to infer long-term changes in shallow lake food webs. Freshw. Biol. 55, 600–613 (doi:10.1111/j.1365-2427.2009.02280.x) [Google Scholar]
- Reiss J., Bridle J. R., Montoya J. M., Woodward G.2009Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 24, 505–514 (doi:10.1016/j.tree.2009.03.018) [DOI] [PubMed] [Google Scholar]
- Robertson A. L., Milner A. M.2006The influence of stream age and environmental variables in structuring meiofaunal assemblages in recently deglaciated streams. Limnol. Oceanogr. 51, 1454–1465 [Google Scholar]
- Rooney N., McCann K., Gellner G., Moore J. C.2006Structural asymmetry and the stability of diverse food webs. Nature 442, 265–269 (doi:10.1038/nature04887) [DOI] [PubMed] [Google Scholar]
- Rouse W. R., et al. 1997Effects of climate change on the freshwaters of arctic and subarctic North America. Hydrol. Process. 11, 873–902 (doi:10.1002/(SICI)1099-1085(19970630)11:8<873::AID-HYP510>3.0.CO;2-6) [Google Scholar]
- Schmid B., Hector A., Huston M. A., Inchausti P., Nijs I., Leadley P. W., Timan D.2002The design and analysis of biodiversity experiments. In Biodiversity and ecosystem functioning: synthesis and perspectives (eds Loreau M., Naeem S., Inchausti P.), pp. 61–75 Oxford, UK: Oxford University Press [Google Scholar]
- Schwartz M. W., Brigham C. A., Hoeksema J. D., Lyons K. G., Mills M. H., van Mantgem P. J.2000Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122, 297–305 (doi:10.1007/s004420050035) [DOI] [PubMed] [Google Scholar]
- Smol J. P., et al. 2005Climate-driven regime shifts in the biological communities of arctic lakes. Proc. Natl Acad. Sci. USA 102, 4397–4402 (doi:10.1073/pnas.0500245102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvari S., Korhola A., Thompson R.2002Lake diatom response to recent Arctic warming in Finnish Lapland. Global Change Biol. 8, 171–181 (doi:10.1046/j.1365-2486.2002.00463.x) [Google Scholar]
- Spooner D. E., Vaughn C. C.2008A trait-based approach to species' roles in stream ecosystems: climate change, community structure, and material cycling. Oecologia 158, 307–317 (doi:10.1007/s00442-008-1132-9) [DOI] [PubMed] [Google Scholar]
- Sterner R. W., Elser J. J.2002‘Ecological stoichiometry: the biology of elements from molecules to the biosphere’ Princeton, NJ: Princeton University Press [Google Scholar]
- Strecker A. L., Cobb T. P., Vinebrooke R. D.2004Effects of experimental greenhouse warming on phytoplankton and zooplankton communities in fishless alpine ponds. Limnol. Oceanogr. 49, 1182–1190 [Google Scholar]
- Sweeney B. W., Schanck J. A.1977Egg development growth and metabolism of Sigara alternata (Say) (Hemiptera-Corixidae) in fluctuating thermal environments. Ecology 58, 265–277 (doi:10.2307/1935602) [Google Scholar]
- Sweeney B. W., Jackson J. K., Newbold J. D., Funk D. H.1992Climate change and the life histories and biogeography of aquatic insects in eastern North America. In Global Climate Change and Freshwater Ecosystems (eds Firth P., Fisher S. G.) pp. 143–176 New York, NY: Springer Verlag [Google Scholar]
- Tuchman N. C., Wetzel R. G., Rier S. T., Wahtera K. A., Teeri J. A.2002Elevated atmospheric CO2 lowers leaf litter nutritional quality for stream ecosystem food webs. Global Change Biol. 8, 163–170 (doi:10.1046/j.1365-2486.2002.00460.x) [Google Scholar]
- Walther G.-R.2010Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019–2024 (doi:10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B. W., Hannah D. M., Moore R. D., Brown L. E., Nobilis F.2008Recent advances in stream and river temperature studies. Hydrol. Process. 22, 902–918 (doi:10.1002/hyp.6994) [Google Scholar]
- Wilcock H. R., Bruford M. W., Nichols R. A., Hildrew A. G.2007Landscape, habitat characteristics and the geographical population structure of two caddisflies. Freshw. Biol. 52, 1907–1929 (doi:10.1111/j.1365-2427.2007.01818.x) [Google Scholar]
- Winder M., Schindler D. E.2004Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85, 2100–2106 (doi:10.1890/04-0151) [Google Scholar]
- Woodward G.2009Biodiversity, ecosystem functioning and food webs in fresh waters: assembling the jigsaw puzzle. Freshw. Biol. 54, 2171–2187 (doi:10.1111/j.1365-2427.2008.02081.x) [Google Scholar]
- Woodward G., Hildrew A. G.2002Differential vulnerability of prey to an invading top predator: integrating field surveys and laboratory experiments. Ecol. Entomol. 27, 732–744 (doi:10.1046/j.1365-2311.2002.00462.x) [Google Scholar]
- Woodward G., Warren P. H.2007Body size and predatory interactions in freshwaters: scaling from individuals to communities. In Body size: the structure and function of aquatic ecosystems (eds Hildrew A. G., Raffaelli D., Edmonds-Brown R.), pp. 98–117 Cambridge, UK: Cambridge University Press [Google Scholar]
- Woodward G., Ebenman B., Emmerson M., Montoya J. M., Olesen J. M., Valido A., Warren P. H.2005Body-size in ecological networks. Trends Ecol. Evol. 20, 402–409 (doi:10.1016/j.tree.2005.04.005) [DOI] [PubMed] [Google Scholar]
- Woodward G., Friberg N., Hildrew A. G.2009The need for scientific rigour in biomonitoring and conservation of fresh waters. Freshwater ecosystems: biodiversity, management and conservation Hauppage, NY: Nova [Google Scholar]
- Woodward G., Christensen J. B., Olafsson J. S., Gislason G. M., Hannesdottir E. R., Friberg N.In press Sentinel systems on the razor's edge: effects of warming on Arctic stream ecosystems. Global Change Biol. (doi:10.1111/j.1365-2486.2009.02052.x) [Google Scholar]
- Wrona F. J., Prowse T. D., Reist J. D., Hobbie J. E., Lévesque L. M. J., Vincent W. F.2006Climate change effects on aquatic biota, ecosystem structure and function. Ambio 35, 359–369 (doi:10.1579/0044-7447(2006)35[359:CCEOAB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Yvon-Durocher G., Jones J. I., Trimmer M., Woodward G., Montoya J. M.2010Warming alters the metabolic balance of ecosystems. Phil. Trans. R. Soc. B 365, 2117–2126 (doi:10.1098/rstb.2010.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick P.1992Stream habitat fragmentation—a threat to biodiversity. Biodiversity Conserv. 1, 80–97 [Google Scholar]